Abstract
The anatomic basis and articulatory features of speech production are often studied with imaging studies that are typically acquired in the supine body position. It is important to determine if changes in body orientation to the gravitational field alter vocal tract dimensions and speech acoustics. The purpose of this study was to assess the effect of body position (upright versus supine) on (1) oral and pharyngeal measurements derived from acoustic pharyngometry and (2) acoustic measurements of fundamental frequency (F0) and the first four formant frequencies (F1–F4) for the quadrilateral point vowels. Data were obtained for 27 male and female participants, aged 17 to 35 yrs. Acoustic pharyngometry showed a statistically significant effect of body position on volumetric measurements, with smaller values in the supine than upright position, but no changes in length measurements. Acoustic analyses of vowels showed significantly larger values in the supine than upright position for the variables of F0, F3, and the Euclidean distance from the centroid to each corner vowel in the F1-F2-F3 space. Changes in body position affected measurements of vocal tract volume but not length. Body position also affected the aforementioned acoustic variables, but the main vowel formants were preserved.
I. INTRODUCTION
Speech and singing can be produced in a variety of body positions, such as upright, supine, prone, and at various angles of recline. This report specifically addresses speech in the upright and supine positions. Although most laboratory studies of speech pertain to the upright position, imaging methods often place the speaker in a supine position, or more rarely, a prone position. Studies of vocal tract (VT) anatomy or function using magnetic resonance imaging (MRI) generally have placed the speaker in a supine position (Fitch and Giedd, 1999; Steiner et al., 2012; Vorperian et al., 2009). The question arises if differences in body position affect the configuration and/or function of the articulators while producing speech or at rest. If so, then generalization of imaging results across body positions may be limited.
Changes that occur with altered body position may be attributable to gravitational effects exerted on the tissues of the VT. There may also be changes in musculoskeletal relationships that are at least partly accommodations to gravitational effects but may reflect other aspects of structural relationships such as head–body alignment. The observed effects of body position on the speech articulators can be compensatory (the speaker makes adjustments in response to altered position) or non-compensatory (no adjustments are made to different orientations to gravity). The effects of body position on VT adjustments during speech and singing have been determined primarily by the methods of imaging, acoustic pharyngometry (APh) (also referred to as acoustic reflectometry), electromyography, tongue pressure recordings, and acoustic measurements (primarily of formant frequencies). Published studies have been conducted almost exclusively on adults, the assumed speakers in the following summary unless specified otherwise.
The results of imaging and APh investigations into positional effects vary somewhat across studies and across individuals. One of the most consistent observations is that the pharyngeal (nasopharyngeal and/or oropharyngeal) cavity is smaller in the supine as opposed to the upright position during speech, as determined from APh (Jan et al., 1994), computed tomography (CT; Sutthiprapaporn et al., 2008), MRI (Engwall, 2006; Kitamura et al., 2005; Traser et al., 2014), ultrasound (Stone et al., 2007; Wrench et al., 2011), x-ray microbeam (Tiede et al., 2000), and videofluoroscopy (Bae et al., 2014). This effect can be explained by gravity pulling the tongue root posteriorly when the subject is in the supine position, thereby reducing the size of the pharynx. Similar effects have been observed during respiration. In a study of tidal nasal breathing with CT (supine body position) and cone beam CT (upright body position), the cross-sectional area of the upper airway was larger, especially in the region between the hard palate and bottom of the uvula in the upright than in the supine body position (Van Holsbeke et al., 2014). This difference in airway geometry was accompanied by functional changes in airway resistance, with larger effects for men than women.
The gravitational influences may not have general effects on lingual function. In a recent study of tongue pressure generation in speech tasks (phoneme repetitions) and nonspeech tasks (isometric contractions), no significant differences were observed between upright and supine body positions (Dietsch et al., 2013). Either muscle activation for these tasks was unaffected by body position, or the degree of activation was adjusted for configurational differences related to body position.
Studies of the effects of body position on velar position and function are not so easily summarized. First, as Perry (2011) points out, most studies were concerned with sleep apnea and not with speech or singing. Vocal activities may result in a different response to gravity than is observed during rest breathing or a task such as swallowing. A further complication is that results vary across studies and sometimes across subjects within a study. The results are summarized here by task: rest breathing, swallowing, and vocal tasks of speech or singing. Studies of rest breathing have shown that the supine position resulted in (1) a thicker and longer velum, along with an increase in uvular width (Pae et al., 1994; Yildirim et al., 1991), (2) a more posterior position of the velum (Bae et al., 2014; Smith and Battagel, 2004; Sutthiprapaporn et al., 2008), or (3) no significant differences in several measures of velopharyngeal structures (Kollara and Perry, 2014; Perry, 2011). For swallowing, changes in body position have been reported to affect the temporal pattern of movement (Perry et al., 2012) and velopharyngeal closing pressure (Nakayama et al., 2013). For speech, recent MRI studies by Perry (2011), reporting on adults, and Kollara and Perry (2014), reporting on children between 4 and 8 yrs old, concluded that there were minimal effects of gravity on measures of velar thickness, velar height, levator muscle length, angles of origin, and pharyngeal dimensions. In an electromyographic study, Moon and Canady (1995) observed a reduced level of muscle activity in the levator veli palatine for phonation in the supine as opposed to the upright position. In a MRI study of untrained singers, Traser et al. (2014) observed elongation of the uvula in the upright position.
Effects of body position also have been reported for the jaw and larynx. Compared to the upright position, the jaw is more protruded in the supine position during speech (Shiller et al., 1999) and singing (Traser et al., 2013), which may be a compensation for pharyngeal constriction, similar to the way in which mandibular advancement is used as a treatment for obstructive sleep apnea (Ferguson et al., 2006). The larynx is elevated (i.e., more rostral or shorter distance from cranial reference point to laryngeal reference point) in the supine position during speech (Kitamura et al., 2005) and singing (Traser et al., 2013) but apparently not during nonvocal states (Yildirim et al., 1991). Laryngeal elevation is related to several factors, including activation of the suprahyoidal and infrahyoidal muscles, neck and head posture, and tracheal pull on laryngeal structures (Traser et al., 2013). At least some of these effects are responses to the gravitational field, but some may be associated with musculo-skeletal repositioning during postural changes.
In summary, there is general consensus that a supine body position differs from an upright position where during respiration, the pharyngeal cavity (PC) is smaller in the supine than in the upright position; and where during speech and singing, the tongue root is retracted (reducing the lumen of the pharynx), the jaw protruded, the larynx elevated, and the effects on the velopharynx variable (ranging from no effect to velar thickening and lengthening, and a posterior position of the velum).
Generally, acoustic studies have measured vowel formant frequencies (or more rarely, formant bandwidth) for speech produced in upright versus supine position (cf. Steiner et al., 2012). Weir et al. (1993) measured the first three formant frequencies for the four corner vowels (/i/ /u/ /ae/ /a/) produced by ten male speakers. The sole significant difference between upright and supine positions was a higher F1 frequency for the vowel /i/ in the supine position. Stone et al. (2007) measured the F1 and F2 frequencies of three vowels (/i/ as in “feet,” /ae/ as in “hat,” and /a/ as in “hot”) produced by 13 speakers. They reported no significant differences for words of continuous speech but some significant differences for isolated production of sustained consonants and vowels. Shiller et al. (1999) also measured F1 and F2 frequencies, focusing on two vowels in a consonant-vowel-consonant context with a carrier phrase produced by six speakers. Both F1 and F2 were higher in supine than upright body position for vowel /ae/ and less reliably for /e/. In another study of F1 and F2 frequencies for the vowels /i/ /u/ and /a/, no significant differences were observed between vowels produced in upright versus supine positions by 12 subjects, although F1 frequencies tended to be higher in the supine position (Bae et al., 2014). Tiede et al. (2000) reported acoustic data from two Japanese male speakers for sustained vowels (/i/ /u/ /a/ /e/ /o/) and running speech in upright and supine positions. No consistent differences were found in formant frequencies or bandwidths between body positions or type of speech material. Stone et al. (2007) and Tiede et al. (2000) concluded from their data that the physiological effects of gravity on the acoustic properties of speech are negligible. Buchaillard et al. (2009) concluded similarly from a biomechanical model of the tongue and oral cavity (OC). However, Shiller et al. (1999) reached the very different conclusion that “subjects do not completely compensate for differences in gravitational load” (p. 9079) and contrasted the motor control of speech with arm movements, for which such compensation has been demonstrated.
In summary, acoustic differences between upright and supine body positions are small or variable across studies. A general conclusion is hindered by the differences in speech sounds used across studies and by the limitation of the acoustic data to the first two or three formants.
Questions remain on the anatomic and acoustic consequences of positional change, especially related to the degree to which subjects compensate for changes in orientation relative to gravity. Particularly needed is a combination of anatomic and acoustic measurements for both sexes. Therefore, the purpose of this study was to assess the effect of body position (upright versus supine) on (1) oral and pharyngeal anatomic measurements derived from APh (OC and PC length and volume of the VT), and (2) acoustic measurements for the extreme vowels of the traditional quadrilateral, of fundamental frequency (F0), the first four formant frequencies (F1, F2, F3, and F4), formant bandwidths (B2 for F2, and B3 for F3), and computed Euclidean distances from a neutral vowel (in the F1-F2-F3 acoustic space). The centroid was calculated to be an approximation of a neutral vowel. Data were obtained for both male and female adults to assess possible sex differences. For the APh portion of the study, we hypothesized that the supine body position would yield smaller anatomic measures of the pharyngeal cavity volume (PCV) but not its length (a pure effect of gravity pulling the tongue root posteriorly). For the acoustic portion of the study, we hypothesized that body position will not affect any of the acoustic measurements (F0, F1, F2, F3, F4, B2, and B3) due to compensation in speech production.
II. METHODS
A. Participants
Participants in this study included 27 adults (13 males and 14 females) between the ages of 17 and 35 yrs. All participants were white native speakers of American English with no known difficulties with voice, speech, language, or hearing as determined by self-report during a phone interview, except for one female participant with a mild high frequency hearing loss. Participants were recruited with Institutional Review Board approved flyers posted on University campus.
B. Procedures
After participants gave consent to participate, anthropometric data were collected including participants' sex, race, age, weight, and height (Table I). Next, speech audio recordings followed by APh measurements were collected first in the upright body position and then in the supine body position for participant ease. Despite data collection order of speech acoustics recordings followed by APh, the description of methods is reversed for expository and explanatory convenience in describing the results; therefore, APh is presented first, followed by the speech acoustics thus maintaining the anatomic–acoustic presentation order throughout this paper. All data collection was performed with two examiners present in the testing room. Participants received monetary compensation.
TABLE I.
Demographic data for male and female participants.
| n | Age (years) | Height (cm) | Weight (kg) | BMI | |
|---|---|---|---|---|---|
| Mean (SDa) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| [Range] | [Range] | [Range] | [Range] | ||
| Males | 13 | 22.2 (4.47) | 181 (7.45) | 88.3 (21.05) | 26.70 (5.47) |
| [17–35] | [166–190] | [71–147] | [21.36–42.9] | ||
| Females | 14 | 21.7 (2.99) | 165 (4.92) | 65.0 (8.16) | 23.63 (2.98) |
| [19–29] | [159–173] | [53–76.25] | [19.1–30.1] |
SD = standard deviation.
1. APh—Anatomic measurements
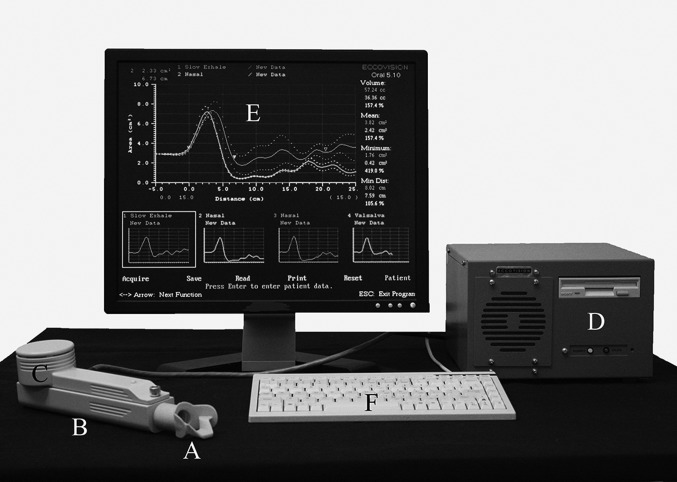
The Eccovision® acoustic pharyngometer (Sleep Group Solutions, 2009) was used to obtain anatomic measurements of the VT in the upright and supine body positions. The APh, also known as an acoustic reflectometer, is a noninvasive Food and Drug Administration approved unit that uses sound echoes to measure the geometry of the oral and pharyngeal cavities/upper airway. It has been in use for several decades as an accurate, non-invasive method of examining the upper airway (Hoffstein and Zamel, 1984; D'Urzo et al., 1987; D'Urzo et al., 1988; Hoffstein and Fredberg 1991; Marshall et al., 1993) both clinically to investigate adults and children with sleep related disorders (Brown et al., 1987; Gelardi et al., 2007; Jung et al., 2004; Marshall et al., 1993; Monahan et al., 2005), and more recently in speech research to study changes or differences in VT dimensions related to aging (Xue et al., 1999), race (Xue and Hao, 2006;), as well as in atypically developing speakers with Down Syndrome (Xue et al., 2010). The APh technique is similar to that of an active sonar and entails emitting pulses of sounds of known frequency and amplitude into the VT, then using the amplitude and arrival times of the reflected acoustic waves to construct the area-distance function of the upper airway, specifically the cross-sectional area of the upper airway as a function of the distance from the glottis to the teeth. Figure 1 shows the APh system consisting of a mouthpiece (A) that is attached to the wave tube (B), which has an integrated electronic platform (C), and in turn is connected to the control unit/a microcomputer (D) that is connected to a monitor (E) to display the pharyngogram, as well as a keyboard (F) to type commands and file names for data acquisition, i.e., perform the acoustic scan. As the participant is gently exhaling through A and B, the electronic platform (C) that contains one sound generator and two microphones emits the acoustic signal (filtered tapping). The resulting acoustic wave, captured by the first of the two microphones, travels down the uniform wave tube (B) and fitted mouthpiece (A) and into the VT lumen. Fractions of the wave are reflected back at each point of discontinuity in the lumen and recorded by the second microphone. The control unit/microcomputer (D), equipped with analog-to-digital and digital-to-analog converters, processes the time of arrival and the amplitude of the reflected sound waves, and uses the monitor (E, as seen in Fig. 1) to display the pharyngogram, a two-dimensional graphic representation of the cross-sectional area of the lumen (cm2) as a function of airway distance (cm). The control unit also calculates volume (cc) from the summation of cross sectional area measurements.
FIG. 1.
The APh system consisting of the mouthpiece (A), wave tube (B), electronic platform (C), control unit/microcomputer (D), monitor (E), and keyboard controls (F). The top half of the monitor display shows a slow exhale condition pharyngogram superimposed with a nose exhale condition pharyngogram. The bottom half of the monitor shows the four different pharyngograms per participant that the system can hold so that each can be superimposed on any of the other pharyngograms to determine consistency of the same exhale condition, or to locate landmarks as described in Fig. 2.
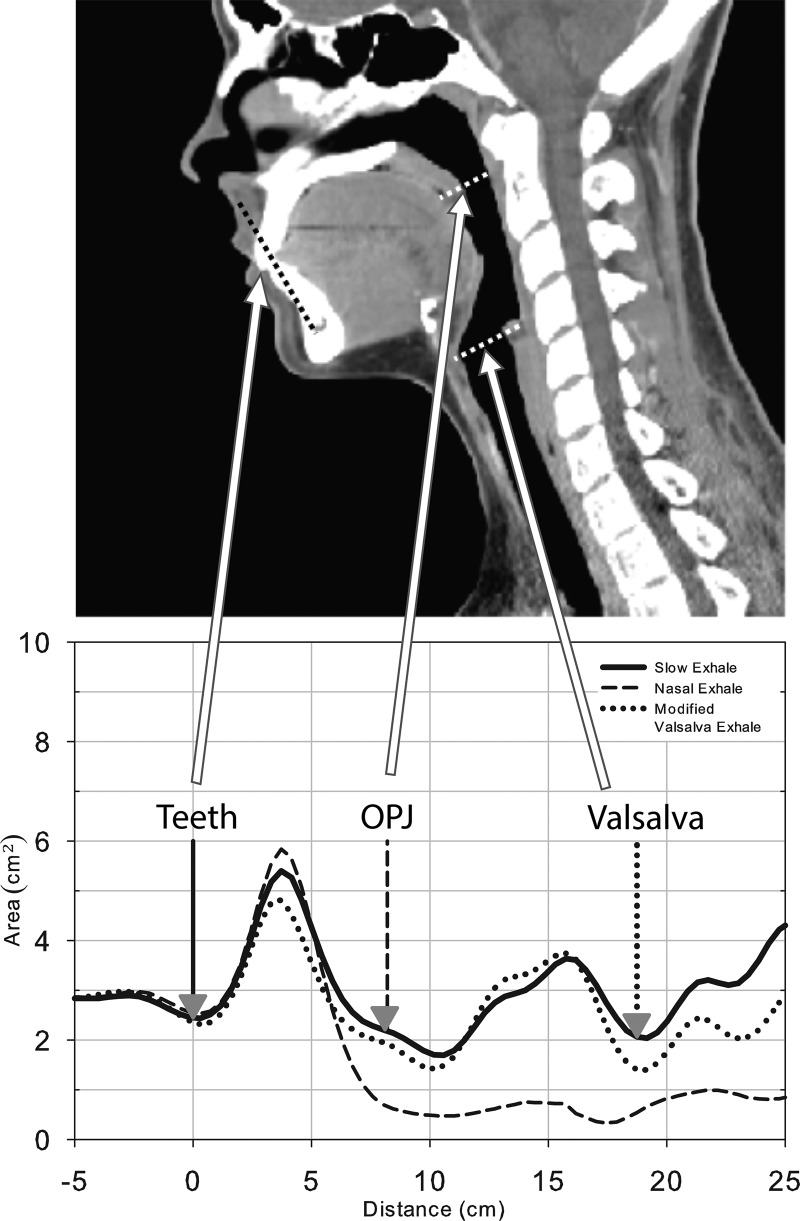
The APh data collection procedure used in this study was one based on the acoustic pharyngometer's operator manual (Sleep Group Solutions, 2009) as well as numerous communications with the manufacturer. A detailed protocol documenting the use of the APh equipment for data acquisition and analysis was developed, and a licensed version of the protocol is available online (Vorperian, 2013). To summarize, APh data collection entailed having the participants position the flange of the mouthpiece against their teeth with their lips over the flange to form an airtight seal (similar to an athletic mouth guard). The mouthpiece also contained a crossbar to guide the tongue to a relaxed, downward position. Each participant was instructed to exhale slowly using each of the following three breathing conditions: (1) Oral exhale condition—participant inhaled through the nose and slowly exhaled through the mouth; this condition was performed several times until the pharyngograms were consistent (<±6% difference between trials) since this slow exhale condition pharyngogram was used to make the at-rest vocal tract length (VTL) and volume measurements. (2) Nose exhale condition—participant inhaled through the nose and slowly exhaled back through the nose; the region of airway constriction seen at the base of the initial peak of this pharyngogram was used to mark the location of the oral pharyngeal junction (OPJ) on the slow oral exhale pharyngogram once the two pharyngograms were superimposed. (3) Modified valsalva condition—participant inhaled through the nose and slowly exhaled through the mouth against a partially constricted glottis; the pharyngogram of this modified valsalva breathing condition was similarly used to mark the location of the glottis on the slow oral exhale pharyngogram once the two pharyngograms were superimposed. The OPJ and glottis marks were used to segment and measure the oral and pharyngeal portions of VTL (OCL-APh and PCL-APh) and volume (OCV-APh and PCV-APh), where the OPJ and glottis marks denoted the endpoints of the oral and pharyngeal cavities respectively (see Fig. 2). Thus, a total of six APh measures were made that included the following three length (L) measurements: VTL-APh, OCL-APh (oral cavity length), PCL-APh (pharyngeal cavity length); and the following three volume (V) measurements: VTV-APh (vocal tract volume), OCV-APh (oral cavity volume), and PCV-APh (pharyngeal cavity volume).
FIG. 2.
A visual representation of the anatomic measures on a pharyngogram (bottom) with corresponding anatomic landmarks on a midsagittal CT scan (top). The CT is for illustrative purposes and representative of normal breathing, not any of the APh breathing conditions. The bottom display is a schematic of three pharyngograms displaying the three different breathing conditions superimposed to illustrate how the nasal and modified valsalva pharyngograms are, respectively, used to locate the OPJ and the glottis on the slow exhale pharyngogram.
2. Speech acoustic recordings and measurements
Speech recordings were made using a SHURE SM48 microphone (Shure Incorporated, Niles, IL) and PMD 660 Marantz solid state recorder (Marantz America, LLC., Mahwah, NJ). The microphone, stabilized with a floor stand, was adjusted to the height of the participant and placed 15 cm from the participant's mouth. The microphone was connected directly to the Marantz recorder, which digitized the speech samples at a 48 kHz sampling rate and 16-bit quantization onto the recording medium, a SanDisk Ultra II flashcard. The sound files were transferred to a computer for editing and analysis using the speech acoustic analysis software package TF32 (Milenkovic, 2010). The stimuli were real words of American English containing the four corner vowels in consonant-vowel-consonant or consonant-vowel context. Words were used, as opposed to sustained vowels, to avoid the problem of hyper-articulation (Engwall, 2006). Stimuli selection took into account word familiarity to younger participants, and high phonological neighborhood density which reportedly maximizes vowel space (Munson and Solomon, 2004). The corner vowels were /u/ as in “hoot,” /a/ as in “hot,” /i/ as in “feet,” and /ae/ as in “hat.” For each corner vowel there were five stimuli (see Appendix A for the full list of word stimuli), with two of these words elicited twice. Thus there were 28 stimuli per body position and 56 stimuli per person (4 vowels × 7 words × 2 body positions). A laptop running the TOCS+ Platform program (Hodge and Daniels, 2007) was used to present the stimuli, both visually and auditorily, where the same set of 28 words was delivered to each participant in both the upright and supine body positions in random order in each body position.
Participants were seated in the upright body position first. One of the two examiners adjusted the microphone to the participant's seated height and distance from the lips as specified above. Each participant was instructed to keep his or her loudness at the same level throughout the speech recording session, to refrain from smiling or laughing, and to count from one to ten in a natural voice while the same examiner adjusted the recording level on the Marantz. Feedback regarding production loudness was given and the target recording level was adjusted to be between 6 and 12 dB below the maximum level. Participants were then instructed to repeat the words they heard. The targeted recording levels were maintained throughout the recording sessions. In the upright body position, the stimuli were photographs presented to each participant visually on the computer monitor with the stimulus word written under it while an adult male audio recording of the stimulus word played over the computer's external speakers. For speech recordings in the supine body position, participants lay on a cot with a supportive neck pillow. The microphone setup and recording process was the same as in the upright position except that in the supine position, the stimuli were presented only in audio form to ensure that each participant maintained the desired body position with a line of vision straight up at the ceiling. Participants were instructed to inform the examiner if they were unsure of a stimulus and the examiner presented it again as needed.
The continuous speech recording from each participant was segmented into separate wave files for each stimulus word. A total of 1489 words (out of a possible 1512) were segmented. The 23 missing stimuli were due to participant error using the TOCS + Platform program. Each of the segmented word files was analyzed using the acoustic analysis software TF32 and the following measurements were made: fundamental frequency, formant frequencies F1–F4, and formant bandwidths B2 and B3.
The acoustic measurements of formant frequencies F1–F4 were made from a 100 ms segment that began at 10% of the total vowel duration. The analysis segment was selected for each word/waveform to capture the steady state of the vowel. Software output values using linear predictive coding (LPC) formant tracking for the 100 ms segment were edited manually for formant tracking errors and the average per formant was used as the F1 through F4 values. The formant values of all seven words per vowel were then averaged, such that each participant had an average F1 for /a/, /ae/, /i/, and /u/ in the upright body position and an average F1 for each of the four vowels in the supine body position, and so on for the other formant frequencies. Fundamental frequency measurement was accomplished with TF32's pitch extraction performed on the selected 100 ms segment.
Formant bandwidth was measured manually from LPC spectra with a Hamming window placed over the middle 20 ms interval of the 100 ms analysis segment. Manual measurements were used because of questionable accuracy of formant bandwidths derived from LPC analyses (Burris et al., 2014). The bandwidths of only the second and third formant bandwidths (B2 and B3) were measured because we believed that these could be measured with satisfactory reliability and would be sufficient to detect bandwidth changes related to differences in body position. Formant bandwidths are determined by several factors that conceivably could be affected by the gravitational orientation of the VT. These include radiation, friction, and heat conduction losses, and dissipation in the walls of the VT (Fant, 1972; Fleischer et al., 2014). We assumed that measurement of two formant bandwidths would be adequate to determine if bandwidths are affected by body position although it is possible that such effects are formant specific. The bandwidths B2 and B3 were manually calculated as the width between ±3 dB of the F2 and F3 formant peaks, respectively, allowing a ±0.5 dB margin of error. Due to the narrowness of the formant peak or weakness of the formant, the bandwidth sometimes could not be measured, or the bandwidth could be measured only on one side. In the latter case, one side's value was doubled for the recorded bandwidth value. For each participant, the measured formant bandwidth values of the seven words per vowel were also averaged, so each participant had an average B2 for /a/, /ae/, /i/, and /u/ in the upright body position and an average B2 for each of the four vowels in the supine body position, and so on for B3.
A centroid was calculated as a measure representing the neutral vowel values for each participant using the average F1, F2, and F3 values of the four vowels in each body position. The Euclidean distance from the centroid to each corner vowel in the F1-F2-F3 acoustic space was calculated for all vowels in both upright and supine positions for each participant. All acoustic measurements were assessed for reliability. Duplicate measurements of the first and second formant frequencies, and the third and fourth formant frequencies were made by two different trained assistants using a random selection of 10% of the total 1489 waveforms (149 words). Paired t-tests between the original measurements and the duplicate measurements for each formant, for each group (male and female), and for each position (upright and supine) revealed no significant differences taking into account the Bonferroni correction (p ≥ 0.0125, see Appendix B).
C. Statistical treatment of data
Statistical analysis of data was performed with SAS software, version 9.4 for Windows. Mixed modeling techniques were used to account for within-subject correlation. The six APh variables were VTL-APh, subdivided into OCL-APh and PCL-APh, and VTV-APh, subdivided into OCV-APh and PCV-APh. All models included fixed effects for sex (male, female) and body position (upright, supine) and a random effect for subject to account for within-subject correlation. The significance level was adjusted using a Bonferroni correction to account for the six measurements for each subject (p = 0.05/6 = 0.0083). Residuals were examined to check model assumptions.
The eight speech acoustic variables were fundamental frequency (F0), formant frequencies (F1–F4), formant bandwidths (B2-B3), and computed Euclidean distance from the centroid in F1-F2-F3 acoustic space. Means and variances of all acoustic measures were calculated for each person by body position and vowel type. These means were used for modeling and to calculate Euclidean distance. All models included fixed effects for sex (male, female), body position (upright, supine), and vowel type (/i/ high-front, /ae/ low-front, /u/ high-back, /a/ low-back). An unstructured covariance matrix was used to model within-subject correlation and to accommodate unequal vowel type and body position variances. The significance level was adjusted using a Bonferroni correction to account for the eight measurements for each subject (p = 0.05/8 = 0.0063).
To examine whether the variability of acoustic measures differed by body position, within-person variances of F0, F1, F2, F3, and F4 were calculated for each person by body position and vowel type. Standard deviations were estimated by taking the square root of the mean of the individual variances grouped by sex, vowel type, and body position. Differences between supine and upright standard deviations were calculated.
III. RESULTS
A. Anatomy: APh
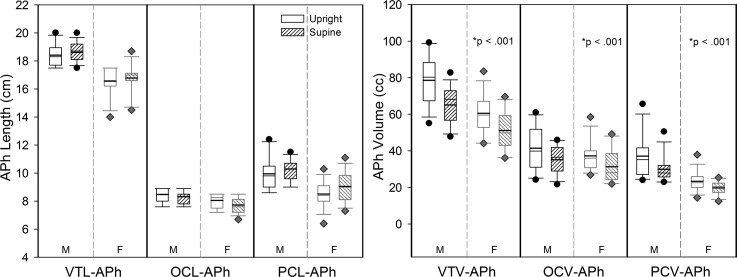
Figure 3 is a boxplot display of the anatomic data on length and volume of the oral, pharyngeal, and VT cavities as derived from APh for all participants in the upright and supine body positions. Aside from the apparent and expected sex differences in anatomic measures where male participants' length and volume values were larger than female participants' values, Fig. 3 shows that body position affects volume but not length measurements where volumetric measurements are smaller in the supine position (hashed boxes) than in the upright position (open boxes).
FIG. 3.
Box plots of APh data for length (left figure) and volume (right figure) for the VT, OC, and PC in males (M) and females (F) in the upright (no fill) and supine (hashed) body positions. The upper and lower bounds of the box are the 75th and 25th percentiles, respectively. The solid black line represents the mean, and the solid gray line represents the median. The upper and lower whiskers represent the 90th and 10th percentiles, respectively, with the black dots and gray diamonds representing outlying data for males and females, respectively. Asterisk denotes significance for body position.
Results of the mixed models with the fixed effects of sex and body position confirm the findings displayed in Fig. 3. There was a significant effect of body position for all three volumetric measurements with volumes being smaller in supine than upright: VTV-APh [F(1,25) = 79.52 (p = <0.0001)], OCV-APh [F(1,25) = 25.66 (p < 0.0001)], and PCV-APh [F(1,25) = 22.95 (p < 0.0001)]. As seen in Fig. 3, OCL-APh was greater in upright than supine and PCL-APh showed the opposite trend; however, none of the length measurements differed significantly by position at the adjusted alpha-level (p < 0.0083). As expected, there was a significant effect of sex (M > F) on 4 of the 6 APh measurements of length and volume (VTL-APh, PCL-APh, VTV-APh, PCV-APh, p < 0.0083; but not OCL-APh, p = 0.0085 or OCV-APh, p = 0.253), consistent with the data in Fig. 3. There was no significant interaction of Body Position × Sex (p > 0.10) for any of the variables (see Appendix C). We conclude that the three volumetric measurements are smaller in the supine than upright body position for individuals of both sexes.
B. Speech acoustics
The mixed effects models for the 8 acoustic variables, had 3 significant main effects for body position: F0 [F(1,25) = 18.04; p = 0.0003], F3 [F(1,25) = 35.65; p < 0.0001], and Euclidean distance [F(1,25) = 26.02; p < 0.0001]. Two variables had significant Position × Vowel interactions: F2 [F(3,23) = 10.43; p = 0.0002] and Euclidean distance [F(3,23) = 5.31; p = 0.0063]. Mixed model findings also confirmed significant sex differences with measurements being lower or smaller for male than female participants, as well as significant vowel differences and Sex × Vowel Type interactions. There were no significant interactions between Sex × Body Position or Sex × Body Position × Vowel Type, i.e., the effect of body position on speech acoustics was the same for male and female participants (see Appendix D).
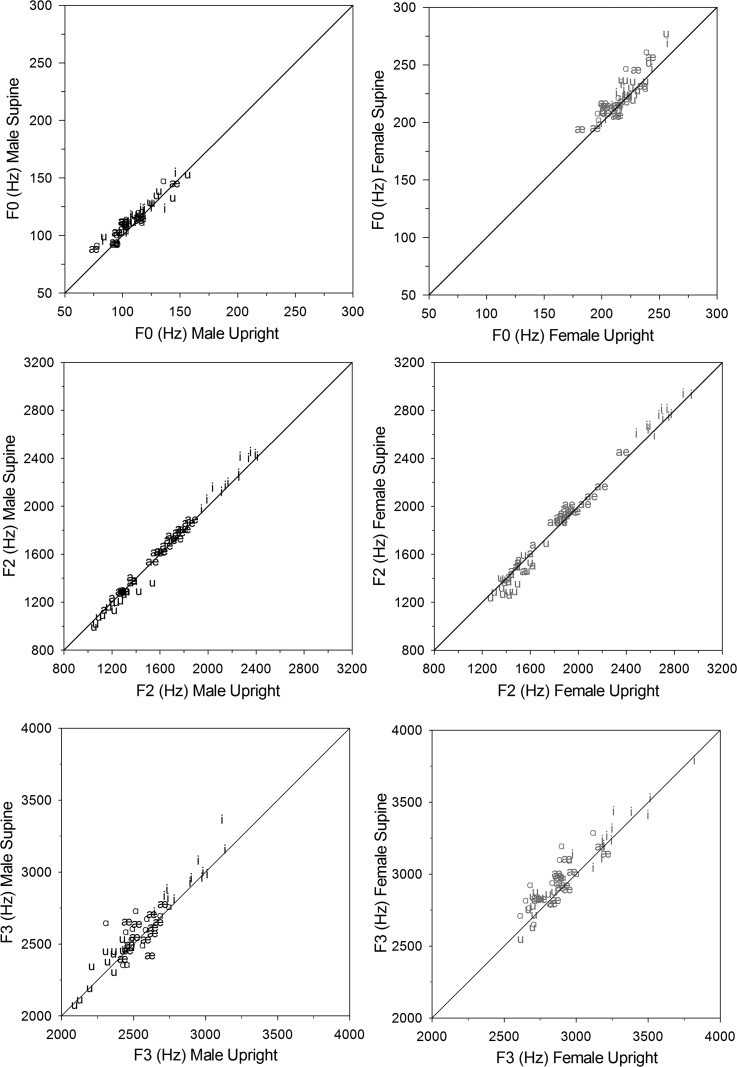
To illustrate these effects, findings are plotted in separate panels for males and females in Figs. 4 and 5. Figure 4 displays vowel specific values for F0, F2, and F3 in upright versus supine positions. There is an apparent trend for F0 and F3 measurements, across all vowels and for both males and females, to be greater in the supine position than in the upright position with more vowels plotted above the diagonal reference line. The Body Position × Vowel Type interaction for F2 can be seen in the middle panels, with more /i/, /a/, and /ae/ vowels above the line (supine > upright) and more /u/ vowels below the line (supine < upright) for both sexes.
FIG. 4.
Scatterplots of the acoustic variables with significant effects for body position: F0 main effect (top panel), F3 main effect (lower panel), and F2 interaction effect (middle panel. The diagonal solid lines (y = x) indicate the reference of perfect consistency between the two body positions.
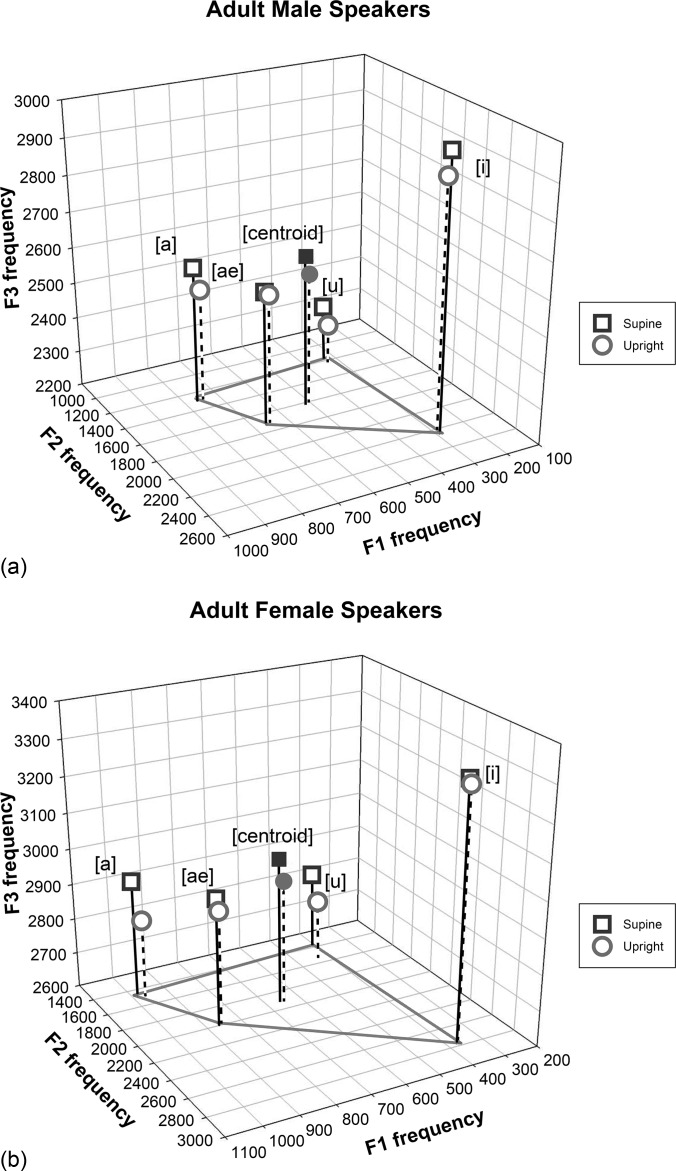
FIG. 5.
3D display of the vowel quadrilateral using the first three formants, as well as the 3D Euclidean distance from the centroid to each of the corner vowels in the F1-F2-F3 vowel acoustic space for male speakers (top) and female speakers (bottom).
Figure 5 is a three-dimensional (3D) display of the vowel quadrilateral using the first three formants and showing the centroid for each position in males [Fig. 5(a)] and females [Fig. 5(b)]. Aside from further illustrating that F3 frequencies were higher in the supine than the upright body position, it shows that the Euclidean distance was greater in supine than upright body position for all vowels and both sexes (significant effect of body position). The magnitude of the differences from the centroid varied by vowel type (significant Body Position × Vowel Type interaction), with /u/ showing the largest differences and /a/ showing the smallest. The significant Sex × Vowel Type interaction [F(3,23) = 9.31; p = 0.0004] is due to differences in distances between vowels for males and females.
Differences in variability in supine versus upright productions for F0 and F1-F4 were examined by taking the difference of the average standard deviation by sex, vowel, and body position for each measure. Women tended to have larger standard deviations than men. Also noted were differences in the variability by vowel type. However, there were no systematic trends for standard deviations to be greater in one body position than another (see Appendix E).
To summarize, the supine body position resulted in an increased F0, F3, and Euclidean distance in the F1-F2-F3 acoustic space, with a significant Vowel Type × Body Position interaction for F2 and Euclidean distance. There were no systematic differences in variability of F0 or F1–F4 by body position.
IV. DISCUSSION
This study examined the effect of body position on VT acoustics using the methods of APh and acoustic measures of vowel production, as an approach to assess the feasibility of using data from medical imaging studies obtained in the supine body position to address speech production in the upright body position. We hypothesized that for the APh portion of the study, the supine body position would yield smaller anatomic measures of the PCV but not its length. For the acoustic portion of the study, we hypothesized that body position would not affect any of the measurements of vowel production (F0, F1, F2, F3, F4, B2, and B3). Findings for APh revealed all three oral, pharyngeal, and VTVs to be significantly smaller in the supine position compared to the upright position. As for acoustic analyses of vowels, findings showed statistically significant effects of body position for three of the eight acoustic measures, with higher frequencies in the supine than upright position for the variables of F0 and F3, and greater Euclidean distance from the centroid to each corner vowel in the F1-F2-F3 space.
The significant effect of body position on VTV, specifically the PCV-APh, is comparable to the findings in several other studies (Bae et al., 2014; Jan et al., 1994; Stone et al., 2007; Sutthiprapaporn et al., 2008; Wrench et al., 2011). However, for the volumes of both the oral and pharyngeal cavities to be smaller in the supine body position makes it difficult to determine the possible effect of one cavity over the other on the acoustic measures. An interpretation of these results is that participants compensate for gravitational effects on the volume of the PC by making compensatory changes in the volume of the OC, thereby preserving the relative dimensions of these portions of the VT. Alternatively, the APh mouthpiece may have interfered with compensation of gravitational effect.
These anatomical results are specific to a VT in the upright versus supine body position, so these results reflect primarily the effect of gravity and not articulatory compensations made to achieve a desired phonetic target in the face of gravitational changes. The acoustic data reflect both gravity and compensations made to achieve vowel production in each gravitational orientation. As noted in the review of previous studies, articulatory adjustments are most likely to be seen in the naso- and oro-pharyngeal region, the jaw, and the larynx. These adjustments are made to preserve the basic acoustic properties of speech.
Concerning the cause of the increased F0 in the supine position, we can only speculate. One possibility is the differential influence of tracheal pull, which is expected to be greater in the upright than supine body position. However, we did not see evidence in either the APh or acoustic data of a change in the position of the larynx that would affect F0 by means such as those described by Honda et al. (1999). Presumably, tracheal pull would draw the larynx caudally, therefore increasing the length of the VT. Apparently, then, the increased F0 in the supine position occurred without an appreciable change in larynx position. Possibly, the reduced tracheal pull in the supine position facilitates a cricoid rotation that lengthens the vocal folds, thereby increasing F0. In addition, upright and supine body positions may have differential effects on the external frame function related to F0 control. Sonninen (1968) described the various extrinsic tension mechanisms operating on the vocal folds, including tracheal pull, the sternothyroid muscle, the thyrohyomandibular muscle chain, and the functional chain composed of the arytenoid cartilage, aryepiglottic muscle, epiglottis, tongue, hyoid, and mandible. These mechanisms may have different influences on F0 control, depending on body position.
The general lack of an effect of body position on the main vowel formants F1 and F2 frequencies agrees with the results of Weir et al. (1993), Tiede et al. (2000), Stone et al. (2007), and Bae et al. (2014) but not those of Shiller et al. (1999). The preponderance of the formant-frequency data from acoustic studies indicates that speakers achieve a high degree of compensation for changes in gravitational orientation. This conclusion is consistent with reports that speech production is little affected by weightlessness as experienced by astronauts (Nixon and Waggoner, 1962) although one report described nasal speech associated with this condition (Kerwin, 1975). Present findings of no main effect of body position on F2, but an interaction of body position and vowel type on F2 indicate that body position perturbs the production of some vowels more than others, and hence there may be more compensation in the production of those vowels.
Body position affected only one formant in particular. F3 had significantly higher values in the supine position than the upright position. Although the observed increase in F3 may be due to chance given that no other formants were affected, it appears from Fig. 4 that many speakers of both sexes have higher F3 frequency values in the supine position where F3 values for most vowels fall above the solid diagonal line that references perfect consistency between the two body positions. Figure 5 also illustrates the higher F3 frequencies in the supine than upright body position, confirming the validity of this result. This increased frequency may reflect an altered configuration in the VT. F3 of the vowel /a/ was most affected of the four corner vowels, which could mean that the back vowel was produced with further backing in the supine position, or there was a constriction in the oral or pharyngeal cavities leading to an increase of this formant value. F3 frequency has been related to the anterior region of the VT (Fant and Pauli, 1974). It would seem that if the tongue is retracted in the supine position, the effect most likely would be to increase the size of the front cavity, which would be expected to decrease rather than increase the F3 frequency. Additional imaging studies are warranted to assess changes in VT configuration in the upright and supine positions for the articulation of specific vowels and to determine the bases for changes in F3 frequency.
The Euclidean distance from the centroid to the corner vowels in the F1-F2-F3 space was significantly greater in the supine than in the upright position, but the differences between upright and supine productions varied by vowel and sex, with /u/ showing the greatest difference and /a/ the smallest. Body position especially affected the high vowels /i/ and /u/ for both sexes, suggesting that tongue height was accomplished somewhat differently between the upright and supine positions. Alternatively, if the same muscle forces were used to produce the high vowels in both body positions, the reduced gravitational effect in the supine position may have allowed a more extreme position of the tongue.
To our knowledge, previous acoustic studies of the effects of body position on vowel production have not addressed whether the variability of F0 or formant measures is affected. Given that speech is routinely produced in the upright position, it would not be surprising if the acoustic measures showed greater variability in the supine than upright position. However, we observed no consistent differences in variability, which can be interpreted to mean that the precision of speech motor control for vowel production is not affected by alteration in body position, at least for the two positions studied here. This finding also implies that there is no order effect (upright recording always preceding supine recordings), which was a limitation in study design.
It appears from our data and from most previous reports that adult speakers of both sexes can compensate fairly effectively for changes in orientation to gravity, thereby achieving main vowel formant patterns that do not change significantly with body position. Previous studies using a variety of techniques have shown that articulatory adjustments are most likely to be seen in the naso- and oro-pharyngeal region, the jaw, and the larynx. These adjustments apparently are made to preserve the basic acoustic properties of speech and are interpreted as evidence of compensation for gravitational effects. The adjustments are likely to be interdependent; for example, in the supine body position, protrusion of the mandible may help to counteract the gravitational pull on the tongue that reduces the pharyngeal lumen.
Given the methodologies used in this study, it is not feasible to assert Kollara and Perry's (2014) findings that imaging data obtained in the supine body position can be translated to speech production in the upright position. However, a major conclusion is that data obtained from one body position can be generalized with caution to another body position, and that additional investigations are warranted on the following issues:
-
(1)
Determining if and how children compensate for gravitational effects on the various components of the VT. The only published data for children pertain to velar function (Kollara and Perry, 2014). Possibly, compensation for changes in orientation to gravitational field requires motor control strategies that are developed with experience.
-
(2)
Establishing the extent to which data from the vocal activities of speech and singing are comparable to nonvocal activities such as rest breathing and swallowing. As indicated in Sec. I, somewhat different results have been reported for vocal versus nonvocal tasks. By virtue of their external target of an acoustic product, vocal tasks may involve specialized strategies that may not be used in nonvocal tasks.
-
(3)
Determining if individuals with compromised sensory or motor function of the aerodigestive tract are limited in compensation for changes in the gravitational field.
-
(4)
Exploring the effect of body position on F3 using the rhotic consonant /r/ and related r-colored vowels (such as the vowel in the General American pronunciation of the word bird). The acoustic marker of /r/ and r-colored vowels is a marked decrease in F3 frequency; for example, the F3 for /r/ is about 80% of the value averaged over a speaker's vowels (Hagiwara, 1995; Hamilton et al., 2014). This phonetically specific lowering of F3, in combination with the variability of /r/ articulation (Alwan et al., 1997; Westbury et al., 1998; Zhou et al., 2008), may be useful in further determining the effects of body position on this particular formant.
This study using APh and measures of vowel acoustics shows that the VT configuration is affected by body position, presumably because of adjustments to orientation in the gravitational field. Information on these adjustments is needed to allow comparisons of data obtained in supine and upright positions, especially because imaging data often are obtained in the supine position.
ACKNOWLEDGMENTS
This work was supported, in part, by National Institutes of Health Grant No. R01 DC006282 from the National Institute on Deafness and Other Communicative Disorders, and Core Grant No. P-30 HD0335 from the National Institute of Child Health and Human Development. S.L.K. was a former undergraduate student at the University of Wisconsin-Madison. We thank Carlyn Burris, Erin H. Douglas, and Jennifer J. Lewandowski for assistance with data collection and analysis, Sevahn K. Vorperian for Fig. 1 photograph, and Reid B. Durtschi, Daniel C. Reilly, Simon M. Lank, and Benjamin M. Doherty for assistance with the figures.
Appendix A
Stimuli for speech recordings.
| Vowel | ||||
|---|---|---|---|---|
| /i/ | /u/ | /a/ | /ae/ | |
| Stimulus | bee | boo | dot | bath |
| feet | shoe | hop | cat | |
| sheep | zoo | top | sad | |
| beada | hoota | hota | bata | |
| eata | boota | pota | hata | |
Word presented twice.
Appendix B
Inter-rater reliability: Paired t-test results of first-to-fourth formant frequency measurements (F1–F4) by two trained assistants, evaluated by sex and body position.
| Males | Females | |||
|---|---|---|---|---|
| Formant | t (df = 28) | p-value | t (df = 41) | p value |
| Upright | ||||
| F1 | −1.12 | 0.272 | −1.51 | 0.0139 |
| F2 | 1.96 | 0.061 | 0.07 | 0.947 |
| F3 | −1.217 | 0.234 | 0.193 | 0.848 |
| F4 | −0.787 | 0.439 | −0.615 | 0.542 |
| t (df = 40) | p-value | t (df = 40) | p-value | |
| Supine | ||||
| F1 | −0.34 | 0.738 | −0.85 | 0.402 |
| F2 | 2.44 | 0.019 | −1.38 | 0.177 |
| F3 | −1.12 | 0.270 | −0.037 | 0.971 |
| F4 | 0.57 | 0.575 | 0.643 | 0.525 |
Appendix C
Results of mixed effects models for six APh length and volume measurements. All models included a random effect for subject. N = 27 subjects (14 female, 13 male), with 2 observations per subject (2 positions). VTL-APh = OCL-APh + PCL-APh; VTV-APh = OCV-APh + PCV-APh.
| Sex | Body position | Sex × Body position | ||||
|---|---|---|---|---|---|---|
| Variable | F(1,25) | p-value | F(1,25) | p-value | F(1,25) | p-value |
| VTL-APh | 31.46 | <0.0001a | 2.65 | 0.1162 | 0.01 | 0.9097 |
| OCL-APh | 8.15 | 0.0085 | 6.50 | 0.0173 | 0.81 | 0.3772 |
| PCL-APh | 14.33 | 0.0009a | 7.72 | 0.0102 | 0.38 | 0.5442 |
| VTV-APh | 14.86 | 0.0007a | 79.62 | <0.0001a | 2.45 | 0.1302 |
| OCV-Aph | 1.37 | 0.2532 | 25.66 | <0.0001a | 0.04 | 0.8513 |
| PCV-APh | 19.88 | 0.0002a | 22.95 | <0.0001a | 2.52 | 0.1249 |
Significant at Bonferroni-adjusted alpha level, p = 0.0083.
Appendix D
Results of F-tests for fixed effects in mixed effects models for the eight speech acoustics variables: Fundamental frequency (F0); first-to-fourth formant frequencies (F1–F4); second and third formant bandwidths (B2–B3); Euclidean distance from centroid in F1-F2-F3 space (EucDist). Models were fit using an unstructured covariance matrix to account for within-subject correlation and unequal variance by vowel and position. Denominator df was estimated using the Kenward-Roger method. N = 27 subjects, with 8 observations per subject (2 positions × 4 vowels). There were five missing data points for B2.
| Sex | Body position | Vowel type | Sex × Body position | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | F(1,25) | p-value | F(1,25) | p-value | F(3,23) | p-value | F(1,25) | p-value |
| F0 | 342.98 | <0.0001a | 18.04 | 0.0003a | 35.14 | <0.0001a | 0.17 | 0.6825 |
| F1 | 112.45 | <0.0001a | 7.31 | 0.0122 | 444.91 | <0.0001a | 0.07 | 0.7994 |
| F2 | 93.89 | <0.0001a | 1.96 | 0.1735 | 450.02 | <0.0001a | 0.52 | 0.4757 |
| F3 | 90.05 | <0.0001a | 35.65 | <0.0001a | 89.02 | <0.0001a | 0.49 | 0.4919 |
| F4 | 177.09 | <0.0001a | 0.64 | 0.4327 | 55.49 | <0.0001a | 2.45 | 0.1300 |
| B2b | 35.83 | <0.0001a | 0.02 | 0.8914 | 4.02 | <0.0194 | 2.46 | 0.1296 |
| B3 | 0.32 | 0.5766 | 0.02 | 0.8995 | 18.99 | <0.0001a | 3.31 | 0.0810 |
| EucDist | 12.88 | 0.0014a | 26.02 | <0.0001a | 464.77 | <0.0001a | 0.09 | 0.7668 |
| Sex × Vowel type | Body position × Vowel type | Sex × Body position × Vowel type | ||||
|---|---|---|---|---|---|---|
| Variable | F(3,23) | p-value | F(3,23) | p-value | F(3,23) | p-value |
| F0 | 0.22 | 0.8807 | 2.07 | 0.1315 | 1.34 | 0.2867 |
| F1 | 9.06 | 0.0004a | 4.01 | 0.0197 | 1.00 | 0.4118 |
| F2 | 12.12 | <0.0001a | 10.43 | 0.0002a | 0.44 | 0.7287 |
| F3 | 1.70 | 0.1954 | 2.57 | 0.0787 | 1.92 | 0.1543 |
| F4 | 8.42 | 0.0006a | 0.64 | 0.5981 | 3.26 | 0.0399 |
| B2b | 5.17 | 0.0070 | 0.10 | 0.9608 | 0.76 | 0.5300 |
| B3 | 1.97 | 0.1459 | 0.10 | 0.9583 | 1.66 | 0.2033 |
| EucDist | 9.13 | 0.0004a | 5.31 | 0.0063a | 0.76 | 0.5261 |
Significant at Bonferroni-adjusted alpha level p = 0.0063.
B2 estimates for denominator df (DDF) differed due to five missing observations: Body position, Sex × Body position: DDF = 24.5; Vowel type, Sex × Vowel type: DDF = 23.2; Position × Vowel type, Sex × Body position × Vowel type: DDF = 22.8.
Appendix E
Average within-subject standard deviations by sex, vowel, and body position for F0, F1–F4. Differences are calculated as supine SD—upright SD.
| F0 SD | ||||
|---|---|---|---|---|
| Sex | Vowel | Upright | Supine | Difference |
| F | a | 11.50 | 6.95 | −4.55 |
| ae | 7.59 | 10.79 | 3.19 | |
| i | 8.68 | 7.40 | −1.28 | |
| u | 8.68 | 9.68 | 0.99 | |
| M | a | 8.67 | 5.81 | −2.86 |
| ae | 4.91 | 5.90 | 0.99 | |
| i | 5.56 | 5.32 | −0.23 | |
| u | 6.97 | 7.33 | 0.36 | |
| F1 SD | F2 SD | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Vowel | Upright | Supine | Difference | Upright | Supine | Difference |
| F | a | 45.05 | 47.92 | 2.88 | 62.43 | 47.22 | −15.21 |
| ae | 65.54 | 70.56 | 5.02 | 67.33 | 99.97 | 32.64 | |
| i | 22.07 | 18.51 | −3.56 | 85.14 | 107.46 | 22.32 | |
| u | 21.40 | 18.08 | −3.32 | 280.74 | 284.96 | 4.22 | |
| M | a | 25.12 | 29.44 | 4.32 | 56.96 | 52.29 | −4.67 |
| ae | 29.96 | 37.32 | 7.35 | 43.19 | 56.59 | 13.40 | |
| i | 15.25 | 14.62 | −0.63 | 73.36 | 65.68 | −7.68 | |
| u | 20.12 | 19.07 | −1.05 | 227.13 | 233.16 | 6.03 | |
| F3 SD | F4 SD | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Vowel | Upright | Supine | Difference | Upright | Supine | Difference |
| F | a | 84.26 | 98.97 | 14.71 | 144.75 | 165.01 | 20.27 |
| ae | 82.42 | 82.24 | −0.18 | 135.98 | 114.52 | −21.46 | |
| i | 114.00 | 127.84 | 13.84 | 93.53 | 71.25 | −22.28 | |
| u | 89.34 | 93.80 | 4.46 | 122.94 | 128.46 | 5.52 | |
| M | a | 54.03 | 81.65 | 27.62 | 119.54 | 175.03 | 55.49 |
| ae | 85.73 | 85.39 | −0.33 | 165.72 | 166.76 | 1.04 | |
| i | 88.20 | 100.82 | 12.61 | 100.99 | 216.04 | 115.05 | |
| u | 79.03 | 91.28 | 12.25 | 119.25 | 116.32 | −2.93 | |
References
- 1. Alwan, A. , Narayanan, S. , and Haker, K. (1997). “ Toward articulatory-acoustic models for liquid approximants based in MRI and EPG data. Part II. The rhotics,” J. Acoust. Soc. Am. 101, 1078–1089. 10.1121/1.417972 [DOI] [PubMed] [Google Scholar]
- 2. Bae, Y. , Perry, J. L. , and Kuehn, D. P. (2014). “ Videofluoroscopic investigation of body position on articulatory positioning,” J. Speech, Lang., Hear. Res. 57, 1135–1147. 10.1044/2013_JSLHR-S-12-0235 [DOI] [PubMed] [Google Scholar]
- 3. Brown, I. B. , McClean, P. A. , Boucher, R. , Zamel, N. , and Hoffstein, V. (1987). “ Changes in pharyngeal cross-sectional area with posture and application of continuous positive airway pressure in patients with obstructive sleep apnea,” Am. Rev. Respir. Dis. 136, 628–632. 10.1164/ajrccm/136.3.628 [DOI] [PubMed] [Google Scholar]
- 4. Buchaillard, S. , Perrier, P. , and Payan, Y. (2009). “ A biomechanical model of cardinal vowel production: Muscle activations and the impact of gravity on tongue positioning,” J. Acoust. Soc. Am. 126, 2033–2051. 10.1121/1.3204306 [DOI] [PubMed] [Google Scholar]
- 5. Burris, C. , Vorperian, H. K. , Fourakis, M. , Kent, R. D. , and Bolt, D. M. (2014). “ Quantitative and descriptive comparison of four acoustic analysis systems: Vowel measurements,” J. Speech, Lang., Hear. Res. 57, 26–45. 10.1044/1092-4388(2013/12-0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dietsch, A. M. , Cirstea, C. M. , Auer, E. T., Jr. , and Searl, J. P. (2013). “ Effects of body position and sex group on tongue pressure generation,” Int. J. Oralfac. Myology 39, 12–22. [PubMed] [Google Scholar]
- 7. D'Urzo, A. D. , Lawson, V. G. , Vassal, K. P. , Rebuck, A. S. , Slutsky, A. S. , and Hoffstein, V. (1987). “ Airway area by acoustic response measurements and computerized tomography,” Am. Rev. Respir. Dis. 135, 392–395. [DOI] [PubMed] [Google Scholar]
- 8. D'Urzo, A. D. , Rubinstein, I. , Lawson, V. G. , Vassal, K. P. , Rebuck, A. S. , Slutsky, A. S. , and Hoffstein, V. (1988). “ Comparison of glottic areas measured by acoustic reflections vs. computerized tomography,” J. Appl. Physiol. 64(1), 367–370. [DOI] [PubMed] [Google Scholar]
- 9. Engwall, O. (2006). “ Assessing magnetic resonance imaging measurements: Effects of sustenation, gravitation, and coarticulation,” in Speech Production: Models, Phonetic Processes, and Techniques, edited by Harrington J. and Tabain M. ( Psychology Press, New York: ), pp. 301–314. [Google Scholar]
- 10. Fant, G. (1972). “ Vocal tract wall effects, losses, and resonance bandwidths,” in Speech Transmission Laboratory Quarterly Progress and Status Reports ( KTH, Royal Institute of Technology, Stockholm: ), Vol. 13(2–3), pp. 28–52. [Google Scholar]
- 11. Fant, G. , and Pauli, S. (1974). “ Spatial characteristics of vocal tract resonance modes,” in Proceedings of Speech Communications Seminar: Speech communication, edited by G. Fant, Stockholm, SCS-74, Vol. 2, pp. 121–132. [Google Scholar]
- 12. Ferguson, K. A. , Cartwright, R. , Rogers, R. , and Schmidt-Nowara, W. (2006). “ Oral appliances for snoring and obstructive sleep apnea: A review,” Sleep 29, 244–262. [DOI] [PubMed] [Google Scholar]
- 13. Fitch, W. T. , and Giedd, J. (1999). “ Morphology and development of the human vocal tract: A study using magnetic resonance imaging,” J. Acoust. Soc. Am. 106, 1511–1522. 10.1121/1.427148 [DOI] [PubMed] [Google Scholar]
- 14. Fleischer, M. , Pinkert, S. , Mattheus, W. , Mainka, A. , and Murbe, D. (2014). “ Formant frequencies and bandwidths of the vocal transfer function are affected by the mechanical impedance of the vocal tract wall,” Biomech. Model Mechanobiol. 14(4), 719–733. 10.1007/s10237-014-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gelardi, M. , Del Giudice, A. M. , Cariti, F. , Cassano, M. , Farras, A. C. , Fiorella, M. L. , and Cassano, P. (2007). “ Acoustic pharyngometry: Clinical and instrumental correlations in sleep disorders,” Braz. J. Otorhinolaryngol. 73(2), 257–265. 10.1590/S0034-72992007000200018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagiwara, R. (1995). “ Acoustic realizations of American /r/ as produced by women and men” (Working Papers in Phonetics No. 90), University of California, Department of Linguistics, Los Angeles, CA, https://escholarship.org/uc/item/8779b7gq (Last viewed June 14, 2014).
- 17. Hamilton, S. , Boyce, S. , Scholl, L. , and Douglas, K. (2014). “ An acoustic threshold for third formant in American English,” J. Acoust. Soc. Am. 135, 2389. 10.1121/1.4877899 [DOI] [Google Scholar]
- 18. Hodge, M. , and Daniels, J. (2007). TOCS+ Intelligibility Measures ( University of Alberta, Edmonton, Alberta, Canada: ). [Google Scholar]
- 19. Hoffstein, V. , and Fredberg, J. J. (1991). “ The acoustic reflection technique for non-invasive assessment of upper airway area,” Eur. Respir. J. 4(5), 602–611. [PubMed] [Google Scholar]
- 20. Hoffstein, V. , and Zamel, N. (1984). “ Tracheal stenosis measured by the acoustic reflection technique,” Am. Rev. Respir. Dis. 130(3), 472–475. [DOI] [PubMed] [Google Scholar]
- 21. Honda, K. , Hirai, H. , Masaki, S. , and Shimada, Y. (1999). “ Role of vertical larynx movement and cervical lordosis in F0 control,” Lang. Speech 42, 401–411. 10.1177/00238309990420040301 [DOI] [PubMed] [Google Scholar]
- 22. Jan, M. A. , Marshall, I. , and Douglas, N. J. (1994). “ Effect of posture on upper airway dimensions in normal human,” Am. J. Respir. Crit. Care Med. 149, 145–148. 10.1164/ajrccm.149.1.8111573 [DOI] [PubMed] [Google Scholar]
- 23. Jung, D. G. , Cho, H. Y. , Grunstein, R. R. , and Yee, B. (2004). “ Predictive value of Kushida index and acoustic pharyngometry for the evaluation of upper airway in subjects with or without obstructive sleep apnea,” J. Korean Med. Sci. 19(5), 662–667. 10.3346/jkms.2004.19.5.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerwin, J. P. (1975). “ Weightlessness: A case history,” Acta Astronaut. 2, 85–87. 10.1016/0094-5765(75)90045-4 [DOI] [PubMed] [Google Scholar]
- 25. Kitamura, T. , Takemoto, H. , Honda, K. , Shimada, Y. , Fujimoto, I. , Syakudo, Y. , Masaki, S. , Kuroda, K. , Oku-uchi, N. , and Senda, M. (2005). “ Difference in vocal tract shape between upright and supine postures: Observations by an open-type MRI scanner,” Acoust. Sci. Technol. 26, 465–468. 10.1250/ast.26.465 [DOI] [Google Scholar]
- 26. Kollara, L. , and Perry, J. L. (2014). “ Effects of gravity on the velopharyngeal structures in children using upright magnetic resonance imaging,” Cleft Palate Craniofac. J. 51(6), 669–676. 10.1597/13-107 [DOI] [PubMed] [Google Scholar]
- 27. Marshall, I. , Maran, N. J. , Martin, S. , Jan, M. A. , Rimmington, J. E. , Best, J. J. K. , Drummond, G. B. , and Douglas, N. J. (1993). “ Acoustic reflectometry for airway measurements in man: Implementation and validation,” Physiol. Meas. 14(2), 157–169. 10.1088/0967-3334/14/2/007 [DOI] [PubMed] [Google Scholar]
- 28. Milenkovic, P. (2010). “ TF32, Time-frequency analysis software program for 32-bit Windows” (Alpha), Madison, WI. http://userpages.chorus.net/cspeech/ (Last viewed January 21, 2010).
- 29. Monahan, K. , Kirchner, H. L. , and Redline, S. (2005). “ Oropharyngeal dimensions in adults: Effect of ethnicity, gender, and sleep apnea,” J. Clin. Sleep Med. 1(3), 257–263. [PubMed] [Google Scholar]
- 30. Moon, J. , and Canady, J. (1995). “ Effects of gravity on velopharyngeal muscle activity during speech,” Cleft Palate Craniofac. J. 32, 371–375. [DOI] [PubMed] [Google Scholar]
- 31. Munson, B. , and Solomon, N. P. (2004). “ The effect of phonological neighborhood density on vowel articulation,” J. Speech, Lang., Hear. Res. 47, 1048–1058. 10.1044/1092-4388(2004/078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakayama, E. , Tohara, H. , Hiraba, H. , Sanpei, R. , Wakasa, H. , Ohno, S. , Kumakura, A. , Gora, K. , Abe, K. , and Ueda, K. (2013). “ Effects of reclining posture on velopharyngeal closing pressure during swallowing and phonation,” J. Oral Rehabil. 40, 450–456. 10.1111/joor.12050 [DOI] [PubMed] [Google Scholar]
- 33. Nixon, C. W. , and Waggoner, C. R. (1962). “ Speech during weightlessness,” MLR Technical Documentary Report 62-45, Wright-Patterson Air Force Base, OH.
- 34. Pae, E. K. , Lowe, A. A. , Sasaki, K. , Price, C. , Tsuchiya, M. , and Fleetham, J. A. (1994). “ A cephalometric and electromyographic study of upper airway structures in the upright and supine positions,” Am. J. Orthod. 106, 52–59. 10.1016/S0889-5406(94)70021-4 [DOI] [PubMed] [Google Scholar]
- 35. Perry, J. L. (2011). “ Variations in velopharyngeal structures between upright and supine positions using upright magnetic resonance imaging,” Cleft Palate Craniofac. J. 48, 123–133. 10.1597/09-256 [DOI] [PubMed] [Google Scholar]
- 36. Perry, J. L. , Bae, Y. , and Kuehn, D. P. (2012). “ Effect of posture on deglutitive biomechanics in healthy individuals,” Dysphagia 27, 70–80. 10.1007/s00455-011-9340-6 [DOI] [PubMed] [Google Scholar]
- 37. Shiller, D. M. , Ostry, D. J. , and Gribble, P. L. (1999). “ Effects of gravitational load on jaw movements in speech,” J. Neurosci. 19, 9073–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleep Group Solutions (2009). Acoustic Pharyngometer Operator Manual, North Miami Beach, FL, pp. 1–26.
- 39. Smith, A. , and Battagel, J. (2004). “ Non-apneic snoring and the orthodontist: Radiographic pharyngeal dimension changes with supine posture and mandibular protrusion,” J. Orthodon. 31, 124–131. 10.1179/146531204225020418 [DOI] [PubMed] [Google Scholar]
- 40. Sonninen, A. (1968). “ The external frame function in the control of pitch in the human voice,” Ann. N.Y. Acad. Sci. 155, 68–90. 10.1111/j.1749-6632.1968.tb56750.x [DOI] [Google Scholar]
- 41. Steiner, I. , Richmond, K. , Marshall, I. , and Gray, C. D. (2012). “ The magnetic resonance imaging subset of the mngu0 articulatory corpus,” J. Acoust. Soc. Am. 131, 106–111. 10.1121/1.3675459 [DOI] [PubMed] [Google Scholar]
- 42. Stone, M. , Stock, G. , Bunin, K. , Kumar, K. , Epstein, M. , Kambhamettu, C. , Li, M. , Parthasarathy, V. , and Prince, J. (2007). “ Comparison of speech production in upright and supine position,” J. Acoust. Soc. Am. 122, 532–541. 10.1121/1.2715659 [DOI] [PubMed] [Google Scholar]
- 43. Sutthiprapaporn, P. , Tanimoto, K. , Ohtsuka, M. , Nagasaki, T. , Iida, Y. , and Katsumata, A. (2008). “ Positional changes of oropharyngeal structures due to gravity in the upright and supine body positions,” Dentomaxillofac. Radiol. 37, 130–136. 10.1259/dmfr/31005700 [DOI] [PubMed] [Google Scholar]
- 44. Tiede, M. K. , Masaki, S. , and Vatikiotis-Bateson, E. (2000). “ Contrasts in speech articulation observed in sitting and supine conditions,” in Proceedings of the 5th Speech Production Seminar, May 1–4, Kloster Seeon, Bavaria, Germany, pp. 25–28. [Google Scholar]
- 45. Traser, L. , Burdumy, M. , Richter, B. , Vicari, M. , and Echtenach, M. (2013). “ The effect of supine and upright position on vocal tract configurations during singing: A comparative study in professional tenors,” J. Voice 27, 141–148. 10.1016/j.jvoice.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 46. Traser, L. , Burdumy, M. , Richter, B. , Vicari, M. , and Echternach, M. (2014). “ Weight-bearing MR imaging as an option in the study of gravitational effects on the vocal tract of untrained subjects in singing phonation,” PLoS One 9, e112405. 10.1371/journal.pone.0112405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Holsbeke, C. S. , Verhulst, S. L. , Vos, W. G. , De Backer, J. W. , Vinchurkar, S. C. , Verdonck, P. R. , van Doorn, J. W. D. , Nadjmi, N. , and De Backer, W. A. (2014). “ Change in upper airway geometry between upright and supine position during tidal nasal breathing,” J. Aerosol Med. Pulm. Drug Delivery 27, 51–57. 10.1089/jamp.2012.1010 [DOI] [PubMed] [Google Scholar]
- 48. Vorperian, H. K. (2013). “ VTLab acoustic pharyngometry (APh) protocol,” Waisman Center, University of Wisconsin-Madison, http://www.waisman.wisc.edu/vocal/links.html (Last viewed January 15, 2014).
- 49. Vorperian, H. K. , Wang, S. , Chung, M. K. , Schimek, E. M. , Durtschi, R. B. , Kent, R. D. , Ziegert, A. J. , and Gentry, L. R. (2009). “ Anatomic development of the oral and pharyngeal portions of the vocal tract: An imaging study,” J. Acoust. Soc. Am. 125, 1666–1678. 10.1121/1.3075589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weir, A. D. , McCutcheon, M. J. , and Flege, J. E. (1993). “ A comparison of formant frequencies for vowels pronounced in the supine and upright positions,” in Proceedings of the 12th Southern Biomedical Engineering Conference, pp. 188–190. [Google Scholar]
- 51. Westbury, J. R. , Hashi, M. , and Lindstrom, M. J. (1998). “ Differences among speakers in lingual articulation for American English / ɹ /,” Speech Commun. 26, 203–226. 10.1016/S0167-6393(98)00058-2 [DOI] [Google Scholar]
- 52. Wrench, A. , Cleland, H. , and Scobbie, J. M. (2011). “ An ultrasound protocol for comparing tongue contours: Upright vs. supine,” in Proceedings of the 17th International Congress of Phonetic Sciences, edited by Lee W. S. and Zee E., Hong Kong, pp. 17–21. [Google Scholar]
- 53. Xue, A. , Jiang, J. , Lin, E. , Glassenberg, R. , and Mueller, P. B. (1999). “ Age-related changes in human vocal tract configurations and the effects on speakers' vowel formant frequencies: A pilot study,” Logoped. Phoniatr. Vocol. 24, 132–137. 10.1080/140154399435084 [DOI] [Google Scholar]
- 54. Xue, S. A. , and Hao, J. G. (2006). “ Normative standards for vocal tract dimensions by race as measured by acoustic pharyngometry,” J. Voice 20(3), 391–400. 10.1016/j.jvoice.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 55. Xue, S. A. , Kaine, L. , and Ng, M. L. (2010). “ Quantification of vocal tract configuration of older children with Down syndrome: A pilot study,” Int. J. Ped. Otorhinolaryng. 74, 378–383. 10.1016/j.ijporl.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 56. Yildirim, N. , Fitzpatrick, M. , Whyte, K. , Jalleh, R. , Wightman, A. , and Douglas, N. (1991). “ The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome,” Am. Rev. Respir. Disord. 144, 845–847. 10.1164/ajrccm/144.4.845 [DOI] [PubMed] [Google Scholar]
- 57. Zhou, X. , Espy-Wilson, C. Y. , Boyce, S. , Tiede, M. , Holland, C. , and Choe, A. (2008). “ A magnetic resonance imaging-based articulatory and acoustic study of ‘retroflex’ and ‘bunched’ American English /r/,” J. Acoust. Soc. Am. 123, 4466–4481. 10.1121/1.2902168 [DOI] [PMC free article] [PubMed] [Google Scholar]