Abstract
In June 2008, a 25-year-old man presented to the Royal Adelaide Hospital, Australia, with Candida dubliniensis leptomeningeal disease. On examination the patient had focal neurological deficits, both sensory and motor. He tested negative for HIV and had normal T cell subsets. The patient was treated with 6 weeks of intravenous liposomal amphotericin B and oral flucytosine, and had a full recovery.
Background
To the authors’ knowledge, this is the first reported case of meningeal involvement by C. dubliniensis in an apparently immunocompetent patient. It is also the first reported case of focal neurological deficits being caused by C. dubliniensis leptomeningeal disease.
Case presentation
In June 2008, a 25-year-old man presented to the Royal Adelaide Hospital, Australia, with the first ever case of C. dubliniensis leptomeningeal disease in an immunocompetent host.
The patient presented to the emergency department with progressive pain and neurological deficits. Symptoms began 12 months prior with the onset of intermittent headaches associated with nausea, vomiting and photophobia. The headache was felt as a ‘pressure’ sensation localised to the frontal and occipital regions, and was exacerbated by coughing, vomiting and bending forwards. The headache would typically persist for 3 days, and recur every 2 to 4 weeks.
In addition to headache, 6 months prior to presentation the patient began experiencing stiffness and pain in his cervical and lumbar spine. The pain did not radiate, but was accompanied by the sensation of bilateral leg ‘heaviness’. He reported having one fall when he had tried to run. He also reported numbness in the S1 dermatome of his left foot, which developed into a severe pain over several months.
The patient denied any significant weight loss or profuse night sweats. He also denied bowel or bladder disturbance, seizures, cough, or shortness of breath. For 3 years he worked cleaning shipping containers with exposure to ageing vegetable and meat produce.
The patient had an extensive history of illicit drug use. Since the age of 14, he predominately intravenously injected amphetamines and also used heroin on occasions. He reused needles after dipping them in boiling water, but never tried to ‘sterilise’ them in lime juice (a known culture medium for candida species). He denied sharing injecting equipment. His drug use history also included sublingual buprenorphine, and heavy use of cannabis and tobacco. He had previously consumed large amounts of alcohol, but had stopped drinking 3 years prior to presentation.
Investigations
On examination the patient had a Glasgow Coma Score of 15, and his vital signs were: temperature 37.0°C, blood pressure 130/80 mm Hg, heart rate 70 beats per min, oxygen saturation 98% breathing room air. Pupils were symmetric and reactive and visual acuity was normal, however funduscopy revealed blunting of the temporal borders of the optic discs bilaterally. On examination of the lower limbs he had some focal motor deficits involving the left leg (reduced power to 4/5 on left hip flexion and left knee extension, and absent left knee jerk), as well as sensory impairments on the left side (ataxia on heel-shin testing, and reduced pin-prick sensation in the L4 dermatome). Examination of the right leg and upper limbs was normal.
Blood tests for complete blood examination and serum biochemistry were unremarkable and a vasculitic screen with antinuclear antibody, anti-neutrophil cytoplasmic antibodies, extractable nuclear antigen and dsDNA was negative. C-Reactive Protein was less than four.
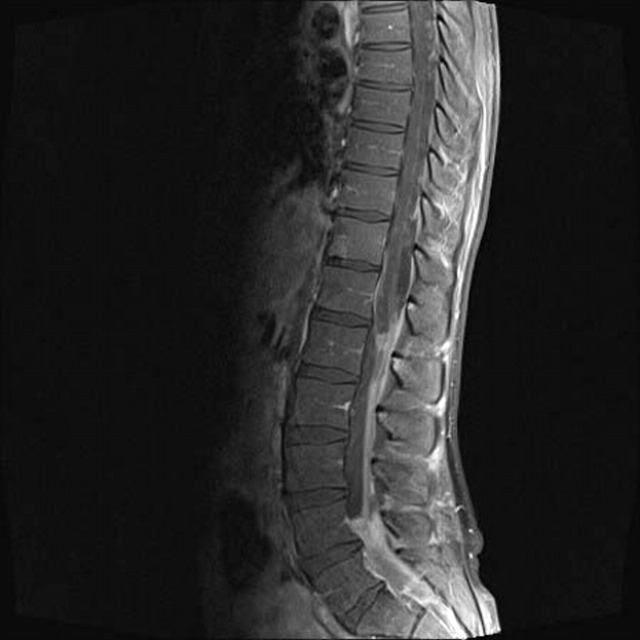
Gadolinium contrast MRI of the brain and spine showed extensive leptomeningeal T2 enhancing lesions of the lumbar spine, particularly of the cauda equina (figure 1). No evidence of intracranial disease was noted.
Figure 1.
A sagittal gadolinium contrast MRI of the patient’s lumbosacral spine showing extensive fungal leptomeningeal disease.
Lumbar puncture showed an elevated opening pressure of 28.5 cm H2O with a raised protein of 4.68 g/l (0.10–0.65 g/l) and a cerebrospinal fluid (CSF) glucose of 2.3 mmol/l with a concurrent serum glucose of 5.0 mmol/l. CSF IgG was elevated at 1.49 g/l (0.01–0.03 g/l), as was albumin at 2.38 g/l (0.10–0.25 g/l). CSF IgG:albumin ratio was 63% (<12%). Oligoclonal bands were detected in the CSF but not in the patient’s blood.
Culture of CSF grew C. dubliniensis which was susceptible to amphotericin B, fluconazole, voriconazole, caspofungin and posaconazole. Repeated blood cultures showed no growth.
Open biopsy of lumbar spine dural tissue revealed a perivascular infiltrate of both acute and chronic inflammatory cells, predominately neutrophils and lymphoyctes. Occasional scattered multinucleate giant cells were present but there was no granuloma formation. Fibroblastic proliferation was prominent, but no malignant cells seen. Gram stains and fungal stains (periodic acid shift-diastase, Grocott methenamine silver) were all negative, as was a Ziehl-Neelsen stain for acid-fast bacilli. Culture of intradural tissue grew C. dubliniensis. The species was identified by conventional methods as described by the Centraalbureau voor Schimmelcultures.1 These methods included carbohydrate assimilation tests by using a ID32C strip (bioMérieux), cycloheximide resistance, nitrate assimilation, growth at 37°C and 40°C, and hydrolysis of urea and yeast morphology studies performed using a cornmeal/tween 80 Dalmau plate. Antifungal susceptibility testing was performed in accordance with the NCCLS M27-A2 reference method for testing of yeasts,2 which was in use in 2008.
Screening tests for HIV, hepatitis B and hepatitis C were negative. Immunophenotyping of lymphocyte surface markers showed a normal CD4/CD8 ratio and normal T cell subsets.
Treatment
The patient was treated with intravenous liposomal amphotericin B (3 mg/kg once daily) and oral flucytosine for 6 weeks. Flucytosine was given at an initial dose of 25 mg/kg every 6 h, with the dose adjusted according to blood levels. Levels were taken on day 5, and then weekly thereafter; more frequently if the renal function became unstable. The target trough level was >25 mg/l to ensure efficacy; the target peak level was <100 mg/l to reduce toxicity. The patient was discharged home on fluconazole 400 mg daily which was intended to be continued for 1 year, but was stopped prematurely after 2 months due to poor patient attendance at the infectious diseases clinic.
Outcome and follow-up
The patient was presumed cured following complete resolution of symptoms and no growth on repeat CSF culture. He remains symptom free after 3 years.
Discussion
To the authors’ knowledge, this is the first reported case of meningeal involvement by C. dubliniensis in an apparently immunocompetent patient. It is also the first time that meningeal infection with this organism has caused neurological deficits.
C. dubliniensis is a pathogenic yeast species that has remarkable phenotypic similarities to Candida albicans, and was not recognised as a distinct species until 1995 when gene analysis was performed at the University of Dublin, from where the yeast derives its name.3 4 It has been isolated from a wide array of anatomical sites and from almost every continent on earth, however it is primarily found in the oral cavities of HIV and AIDS patients where it may be carried asymptomatically or cause symptomatic oropharyngeal candidiasis.3 In immunocompetent individuals, C. dubliniensis is an uncommon commensal organism in the oral cavity and is an extremely rare cause of candidemia and invasive infections.3
Although C. dubliniensis is typically less pathogenic than its more common relation, C. albicans, its incidence has greatly increased in recent times. Epidemiological studies have demonstrated that it was exquisitely rare prior to the early 1990s, but that its prevalence dramatically increased during this period predominately among HIV-infected patients.3 Possible explanations for the emergence of C. dubliniensis at this time include the increasing prevalence of HIV and AIDS, and positive selection pressure following the introduction of azole antifungal treatment for oral candidiasis in this cohort. Many isolates of C. dubliniensis are resistant to azoles, particularly fluconazole, and Moran et al demonstrated that fluconazole-susceptible isolates could readily be made resistant by exposing them to fluconazole in vitro.5 6 In addition to applying a positive selection pressure, there is also evidence that fluconazole increases the pathogenicity of C. dubliniensis by enhancing adherence to epithelial cells and inducing secretion of proteinase.7 More recent data suggest that the incidence of C. dubliniensis is now decreasing possibly due to the use of highly active anti retro-viral therapy (HAART) and reducing rates of oropharyngeal candidiasis in the HIV-infected community.3
Invasive infection by C. dubliniensis is extremely uncommon, and is usually associated with severe immunosuppression in organ transplant patients.8–10 Most invasive infections are cases of fungemia alone, with no distant seeding or organ involvement. Meningeal infection from C. dubliniensis has only been documented once previously in the medical literature. This case involved a heart and lung transplant recipient in Australia who was immunosuppressed with cyclosporine, methyprednisolone and azathioprine.11 In contrast to the patient that we present here who had focal neurological deficits, that case featured only headache and early morning nausea, but no neck stiffness, photophobia, or focal neurological signs.
Of all the reports of candida fungemia, intravenous drug use appears to be an important risk factor.12–14 In the vast majority of these cases, the infectious strain has been C. albicans, not C. dubliniensis, and there are only a few reports of C. dubliniensis fungemia in HIV-negative, non-transplant patients.10 15
This case is unique in that it is the first documented report of meningeal involvement by C. dubliniensis in an apparently immunocompetent patient, and also because the infection manifested with focal neurological signs. This suggests that the pathogenicity of this organism may be increasing, which underlines the importance of increased awareness of the species and the need for on-going epidemiological surveillance. This case also highlights two other important points for clinicians: the importance of obtaining tissue specimens in addition to fungal blood cultures when a fungal infection is suspected, and the need to maintain a high index of suspicion for fungemia and distant seeding in injecting drug users.
Learning points.
-
▶
The pathogenicity of C. dubliniensis may be increasing, and it’s therefore important for clinicians to have heightened awareness of the organism.
-
▶
When a case of fungal infection is suspected, tissue samples should be taken in addition to fungal blood cultures, as the yield from blood cultures can be low. Identification of C. dubliniensis will require specialised laboratory techniques.
-
▶
Clinicians need to maintain a high index of suspicion for fungemia and distant seeding in injecting drug users.
-
▶
The prevalence of C. dubliniensis has increased in parallel with an increase in the prevalence of HIV/AIDS and the use of azoles, but now may be decreasing due to HAART.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Kurtzman CP, Fell JW. The Yeasts, a Taxonomic Study. Fourth edition Amsterdam: Elsevier Science BV; 1998. [Google Scholar]
- 2.National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. Pennsylvania: NCCLS document M27-A. NCCLS. [Google Scholar]
- 3.Sullivan DJ, Moran GP, Pinjon E, et al. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res 2004;4:369–76. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan DJ, Westerneng TJ, Haynes KA, et al. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology (Reading, Engl) 1995;141 (Pt 7):1507–21. [DOI] [PubMed] [Google Scholar]
- 5.Moran GP, Sullivan DJ, Henman MC, et al. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother 1997;41:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol 1998;36:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg-von Zepelin M, Niederhaus T, Gross U, et al. Adherence of different Candida dubliniensis isolates in the presence of fluconazole. AIDS 2002;16:1237–44. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb GS, Limaye AP, Chen YC, et al. Candida dubliniensis fungemia in a solid organ transplant patient: case report and review of the literature. Med Mycol 2001;39:483–5. [DOI] [PubMed] [Google Scholar]
- 9.Meis JF, Ruhnke M, De Pauw BE, et al. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerging Infect Dis 1999;5:150–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebti A, Kiehn TE, Perlin D, et al. Candida dubliniensis at a cancer center. Clin Infect Dis 2001;32:1034–8. [DOI] [PubMed] [Google Scholar]
- 11.van Hal SJ, Stark D, Harkness J, et al. Candida dubliniensis meningitis as delayed sequela of treated C. dubliniensis fungemia. Emerging Infect Dis 2008;14:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odds FC, Palacio-Hernanz A, Cuadra J, et al. Disseminated Candida infection syndrome in heroin addicts–dominance of a single Candida albicans biotype. J Med Microbiol 1987;23:275–7. [DOI] [PubMed] [Google Scholar]
- 13.Collignon PJ, Sorrell TC. Disseminated candidiasis: evidence of a distinctive syndrome in heroin abusers. Br Med J (Clin Res Ed) 1983;287:861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt ME, Harrison LH, Pass M, et al. Candida dubliniensis fungemia: the first four cases in North America. Emerging Infect Dis 2000;6:46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JO, Garofalo L, Blecker-Shelly D, et al. Candida dubliniensis infections in a pediatric population: retrospective identification from clinical laboratory isolates of Candida albicans. J Clin Microbiol 2003;41:3354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]