Abstract
To improve the therapeutic effectiveness of cell transplantation, a transplantation system of genetically modified, injectable spheroids was developed. The cell spheroids are prepared in a culture system on micropatterned plates coated with a thermosensitive polymer. A number of spheroids are formed on the plates, corresponding to the cell adhesion areas of 100 µm diameter that are regularly arrayed in a two-dimensional manner, surrounded by non-adhesive areas that are coated by a polyethylene glycol (PEG) matrix. The spheroids can be easily recovered as a liquid suspension by lowering the temperature of the plates, and their structure is well maintained by passing them through injection needles with a sufficiently large caliber (over 27 G). Genetic modification is achieved by gene transfection using the original non-viral gene carrier, polyplex nanomicelle, which is capable of introducing genes into cells without disrupting the spheroid structure. For primary hepatocyte spheroids transfected with a luciferase-expressing gene, the luciferase is sustainably obtained in transplanted animals, along with preserved hepatocyte function, as indicated by albumin expression. This system can be applied to a variety of cell types including mesenchymal stem cells.
Keywords: Bioengineering, Issue 101, Spheroid, Cell transplantation, Gene transfection, Non-viral carrier, Micropatterned plate, Genetic modification
Introduction
Cell transplantation therapy has attracted widespread attention for treating various intractable diseases. The activity and half-life of bioactive factors that are secreted by the transplanted cells are essential for improved therapeutic effectiveness of a cell transplantation system. Genetic modification of the cells prior to transplantation is a beneficial technique to regulate and manipulate cellular functions, including the secretion of the bioactive factors. It is also important to maintain a favorable microenvironment for the cells for avoiding cell death or loss of cell activity. Three-dimensional (3D) spheroid cell culture, in which cell-to-cell interactions are well preserved, is promising for this purpose, for example, for improving albumin secretion from primary hepatocytes and promoting multi-lineage differentiation from mesenchymal stem cells (MSCs) 1-7.
In this study, a novel combination system of spheroid culture and gene transfection is used to serve as a platform for genetically modified cell transplantation. For creating spheroid cells, a spheroid culture system on micropatterned culture plates is used. On these plates, cell adhesion areas of 100 µm diameter are regularly arrayed in a two-dimensional manner and are surrounded by non-adhesive areas coated by a PEG matrix3. By seeding an adequate number of cells, arrays of 3D spheroids of 100 µm in diameter are formed corresponding to the micropatterned culture bed.
The spheroids are recovered without disrupting their 3D structure by using thermosensitive cell-culture plates, that were coated with a thermosensitive polymer, poly(iso-propylacrylamide) (PIPAAm) 8-10. The micropatterned architecture is constructed on the thermosensitive plates (custom-built). By simply lowering the temperature of the plates, the spheroids are detached from the culture bed and dispersed in phosphate buffered saline (PBS). Thus, a large number of spheroids with a uniform size of 100 µm can be obtained in the form of an injectable suspension.
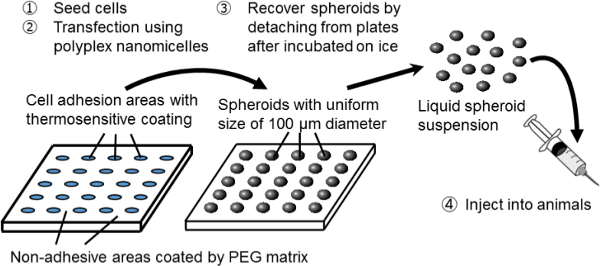 Figure 1. Schematic representation of the spheroid culture system on a micropatterned plate. Genetic modification is achieved by gene transfection using the original non-viral gene carrier, polyplex nanomicelle. It is composed of plasmid DNA (pDNA) and polyethylene glycol (PEG)-polycation block copolymers11. These have a characteristic core-shell structure consisting of a PEG shell and an inner core of condensed pDNA, allowing safe and effective gene introduction into cells for therapeutic purposes11. Please click here to view a larger version of this figure.
Figure 1. Schematic representation of the spheroid culture system on a micropatterned plate. Genetic modification is achieved by gene transfection using the original non-viral gene carrier, polyplex nanomicelle. It is composed of plasmid DNA (pDNA) and polyethylene glycol (PEG)-polycation block copolymers11. These have a characteristic core-shell structure consisting of a PEG shell and an inner core of condensed pDNA, allowing safe and effective gene introduction into cells for therapeutic purposes11. Please click here to view a larger version of this figure.
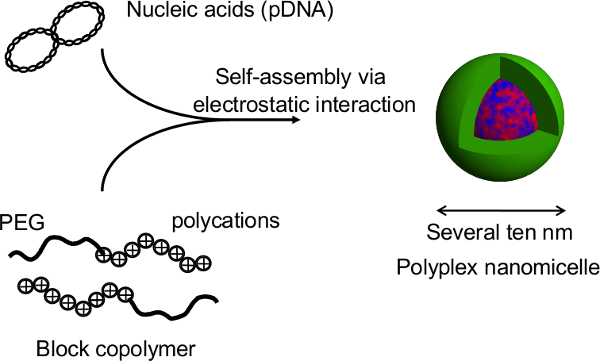 Figure 2. Structure of the polyplex nanomicelle formed by the complex of nucleic acids and PEG-block-polycation block copolymers. In this study, the primary advantage of this technique is that the spheroid structure is not disrupted during gene transfection by the nanomicelles. After nanomicelle-mediated transfections of rat primary hepatocyte spheroids, prolonged transgene expression is obtained for more than a month with continuous albumin secretion from the hepatocytes at a level comparable to that of untransfected spheroids12. The transgene expression and albumin secretion from the spheroids are also maintained after recovery from the thermosensitive plates. It is evident that nanomicelles can safely facilitate gene introduction without impairing the innate functions of the hepatocytes. Thus, the combination of spheroid cells cultured on thermosensitive micropatterned plates with gene introduction using nanomicelles is a promising platform for genetically modified cell transplantation. Please click here to view a larger version of this figure.
Figure 2. Structure of the polyplex nanomicelle formed by the complex of nucleic acids and PEG-block-polycation block copolymers. In this study, the primary advantage of this technique is that the spheroid structure is not disrupted during gene transfection by the nanomicelles. After nanomicelle-mediated transfections of rat primary hepatocyte spheroids, prolonged transgene expression is obtained for more than a month with continuous albumin secretion from the hepatocytes at a level comparable to that of untransfected spheroids12. The transgene expression and albumin secretion from the spheroids are also maintained after recovery from the thermosensitive plates. It is evident that nanomicelles can safely facilitate gene introduction without impairing the innate functions of the hepatocytes. Thus, the combination of spheroid cells cultured on thermosensitive micropatterned plates with gene introduction using nanomicelles is a promising platform for genetically modified cell transplantation. Please click here to view a larger version of this figure.
Protocol
All the animal studies were conducted with the approval of the Animal Care and Use Committee of the University of Tokyo, Tokyo, Japan.
1. Cell Preparation
- For primary hepatocytes, follow the protocol for hepatocyte isolation from rat by a modified two-step collagenase digestion process13,14.
- Anesthetize Sprague Dawley (SD) rats (male, 5 weeks old) under inhalation anesthesia with isoflurane. Place a rat in a chamber connected to an anesthesia machine to provide isoflurane for the chamber. Take out the rat after falling asleep, and set it on the operating table with ventilation using a mask. Control the isoflurane flow approximately at 0.4 – 0.7 L/min by checking the conditions of the rat. Perfuse the livers of Sprague Dawley (SD) rats (male, 5 weeks old) from the hepatic portal vein with a special solution composed of 8 g/L sodium chloride (NaCl), 400 mg/L potassium chloride (KCl), 78 mg/L sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O), 151 mg/L disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O), 2.38 g/L 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES), 190 mg/L ethylene glycol tetraacetic acid (EGTA), 350 mg/L sodium hydrogen carbonate (NaHCO3), and 900 mg/L glucose.
- Circulate collagenase solution through the liver. NOTE: The solution is composed of 500 mg/L collagenase, 9.8 g/L Hank’s buffered salt, 2.38 g/ml HEPES, 556 mg/ml calcium chloride hydrate (CaCl2·H2O), 350 mg/L NaHCO3, and 50 mg/L trypsin inhibitor, with the pH adjusted to 7.2.
- Remove the liver carefully, and mince it gently on a dish using a scalpel blade, add Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and filter the cell suspension through a 100 μm nylon mesh. To remove additional debris, centrifuge the cell suspension at 20 × g for 1 min. At this step, hepatocytes are in the supernatant.
- Repeat the centrifugation step twice (a total of three centrifugations). Finally, centrifuge the hepatocytes at 50 × g for 3 min to recover them in the form of a pellet.
- Re-suspend the hepatocytes to a concentration of 4 × 105 cells/ml in a special culture medium composed of DMEM supplemented with 10% FBS, 1% Pen-Strep-Glut (PSQ), 1% dimethyl sulfoxide (DMSO), 0.1 µmol/L dexamethasone, 0.5 µg/ml insulin, 10 mmol/L nicotinamide, 0.2 mmol/L phosphorylated ascorbate (Asc-2P), and 10 ng/ml human epidermal growth factor (hEGF)15. This special medium is mandatory for preserving the hepatic function under in vitro conditions.
For obtaining rat MSCs, euthanize Sprague Dawley (SD) rats (male, 5 weeks old) by excessive administration of isoflurane. Resect the femurs and tibias, and collect the bone marrows by inserting a 22 G needle into the shaft of the bone to flush it out with 10 ml of DMEM supplemented with 10% FBS. Collect the cells by filtration through a 100 µm nylon mesh.
Seed the cells onto 10 cm culture dishes using DMEM containing 10% FBS and 1% penicillin / streptomycin. For the spheroid experiments, use MSCs within 5 passages.
2. Preparation of 3D Cell Spheroids
Commercially obtain micropatterned culture plates, on which the cell adhesive areas are regularly arrayed at 100 µm diameter in a two-dimensional manner, surrounded by non-adhesive areas coated by the PEG matrix. NOTE: For cell transplantation, an additional coating with the thermosensitive polymer PIPAAm is necessary to allow cell detachment by cooling the plates (see step 5.1). Temperature fluctuations can induce changes in the chemistry of this polymer8-10. At 37 °C, PIPAAm is slightly hydrophobic, allowing the cells to be cultured under normal conditions. A decrease in the temperature below 32 °C results in rapid hydration of the polymer, leading to the spontaneous detachment of the cells.
Seed the hepatocytes or MSCs on 12-well micropatterned plates at a density of 4 × 105 cells/well andincubate them at 37 °C in a humidified atmosphere containing 5% CO2. The cells will accumulate on the micropatterned adhesion areas and gradually form round spheroids in 2 days. NOTE: For preparing control cells in a monolayer culture, use normal 12-well plates and seed the cells at an identical density, following a similar procedure as described above.
3. Preparation of Polyplex Nanomicelles
Synthesize a block copolymer, PEG-PAsp(DET) (poly[N’-[N-(2-aminoethyl)-2-aminoethyl] aspartamide]) (PEG Mw = 12,000, polymerization degree (DP) of PAsp(DET) segment = 59), and a homopolymer that is composed of only the cationic segment [PAsp(DET)] (DP = 55), following the procedures previously described by the authors11,16,17.
Prepare a mixed solution of the PEG-PAsp(DET) block copolymer and the PAsp(DET) homopolymer at an equal molar ratio of residual amino groups in 10 mM HEPES buffer (pH 7.3) by adjusting the polymer concentration to 33.3 µg/ml and 19.1 µg/ml, respectively. NOTE: The polymer concentrations described above are representative values for preparing nanomicelles with a residual molar ratio of the total amino groups in the two polymers to the phosphate groups in the pDNA (N/P ratio) of 10. The N/P ratio can vary depending on the cell type and the purpose (for details, see Discussion). The combined use of the two polymers can achieve both effective PEG shielding and functioning of PAsp(DET) to enhance endosomal escape (for details, see Discussion)18.
Prepare the pDNA encoding Gaussia luciferase, GL4 luciferase, or erythropoietin by cloning the segment expressing the respective genes into the pCAG-GS plasmid (http://www.cdb.riken.jp/pcs/protocol/vector/map/m36.html) to obtain expression under the CAG promoter/enhancer using a commercial kit according to manufacturer’s protocol. Amplify the pDNA in a competent Escherichia coli strain and purify it using an endotoxin-free plasmid DNA purification system. Determine the pDNA concentration at an absorbance of 260 nm to obtain a 150 µg/ml solution in 10 mM HEPES buffer (pH 7.3).
To prepare the polyplex nanomicelles, thoroughly mix the pDNA solution (150 µg/ml in 10 mM HEPES buffer) and the premixed solution of the two polymers at a ratio of 2:1 (by volume).
4. Gene Transfection into Spheroids
Incubate the cells (hepatocytes or MSCs) for 72 hrs after seeding onto the micropatterned plates to allow for the formation of mature spheroids. For gene transfection, add 100 µl of the polyplex nanomicelle solution (containing 10 µg of pDNA) to each well after replacing the culture medium with 1 ml of fresh medium. Continue the incubation with the nanomicelle solution for 24 hrs.
For a control using a lipid-based transfection reagent, mix pDNA solutions with the reagent at a weight ratio reagent / pDNA of 3. Adjust the final dose of pDNA to be equal for both the lipid-based reagent and nanomicelle methods.
5. Recovery and Transplantation of Cell Spheroids
Replace the culture medium with 200 µl of chilled PBS and place the plates on ice. NOTE: Generally, the spheroids can detach in about 15 min and can be recovered in the form of a suspension for transplantation.
Gently aspirate the cells in the 200 µl suspension using a syringe with a 23 G or 27 G needle for in vivo injections.
6. Evaluation of Transgene Expression
For the in vitro evaluation of the expression of Gaussia luciferase secreted in the culture medium, collect 50 – 100 µl of the medium precisely 24 hrs after replacing with fresh medium. Estimate the luciferase expression using a commercial renilla luciferase assay system and a luminometer according to manufacturer’s protocol. NOTE: The Gaussia luciferase remains stable in the culture medium for more than a week. Thus, to evaluate the real-time efficiency of the transgene expression, replace the medium with fresh one prior to collecting the sample medium. The timing of the medium change can be flexible.
- For the in vivo evaluation of the transgene expression in host animals following cell transplantation, anesthetize BALB/c nude mice (female; 7 weeks old) under inhalation anesthesia with isoflurane.
- Place a mouse in a chamber connected to an anesthesia machine to provide isoflurane for the chamber. Take out the mouse after falling asleep, and set it on the operating table with ventilation using a mask. Control the isoflurane flow approximately at 0.2 – 0.5 L/min by checking the conditions of the mouse.
- Inject 200 µl of the cell suspension containing spheroids transfected with the GL4 luciferase-expressing pDNA (as described in 4.1) into the subcutaneous tissue of the abdominal region.
- Immediately after injecting D-luciferin (150 mg/kg; intravenous route), measure the luciferase expression using IVIS imaging system according to manufacturer’s protocol.
- For evaluating the therapeutic effects of the cell transplantation, inject 200 µl of the cell suspension containing spheroids transfected with the erythropoietin-encoding pDNA into the subcutaneous tissue of the abdominal region.
- Collect blood samples by submandibular bleeding to obtain approximately 200 µl of blood19. Measure the hemoglobin and hematocrit using a blood sample analyzer. NOTE: The cell number for transplantation is regulated by the number seeded on the plates. Unfortunately, it is difficult to determine the exact cell number because the number inside spheroids cannot be measured.
After experiments, place the mice on a heating pad connected with a temperature controller until awakening from anesthesia.
Representative Results
Gene transfection of the Gaussia luciferase-expressing pDNA was performed in the spheroids formed by the hepatocytes or MSCs using polyplex nanomicelles or the control lipid-based transfection reagent12. The nanomicelles induced almost no change in the spheroid structure compared with non-transfected spheroids on the micropatterned plates, whereas the control reagent significantly disrupted the structure a day after the transfection (Figure 3). After transfection using the nanomicelles, consistent albumin secretion and the transgene (Gaussia luciferase) expression were well maintained for almost a month. However, when the control reagent was used, no albumin secretion was observed, although the degree of the transgene expression was similar to that obtained from the nanomicelles (Figure 4) 12.
During spheroid recovery using the thermosensitive micropatterned plates, the 3D structure of the spheroids was well preserved for both the primary hepatocytes and the MSCs in suspension after recovery from the cooled plates (Figure 5). The structure was also well maintained after passing the spheroids through injection needles with a sufficiently large caliber, such as the 23 (400 µm) and 27 G (220 µm) needles.
After subcutaneous injections of the recovered hepatocyte spheroids, luciferase expression was detected in the animals for more than a few weeks (Figure 6) 20. Albumin expression from transplanted hepatocytes was observed in the host tissue20, suggesting that the cell function was preserved through the process of spheroid recovery and transplantation. To test their potential for therapeutic applications, the hepatocyte spheroids were also transfected with erythropoietin-encoding pDNA. After subcutaneous transplantation of the spheroids expressing erythropoietin, a significant hematopoietic effect was obtained in the animals for one month (Figure 7) 20.
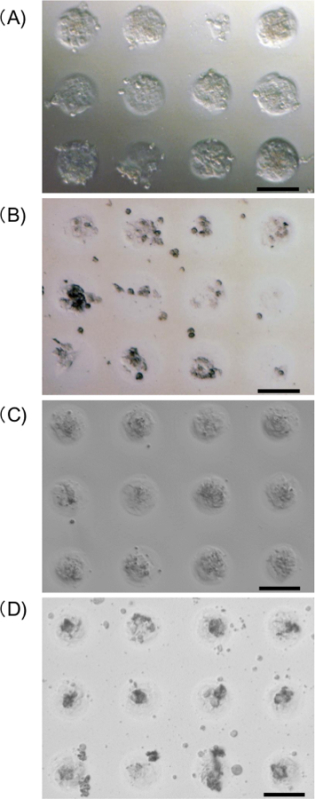 Figure 3. Microscopic images of hepatocyte spheroids (A, B) and MSC spheroids (C, D) on a micropatterned plate a day after gene transfection, using polyplex nanomicelles (A, C) or lipid-based reagent (B, D). Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 3. Microscopic images of hepatocyte spheroids (A, B) and MSC spheroids (C, D) on a micropatterned plate a day after gene transfection, using polyplex nanomicelles (A, C) or lipid-based reagent (B, D). Scale bar: 100 µm. Please click here to view a larger version of this figure.
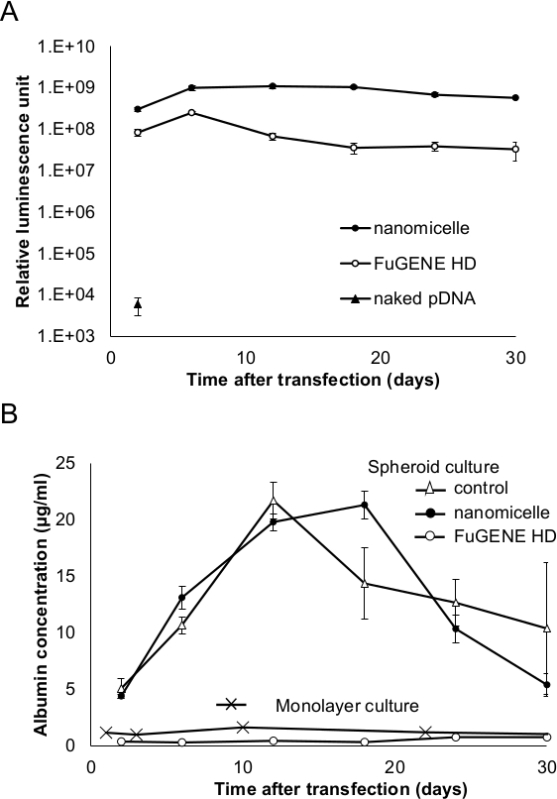 Figure 4. (A) Transgene expression of Gaussia luciferase after transfecting the hepatocyte spheroids. (B) Albumin secretion from the hepatocyte spheroids or hepatocytes in a monolayer culture after the transfection. Spheroids were transfected using nanomicelle (●) or control lipid-based transfection reagent (○), or naked pDNA (▲). The albumin secretion from the control (untransfected) hepatocytes (in spheroids (△) or monolayer culture (×)) is also shown. The results are represented as mean ± SEM; n = 4 for monolayer culture and n = 6 for spheroids. (Reprinted with permission from reference 12). Please click here to view a larger version of this figure.
Figure 4. (A) Transgene expression of Gaussia luciferase after transfecting the hepatocyte spheroids. (B) Albumin secretion from the hepatocyte spheroids or hepatocytes in a monolayer culture after the transfection. Spheroids were transfected using nanomicelle (●) or control lipid-based transfection reagent (○), or naked pDNA (▲). The albumin secretion from the control (untransfected) hepatocytes (in spheroids (△) or monolayer culture (×)) is also shown. The results are represented as mean ± SEM; n = 4 for monolayer culture and n = 6 for spheroids. (Reprinted with permission from reference 12). Please click here to view a larger version of this figure.
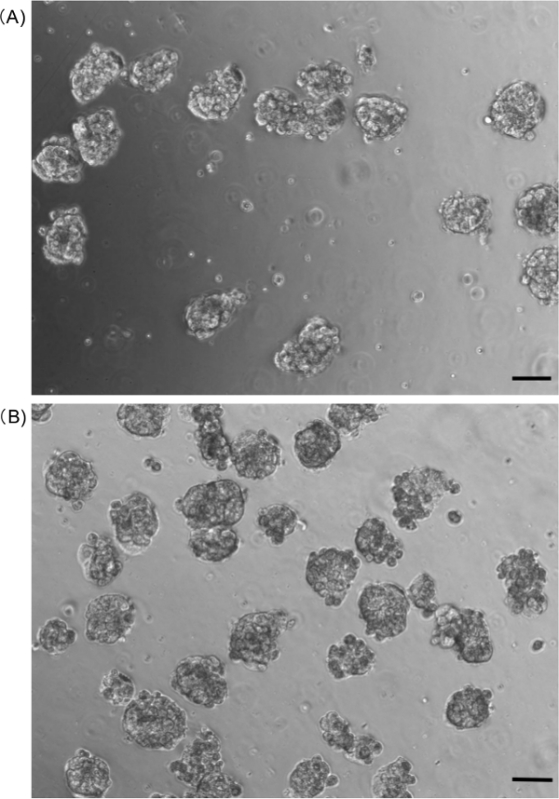 Figure 5. Microscopic images of the hepatocyte spheroids in suspension after passing them through (A) 23- or (B) 27 G injection needles. Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 5. Microscopic images of the hepatocyte spheroids in suspension after passing them through (A) 23- or (B) 27 G injection needles. Scale bar: 100 µm. Please click here to view a larger version of this figure.
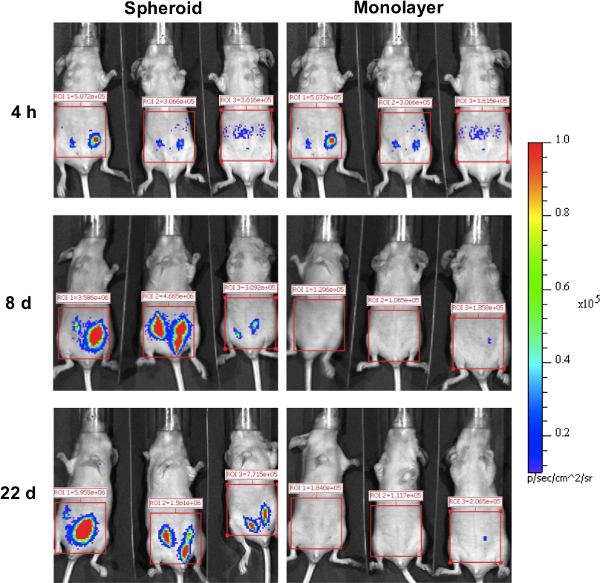 Figure 6. Luciferase expression in host mice after hepatocyte transplantation. After 24 hrs after transfection with luciferase (GL4)-encoding pDNA, the hepatocyte spheroids and single-cell suspension from the monolayer cultures were transplanted into the subcutaneous tissue of the abdominal region. The luciferase expression in the host mice was evaluated using an IVIS Imaging System (Reprinted with permission from reference 20).Please click here to view a larger version of this figure.
Figure 6. Luciferase expression in host mice after hepatocyte transplantation. After 24 hrs after transfection with luciferase (GL4)-encoding pDNA, the hepatocyte spheroids and single-cell suspension from the monolayer cultures were transplanted into the subcutaneous tissue of the abdominal region. The luciferase expression in the host mice was evaluated using an IVIS Imaging System (Reprinted with permission from reference 20).Please click here to view a larger version of this figure.
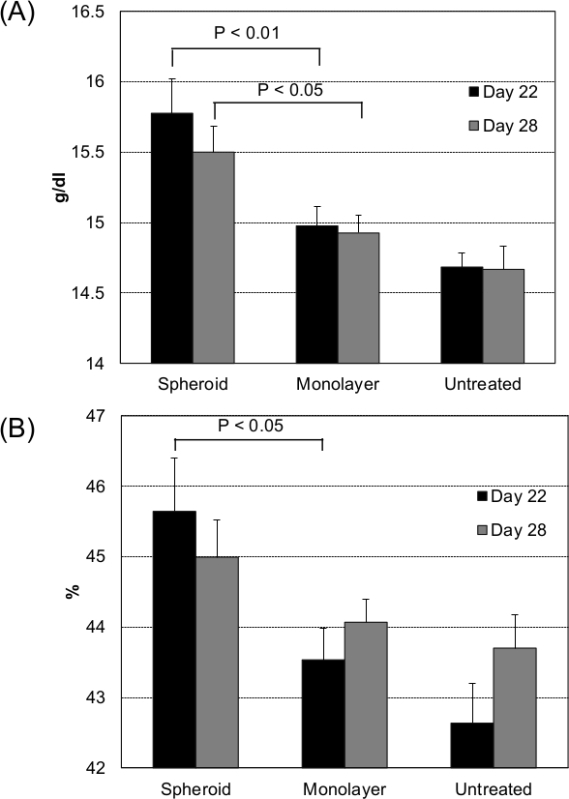 Figure 7. Hematopoiesis after transplantation of the erythropoietin-expressing hepatocytes. Hepatocytes in the spheroid and monolayer cultures were transfected with erythropoietin-encoding pDNA. After 24 hrs of transfection, the spheroids and single-cell suspensions from the monolayer cultures were subcutaneously transplanted into the mouse abdomen. At 22 and 28 days after transplantation, (A) hemoglobin and (B) hematocrit levels were measured from the blood samples. Data are presented as the mean ± SEM; n = 12 for spheroid and monolayer groups and n = 6 for untreated controls. Statistical significance was determined by a 2-tailed Student's t-test (Reprinted with permission from reference 20). Please click here to view a larger version of this figure.
Figure 7. Hematopoiesis after transplantation of the erythropoietin-expressing hepatocytes. Hepatocytes in the spheroid and monolayer cultures were transfected with erythropoietin-encoding pDNA. After 24 hrs of transfection, the spheroids and single-cell suspensions from the monolayer cultures were subcutaneously transplanted into the mouse abdomen. At 22 and 28 days after transplantation, (A) hemoglobin and (B) hematocrit levels were measured from the blood samples. Data are presented as the mean ± SEM; n = 12 for spheroid and monolayer groups and n = 6 for untreated controls. Statistical significance was determined by a 2-tailed Student's t-test (Reprinted with permission from reference 20). Please click here to view a larger version of this figure.
Discussion
In this protocol, it is critical to maintain the 3D structure of spheroids during the steps of gene introduction and spheroid recovery. It is essential to maintain a favorable microenvironments for the cells to avoid cell death or loss of cell activity. For example, albumin secretion, a representative innate function of hepatocytes, is well preserved in the hepatocyte spheroids, while the hepatocytes in the conventional monolayer culture rapidly lose their secretory capacity a few days after seeding12. For MSCs, the spheroids significantly enhance their efficiency of differentiation into adipocytes or osteoblasts, with increased expression levels of genes involved in adipogenesis and osteogenesis, and down-regulation of genes that maintain the MSC self-renewal phenotype4.
For gene introduction, various transfection reagents had been tested previously, including lipid-based ones (Figure 3, 4) and polymer-based ones, such as polyethylenimine. Unfortunately, none of the reagents provided successful gene introduction without disrupting the spheroid structure. Especially, the lipid-based reagents tend to induce complete disruption of the spheroids, although the transgene expression is efficient, even in the remaining cells on the micropatterned plates. It is likely that the lipid-based reagents induce significant membrane destabilization, leading to high transgene expression, but disrupt the spheroids by rendering the cell-to-cell interactions unstable.
In contrast, the cationic polymer disrupted the spheroids to a lesser extent than the lipids, especially in the conditions of low N/P ratios, to form the polyplexes. The standard protocol shown here uses polyplex nanomicelles that possess PEG shielding. Alternatively, polyplexes with cationic homopolymers, such as polyethylenimine, can also be used for gene introduction into the spheroids. Indeed, cationic polyplexes without the PEG surface generally provide high transgene expression in in vitro transfections, and are attractive options depending on the cell type and purpose of the spheroid transplantation.
A characteristic feature of this protocol is the use of micropatterned culture plates coated with a thermosensitive polymer. The micropatterned arrays can produce a large number of cell spheroids with a uniform diameter. This protocol uses 100 µm diameter arrays, but this aspect is flexible; however, very large diameters, such as 500 µm, often cause cell necrosis in the core of the spheroids (unpublished data). By simply cooling the plates on ice, the spheroids can be obtained in the form of an injectable suspension by gentle aspiration with a syringe. A potential limitation of this technique is the size of the needle used to inject the spheroids. It was confirmed that a 27-G needle can be safely used for spheroids with 100 µm diameter. However, narrow needles cannot be applied for these spheroids. Since a 27-G needle is too large, especially for transplanting spheroids into mouse spinal cords, a possible alternative is to use scaffolds to retain cells inside them.
This protocol can be potentially applied for several purposes of cell transplantation. Compared to the suspension spheroid culture system, the micropatterned plates have the advantage that a sufficient number of spheroids with uniform diameter can be easily obtained. Although the method of gene introduction can be flexible, as mentioned previously, polyplex nanomicelles with PEG shielding are particularly effective for avoiding spheroid disruption. Hopefully, this system will improve the therapeutic effects of transplanted cells.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We deeply appreciate Dr. Takeshi Ikeya and technical staff in Toyo Gosei, Tokyo, Japan for providing thermosensitive micropatterned culture plates as well as scientific advice. We also thank Ms. Satomi Ogura, Ms. Sae Suzuki, Ms. Asuka Miyoshi and Ms. Katsue Morii for technical assistance with animal experiments. This work was financially supported in part by the JSPS KAKENHI Grant-in-Aid for Scientific Research, the Center of Innovation (COI) Program and the S- innovation program from the Japan Science and Technology Agency (JST), and the JSPS Core-to-Core Program, A. Advanced Research Networks.
References
- Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101(3):914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa C, Tomita Y, Shono M, Ishimura K, Ichihara A. Importance of cell aggregation for expression of liver functions and regeneration demonstrated with primary cultured hepatocytes. J Cell Physiol. 1993;156(3):522–530. doi: 10.1002/jcp.1041560311. [DOI] [PubMed] [Google Scholar]
- Otsuka H, et al. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. Chembiochem. 2004;5(6):850–855. doi: 10.1002/cbic.200300822. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30(14):2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Bartosh TJ, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16(4):735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- Nakasone Y, Yamamoto M, Tateishi T, Otsuka H. Hepatocyte spheroids underlayered with nonparenchymal cells for biomedical applications. IEICE Transactions on Electronics. 2011;E94:176–180. [Google Scholar]
- Nishida K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Ohashi K, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- Sekine H, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17(23-24):2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- Itaka K, Kataoka K. Progress and prospects of polyplex nanomicelles for plasmid DNA delivery. Curr Gene Ther. 2011;11(6):457–465. doi: 10.2174/156652311798192879. [DOI] [PubMed] [Google Scholar]
- Endo T, Itaka K, Shioyama M, Uchida S, Kataoka K. Gene transfection to spheroid culture system on micropatterned culture plate by polyplex nanomicelle: a novel platform of genetically-modified cell transplantation. Drug Deliv and Transl Res. 2012;2(5):398–405. doi: 10.1007/s13346-012-0091-1. [DOI] [PubMed] [Google Scholar]
- Howard RB, Christensen AK, Gibbs FA, Pesch LA. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967;35(3):675–684. doi: 10.1083/jcb.35.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno C, Yoshizato K. Long-term cultivation of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. Am J Pathol. 1996;148(2):383–392. [PMC free article] [PubMed] [Google Scholar]
- Kanayama N, et al. A PEG-based biocompatible block catiomer with high buffering capacity for the construction of polyplex micelles showing efficient gene transfer toward primary cells. ChemMedChem. 2006;1(4):439–444. doi: 10.1002/cmdc.200600008. [DOI] [PubMed] [Google Scholar]
- Itaka K, Ishii T, Hasegawa Y, Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials. 2010;31(13):3707–3714. doi: 10.1016/j.biomaterials.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. PEGylated Polyplex With Optimized PEG Shielding Enhances Gene Introduction in Lungs by Minimizing Inflammatory Responses. Mol Ther. 2012;20(6):1196–1203. doi: 10.1038/mt.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Uchida S, et al. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35(8):2499–2506. doi: 10.1016/j.biomaterials.2013.12.012. [DOI] [PubMed] [Google Scholar]


