Abstract
Robust protocols to test putative herbicide resistant weed populations at whole plant level are essential to confirm the resistance status. The presented protocols, based on whole-plant bioassays performed in a greenhouse, can be readily adapted to a wide range of weed species and herbicides through appropriate variants. Seed samples from plants that survived a field herbicide treatment are collected and stored dry at low temperature until used. Germination methods differ according to weed species and seed dormancy type. Seedlings at similar growth stage are transplanted and maintained in the greenhouse under appropriate conditions until plants have reached the right growth stage for herbicide treatment. Accuracy is required to prepare the herbicide solution to avoid unverifiable mistakes. Other critical steps such as the application volume and spray speed are also evaluated. The advantages of this protocol, compared to others based on whole plant bioassays using one herbicide dose, are related to the higher reliability and the possibility of inferring the resistance level. Quicker and less expensive in vivo or in vitro diagnostic screening tests have been proposed (Petri dish bioassays, spectrophotometric tests), but they provide only qualitative information and their widespread use is hindered by the laborious set-up that some species may require. For routine resistance testing, the proposed whole plant bioassay can be applied at only one herbicide dose, so reducing the costs.
Keywords: Environmental Sciences, Issue 101, Weed science, resistant biotypes, monitoring, seed germination, weed control, herbicide efficacy, herbicide treatment, resistance level.
Introduction
Herbicides are the most extensively used weed control measure, accounting for up to 50% of the global plant protection market 1. They are relatively cheap tools, avoid labor-intensive and time-consuming soil cultivation practices, and ultimately result in cost-effective, safe and profitable food production 2. However, the great phenological and genetic variability present in many weed species, together with an over-reliance on herbicide use, frequently results in the selection of herbicide-resistant weed populations. The introduction of selective herbicides with a very specific metabolic target 3-5 has dramatically increased the number of resistance cases over the years. To date, 240 weed species (140 dicots and 100 monocots) worldwide have evolved resistance to different herbicide Sites of Action (SoA) 4. This is a major concern for weed management and more in general for sustainable crop production.
Early detection of resistance, based on reliable tests, frequently performed in a greenhouse, is a key step to manage herbicide resistant weeds. Different approaches have been developed according to the aims, required level of accuracy, time and resources available, as well as the weed species considered 6-12. However, when confirmation of the resistance status of a new weed biotype is required (i.e., a group of individuals that share several physiological characteristics, including the ability to survive one or more herbicides belonging to a particular group used at a dose that would normally control them), a robust whole plant bioassay needs to be performed in a controlled environment 4, 11.
A biotype is seldom resistant to just one herbicide. Each biotype is therefore characterized by a certain resistance pattern, i.e., number and type of SoA of the herbicides it is resistant to, and by a given resistance level to each herbicide 13. The early and reliable determination of the pattern of cross or multiple resistance 5, 14 is important for field resistance management.
It is worth mentioning that herbicide resistance has nothing to do with the natural tolerance that some weed species exhibit towards some herbicides, e.g., dicot species vs. ACCase-inhibiting herbicides, monocot species vs. 2,4-D, Equisetum arvense vs. glyphosate.
This paper presents a robust approach for testing putative herbicide resistant biotypes sampled in fields where poor control by herbicide(s) had been reported. Relevant variants to the standard protocols in relation to the weed species involved are presented. The advantages over alternative techniques/protocols based on either whole plant bioassays using only one herbicide dose 15,or treating seeds in Petri dishes 8 are related to the higher reliability and the possibility of inferring the resistance level because of the inclusion of two herbicide doses in the experiments. However, for routine resistance testing, the same methods can be applied at only one herbicide dose, so reducing the costs.
As well as allowing confirmation of the resistance status, the information obtained can be used for both optimizing the following research steps and/or devising sound resistance management strategies.
Protocol
1. Seed Sampling and Storage
Monitor cultivated fields for unjustified poor herbicide performance, i.e., not due to adverse climatic conditions or low quality herbicide treatments.
Collect a seed sample from one species at a time and assign a unique code. Mature seeds are usually collected before crop harvesting from plants that had survived the herbicide treatment(s). Timely monitor to observe if the seeds are shed by mother plant when mature.
Fill in a form for each sample indicating the assigned unique code, name of species, collection date, GPS coordinates, municipality, farmer’s name, field size, infestation level, crop, herbicide(s) used during the season and historical records of the field.
Collect seeds from at least 30 randomly selected plants that are representative of the field infestation. Ensure that the seed sample contains at least 5,000 mature seeds. For an obligate outcrossing weed species (e.g., Lolium spp. or Amaranthus spp.), reduce the number of plants to 10-15, keeping the total number of seeds around 5,000 11.
Sub-sample the field if patches of weeds are scattered over large areas (more than a hectare) as different herbicide resistant biotypes are selected.
Store seeds in unsealed paper bags labeled with the unique code assigned.
Allow moisture to evaporate but do not expose seeds to high temperature (i.e., avoid leaving them in a car under the sun) or to extreme temperature fluctuations to avoid induction of secondary dormancy.
Clean (remove chaff, de-hull seeds, etc.) and store them at ambient temperature in a dry room. After conducting the first resistance tests, store the seeds for long periods of time in a dark room at 4 °C, preferably in vacuum-sealed plastic bags. In this way seeds preserve their viability for a significantly longer time.
2. Seed Dormancy Breaking
NOTE: Seed dormancy provides a flexible and efficient mechanism that enables weeds to adapt and persist in agro-ecosystems. To break dormancy and allow seed germination, different protocols have to be used depending on weed species, i.e., the type of dormancy 16. There are three main ways to remove dormancy:
- Vernalization NOTE: To obtain simultaneous germination and seedling emergence, a period of seed vernalization ranging from a few days to a week is required to remove physiological dormancy from many species: e.g., Amaranthus retroflexus, Chenopodium album, Lolium spp., Avena fatua, Polygonum persicaria, Phalaris paradoxa17-19. A longer period of up to 15 days is required for Papaver rhoeas, Cyperus difformis and Ammania coccinea and up to 30 days for Schoenoplectus mucronatus20.
- Put some deionized water in plastic dishes. Cut two layers of filter paper and soak them in the water, remove any excess. Place the air-dried seeds on the paper. Transfer the plastic dishes to a fridge at 4 °C for the required period of time.
- Scarification NOTE: Some weed species are more recalcitrant to germination than others due to mechanical dormancy, i.e. seed coat characteristics, and necessitate the use of a chemical scarification using sulfuric acid to germinate 21.
- Prepare a beaker with concentrated sulfuric acid (95-98%). Prepare a beaker full of water. Put the seeds in an envelope of non-woven fabric.
- Soak e.g., Echinochloa spp. or Sorghum halepense seeds for 20 min or 5 min, respectively, in concentrated sulfuric acid.
- Take the envelope out of the beaker using a pair of tweezers and put it in the beaker full of water. Open the envelope, put the seeds in a small colander and rinse them thoroughly under running water.
- Soak the seeds for 2 min in chloroform. Rinse the seeds with deionized water and dry them with absorbent paper. Dunk the seeds in 80% sulfuric acid for 5 min.
- Put the seeds in a small colander and rinse them thoroughly under running water.
- Post-harvest seed maturation NOTE: Seeds of other weed species do not germinate at all for a few months after maturity, regardless of the method used to break dormancy.
- Store the seeds for a period of at least 3-4 months at RT and low humidity and then follow the above protocols for dormancy breaking (e.g., Oryza sativa var. sylvatica or P. rhoeas).
3. Seed Germination
- Place seeds to be germinated in plastic dishes containing 0.6% (m/v) agar with 0.1% potassium nitrate (KNO3) added:
- Prepare a solution of agar at 0.6% + 0.1% KNO3 using deionized water. Dissolve the agar in a microwave oven.
- Pour the agar solution into plastic dishes. Cool the substrate and then put in the seeds.
Place plastic dishes in a germination cabinet for about a week with light and temperature conditions depending on the optimum conditions for each weed species. For most winter species, the temperature range is 15/25 °C night/day and 12 hr photoperiod with neon tubes providing a Photosynthetic Photon Flux Density (PPFD) of 15-30 µmol m-2 sec-1. For many summer species, the temperature range is 15/30 °C night/day. Variant: Some species, such as S. halepense, need a heat treatment. Therefore after the scarification, seeds of S. halepense are subjected to the following conditions: cycles of 4 hr at 45 °C and 20 hr at 24 °C for three days in the germination cabinet, and then three days in normal conditions.
4. Seedling Transplanting and Growth
Transplant fifteen to twenty seedlings into plastic trays (325 x 265 x 95 mm) filled with a standard potting mix (60% silty loam soil, 15% sand, 15% agriperlite and 10% peat — by volume). NOTE: Transplanting, instead of direct sowing, allows a uniform stand of plants at the same growth stage to be obtained, which is an important pre-condition to optimize the performance of the herbicide treatment.
Identify each tray with a barcode including all information for the unique identification: population code, herbicide being tested, replicate number and progressive tray number.
Place trays in a heated greenhouse and water plants as needed to maintain the substrate at or near field capacity. NOTE: The growth temperature varies depending on the weed species. Often tests are done during autumn/winter/spring, so light is supplemented using 400 W metal-halide lamps, which provide a PPFD of about 150 µmol m-2 sec-1 and a 12 hr photoperiod 24, 19. Summer weed species with C4 photosynthetic cycle usually require higher light intensity and therefore tests are done in late spring-summer or the supplemented light intensity is about 400 µmol m-2 sec-1 with a 14 hr photoperiod.
- Use a different protocol for some weed species infesting paddy rice, e.g., A. plantago-aquatica, S. mucronatus and C. difformis as described in 22.
- Transplant the seedlings into polystyrene trays with 24 round cells (55 mm diameter, 64 mm deep) filled with 60% silty loam soil, 30% sand and 10% peat (by volume).
- Set the trays in 12 cm deep plastic containers filled with water and battened down by screwed stainless steel rods to prevent them floating (Figure 1).
- Maintain water level in the containers at 1-2 cm below the level of the soil surface and add 1.5 g of copper sulfate to each container (which contains 10-12 L of water) to avoid the proliferation of algae.
5. Herbicide Treatments
- Treatments with pre-emergence herbicides:
- After about three days in the germination cabinet as described in section 3, transplant the germinating seeds into plastic trays containing the substrate described above and cover with a layer of soil (about 1 cm). This is a critical step in order to ensure that seedlings will not emerge due to the herbicide effect rather than an excessive burial depth.
- Take the substrate to field capacity by placing the trays, which have a few holes at the bottom, on saucers filled with water.
- One day after transplanting, treat the trays with the pre-emergence herbicide 25.
- Keep the substrate at or near field capacity by adding water as needed both from above and below by capillarity from the saucer. This procedure favors the permanence of the herbicide at the proper depth (i.e., where the germinating seeds are) for a good treatment efficacy.
- Treatments with post-emergence herbicides:
- Spray plants when they reach the 2-3 leaf stage (i.e., growth stage 12-13 of the Extended BBCH growth scale 26).
- Starting from the day after the treatment, set the irrigation system according to the water requirements of the weed species and the season (e.g., for Echinochloa spp. it delivers water for 3 min 4 times per day, at regular intervals from 9 am to 9 pm). Water is distributed using an automatic sprinkler irrigation system. Variant: glyphosate is applied at plant stage BBCH 14–21.
- Herbicide preparation and distribution. NOTE: All herbicides (pre- and post-emergence) are applied as commercial formulations with recommended surfactants at two doses, recommended field dose (1x) and three times that (3x).
- If needed, prepare the surfactant solution in bulk according to the label instructions; the final concentration is usually expressed as a percentage of the final volume (e.g., 0.3%) or as volume to be distributed per unit area (e.g., 1 L ha-1).
- Use the surfactant solution as solvent for the herbicide (solute) solution in order to keep the right concentration of the active ingredient. Prepare the most concentrated herbicide solution first (3x). Calculate the quantity of commercial product to be dissolved in the surfactant solution (or in deionized water if a surfactant is not necessary) using the following equation: Doseherb = [(Dosefield x Dosemax) x Vfin] / Vdel Where: Doseherb = herbicide dose (ml), Dosefield = herbicide field dose (ml ha-1), Dosemax = maximum dose delivered, Vfin = final volume of the solution (L), Vdel = volume delivered by the bench sprayer (L ha-1).
- Dilute (2:1, v/v) the herbicide solution 3x to prepare the less concentrated one (1x). This procedure reduces the chance of making mistakes when weighing or pipetting the herbicides. Herbicide solution concentration is expressed as volume to be distributed per unit area (L ha-1).
- Start the sequence of treatment with the lower herbicide dose (1x). In this way there is no need to wash the spraying cabinet between two treatments with the same herbicide.
- Distribute the herbicide solution using a precision bench sprayer delivering 300 L ha-1 (±1%), at a pressure of 215 kPa, and a speed of 0.75 m sec-1, with a boom equipped with three flat-fan (extended range) hydraulic nozzles.
- Wash the spraying cabinet twice when the herbicide is changed using bleach 1% (v/v) and then rinse. Variant: glyphosate is applied with a spray volume of 200 L ha-1 27. NOTE: Particular attention has to be paid when highly biological herbicides, such the sulfonylureas sulfometuron or flazasulfuron, are used. In the latter case do one washing with a bleach solution and another with ammonia (2.5% v/v), followed by a careful rinse with water.
6. Collection and Analysis of the Data
- Through a barcode reader, which automatically identifies each tray, record the number of plants that survived the treatment as well as the Visual Estimated Biomass (VEB). Plants are assessed as being dead if they show no active growth regardless of color or other appearance.
- Make the assessment three or four weeks after treatment (WAT) depending on the herbicides tested (e.g., three WAT for ACCase inhibitors and four WAT for ALS inhibitors or glyphosate).
- Evaluate the general treatment efficacy by including a susceptible population (check S) in all experiments, i.e., a population collected in a site which was never or seldom treated with herbicides.
- Express plant survival as percentage of the number of treated plants, counted just before the herbicide treatment, and calculate the standard error (S.E.) per mean value (mean value of the two replicates).
- The VEB is obtained through a visual comparison of plant biomass between treated and not-treated check of the same population 25, 28. A score, ranging from 10 for plants not affected by the herbicide (compared with the not-treated check) to 0 when the plants are clearly dead, is given to each treated tray.
Ascribe populations to four categories based on the results obtained from treatments with two herbicide doses: S when less than 5% of plants survived the herbicide dose 1x, SR when survivors ranged from 5% to 20% at herbicide dose 1x, R when more than 20% of plants survived the herbicide dose 1x and RR when survivors are more than 20% at herbicide dose 1x and more than 10% at herbicide dose 3x 17.
Representative Results
To assess the resistance status of a putative resistant population, it is fundamental to include a susceptible check in the assay in order to verify the herbicide efficacy. The results of a screening test conducted on P. rhoeas populations, a weed infesting wheat fields, are reported in Figure 2, where the efficacy of four post-emergence herbicides on a susceptible check (09-36) and on the suspected resistant one (10-91) are presented. Population 09-36 was completely controlled by the ALS inhibitor iodosulfuron while only one plant survived dose 1x of the other two ALS herbicides tested, florasulam and tribenuron-methyl (Figure 2). Instead, around 60% of the plants of population 10-91 survived both herbicide doses of iodosulfuron and tribenuron-methyl and around 50% survived the 1x dose of florasulam. These results confirm that population 10-91 is highly resistant (RR) to iodosulfuron and tribenuron-methyl and resistant (R) to florasulam. A different response was observed with 2,4-D, a herbicide having a different SoA (i.e., it mimics endogenous auxin), widely used to control dicot weeds in wheat. Only 33% of plants of the S check were killed with this herbicide at dose 1x and the VEB value was > 20% (Figure 2). The lack of efficacy on the check population does not confirm if population 10-91 is resistant to this herbicide or not. In this case it is recommended to perform the experiment again and if the results are confirmed, to change the S population. An example of good control of the susceptible check is reported in Figure 3. The Echinochloa spp. population 07-16L was completely controlled by all herbicides at the recommended field dose (1x). In this case, it is possible to state that population 08-42 is highly cross-resistant to all ALS inhibitors tested, i.e., azimsulfuron, bispyribac-Na, imazamox and penoxsulam. The not-treated check of both populations is reported on the left. These plants are used to calculate the VEB; the amount of biomass is visually estimated tray by tray giving a score of 10 to the not-treated check and then assigning a score from 0, for the replicate without any green plant tissue, to 10 when the biomass is comparable to the not-treated check (Figure 3).
Another example of output is reported in Figure 4, where plant survival of Lolium spp. to glyphosate is shown. The populations tested were collected in wheat-based cropping systems where glyphosate is exerting different selection pressure (i.e., occasionally or 1-2 treatments per year or 3-more times per year). Plants were sprayed at early tillering stage (BBCH 14-21) using two doses of glyphosate: 480 and 1440 g a.e. ha-1, which represent the minimum and maximum recommended field dose for annual weeds (i.e., therophytes) at that growth stage. Data were collected four weeks after treatment. Based on both experiments, seven of the tested populations had a survival rate of 80% or more (populations 343, 383, 384, 403, 509, 512 and 537) at the lowest dose applied but only 50% of plants of populations 403 and 509 survived the highest glyphosate dose. One population had a survival rate of around 40% at 1x dose, whereas just a few plants of population 509 survived the lowest dose and population 508 was fully controlled at any dose. In summary, these experiments showed different levels of resistance to glyphosate that often reflected the field history of herbicide usage. The level of glyphosate resistance was higher for the populations that had been more intensely treated: i.e., the number of field applications per year and number of years of selection pressure was higher.
The protocol described for one herbicide (Figure 4) can be applied to numerous others having different SoA; in this way the resistance pattern of one or more populations can be determined. An example of resistance pattern variability of Echinochloa spp. populations is reported in Table 1. Historical records of herbicide use and crop management obtained from the farmer indicated that ALS-inhibiting herbicides were the selecting agent (i.e., penoxsulam or imazamox). The resistance test was therefore performed with three ALS-inhibiting herbicides (azimsulfuron, penoxsulam and imazamox) belonging to different chemical families, and one herbicide having another SoA, the ACCase-inhibiting herbicide profoxydim. The susceptible check (07-16L) was completely controlled by all herbicides tested (Table 1). Three resistance patterns were detected: thirteen populations resulted as being resistant only to ALS inhibitors, four populations resulted as being resistant only to the ACCase inhibitor profoxydim, and three populations showed a multiple resistance pattern to both the ACCase inhibitor profoxydim and ALS inhibitors. Within each resistance pattern it is possible to distinguish different biotypes, e.g., four populations resistant to ALS inhibitors survived only treatments with the sulfonylurea azimsulfuron while two of the multi-resistant populations survived only treatment with the ALS inhibitor azimsulfuron but were quite controlled by penoxsulam and imazamox.
 Figure 1. Example of C. difformis, a weed species infesting paddy rice, experiment set-up. Polystyrene trays are put into plastic containers and blocked by screwed stainless steel rods to prevent them floating. Water is maintained at 1-2 cm below the level of the soil surface to mimic paddy rice conditions. Photograph was taken four weeks after the treatment. Please click here to view a larger version of this figure.
Figure 1. Example of C. difformis, a weed species infesting paddy rice, experiment set-up. Polystyrene trays are put into plastic containers and blocked by screwed stainless steel rods to prevent them floating. Water is maintained at 1-2 cm below the level of the soil surface to mimic paddy rice conditions. Photograph was taken four weeks after the treatment. Please click here to view a larger version of this figure.
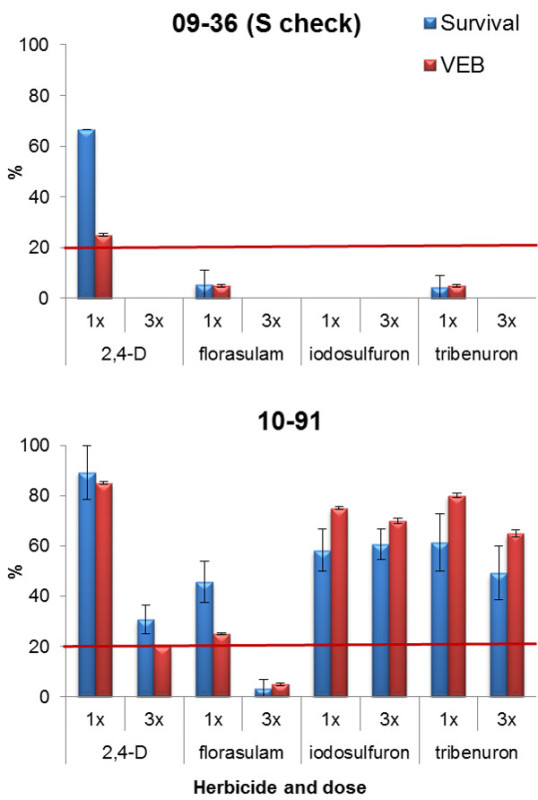 Figure 2. Response of two P. rhoeas populations to post-emergence herbicides. Effect of iodosulfuron, tribenuron, florasulam and 2,4-D tested at the recommended field dose (1x) and at three times that (3x) on plant survival (blue bars) and visual estimation biomass (VEB; red bars) of the susceptible check (09-36) and of a resistant population (10-91). The assessment was done four weeks after the herbicide treatment. Plant survival and VEB are expressed as percentage of the number of treated plants and the VEB of the not-treated checks (%). The horizontal line at 20% of plant survival represents the discriminating threshold between resistant and susceptible populations when plants are treated at dose 1x. Vertical bars represent standard errors calculated on the mean value of the two replicates. Please click here to view a larger version of this figure.
Figure 2. Response of two P. rhoeas populations to post-emergence herbicides. Effect of iodosulfuron, tribenuron, florasulam and 2,4-D tested at the recommended field dose (1x) and at three times that (3x) on plant survival (blue bars) and visual estimation biomass (VEB; red bars) of the susceptible check (09-36) and of a resistant population (10-91). The assessment was done four weeks after the herbicide treatment. Plant survival and VEB are expressed as percentage of the number of treated plants and the VEB of the not-treated checks (%). The horizontal line at 20% of plant survival represents the discriminating threshold between resistant and susceptible populations when plants are treated at dose 1x. Vertical bars represent standard errors calculated on the mean value of the two replicates. Please click here to view a larger version of this figure.
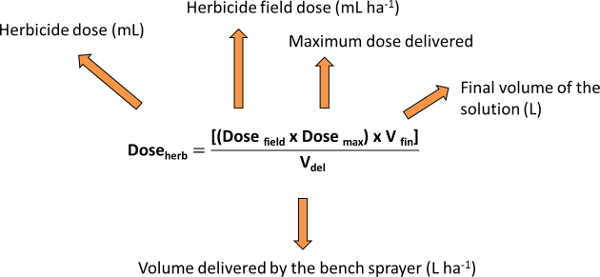 Figure 3. Visual results of a screening on two Echinochloa spp. populations. The susceptible check, 07-16L (S), and the resistant population, 08-42, were tested with four ALS inhibitors (reported on the right) at two doses, 1x and 3x, (reported at the bottom). For the S check only results of dose 1x are reported, because all plants were already controlled at that dose. Three examples of VEB score are reported in red: 0 = all plants dead, 10 = all plants survived and biomass is comparable to the not-treated (NT) check (reported on the left), 5 = biomass is about half of that in a tray of the not-treated check. Please click here to view a larger version of this figure.
Figure 3. Visual results of a screening on two Echinochloa spp. populations. The susceptible check, 07-16L (S), and the resistant population, 08-42, were tested with four ALS inhibitors (reported on the right) at two doses, 1x and 3x, (reported at the bottom). For the S check only results of dose 1x are reported, because all plants were already controlled at that dose. Three examples of VEB score are reported in red: 0 = all plants dead, 10 = all plants survived and biomass is comparable to the not-treated (NT) check (reported on the left), 5 = biomass is about half of that in a tray of the not-treated check. Please click here to view a larger version of this figure.
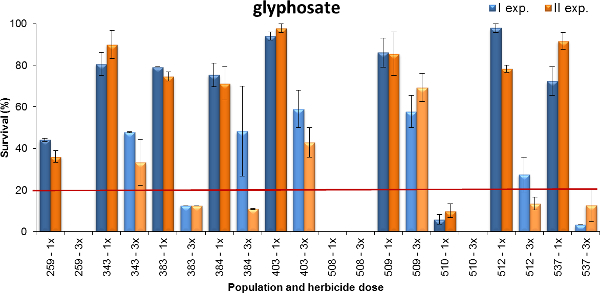 Figure 4. Percentage of plant survival for ten Lolium spp. populations tested with glyphosate. Plant survival recorded in two experiments (blue bars and orange bars for exp. I and II, respectively). Data are expressed as a percentage (%) of the number of treated plants. Two susceptible checks were fully controlled at dose 1x and are therefore not reported in the graph. Two doses were tested, the minimum (1x = 480 g a.e. ha-1) and maximum (3x = 1440 g a.e. ha-1) doses reported on the product label. The horizontal line at 20% of plant survival represents the discriminating threshold between resistant and susceptible populations when plants were treated at dose 1x. Vertical bars represent standard errors calculated on the mean value of the two replicates. Please click here to view a larger version of this figure.
Figure 4. Percentage of plant survival for ten Lolium spp. populations tested with glyphosate. Plant survival recorded in two experiments (blue bars and orange bars for exp. I and II, respectively). Data are expressed as a percentage (%) of the number of treated plants. Two susceptible checks were fully controlled at dose 1x and are therefore not reported in the graph. Two doses were tested, the minimum (1x = 480 g a.e. ha-1) and maximum (3x = 1440 g a.e. ha-1) doses reported on the product label. The horizontal line at 20% of plant survival represents the discriminating threshold between resistant and susceptible populations when plants were treated at dose 1x. Vertical bars represent standard errors calculated on the mean value of the two replicates. Please click here to view a larger version of this figure.
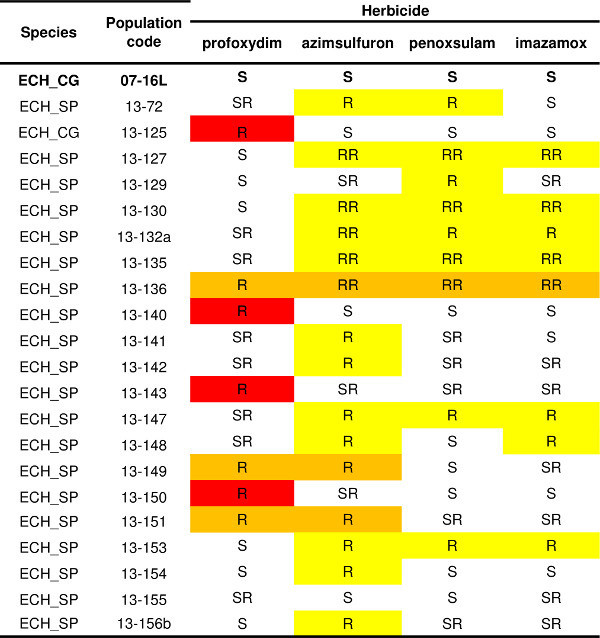 Table 1. Resistance status of twenty one populations of Echinochloa spp. Susceptible check (07-16L) is reported in bold. Resistance levels are reported for each of the four herbicides tested (one ACCase inhibitor, profoxydim, and three ALS inhibitors, azimsulfuron, penoxsulam and imazamox) according to four categories: S = less than 5% of plants survived the herbicide dose 1x, SR = plant survival ranged from 5% to 20% at herbicide dose 1x, R = more than 20% of plants survived the herbicide dose 1x, RR = plant survival was more than 20% at herbicide dose 1x and more than 10% at dose 3x. Different resistance patterns are highlighted: red = resistance only to ACCase inhibitor, yellow = resistance only to ALS inhibitor(s), orange = resistance to the ACCase inhibitor and to at least one ALS inhibitor.
Table 1. Resistance status of twenty one populations of Echinochloa spp. Susceptible check (07-16L) is reported in bold. Resistance levels are reported for each of the four herbicides tested (one ACCase inhibitor, profoxydim, and three ALS inhibitors, azimsulfuron, penoxsulam and imazamox) according to four categories: S = less than 5% of plants survived the herbicide dose 1x, SR = plant survival ranged from 5% to 20% at herbicide dose 1x, R = more than 20% of plants survived the herbicide dose 1x, RR = plant survival was more than 20% at herbicide dose 1x and more than 10% at dose 3x. Different resistance patterns are highlighted: red = resistance only to ACCase inhibitor, yellow = resistance only to ALS inhibitor(s), orange = resistance to the ACCase inhibitor and to at least one ALS inhibitor.
Discussion
Several steps within the protocols are critical for a successful assessment of herbicide resistance in a population: 1) seeds should be collected when mature from plants that had survived the herbicide treatment(s). Maturation of the seeds on the mother plant is crucial to avoid difficulties in seed germination later; 2) the proper storage of seeds is recommended to avoid proliferation of molds that would prevent germination; 3) seedlings should be treated at the right growth stage, as reported on the label of the herbicide package. Care must be taken so that plants to be treated have reached approximately the same growth stage; 4) the herbicide solutions should be prepared and handled with accuracy so that plants are sprayed with the correct concentration of active ingredient therefore avoiding unverifiable mistakes; 5) after each herbicide treatment it is recommended to thoroughly clean the spraying cabinet and glassware used to prepare the solutions to avoid contamination in the following herbicide treatment, especially when highly biologically active herbicides are involved.
The protocols presented herein can be readily adapted to a wide range of weed species with the necessary modifications according to species and herbicides of interest. In particular, methods to break seed dormancy and for germination are steps that should be reconsidered for each new weed species (see sections 2 and 3). Spraying equipment sometimes needs adjustments when different herbicides are used, e.g., glyphosate requires different settings of the spraying cabinet (see section 5.3) and plants are treated at a later growth stage than with most herbicides.
The time and space required to perform these protocols can be a limiting factor and may not be suitable for routine testing. However, to limit the costs, only one herbicide dose may be used. In this way information can still be obtained on whether the population is resistant. A potential limitation of the approach is related to the fact that no resistant checks are included in the experiments. In fact, due to the large number of biotypes evaluated (i.e., different species and herbicides involved), many checks should be included in each experiment, so increasing the costs.
However, the advantages over alternative techniques/protocols based on whole plant bioassays using only one herbicide dose 15 are related to the higher reliability and the possibility of inferring the resistance level. Quicker and less expensive diagnostic screening tests have also been devised, in vivo or in vitro (e.g., Petri dish bioassays 8, spectrophotometric tests on herbicide target enzyme 29). However, they only provide qualitative information and require some preliminary work, sometimes laborious, to identify the herbicide dose for discriminating between resistant and susceptible plants. The in vitro assays also need to be adapted according to the active ingredient used.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The research was supported by the National Research Council (CNR) of Italy. The authors thank GIRE members for collecting seed samples and are grateful to Alison Garside for revising the English.
References
- Massa D, Kaiser YI, Andújar-Sánchez D, Carmona-Alférez R, Mehrtens J, Gerhards R. Development of a geo-referenced database for weed mapping and analysis of agronomic factors affecting herbicide resistance in Apera spica-venti L. Beauv. (Silky Windgrass) Agronomy. 2013;3(1):13–27. [Google Scholar]
- Powles SB, Shaner DL. Herbicides Resistance and World Grains. Raton, FL: CRC Press LLC; 2001. p. 308. [Google Scholar]
- Sattin M. Proc. BCPC International Congress. UK: Crop Science & Technology; 2005. Herbicide resistance in Europe: an overview; pp. 131–138. [Google Scholar]
- Heap IM. International Survey of Herbicide Resistant Weeds. 2015. [2015 Jan 15]. Available from: http://www.weedscience.org.
- Jasieniuk M, Le Corre V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013;29(11):649–658. doi: 10.1016/j.tig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Heap IM. Identification and documentation of herbicide resistance. Phytoprotection. 1994;75(4):85–90. [Google Scholar]
- Beckie HJ, Heap IM, Smeda RJ, Hall LM. Screening for herbicide resistance in weeds. Weed Technol. 2000;14(2):428–445. [Google Scholar]
- Tal A, Kotoula-Syka E, Rubin B. Seed-bioassay to detect grass weeds resistant to acetyl coenzyme A carboxylase inhibiting herbicides. Crop Prot. 2000;19:467–472. [Google Scholar]
- Boutsalis P. Syngenta Quick-Test: a rapid whole-plant test for herbicide resistance. Weed Technol. 2001;15(2):257–263. [Google Scholar]
- Menchari Y, et al. Weed response to herbicides: regional-scale distribution of herbicide resistance alleles in the grass weed Alopecurus myosuroides. New Phytol. 2006;171(4):861–874. doi: 10.1111/j.1469-8137.2006.01788.x. [DOI] [PubMed] [Google Scholar]
- Burgos NR, et al. Review: confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013;61(1):4–20. [Google Scholar]
- Owen MJ, Martinez NJ, Powles SB. Multiple herbicide-resistant Lolium rigidum. (annual ryegrass) now dominates across the Western Australian grain belt. Weed Res. 2014;54(3):314–324. [Google Scholar]
- Herbicide Resistance Action Committee. Classification of herbicides according to site of action. 2015. [2015 Jan 19]. Available from: http://www.hracglobal.com/Education/ClassificationofHerbicideSiteofAction.aspx.
- Beckie HJ, Tardif FJ. Herbicide cross resistance in weeds) Crop Prot. 2012;35:15–28. [Google Scholar]
- Moss SR, et al. Proc. BCPC Weeds. Brighton: 1999. The occurrence of herbicide-resistant grass-weeds in the United Kingdom and a new system for designating resistance in screening assays; pp. 179–184. [Google Scholar]
- Baskin CC, Baskin JM. Seeds, Ecology, Biogeography and Evolution of dormancy and Germination. San Diego, USA: Academic Press; 1998. pp. 27–42. [Google Scholar]
- Sattin M, Gasparetto MA, Campagna C. Proc. BCPC - Weeds. Brighton, UK: 2001. Situation and management of Avena sterilis. ssp. ludoviciana. and Phalaris paradoxa. resistant to ACCase inhibitors in Italy; pp. 755–762. [Google Scholar]
- Scarabel L, Varotto S, Sattin M. A European biotype of Amaranthus retroflexus. cross-resistant to ALS inhibitors and response to alternative herbicides. Weed Res. 2007;47(6):527–533. [Google Scholar]
- Collavo A, Panozzo S, Lucchesi G, Scarabel L, Sattin M. Characterisation and management of Phalaris paradoxa. resistant to ACCase-inhibitors. Crop Prot. 2011;30(3):293–299. [Google Scholar]
- Scarabel L, Carraro N, Sattin M, Varotto S. Molecular basis and genetic characterisation of evolved resistance to ALS-inhibitors in Papaver rhoeas. Plant Sci. 2004;166(3):703–709. [Google Scholar]
- Panozzo S, Scarabel L, Tranel PJ, Sattin M. Target-site resistance to ALS inhibitors in the polyploid species Echinochloa crus-galli. Pestic. Biochem. Phys. 2013;105(2):93–101. [Google Scholar]
- Sattin M, Berto D, Zanin G, Tabacchi M. Proc. BCPC. Weeds. Brighton: 1999. Resistance to ALS inhibitors in rice in north-western Italy; pp. 783–790. [Google Scholar]
- Scarabel L, Berto D, Sattin M. Aspects of Applied Biology. Vol. 69. Wellesbourne, UK: 2003. Dormancy breaking and germination of Alisma plantago-aquatica. and Scirpus mucronatus; pp. 285–292. [Google Scholar]
- Collavo A, Strek H, Beffa R, Sattin M. Management of an ACCase-inhibitor-resistant Lolium rigidum. population based on the use of ALS inhibitors: weed population evolution observed over a 7 years field-scale investigation. Pest Manag. Sci. 2013;69(2):200–208. doi: 10.1002/ps.3449. [DOI] [PubMed] [Google Scholar]
- Scarabel L, Panozzo S, Savoia W, Sattin M. Target-site ACCase-resistant Johnsongrass (Sorghum halepense). selected in summer dicot crops. Weed Technol. 2014;28(2):307–315. [Google Scholar]
- Hess M, Barralis H, Bleiholder H, Buhur L, Eggers T, Hack H, Strauss R. Use of the extended BBCH scale - general for the description of the growth stages of mono- and dicotyledonous weed species. Weed Res. 1997;37(6):433–441. [Google Scholar]
- Collavo A, Sattin M. First glyphosate-resistant Lolium. spp. biotypes found in a European annual arable cropping system also affected by ACCase and ALS resistance. Weed Res. 2014;54(4):325–334. [Google Scholar]
- Scarabel L, Cenghialta C, Manuello D, Sattin M. Monitoring and management of imidazolinone-resistant red rice (Oryza sativa. L., var. sylvatica.) in Clearfield® Italian paddy rice. Agronomy. 2012;2(4):371–383. [Google Scholar]
- Zelaya IA, Anderson JAH, Owen MDK, Landes RD. Evaluation of spectrophotometric and HPLC methods for shikimic acid determination in plants: Models in glyphosate-resistant and-susceptible crops. J. Agric. Food Chem. 2011;59(6):2202–2212. doi: 10.1021/jf1043426. [DOI] [PubMed] [Google Scholar]


