Abstract
Diseases affecting the integrity of spinal cord motor neurons are amongst the most debilitating neurological conditions. Over the last decades, the development of several animal models of these neuromuscular disorders has provided the scientific community with different therapeutic scenarios aimed at delaying or reversing the progression of these conditions. By taking advantage of the retrograde machinery of neurons, one of these approaches has been to target skeletal muscles in order to shuttle therapeutic genes into corresponding spinal cord motor neurons. Although once promising, the success of such gene delivery approach has been hampered by the sub-optimal number of transduced motor neurons it has so far shown to yield. Motor end plates (MEPs) are highly specialized regions on the skeletal musculature that are in direct synaptic contact to the spinal cord α motor neurons. In this regard, it is important to note that, so far, the efforts to retrogradely transfer genes into motor neurons were made without reference to the location of the MEP region in the targeted muscles. Here, we describe a simple protocol 1) to reveal the exact location of the MEPs on the surface of skeletal muscles and 2) to use this information to guide the intramuscular delivery and subsequent optimal retrograde transport of retrograde tracers into motor neurons. We hope to utilize the results from these tracing experiments in further studies into investigating retrograde transport of therapeutic genes to spinal cord motor neurons through the targeting of MEPs.
Keywords: Neurobiology, Issue 101, Motor neurons, motor end plates, retrograde transport, striated muscles, mouse, rat, hindlimb, forelimb
Introduction
The loss of control of voluntary movement that results from neurological conditions such as motor neuron disease and spinal- as well as Duchenne muscular atrophy is a debilitating condition that has high and long lasting impact on the every day life of affected individuals. Over the last decade, research efforts aiming to stop or at least delay the deleterious effects of these neuromuscular diseases has been a priority for many clinicians and scientist around the world. In this regard, the recent generation of animal models that mimic these neuromuscular diseases has been instrumental in obtaining fundamental insights into the physiological mechanisms underlying the development and progression of these conditions 1-13. Treatment of these neuromuscular disease requires direct access to the spinal cord and can be achieved by spinal cord injections 14,15. Recent advances in gene therapy have also targeted the striated muscles of the upper and lower limbs to shuttle therapeutic genes to the corresponding α motor neurons that are located within the ventral horn of the spinal cord 1,9-13. However, this once promising strategy has failed to improve the outcome of these neurological conditions. While it is fair to conclude that these poor outcomes could be, at least partly, be attributable to the low efficacy of these protective genes, one cannot exclude the low efficacy of these gene delivery methods.
Motor end plates (MEPs) are specialized regions of the skeletal myofibres that are indented by the axon terminals of large peripheral motor fibres originating from α motor neurons. Together, the peripheral nerve fibre endings and the MEPs form the neuromuscular junction, i.e., the site where synaptic impulses are triggered by the anterograde release of the neurotransmitter, acetylcholine. Importantly, the relationship between peripheral nerve fibres and the MEPs is bi-directional, although different motors are responsible for the transport of molecules and organelles towards as well as away from the neuron somata 16-18. In light of these anatomical considerations, MEPs appear to be the targets of choice for the delivery and subsequent retrograde transport of genetic material to the corresponding motor neurons. In this context, it is not surprising that the success of motor neuron transduction greatly depends on the distance between the intramuscular injection of viral vectors and the muscle’s MEPs 19-20. Surprisingly, however, the exact location of the MEP zones on the myofibres of the laboratory rat and mouse, the two species of choice to model neuromuscular diseases, were not available until recently.
We have produced comprehensive maps of the MEP region for several forelimb muscles in the rat and the mouse 21-22. More recently, we have shown the details of the organization of the MEP region for several muscles of the mouse hindlimb 23 and we are currently analysing the features of the MEPs on the rat hindlimb. In our hands, intramuscular injections of retrograde tracers directed to the entire MEP zones in these muscles gave rise to more labelled motor neurons that are spanning more spinal cord segments than previously reported. Here we present the protocol that has been developed over the last few years to reveal the location of the MEPs on the external surface as well as throughout the depth of hindlimb and forelimb muscles in both the mouse and the rat.
Protocol
All experimental procedures described here complied with the Animal Care and Ethics Committee of UNSW Australia and were performed in accordance with the National Health and Medical Research Council of Australia regulations for animal experimentation. All procedures in this protocol should be performed in accordance with the requirement of the relevant Animal Care and Ethics Committee.
1. Acetylcholinesterase Histochemical Staining
- Prepare the Acetylcholinesterase reaction mixture
- Add 290 mg of Acetylthiocholine iodide to 200 ml of 0.1 M phosphate buffer (PB). Mix with a magnetic stirrer at 900 rpm.
- Add 600 mg of glycine under continuous stirring.
- Slowly add 420 mg of copper sulphate to the reaction mixture under continuous stirring until the product is completely dissolved.
Remove the skin on the animal carcass (obtained through tissue sharing) by making incisions into the skin of a perfused rat or mouse, grasping the skin from the neck area and pulling it past its feet. Ensure that the fascia covering each muscle is either removed or significantly perforated to ensure ample exposure of the muscle fibres to the reaction solution.
Immerse the entire body or limb of interest into the reaction mixture and incubate O/N at 4 °C.
Wash the carcass for 2 min in distilled water. Note: At this stage, the muscles exhibit a blue coloration and the motor end plates (MEPs) can be seen as white dots.
Expose the carcass to a 10% ammonium sulphide solution for 3-5 sec. Note: The muscle fibres will rapidly turn brown and the MEPs will be observable as black speckled dots. If the reaction occurs too quickly, and the muscle fibres are stained too dark to distinguish between the MEPs and the muscle fibres try using lower concentrations of ammonium sulphide solution (e.g., 5%). The reaction time to get the optimal contrast between the MEPs and the muscle fibres varies from one sample to the other. Therefore it is difficult to define the exact exposure time to the reagent. The reaction should be closely monitored and stopped when the contrast is deemed adequate.
Wash the carcass two times, with agitation in distilled water and gently pat the carcass dry.
Photograph both the lateral and medial aspects of the limb, ensuring that the MEPs for all the muscles of interest are captured.
2. Intramuscular Injections at the Motor End Plates
Pull glass micropipettes with a micropipette puller (the use of graded micropipettes with plungers is recommended). With the help of a dissecting microscope, break the tips of the micropipettes with a pair of forceps such that the internal diameter of the lumen of the micropipette is approximately 0.5 mm.
Ensure that all surgical instruments used are freshly autoclaved and that the surgical area is sterile.
Fill the micropipettes with Fluoro-Gold (5% in distilled water).
Induce anaesthetise in the animal in an induction chamber with Isofluorane (4% in O2). Check for righting reflex and ensure its absence before removing it from the induction chamber. Secure a nose cone over the snout of the rat and deliver Isofluorane (2% in O2) for maintenance of anaesthesia. Pinch the animal’s toes and gently touch the animal’s eye to ensure that both the pedal withdrawal and the corneal reflexes are absent. Note: A mixture of ketamine and xylazil can also be used (80 and 10 mg/kg respectively, delivered intraperitoneally). Ketamine is a Schedule 8 (“Controlled”) drug and necessary protocols associated with the purchase, use, storage and disposal of the drug will have to be adhered to.
Apply eye lubricant to prevent drying of the eyes during the course of the procedure.
Shave the targeted limb and use gauze to wipe the shaved area with three alternating scrubs of chlorhexidine and 70% alcohol. Transfer the animal to the surgical area.
Place the animal on a clean underpad and position it appropriately to ensure good access to the targeted muscle(s).
Use a pair of forceps with tooth grips to lift the skin over the targeted muscle away from the underlying musculature and make an incision in the skin with surgical scissors. Ensure that the incision is large enough to completely expose the muscle(s) of interest. Ensure that there is minimal disruption to the fascia.
Use the MEP photographs to mentally transpose the location and shape of the MEP region onto the muscle(s). Note: A fine felt marker could assist to reproduce the MEP region from the photograph onto the muscle(s) of interest.
Perform multiple injections along the full length of the MEP region (for Fluoro-Gold, 3 to 4 injections of 1-2 µl each should be sufficient). Gently wipe the muscle(s) to remove any seepage.
Bring the two ends of the incised skin close together with blunt forceps and close the wound with surgical clips. Infiltrate 0.1 ml Bupivacaine (0.5% in water) (or other local anaesthetics) along the entire span of the wound.
Turn the anaesthetic machine off and monitor the animal until it has is fully recovered from anaesthesia.
Wait for 14 days between administration of intra-muscular injections and the perfusion of the animals.
3. Perfusions
- Administer a lethal dose of pentobarbitone sodium solution (e.g. Lethabarb; 150 mg/kg) to the animal by intraperitoneal injection.
- Closely monitor the animal until a deep level of anaesthesia is reached, as confirmed by the absence of the pedal withdrawal and the corneal reflexes.
Position the animal on its back on a dissecting board placed over a perfusion sink.
Use a pair of forceps with tooth grips to lift the skin covering the base of the sternum. Make a small skin incision with a pair of strong surgical scissors immediately below the sternum and then use the forceps to grip the xiphoid cartilage of the sternum. While holding the xiphoid cartilage, enlarge the incision on both sides of the chest cavity, from the sternum to the armpits.
- Cut the diaphragm to expose the heart.
- Inject 0.ml of heparin directly into the apex of the heart to prevent coagulation of the blood.
- Cut the apex to allow the insertion of a cannula into the left ventricle, and then quickly clamp the cannula in place with the aid of a haemostat.
- Make an incision in the right atrium with a pair of fine scissors and immediately start the peristaltic pump. Perfuse with 0.1 M PB until the liquid flowing out of the atrium is nearly free of blood and the color of the liver becomes light brown. Then, perfuse with a solution of paraformaldehyde (4% in 0.1 M PB) until the entire body of the animal becomes rigid.
4. Cervical Spinal Cord Dissection and Preparation for Histology
Lay the animal carcass on its abdomen.
Cut the skin at the body’s midline with a scalpel and reflect it from the base of the skull to the level of the iliac bone.
Cut through and reflect the paravertebral muscles to expose the dorsal aspect of the vertebral column.
- Identify the bony spinous process of Atlas, the second cervical segment (i.e., C2), and remove it with a pair of surgical rongeurs or forceps to locate the corresponding underlying dorsal root.
- Identify the right C2 root by coloring it with a permanent felt marker.
- Remove the next vertebrae one by one and mark the right dorsal roots with alternating colors (i.e., C2, C4, C6, and C8 can be colored in green and C3, C5, C7, T1 can be colored in blue).
- Use a small surgical needle (e.g., 30 G gauge needle) to gently pierce through the dura covering the lower end of the spinal cord. With the bevel of the needle facing up, lift the dura away from the cord while moving the needle rostrally to make a longitudinal slit.
- Reflect the dura.
- Cut the spinal cord transversally into one- or two-segment blocks with a new scalpel blade.
- Leave the spinal cord segments in situ and, for each block, carefully make a small fiducial mark on the left side of each block with the scalpel blade half way between two adjacent roots. Ensure that the fiducial mark is deep enough through the tissue as to be visible from the ventral aspect of the blocks. Make the fiducial mark at an angle of approximately 45°, pointing either anteriorly or posteriorly with respect to animal anatomy, to aid in the orientation of the tissue segment following dissection.
Collect the individual blocks into small, clearly labelled bottles containing a solution of paraformaldehyde (4% in 0.1 M PB) and leave at RT O/N.
Transfer the blocks into clean, clearly labelled bottles containing a solution of sucrose (30% in 0.1 M PB) and keep at 4 °C for at least two days.
Place the segments in cryo-moulds and covered them with tissue freezing medium. For longitudinal histological preparation, orient the blocks with the dorsal aspect facing up and use the fiducial mark to orient the block in the cryo-moulds.
Freeze the cryo-moulds at -20 °C and cut with a cryostat in 50 µm-thick sections. Note: The sections can be directly mounted on adhesion microscope slides and left to dry O/N. Alternatively, the sections can be floated in 48-well plates filled with 0.1 M PB and subsequently mounted using the fiducial mark to orient the tissue sections on the slides.
5. Lumbar Spinal Cord Dissection and Preparation for Histology
Lay the animal carcass on it’s back.
Perform a midline incision along the abdomen of the animal and remove the viscera. Dissect out the muscles of the posterior abdominal wall to expose the ventral aspect of the vertebral column.
- Locate the short caudal-most rib and its adjoining T13 vertebra where the T13 ventral root exits the bone.
- Consecutively remove one by one the two or three vertebrae rostral to T13 (i.e., T12, T11 and, if needed, T10) with fine surgical rongeurs to follow the T13 ventral root until its point of entry in the ventral aspect of the ventral cord and mark it with a permanent felt marker.
- From this point on, repeat the same procedure to identify the location of the ventral root entry points for L1, L2, L3, L4, L5, L6 and S1, coloring them with alternating colors.
Follow the same procedure as described in Section 4.5-4.10 for lumbar spinal cord dissection.
Representative Results
Acetylcholinesterase histochemical staining reveals the location of the motor end plates across the width of the muscles. Figure 1 illustrates the results of such staining performed on a whole rat forelimb. It is suggested to optimise the concentration of the ammonium sulphide solution (e.g., 5-7% instead of 10%) as well as the time the specimen is immersed in the solution if the non-specific background staining on the muscles fibres is too excessive. Figure 2 illustrates a column of labelled motor neurons in the cervical spinal cord after an injection of retrograde neuronal tracer along the length of the motor end plate region of a mouse triceps brachii muscle. The retrogradely labelled motor neurons typically form a longitudinal column that extends across multiple segments of the spinal cord. Figure 3 illustrates the different numbers of labelled spinal cord motor neurons that was obtained through selective Fluoro-Gold targeting of the motor end plate region in triceps brachii. Maximal uptake was observed following delivery into the entire span of the MEP region, as opposed to targeting specific compartments of the MEP region.
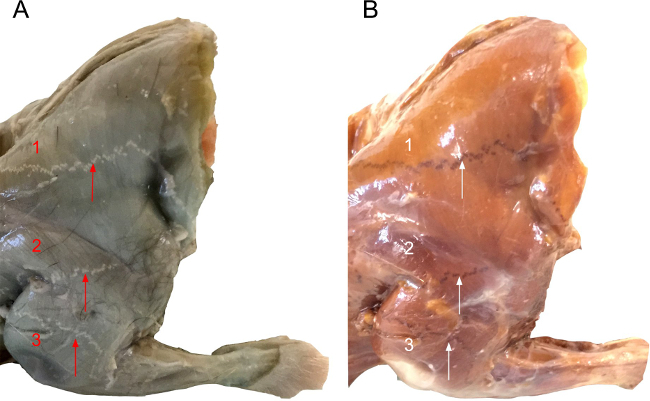 Figure 1: View of the Lateral Forelimb of the Rat after Acetylcholinesterase Histochemical Staining for the Motor End Plates before (A) and after (B) Exposure to the Ammonium Sulphide Solution. In (A), the motor end plates are apparent as white speckled dots that form a continuous line that crosses the entire width of the muscle while the muscle fibres adopt a green-blue hue. In (B), the same forelimb is presented after immersion in the ammonium sulphide solution. After development in the ammonium sulphide solution, the motor end plates turn black over the brown muscle fibres. The arrows in both parts of the figures point to the location of the motor end plates for (1) Acromiotrapezius, (2) Spinodeltoideus and (3) Triceps Brachii.
Figure 1: View of the Lateral Forelimb of the Rat after Acetylcholinesterase Histochemical Staining for the Motor End Plates before (A) and after (B) Exposure to the Ammonium Sulphide Solution. In (A), the motor end plates are apparent as white speckled dots that form a continuous line that crosses the entire width of the muscle while the muscle fibres adopt a green-blue hue. In (B), the same forelimb is presented after immersion in the ammonium sulphide solution. After development in the ammonium sulphide solution, the motor end plates turn black over the brown muscle fibres. The arrows in both parts of the figures point to the location of the motor end plates for (1) Acromiotrapezius, (2) Spinodeltoideus and (3) Triceps Brachii.
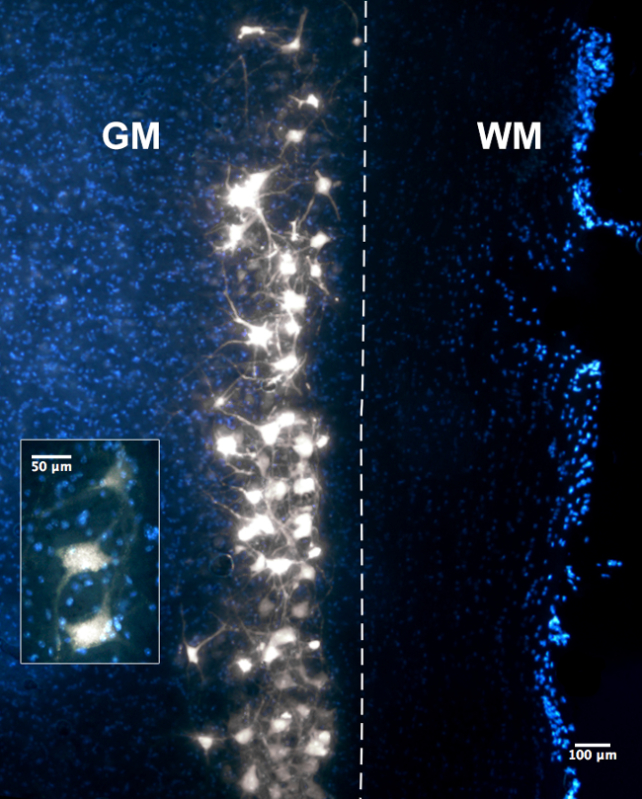 Figure 2: Photomicrographs of 50 µm Longitudinal Section through the Ventral Horn of the Spinal Cord at Cervical Levels Revealing Fluoro-Gold-labelled Motor Neurons. WM represents the white matter and GM represents the grey matter of the spinal cord. The dashed line indicates the boundary between the grey and white matter within the spinal cord section observed in the figure. The photomicrograph was taken with a 10X objective under the DAPI filter whereas the inset was taken with the 20X objective under the same filter. In both panels, Fluoro-Gold labelling can be observed in the motor neuron cytoplasm as well as proximal axon/dendritic processes. Figure as originally published in Tosolini et al. (2013) Targeting the Full Length of the Motor End Plate Regions in the Mouse Forelimb Increases the Uptake of Fluoro-Gold into Corresponding Spinal Cord Motor Neurons Front. Neurol. 4:58. doi: 10.3389/fneur.2013.00058
Figure 2: Photomicrographs of 50 µm Longitudinal Section through the Ventral Horn of the Spinal Cord at Cervical Levels Revealing Fluoro-Gold-labelled Motor Neurons. WM represents the white matter and GM represents the grey matter of the spinal cord. The dashed line indicates the boundary between the grey and white matter within the spinal cord section observed in the figure. The photomicrograph was taken with a 10X objective under the DAPI filter whereas the inset was taken with the 20X objective under the same filter. In both panels, Fluoro-Gold labelling can be observed in the motor neuron cytoplasm as well as proximal axon/dendritic processes. Figure as originally published in Tosolini et al. (2013) Targeting the Full Length of the Motor End Plate Regions in the Mouse Forelimb Increases the Uptake of Fluoro-Gold into Corresponding Spinal Cord Motor Neurons Front. Neurol. 4:58. doi: 10.3389/fneur.2013.00058
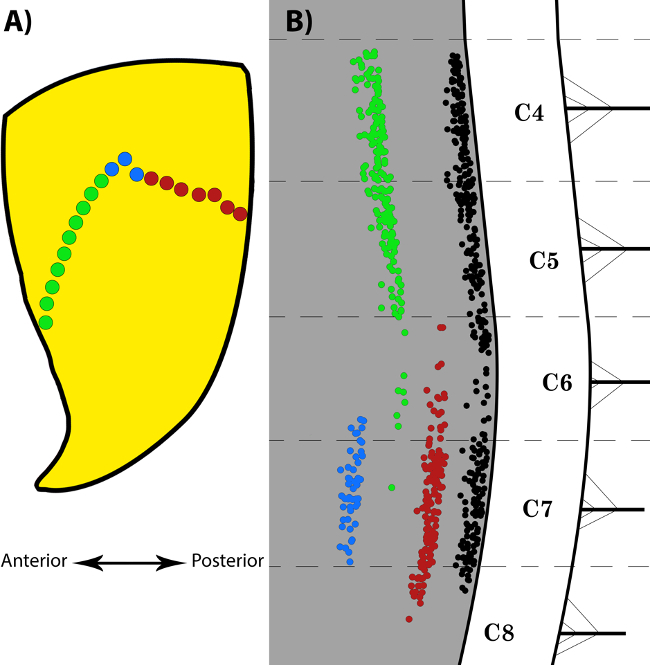 Figure 3:Selective Fluoro-Gold Targeting of the Motor End Plate Region in the Triceps Brachii and the Resulting Labelling in the Spinal Cord Motor Neurons. (A) is a schematic representation of the location of the motor end plates on triceps brachii fibres. The three different sections of the MEP region that were targeted individually are represented by the green, red and blue dots. The green and red dots represent the anterior and posterior halves of the entire MEP region respectively, whilst the blue dots represent the location of the bolus injection of FG into the belly of the muscle. The double-headed arrow orientates the muscle in an anterior-posterior axis. (B) is a composite diagram illustrating the labelling obtained following selective targeting of the different sections of the MEP region of the triceps brachii, as indicated in (A). Each dot in (B) represents a single labelled motor neuron. The black column is representative of typical labelling observed following injection of FG into the entire span of the MEP region of the muscle. The red motor neuron column is obtained following FG injections into the posterior half of the muscle, whilst the green motor neuron column is obtained following FG injections into the anterior half of the muscle. Injections restricted to the “belly” of the muscle yielded motor neurons in only a small number of spinal cord segments, as demonstrated by the blue column. Figure as originally published in Tosolini et al. (2013) Targeting the Full Length of the Motor End Plate Regions in the Mouse Forelimb Increases the Uptake of Fluoro-Gold into Corresponding Spinal Cord Motor Neurons Front. Neurol. 4:58. doi: 10.3389/fneur.2013.00058
Figure 3:Selective Fluoro-Gold Targeting of the Motor End Plate Region in the Triceps Brachii and the Resulting Labelling in the Spinal Cord Motor Neurons. (A) is a schematic representation of the location of the motor end plates on triceps brachii fibres. The three different sections of the MEP region that were targeted individually are represented by the green, red and blue dots. The green and red dots represent the anterior and posterior halves of the entire MEP region respectively, whilst the blue dots represent the location of the bolus injection of FG into the belly of the muscle. The double-headed arrow orientates the muscle in an anterior-posterior axis. (B) is a composite diagram illustrating the labelling obtained following selective targeting of the different sections of the MEP region of the triceps brachii, as indicated in (A). Each dot in (B) represents a single labelled motor neuron. The black column is representative of typical labelling observed following injection of FG into the entire span of the MEP region of the muscle. The red motor neuron column is obtained following FG injections into the posterior half of the muscle, whilst the green motor neuron column is obtained following FG injections into the anterior half of the muscle. Injections restricted to the “belly” of the muscle yielded motor neurons in only a small number of spinal cord segments, as demonstrated by the blue column. Figure as originally published in Tosolini et al. (2013) Targeting the Full Length of the Motor End Plate Regions in the Mouse Forelimb Increases the Uptake of Fluoro-Gold into Corresponding Spinal Cord Motor Neurons Front. Neurol. 4:58. doi: 10.3389/fneur.2013.00058
Discussion
Intramuscular targeting and subsequent retrograde transfer of therapeutic transgenes to the corresponding α motor neurons for the experimental treatment of neuromuscular condition is not a new strategy. For instance, this delivery method has been used to delay neuromuscular degeneration at different stages of the ALS progression in SOD1 mice and rats 1,9-12 as well as in mice with SMA 13. Whilst promising, the efficacy of these gene therapy scenarios has been limited. In this regard, we propose that the uptake of the transgenes by spinal cord motor neurons could be improved by the selective targeting of the motor end plates on the muscle fibres. It is important to note that, in the above-mentioned studies, the intramuscular injections of viral vectors were performed without reference to the location of the MEP region in the targeted muscles.
Throughout our analyses of the distribution of the motor end plates (MEPs) of the hindlimb and the forelimb, a few principles have emerged. First, the MEP region spans across the entire width of a muscle. Moreover, the MEPs are consistently aligned orthogonally with regards to the direction of the myofibres. Notably, the MEP region is not always located within the thickest portion of the muscle, an area often referred to as the ‘belly’ and that is often the target of bolus intramuscular injections. It is worth noting that the current analyses were conducted on C57Bl6 mouse 22-23 and the Long Evans rat 21 and it was found that the distribution of the MEPs is conserved within each of these two strains of animals. However, whether or not the MEP distribution is conserved between different strains of animals within the same species remains to be documented.
Originally, we have documented the location of the MEP region for several muscles of the forelimb and hindlimb in order to guide small, multiple intramuscular injections of the retrograde neuronal tracer Fluoro-Gold. These MEP maps can be used to shuttle transgenes into motor neurons. In such instance, however, it is important to adjust the volumes of injections according to the type of virus used (e.g., adenovirus, lentivirus, etc.) its serotype as well as its titre. Keeping the fascia intact is a good way to keep the virus confined within the limits of the muscle(s) of interest. Another way to avoid spurious transfection of motor neurons innervating neighbour muscles is to wipe the surface of the muscle gently after the injections. These precautions might be irrelevant in some experimental designs where achieving high levels of transduction could be more relevant than containing it within the pool of motor neurons supplying the targeted muscle. Nevertheless, they are good practice for obvious safety reasons. If more than one isolated muscles need to be injected, it might be wise, if possible, to select a group of muscles that are close to each other to minimize the size of the skin incision. Rats and mice have very loose skin (i.e., not attached to the muscles), so it is relatively easy to target several muscles without having to perform huge skin incisions.
Effective gene therapy for ALS and other neuromuscular conditions using the retrograde machinery of neurons remains a challenge as, ideally, such therapy would transfer beneficial genes across the entire population of spinal cord motor neurons. However, it is not necessary to target all motor neurons to achieve sustainable levels of transduction if the therapeutic gene of interest is coding a secretory protein such as a neurotrophins. Indeed, non-transduced motor neurons in the vicinity of those that have been transduced have been shown to internalize exogenous molecules of neurotrophins through a paracrine mechanism 24. This ‘bystander effect’ greatly enhances the efficacy of intramuscular targeting to shuttle secretory protein-encoding genes into corresponding motor neurons. On the other hand, given the fact that the loss of respiratory control is the ultimate cause of death in ALS, intramuscular injections of therapeutic viral constructs could be performed in intercostal muscles 1 or intrapleurally 25 to protect the motor neurons involved in breathing. By describing a method to easily highlight the location and organization of the MEPs on skeletal muscles, the present protocol will prove to be a valuable tool to explore novel gene therapy strategies for neuromuscular dysfunctions. This protocol also provides a minimally invasive way to preserve the integrity of the neuromuscular junction in these lower motor neuron diseases.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by a National Health & Medical Research Centre (NHMRC) project grant to R.M.
References
- Kaspar BK. Retrograde Viral Delivery of IGF-1 Prolongs Survival in a Mouse ALS Model. Science. 2003;301(5634) doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Ishiyama T, Okada R, Nishibe H, Mitsumoto H, Nakayama C. Riluzole slows the progression of neuromuscular dysfunction in the wobbler mouse motor neuron disease. Brain Res. 2004;1019(1-2):226–236. doi: 10.1016/j.brainres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Parkinson NJ, Davies KE, Talbot K. Survival motor neuron deficiency enhances progression in an amyotrophic lateral sclerosis mouse model. Neurobiol Dis. 2009;34(3):511–517. doi: 10.1016/j.nbd.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. P Natl Acad Sci USA. 2009;106(44):18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Li S, Gregorevic P, Fall BM, Chamberlain JS. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol Ther. 2009;18(1):206–213. doi: 10.1038/mt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch L. Genetic rodent models of amyotrophic lateral sclerosis. J Biomed Biotechnol. 2011;2011(6):348765–11. doi: 10.1155/2011/348765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SJP, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 2013;591(Pt 2):559–570. doi: 10.1113/jphysiol.2012.241679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett CA, et al. DYNC1H1 mutation alters transport kinetics and ERK1/2-cFos signalling in a mouse model of distal spinal muscular atrophy) Brain. 2014;137(Pt 7):1883–1893. doi: 10.1093/brain/awu097. [DOI] [PubMed] [Google Scholar]
- Bordet T, et al. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice) Hum Mol Gen. 2001;10(18):1925–1933. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- Acsadi G, et al. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13(9):1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- Azzouz M, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Suzuki M, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16(12):2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkhelifa-Ziyyat S, et al. Intramuscular scAAV9-SMN Injection Mediates Widespread Gene Delivery to the Spinal Cord and Decreases Disease Severity in SMA Mice. Mol Ther. 2013;21(2):282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Büeler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Gen. 2000;9(5) doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Haenggeli C, et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86(5):1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD. The Molecular Motor Toolbox for Intracellular Transport. Cell. 2003;112(4):467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Fainzilber M. Cell biology: Alternative energy for neuronal motors. Nature. 2013;495(7440):178–180. doi: 10.1038/495178a. [DOI] [PubMed] [Google Scholar]
- Gracies J-M, Lugassy M, Weisz DJ, Vecchio M, Flanagan S, Simpson DM. Botulinum Toxin Dilution and Endplate Targeting in Spasticity. A Double-Blind Controlled Study. Arch Phys Med Rehabil. 2009;90(1):9–16. doi: 10.1016/j.apmr.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Van Campenhout A, Molenaers G. Localization of the motor endplate zone in human skeletal muscles of the lower limb: anatomical guidelines for injection with botulinum toxin. Dev Med Child Neurol. 2011;53(2):108–119. doi: 10.1111/j.1469-8749.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Tosolini AP, Mohan R, Morris R. Targeting the full length of the motor end plate regions in the mouse forelimb increases the uptake of fluoro-gold into corresponding spinal cord motor neurons. Front Neurol. 2013;4:58. doi: 10.3389/fneur.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Tosolini AP, Morris R. Targeting the motor end plates in the mouse hindlimb gives access to a greater number of spinal cord motor neurons: an approach to maximize retrograde transport. Neuroscience. 2014;274:318–330. doi: 10.1016/j.neuroscience.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Baumgartner BJ, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 1998;153(1):102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Zhan W-Z, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS ONE. 2013;8(5):e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]


