Abstract
Calcifying fibrous tumour (CFT) is a benign tumour of elusive aetiology and a potential for local recurrence. Despite its peculiar histological characteristics it can still be confused with interrelated differential diagnosis like inflammatory myofibroblastic tumour (IMT) or solitary fibrous tumours. The clinical differential diagnosis is however much wider. To date seven cases of multiple peritoneal CFTs are on record. The authors present a case discovered incidentally during laparoscopic cholecystectomy, with no previous history and no radiological diagnosis achieved despite having undergone magnetic resonance cholangiopancreatography (MRCP) and normal routine perioperative investigation. The patient is disease-free 12 months after diagnosis. The case report is followed by a detailed literature review.
Background
The recognition of calcifying fibrous tumour (CFT) first occurred in 1988 under the name of ‘childhood fibrous tumour with psammoma bodies’, by Rosenthal and Abdul Karim.1 A series of 10 cases by Fetsch et al in 1993 defined the lesion as ‘calcifying fibrous pseudotumour’, as some of the cases had dystrophic calcification, and the age range was no longer confined to childhood.2 The WHO3 since 2004 recognised its potential for local recurrence, as shown in six cases published to date,2 4–6 and renamed the entity CFT.
We present an unsuspected case of multifocal peritoneal CFT presenting incidentally during laparoscopic surgery. This case illustrates how rarely, what initially perioperatively looks like omental metastatic disease can be a benign soft tissue tumour with good outcome and also how this lesion can escape detection by routine pre-operative screening.
Case presentation
A 32-year-old gentleman was being investigated for recurrent bouts of symptomatic cholelithiasis and deranged liver biochemistry compatible with non-alcoholic steatohepatitis, the latter attributed to a significantly raised body mass index. There was no history of previous surgery in the abdomen.
Investigations
A MRCP revealed only cholelithiasis. During elective laparoscopic cholecystectomy, multiple (21) omental solid deposits, as well as nodules adherent to the cystic duct, mesentery of the terminal ileum, tail of pancreas and splenic hilum were noted. The operation was converted to a laparotomy. There were no signs of bowel obstruction or ischaemia. The common bile duct was not compressed by these lesions. Local excision of all lesions was performed.
On gross examination all 21 lesions were of solid consistency, somewhat glistening white cut surface and some possessing a gritty texture on sectioning. The size of these lesions did not exceed 3 cm. Adherence to omental fat could easily be noted, despite the circumscribed nature of these lesions. No haemorrhagic, necrotic or cavitary foci were noted (figure 1A,B). None of the lesions had an identifiable capsule.
Figure 1.
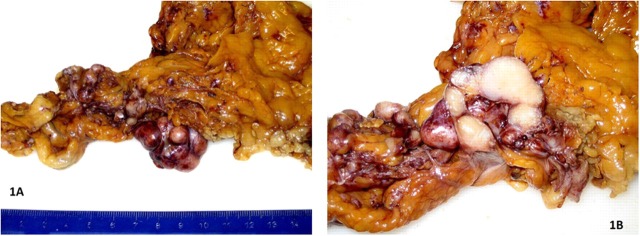
(A,B) Multiple solid omental nodules with white glistening cut surface.
The excised peritoneal nodules were fixed in 10% neutral buffered formalin. The paraffin-embedded tissue sections were stained with H&E. Immunohistochemical staining was performed using the avidin-biotin/horse radish peroxidise complex. The antibodies tested were vimentin (CloneV9 1/350; DAKO – Glostrup, Denmark), factor XIIIa (Clone AC-1A1 1/50; Abcam – Cambridge, UK), CD34 (Clone QBEnd10 1/50; DAKO), CD117 (rabbit polyclonal 1/450; DAKO), smooth muscle actin (SMA) (Clone 1A4 1/70; DAKO), Desmin (Clone D33 1/80; DAKO) and anaplastic lymphoma kinase (Clone ALK-1 1/50; Monosan). Appropriate positive control samples were run concurrently with the above.
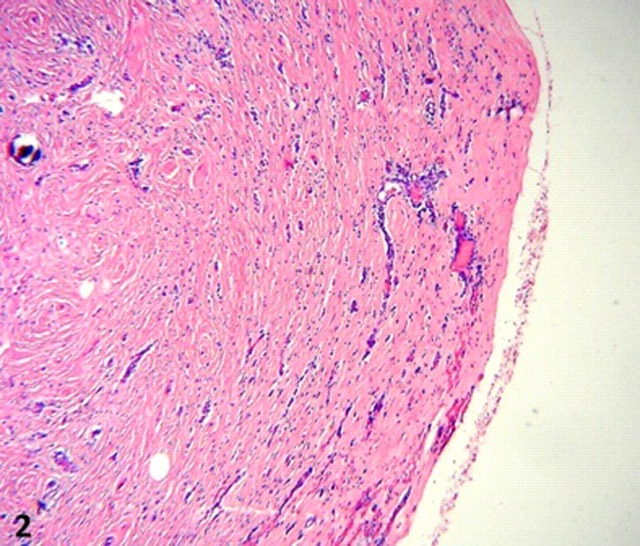
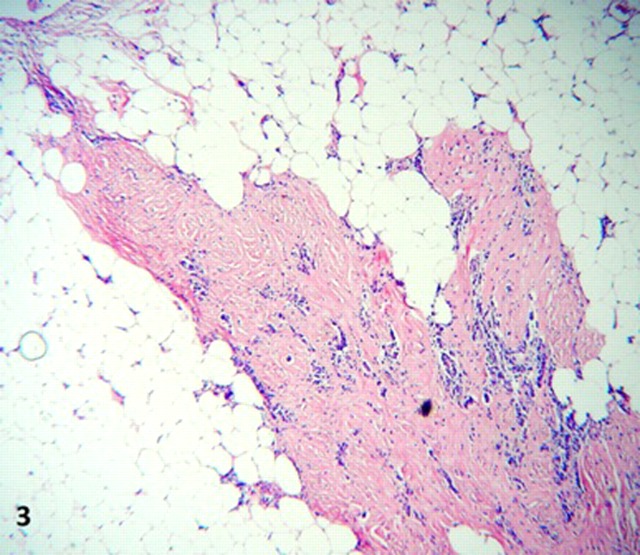
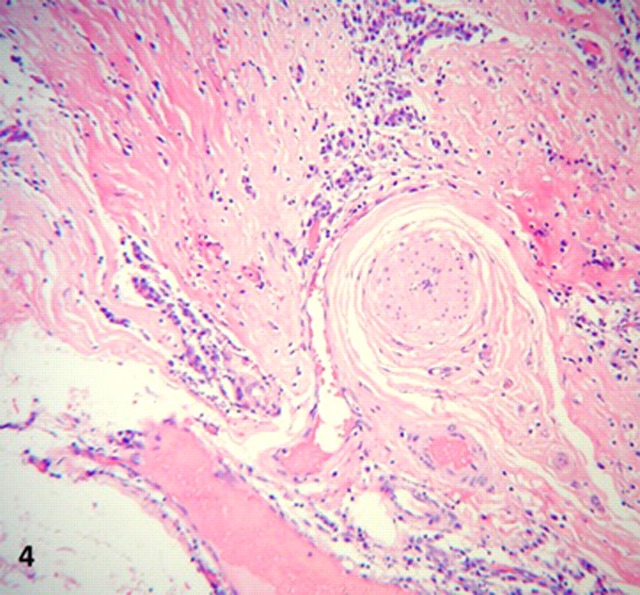
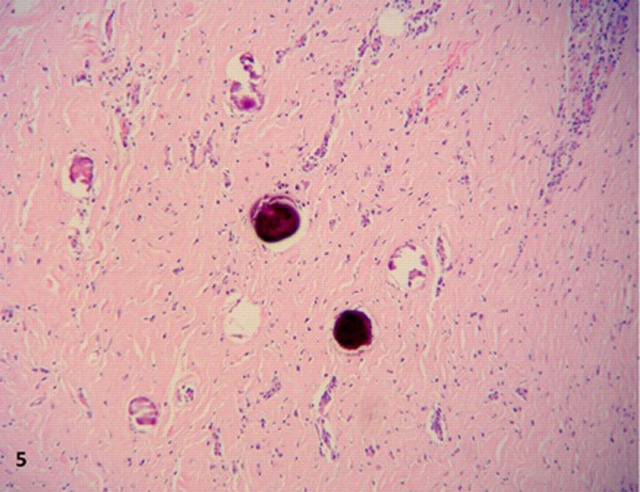
On microscopy, all lesions had similar histology, consisting of unencapsulated nodules with a densely hyalinised fibrosclerotic stroma. (figure 2). Benign slender spindle fibroblast-like cells are embedded within this matrix, with thick collagen bundles sometimes forming whorled arrangements. Most areas were paucicellular, only to be interrupted by haphazardly spaced lymphoplasmacytic infiltrates, mostly perivascular, without germinal centre formation. Other features of note were Russell bodies, entrapped omental adipose tissue and neurovascular bundles (figures 3 and 4), a high microvessel density (MVD), mostly at the periphery of the nodules, focal haemosiderin deposits and numerous CD117 positive mast cells. Also distinctive calcifications, largest measuring 80 µm in diameter were irregularly scattered in the stroma. Most of the calcifications had a typical lamellated psammomatous appearance but minor areas of dystrophic calcification were also seen (figure 5).
Figure 2.
Unencapsulated circumscribed nodule with densely hyaline fibrosclerotic stroma, low power.
Figure 3.
Lymphoplasmacytic infiltrates within stroma and infiltration into adipose tissue, high power.
Figure 4.
Entrapment of neurovascular bundle, high power.
Figure 5.
Psammomatous and dystrophic calcifications, high power.
Mitotic figures were not seen. MVD was calculated by strictly counting CD34 positive vessels in 10 random high power fields (HPF), that is, magnification ×400, total area 3 mm2 and ranged from 77 to 90 vessels per 5 HPF.
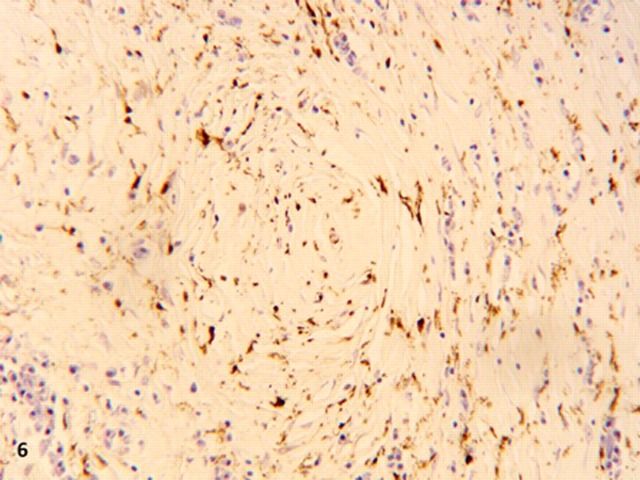
Strong and moderate cytoplasmic staining of the spindle cells was seen with vimentin and factor XIIIa, respectively (figure 6). SMA, CD34, CD117, ALK and desmin were negative. CD34 stained the endothelial cells of the blood vessels and CD117 stained the mast cells. These findings, together with the typical histological features described above led to the diagnosis of CFT, of which a concise discussion about its properties and pathogenesis will ensue.
Figure 6.
Diffuse moderate intensity cytoplasmic positivity of spindle fibre cells for factor XIIIa, high power.
Outcome and follow-up
Postoperative recovery was uneventful and the patient is disease-free 12 months following the intervention.
Discussion
Since its establishment as a benign soft tissue tumour, numerous reports have expanded the range of clinical presentations and properties of this tumour. A total of 109 cases have been reported so far, with almost any organ being affected.2 4–50 Fourteen cases have a multifocal presentation,5 7 10 14–18 20 21 29 50 seven of which are peritoneal.5 10 14 15 21 29
The histological features, when present are now recognised as typical and most of the times the diagnosis is clinched on H&E. These lesions are circumscribed unencapsulated masses composed of a paucicellular population of spindle-shaped fibroblasts in a densely hyalinised, often lamellated collagenous stroma.2 3 Collagen deposition is also noted around small vessels.7 Most contain lymphoplasmacytic infiltrates often in a perivascular location.10 The cellularity of the spindle cells tends to be less in the periphery of the lesions.7 Both psammomatous and dystrophic calcifications are present3 ranging in size from 10 to 110 µm.7 Rare foci of ossification are also described.4 Entrapment of neighbouring structures is a common sight in these tumours.
Less common features are Russell bodies, mast cells, eosinophils, polymorphonuclear leucocytes, xanthoma cells and multinucleated giant cells, often surrounding calcifications.2 9 Some lesions, like in this case, show focal haemosiderin deposition.9 Occasionally the stroma can have a myxoid consistency.10
The lymphoid aggregates can be seen in the stroma or more often perivascular, with or without germinal centre formation.10 When present around hyalinised capillaries, it may be confused with the hyaline vascular variant of Castleman’s disease,9 and to complicate matters a unique CFT was also reported as arising within a node with the hyaline vascular variant of Castleman’s disease.46
Microvascular density in CFT is distributed equally centrally and peripherally, a feature which can also help to distinguish it from (IMT).4 The technique used above is similar to the method described by Weidner et al,51 and results correlate well with published data.4
CFT are now known to have a wide age range, from 1 year of age to 65 years,5 with no gender predilection, although multifocal lesions tend to be commoner in women.15 The size of the lesions is variable, tending to be greater in soft tissues with the largest on record measuring 25 cm.5 Peritoneal nodules tend to be smaller, the largest not exceeding 3.5 cm.21 Most CFT are sessile and broad-based, but pedunculated lesions are also on record.8 Not uncommonly, macroscopic haemorrhage, necrosis and cavitation can be encountered,3 but usually the cut surface is homogenous but in different shades of white, grey or tan-pink.7
MRI imaging shows sharp circumscription of the lesions, especially in the soft tissue, with a signal intensity closer to muscle than to fat.2 MRCP is not sensitive, as evidenced by our case. Contrast enhanced CT scans can show calcifications as areas with higher attenuation than the enhancing vessels.7 On electron microscopy, the spindle cells are shown to be fibroblasts, surrounded by abundant collagen fibrils, where the initial event in intracytoplasmic calcification is organelle degeneration.1 7 Calcification in CFT can be both intracellular and extracellular.
Immunohistochemical staining properties of the fibroblasts do not vary according to region of the body, with the slight exception of CD34. CFT typically shows cytoplasmic positivity for vimentin and factor XIIIa, and are negative for skeletal muscle actin, desmin, ALK and cytokeratins.3 10 SMA is usually negative but is rarely reportedly positive.10 CD34 fibrocyte positivity in CFT was first described in the peritoneum,8 and its expression is now thought to represent early submesothelial fibroblast properties, where the reactive fibroblast can then differentiate into myofibroblasts, myxoid fibroblasts and collagenous fibroblasts.13 Most CD34-positive CFT occur in the peritoneum.13
The aetiological factors behind CFT are unknown, although previous trauma has been reported in some cases.1 7 8 To date, only a single occurrence of familial CFT is described, between two sisters, each presenting at 17 years of age with multiple peritoneal lesions.10
The differential diagnosis of CFT in the abdomen includes pelvic or mesenteric fibromatoses, elastofibroma of the omentum, retractile mesenteritis, smooth muscle tumours, solitary fibrous tumour of the peritoneum, IMT and hyalinising granuloma. These are usually easily distinguished on H&E, except for cases of early IMT and localised solitary fibrous tumour of the peritoneum.8 An inter-relationship and pathological continuum between CFT and IMT was previously proposed, due to the occurrence of these two entities in the same lesion.7 10 12 21 However, large series published in the last decade demonstrate notable differences in immunohistochemical staining properties between the two. IMT almost always stains positive for ALK-1 antibody (a product of its peculiar 2:5 chromosomal translocation) and skeletal muscle actin but only focally for factor XIIIa (less than 10% of area examined), while CFT never stained positive for ALK-1 and skeletal actin but shows diffuse positivity for factor XIIIa.4 5 Thus it is unlikely that there is a pathologic continuum from IMT to CFT, but rather a common earlier, short-lived precursor lesion, as discussed earlier.
So far no mortality has been attributed to CFT. An asymptomatic multifocal peritoneal presentation during elective surgery is uncommon. In most cases, after local excision, a short-term follow-up programme and reassurance are all that is needed. The caring physician should keep in mind the possibility of its benign nature, and the pathologist should not forget other more common differential diagnoses.
Learning points.
-
▶
Single or multiple omental deposits can rarely be inflammatory or low grade benign tumours.
-
▶
In this respect one should look for history of trauma, metabolic disease and concomitant soft tissue lesions after having explored the common sites of origin of a primary carcinoma.
-
▶
CFT is a benign soft tissue tumour which can rarely present as multiple omental solid deposits. No mortalities have been attributed to this entity so far.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med 1988;112:798–800. [PubMed] [Google Scholar]
- 2.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol 1993;17:502–8. [DOI] [PubMed] [Google Scholar]
- 3.Kleihaues P, Sobin LH. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC press, 2002:77–8. [Google Scholar]
- 4.Hill KA, Gonzalez-Crussi F, Chou PM. Calcifying fibrous pseudotumor versus inflammatory myofibroblastic tumor: a histological and immunohistochemical comparison. Mod Pathol 2001;14:784–90. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento AF, Ruiz R, Hornick JL, et al. Calcifying fibrous ‘pseudotumor’: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol 2002;10:189–96. [DOI] [PubMed] [Google Scholar]
- 6.Maeda A, Kawabata K, Kusuzaki K. Rapid recurrence of calcifying fibrous pseudotumor (a case report). Anticancer Res 2002;22:1795–7. [PubMed] [Google Scholar]
- 7.Pinkard NB, Wilson RW, Lawless N, et al. Calcifying fibrous pseudotumor of pleura. A report of three cases of a newly described entity involving the pleura. Am J Clin Pathol 1996;105:189–94. [DOI] [PubMed] [Google Scholar]
- 8.Weynand B, Draguet AP, Bernard P, et al. Calcifying fibrous pseudotumour: first case report in the peritoneum with immunostaining for CD34. Histopathology 1999;34:86–7. [DOI] [PubMed] [Google Scholar]
- 9.Kocova L, Michal M, Sulc M, et al. Calcifying fibrous pseudotumour of visceral peritoneum. Histopathology 1997;31:182–4. [DOI] [PubMed] [Google Scholar]
- 10.Chen KT. Familial peritoneal multifocal calcifying fibrous tumor. Am J Clin Pathol 2003;119:811–15. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Maheshwari V, Alam K, et al. Calcifying fibrous pseudotumor of peritoneum. J Postgrad Med 2007;53:189–90. [DOI] [PubMed] [Google Scholar]
- 12.Pomplun S, Goldstraw P, Davies SE, et al. Calcifying fibrous pseudotumour arising within an inflammatory pseudotumour: evidence of progression from one lesion to the other? Histopathology 2000;37:380–2. [DOI] [PubMed] [Google Scholar]
- 13.Zámecnik M, Michal M, Boudova L, et al. CD34 expression in calcifying fibrous pseudotumours. Histopathology 2000;36:183–4. [DOI] [PubMed] [Google Scholar]
- 14.Sigel JE, Smith TA, Reith JD, et al. Immunohistochemical analysis of anaplastic lymphoma kinase expression in deep soft tissue calcifying fibrous pseudotumor: evidence of a late sclerosing stage of inflammatory myofibroblastic tumor? Ann Diagn Pathol 2001;5:10–4. [DOI] [PubMed] [Google Scholar]
- 15.Farah RB, Dimet S, Bidault AT, et al. Multiple peritoneal calcifying fibrous tumors revealed by ischemic colitis. Ann Diagn Pathol 2007;11:460–3. [DOI] [PubMed] [Google Scholar]
- 16.Suh JH, Shin OR, Kim YH. Multiple calcifying fibrous pseudotumor of the pleura. J Thorac Oncol 2008;3:1356–8. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, Yuki D, Sakata K. Multiple calcifying fibrous pseudotumors disseminated in the pleura. Ann Thorac Surg 2008;85:e3–5. [DOI] [PubMed] [Google Scholar]
- 18.Mito K, Kashima K, Daa T, et al. Multiple calcifying fibrous tumors of the pleura. Virchows Arch 2005;446:78–81. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Izhak O, Itin L, Feuchtwanger Z, et al. Calcifying fibrous pseudotumor of mesentery presenting with acute peritonitis: case report with immunohistochemical study and review of literature. Int J Surg Pathol 2001;9:249–53. [DOI] [PubMed] [Google Scholar]
- 20.Hainaut P, Lesage V, Weynand B, et al. Calcifying fibrous pseudotumor (CFPT): a patient presenting with multiple pleural lesions. Acta Clin Belg 1999;54:162–4. [DOI] [PubMed] [Google Scholar]
- 21.Van Dorpe J, Ectors N, Geboes K, et al. Is calcifying fibrous pseudotumor a late sclerosing stage of inflammatory myofibroblastic tumor? Am J Surg Pathol 1999;23:329–35. [DOI] [PubMed] [Google Scholar]
- 22.Liu DD, Song LH. Calcifying fibrous tumor in gastric wall: report of a case. Zhonghua Bing Li Xue Za Zhi 2009;38:346–7. [PubMed] [Google Scholar]
- 23.Bobbio A, Mazzeo A, Carbognani P, et al. An unusual case of calcifying fibrous pseudotumour of the cervicothoracic junction. Eur J Cardiothorac Surg 2008;34:1123–5. [DOI] [PubMed] [Google Scholar]
- 24.Sudhakar S, Mistry Y, Dastidar A, et al. Calcifying fibrous tumour: an unusual omental lesion. Pediatr Radiol 2008;38:1246–8. [DOI] [PubMed] [Google Scholar]
- 25.Emanuel P, Qin L, Harpaz N. Calcifying fibrous tumor of small intestine. Ann Diagn Pathol 2008;12:138–41. [DOI] [PubMed] [Google Scholar]
- 26.Lakhtakia R, Jambhekar N. Calcifying fibrous pseudotumour: a case report. Indian J Pathol Microbiol 2007;50:831–2. [PubMed] [Google Scholar]
- 27.Kuwata T, Inadome A, Matsumoto K, et al. Case report of calcifying fibrous pseudotumor of the adrenal grand. Nippon Hinyokika Gakkai Zasshi 2008;99:48–51. [DOI] [PubMed] [Google Scholar]
- 28.Lee D, Suh YL, Lee SK. Calcifying fibrous pseudotumour arising in a gastric inflammatory myofibroblastic tumour. Pathology 2006;38:588–91. [PubMed] [Google Scholar]
- 29.Lee JC, Lien HC, Hsiao CH. Coexisting sclerosing angiomatoid nodular transformation of the spleen with multiple calcifying fibrous pseudotumors in a patient. J Formos Med Assoc 2007;106:234–9. [DOI] [PubMed] [Google Scholar]
- 30.Lau SK, Weiss LM. Calcifying fibrous tumor of the adrenal gland. Hum Pathol 2007;38:656–9. [DOI] [PubMed] [Google Scholar]
- 31.Mardi K, Sharma J. Calcifying fibrous pseudotumor of the soft palate–a case report. Indian J Pathol Microbiol 2006;49:394–5. [PubMed] [Google Scholar]
- 32.Kirby PA, Sato Y, Tannous R, et al. Calcifying fibrous pseudotumor of the myocardium. Pediatr Dev Pathol 2006;9:384–7. [DOI] [PubMed] [Google Scholar]
- 33.Shigematsu H, Sano Y, Kasahara S, et al. Calcifying fibrous tumor arising from the heart. J Thorac Cardiovasc Surg 2006;132:e21–2. [DOI] [PubMed] [Google Scholar]
- 34.Attila T, Chen D, Gardiner GW, et al. Gastric calcifying fibrous tumor. Can J Gastroenterol 2006;20:487–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elpek GO, Küpesiz GY, Ogüs M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int 2006;56:227–31. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein EB, Savel RH, Sen F, et al. Calcifying fibrous pseudotumor of the neck: diagnostic challenges of a rare benign lesion. Am Surg 2005;71:1051–4. [PubMed] [Google Scholar]
- 37.Jang KS, Oh YH, Han HX, et al. Calcifying fibrous pseudotumor of the pleura. Ann Thorac Surg 2004;78:e87–8. [DOI] [PubMed] [Google Scholar]
- 38.Soyer T, Ciftci AO, Güçer S, et al. Calcifying fibrous pseudotumor of lung: a previously unreported entity. J Pediatr Surg 2004;39:1729–30. [DOI] [PubMed] [Google Scholar]
- 39.Delbecque K, Legrand M, Boniver J, et al. Calcifying fibrous tumour of the gastric wall. Histopathology 2004;44:399–400. [DOI] [PubMed] [Google Scholar]
- 40.Mourra N, Bell S, Parc R, et al. Calcifying fibrous pseudotumour: first case report in the gallbladder. Histopathology 2004;44:84–6. [DOI] [PubMed] [Google Scholar]
- 41.Ammar A, El Hammami S, Horchani H, et al. Calcifying fibrous pseudotumor of the pleura: a rare location. Ann Thorac Surg 2003;76:2081–2. [DOI] [PubMed] [Google Scholar]
- 42.Lee HY, Chuah KL, Tan PH. Test and teach. Number fifty-three. Diagnosis: calcifying fibrous pseudotumour. Pathology 2003;35:166–9. [PubMed] [Google Scholar]
- 43.Cavazza A, Gelli MC, Agostini L, et al. Calcified pseudotumor of the pleura: description of a case. Pathologica 2002;94:201–5. [DOI] [PubMed] [Google Scholar]
- 44.Eftekhari F, Ater JL, Ayala AG, et al. Case report: calcifying fibrous pseudotumour of the adrenal gland. Br J Radiol 2001;74:452–4. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann H, Beaver ME, Maillard AA. Calcifying fibrous pseudotumor of the neck. Arch Pathol Lab Med 2000;124:435–7. [DOI] [PubMed] [Google Scholar]
- 46.Dargent JL, Delplace J, Roufosse C, et al. Development of a calcifying fibrous pseudotumour within a lesion of Castleman disease, hyaline-vascular subtype. J Clin Pathol 1999;52:547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong HS, Lee GK, Sung R, et al. Calcifying fibrous pseudotumor of mediastinum–a case report. J Korean Med Sci 1997;12:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukunaga M, Kikuchi Y, Endo Y, et al. Calcifying fibrous pseudotumor. Pathol Int 1997;47:812. [DOI] [PubMed] [Google Scholar]
- 49.Reed MK, Margraf LR, Nikaidoh H, et al. Calcifying fibrous pseudotumor of the chest wall. Ann Thorac Surg 1996;62:873–4. [PubMed] [Google Scholar]
- 50.Kawahara K, Yasukawa M, Nakagawa K, et al. Multiple calcifying fibrous tumor of the pleura. Virchows Arch 2005;447:1007–8. [DOI] [PubMed] [Google Scholar]
- 51.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995;36:169–80. [DOI] [PubMed] [Google Scholar]