Abstract
Generation of induced pluripotent stem cell (iPSCs) from adult skin fibroblasts and subsequent differentiation into somatic cells provides fascinating prospects for the derivation of autologous transplants that circumvent histocompatibility barriers. However, progression through a pluripotent state and subsequent complete differentiation into desired lineages remains a roadblock for the clinical translation of iPSC technology because of the associated neoplastic potential and genomic instability. Recently, we and others showed that somatic cells cannot only be converted into iPSCs but also into different types of multipotent somatic stem cells by using defined factors, thereby circumventing progression through the pluripotent state. In particular, the direct conversion of human fibroblasts into induced neural progenitor cells (iNPCs) heralds the possibility of a novel autologous cell source for various applications such as cell replacement, disease modeling and drug screening. Here, we describe the isolation of adult human primary fibroblasts by skin biopsy and their efficient direct conversion into iNPCs by timely restricted expression of Oct4, Sox2, Klf4, as well as c-Myc. Sox2-positive neuroepithelial colonies appear after 17 days of induction and iNPC lines can be established efficiently by monoclonal isolation and expansion. Precise adjustment of viral multiplicity of infection and supplementation of leukemia inhibitory factor during the induction phase represent critical factors to achieve conversion efficiencies of up to 0.2%. Thus far, patient-specific iNPC lines could be expanded for more than 12 passages and uniformly display morphological and molecular features of neural stem/progenitor cells, such as the expression of Nestin and Sox2. The iNPC lines can be differentiated into neurons and astrocytes as judged by staining against TUJ1 and GFAP, respectively. In conclusion, we report a robust protocol for the derivation and direct conversion of human fibroblasts into stably expandable neural progenitor cells that might provide a cellular source for biomedical applications such as autologous neural cell replacement and disease modeling.
Keywords: Neuroscience, Issue 101, Direct conversion, lineage reprogramming, transgene-free reprogrammed cells, neural stem cells, transdifferentiation, neuronal differentiation, glial differentiation, stem cell biology, disease modeling, neural cell replacement, stem cell therapy.
Introduction
In 2006 Yamanaka and colleagues could show for the first time the possibility of reprogramming of somatic cells into a pluripotent state1. This dedifferentiation was achieved by overexpression of four transcription factors Oct4, Sox2, Klf4, and c-Myc in murine fibroblasts. The generated so-called induced pluripotent stem cells (iPSCs) show functional equivalence to embryonic stem cells (ESCs) and can thus be differentiated into all cell types of the adult organism. One year later reprogramming to iPSCs could also be achieved for human fibroblasts2. Experiments in animal models demonstrate that iPSC-derived cells can generally be used for cell replacement therapy, e.g., in Parkinson’s Disease (PD) 3-5. However, several limitations associated with the use of iPSCs represent roadblocks for the full realization of their therapeutic potential. First of all, reprogramming of cells into a pluripotent state and subsequent quality control is generally a time-consuming and inefficient process yielding in extensive and thus costly cell culture procedures. Second, iPSCs need to be re-differentiated into the desired cell type of interest before biomedical application and the probability of residual pluripotent cells in the differentiated population harbors a significant tumorigenic potential and thus displays a high risk after cell transplantation6. Third, the reprogramming process is usually achieved by inducing the reprogramming factors by lenti- or retroviral infection. The integration of these viruses into the host genome might lead to insertional mutagenesis and/or uncontrolled reactivation of the transgenes7,8. Non-integrative systems have been developed to deliver the reprogramming factors to target cells, which minimize the risk of insertional mutagenesis and transgene reactivation. Examples for these transgene-free approaches are the reprogramming of cells using non-integrating Adeno or Sendai virus9,10, DNA-based vectors11 or the application of DNA-free methods, like transfection of synthetic mRNA12 or transduction of recombinant proteins13,14. Another promising method for the derivation of transgene-free iPSC is the use of loxP-modified lentiviral reprogramming constructs and subsequent deletion of transgenes using the Cre-loxP DNA recombination system15,16.
A more straightforward approach to generate neural cells for cell replacement therapy represents direct conversion of fibroblasts into post-mitotic neurons17-20. Vierbuchen et al. reported that the overexpression of transcription factors Ascl1, Brn2 and Myt1l results in the generation of 20% neurons from murine fibroblasts17. In 2011 it was shown, that the same three transcription factors in combination with overexpression of NeuroD1 enable transdifferentiation of human fibroblasts into neurons19. Human induced neurons could also be generated by overexpression of Ascl1 and Ngn2 under dual SMAD- and GSK3β- inhibition20. Notably, direct conversion of fibroblasts into neurons generates a non-proliferative, post-mitotic cell population that does not allow further expansion and biobanking.
Recently, the direct conversion of fibroblasts into a proliferating neural stem/progenitor cell population was reported21-26. For sake of clarity, all these cell types will be named as induced neural progenitor cells (iNPCs) in this report. Han et al. overexpressed Brn4, Sox2, c-Myc and Klf4 to generate iNPCs. Like their neural stem cell counterparts derived from either primary tissue or pluripotent cells these iNPCs are tripotential and could be differentiated into neurons, astrocytes and oligodendrocytes21. Our group reported a slightly different conversion protocol involving overexpression of Sox2, Klf4, and c-Myc and induced Oct4-expression for 5 days only. With this approach we could generate stably proliferating iNPCs from murine embryonic and adult fibroblasts that exhibit complete silencing of the reprogramming factors22. In contrast to iPSCs, iNPCs do not exhibit a tumorigenic potential after transplantation27. We used converted cells successfully in an animal model of demyelination, myelin deficient rats, demonstrating that iNPCs are clinically useful22. Until then, NPCs could be only generated from pluripotent stem cells or primary neural tissue28-32. iNPCs are stably expandable cells that can be cryopreserved and are able to differentiate into neurons, astrocytes and oligodendrocytes. Much effort has been made to adapt the direct conversion protocol from mouse to human cells23,26,33,34. In 2012 it was published that overexpression of the single factor Sox2 in fibroblasts is sufficient to generate murine and human iNPCs33. The authors reported generation of human iNPCs from fetal foreskin fibroblasts and characterized them by staining against Sox2 and Nestin. However, the target cells used for reprogramming represent a very particular cell type that won’t be available in clinical practice and there was no functional characterization of converted cells by transplantation in an animal model performed. A more recent publication describes the generation of neuronal restricted progenitors from human fetal fibroblasts by overexpression of Sox2, c-Myc and either Brn2 or Brn434. The generated cell lines showed self-renewal capacity and could be differentiated into various types of terminal neurons. However, the usage of fetal fibroblasts is unfavorable, as these cells are of heterogeneous origin and one cannot exclude the presence of residual e.g., neural crest stem cells in the preparations. In 2014, Zhu et al. reported the direct conversion of human adult and neonatal fibroblasts into tripotential neural progenitor cells by overexpression of Sox2 together with Oct4 or Oct4 alone and addition of small molecules to the cell culture media. Notably, based on their studies Sox2 alone was insufficient to induce direct conversion26. More recently, Lu et al. reported that the overexpression of the Yamanaka factors Oct4-, Sox2-, Klf4-, c-Myc by Sendai virus for 24 hr and subsequent inactivation of the virus by increased temperature results in the generation of expandable tripotential neural precursor cells23. In conclusion, although the conversion protocols published for human cells thus far have in common the overexpression of at least one or more of the Yamanaka factors, often in a timely restricted manner, there is no clear indication of the minimal molecular factors needed to drive direct conversion into iNPCs. The timely restricted overexpression of Oct4 by either genetic means, transfection with synthetic mRNA, or cell-permeant protein together with constitutive expression of Sox2, Klf-4, and c-Myc did not result in stable human iNPC lines yet. Thus, the application of Sendai virus to overexpress all Yamanaka factors and timely restrict their activity by heat inactivation of the virus23 together with optimized neural media induction conditions22,31 represents the preferred strategy thus far.
Several studies demonstrate the cellular functionality of NSCs or their differentiated counterparts in different animal disease models. Neural progenitors from human pluripotent stem cells have been transplanted in mouse models of the neuroinflammatory disorder multiple sclerosis35,36. The applicability of hESC-derived neural progenitor cells in EAE (experimental autoimmune encephalomyelitis)-mice was first shown in 2008 35. Multipotent neural precursor cells were injected into the ventricles of mouse brain, and the transplantation resulted in the reduction of clinical signs of EAE. Kim et al. generated oligodendroglial precursors from hESCs and transplanted those intracerebroventricularly into EAE-mice. Although transplants did not survive for more than 10 days, mice showed significant improvement of neurological function and reduction of proinflammatory immune cells in the white matter36. Stem cell therapy has also been applied for preclinically targeting of Parkinson’s Disease as NPCs could be successfully employed in the respective animal models. For this, progenitor cells were either derived from fetal brain tissue37 differentiated from pluripotent stem cells38-40 or mesenchymal stem cells41 were used. In 2012 we showed that mouse iNPCs are able to produce proteolipid protein, the major myelin protein component, after transplantation into the brains of myelin-deficient (md) rats22. By that proof-of-principle experiment the therapeutic applicability of iNPCs was firstly proven and soon confirmed by another study32. However, the full potential of therapeutic use of human iNPCs remains to be explored.
Here we show an robust and integrated process of (i) derivation of human primary cells from adult patients via skin biopsy, (ii) direct conversion of human fibroblasts into a neural progenitor state and (iii) the ability of iNPCs to be differentiated into neuronal and glial lineages. Usage of this protocol will help to speed up generation of autologous human cells for therapeutic applications.
Protocol
The human fibroblasts used in this study were obtained from a skin punch biopsy after getting informed consent and ethical clearance by the ethics committee of the University of Würzburg, Germany (ethical report no: 96/11 dated 10.06.2011).
1. Punch Biopsy
Disinfect skin of patient. Anesthetize skin part of which biopsy will be taken from (preferentially a less sun-exposed area) using 0.5 to 1 ml mepivacaine hydrochloride intracutaneously and prepare skin biopsy using a sterile 3mm biopsy punch, rinse biopsy with DPBS + 1 µl/1ml Gentamicin and remove fat. Rinse biopsy twice with DPBS + Gentamicin, aspirate DPBS completely and cover biopsy with Dispase II (2.4 U/ml) and incubate 16 - 18 hr at 4 °C.
Aspirate Dispase completely, wash twice with DPBS and remove epidermis with tweezers. Wash biopsy twice with DPBS, add 2 ml Collagenase Type 2 (500 U/ml CDU), transfer in 15 ml tube and add Collagenase up to 5 ml total volume. Incubate for 45 min at 37 °C, 5% CO2.
Centrifuge 5 min at 180 x g and discard supernatant afterwards. Resuspend pellet in 10 ml DMEM-FCS-Gentamicin-media (10% FCS, 1 µl/ml Gentamicin) and centrifuge again for 5 min at 180 x g. Discard supernatant and resuspend pellet in 1.5 ml DMEM-FCS-Gentamicin-media. Transfer to T25 adherent tissue culture flask and incubate at 37 °C, 5% CO2.
Change media every 3 days.
After approximately 2 weeks, when cells are confluent around skin parts, split cells using Trypsin/EDTA (0.05%): wash cells once with DPBS, incubate 5 min at 37 °C, 5% CO2 after addition of 2 ml Trypsin/EDTA (0.05%), add 2 ml of DMEM-FCS-Gentamicin-media, transfer to 15 ml tube and centrifuge at 180 x g for 5 min; discard supernatant and resuspend pellet in appropriate amount of DMEM-FCS-Gentamicin-media.
Further expand the cells by changing the medium every 3rd day and splitting as described above when cells are confluent; addition of Gentamicin can be stopped after passage 2.
Before using the cells for direct conversion experiments, make sure to exclude mycoplasma contamination by standard assays.
When cells have been expanded, you may directly start conversion or freeze cells down using 10% DMSO and 90% FCS. Cells should be stored in -80 °C for 2 days and then transferred to liquid nitrogen for long-term storage.
For preparation of conversion, wash cells once with DPBS, aspirate DPBS and add 2.5 ml Trypsin/EDTA (0.05%). Keep for 5 min at 37 °C, 5% CO2. Tap flask gently to detach cells, resuspend in 2.5 ml of fibroblast media (DMEM incl. L-Glu + 10% FCS + 1% NEAA + 1% Sodium pyruvate) and transfer to 15 ml tube. Centrifuge at 180 x g for 5 min and aspirate the supernatant. Resuspend cells in 1 ml fibroblast media and pipette up and down to generate single cell suspension.
Count the number of cells using a Fuchs-Rosenthal- or Neubauer-cell counting chamber and plate 3 x 104 cells per well of a 24 well-plate. Incubate O/N at 37 °C, 5% CO2.
2. Infection of Human Fibroblasts with Sendai Virus and Transdifferentiation
NOTE: To ensure safety, the following steps handling virus have to be performed in a biological safety cabinet and a laminar flow hood, and with appropriate personal safety equipment, especially a surgical mask, to prevent mucosal exposure.
Switch cells to 100 µl fibroblast media (Step 1.9). Thaw aliquots of Oct4-, Klf4-, Sox2- and c-myc-Sendai virus and resuspend each virus in 1 ml fibroblast media. Add each virus with a MOI of 3 to cells (virus titer varies from lot to lot, but one aliquot of each virus can generally be used for infection of 10 wells of a 24 well-plate) and mix gently. Incubate O/N at 37 °C, 5% CO2.
After 24 hr aspirate the medium and add 500 µl of neuroinduction media (1:1 DMEM/F-12 : Neurobasal, 1x N2, 1x B27, 1% GlutaMAX, 10 ng/ml hLIF, 3 µM CHIR99021, 2 µM SB431542) and culture at 39 °C, 5% CO2 from now on. Change the media every other day.
On day 6 after infection prepare laminin-coated 6-well-plates: dilute laminin to 1 µg/ml in DPBS, add 1 ml of solution onto 6 well-plates and keep at 4 °C for 24 hr.
On day 7 after infection split the cells using DPBS/EDTA: aspirate the media and wash cells once with DPBS, then add 500 µl of DPBS/EDTA and incubate for 10 min at 37 °C, 5% CO2.
Add 500 µl of DMEM/F12 and remove suspension from plate in a 15 ml tube and centrifuge at 180 x g for 5 min.
Aspirate the supernatant and resuspend the pellet in 1.5 ml of neuroinduction media, plate on laminin-coated 6 well-plates and add ROCK inhibitor Y27632 in a final concentration of 10 µM to the cells, culture at 39 °C, 5% CO2.
Change the media every other day.
From day 14 after infection culture the cells at 37 °C, 5% CO2. Neuroepithelial colonies become apparent around day 17 after infection by phase contrast microscopy. They should be large enough to be picked around day 20 after infection.
3. Isolation of Putative iNPC Colonies
One day before picking, coat one 48 well-plate with laminin: dilute laminin to 1 µg/ml in DPBS, add 300 µl of solution onto plates and keep at 4 °C for 24 hr. One day later aspirate laminin from plates and add 200 µl of neuroinduction media to the wells.
Wash the 6 well-plates containing the colonies to be picked once with DPBS and add 2 ml DPBS per well of 6 well-plate. Pick the colonies mechanically: scrape around colonies using a thin needle to get rid of surrounding cells; set the 200 µl pipetman on 50 µl and pick the colonies using the respective pipette tips.
Transfer one colony each into one well of a laminin-coated 48 well-plate and generate a single cell suspension mechanically by pipetting up and down 10 times. Add ROCK inhibitor Y27632 in a final concentration of 10 µM to the cells.
Grow the cells on the 48 well-plate at 37 °C, 5% CO2 for 2 days. Change the media every second day until cells reach 80 - 90% confluency (approx. 3 - 4 days).
4. Expansion and Cryo-preservation of iNPCs
- Passaging and Expansion of iNPCs
- Aspirate the medium and wash the cells with DPBS. Aspirate the DPBS and add 200 µl DPBS/EDTA and incubate for 10 min at 37 °C, 5% CO2, add 200 µl of DMEM/F12 and remove suspension from plate in a 15 ml tube, centrifuge at 180 x g for 5 min.
- Aspirate the supernatant and resuspend the pellet in 0.5 ml of neuroinduction media (Step 2.2), plate on laminin-coated 12 well-plates and add ROCK inhibitor Y27632 in a final concentration of 10 µM to the cells, culture at 37 °C, 5% CO2. After cells reach 80 - 90% confluency they can be either further expanded or frozen down as follows.
- Freezing of iNPCs
- Aspirate the medium and wash the cells with DPBS. Aspirate the DPBS and add 300µl DPBS/EDTA and incubate for 10 min at 37 °C, 5% CO2, add 300 µl of DMEM/F12 and remove suspension from plate in a 15 ml tube, centrifuge at 180 x g for 5 min.
- Aspirate the supernatant and resuspend the pellet in 0.5 ml of commercial NSC freezing medium. Transfer suspension to freezing vials and put in cell-freezing container. Store at -80 °C for 2 days, then transfer to liquid nitrogen for long-term storage.
5. Differentiation of iNPCs
- Differentiation towards neuronal lineage
- Culture cells on laminin-coated plates as described above. Change media to neuro-diff-media (DMEM/F-12, 1x N2, 1x B27, 300 ng/ml cAMP, 200 µM vitamin C, 10 ng/ml BDNF, 10 ng/ml GDNF) when cells are approximately 70% confluent. Change media every other day for three weeks. Do not split cells during differentiation.
- After three weeks cells should express the neuronal marker TUJ1. To gain more mature neurons and neuronal subtypes continue differentiation up to 3 months.
- Differentiation towards glial lineage
- Change media to glial induction media (1:1 DMEM/F-12 : Neurobasal, 1x N2, 1x B27, 10 µM β-mercaptoethanol, 100 µM non-essential amino acids, 1.5 mM L-Glutamine, 5 µg/ml insulin, 40 ng/ml T3) including 20 ng/ml EGF to induce differentiation into glial precursor cells. Remove half of the media and replace by fresh media every other day for about 2 weeks. During this period cells should be split 1:3 in presence of 10 µM ROCK inhibitor Y27632 when 100% confluency is reached.
- After 2 weeks differentiate cells further into astrocytes: when cells are nearly confluent change media to commercial available astrocyte media, change media every second day. After about 7 days cells start to express astrocyte markers (e.g., GFAP). If cells get 100% confluent during the differentiation protocol, split them in a 1:2 ratio in presence of 10 µM ROCK inhibitor Y27632.
Representative Results
Here we present description of an integrated process that allows generation of induced neural progenitor cells (iNPCs) from human fibroblasts that have been obtained by a punch biopsy from skin within less than 8 weeks (Figure 1). Patient-specifc iNPCs can be further differentiated into neuronal and glial lineages and harbor huge potential for cell replacement therapy and disease modeling.
Infection of the fibroblasts with Oct4-, Klf4-, Sox2- and c-myc- Sendai viruses23 and culture in neuroinductive media conditions22,31 resulted in a change of morphology of the fibroblasts and subsequent emergence of colonies 17 days after infection (Figure 2). Generated monoclonal cell lines can be expanded and stain positive for neural stem cell markers like Sox2, Sox1, Nestin, Vimentin, Otx2 and Pax6, as well as for proliferation marker Ki67, whereas they do not express the pluripotency-associated marker Oct4 (Figure 3).
Moreover, by addition of neuronal growth factors to the cell culture media the neural progenitor cells can be differentiated into neurons. By applying a differentiation protocol based on Izrael et al.42 and subsequent final differentiation with astrocyte induction media cell lines can also be differentiated into glial lineages as judged by analysis of typical morphological changes and staining against glial fibrillary acidic protein (GFAP) (Figure 4).
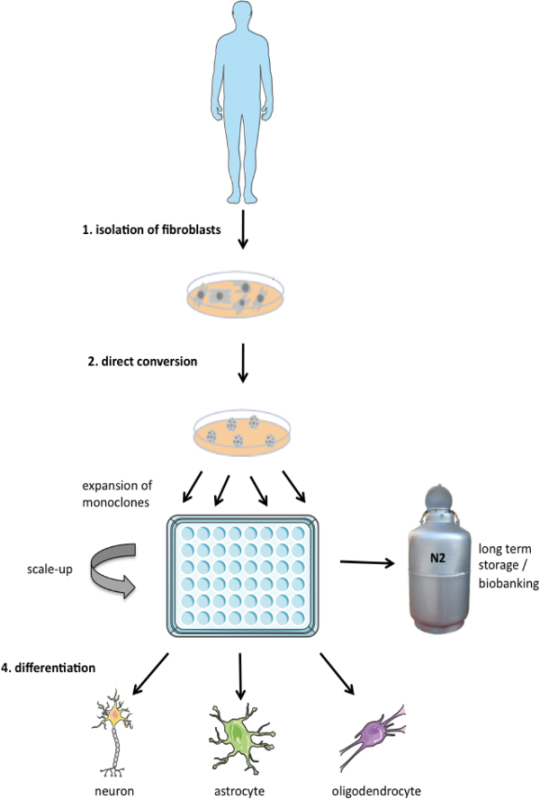 Figure 1. Schematic representation of three-step protocol applied in this study. Fibroblasts can be isolated from patients by a punch biopsy and expanded. These cell lines can then be directly converted into induced multipotent neural progenitor cells (iNPCs) and monoclonally expanded. iNPC lines can be used for long term storage (biobanking for repeated or standardized use) or they can be differentiated into neuronal and glial lineages. Please click here to view a larger version of this figure.
Figure 1. Schematic representation of three-step protocol applied in this study. Fibroblasts can be isolated from patients by a punch biopsy and expanded. These cell lines can then be directly converted into induced multipotent neural progenitor cells (iNPCs) and monoclonally expanded. iNPC lines can be used for long term storage (biobanking for repeated or standardized use) or they can be differentiated into neuronal and glial lineages. Please click here to view a larger version of this figure.
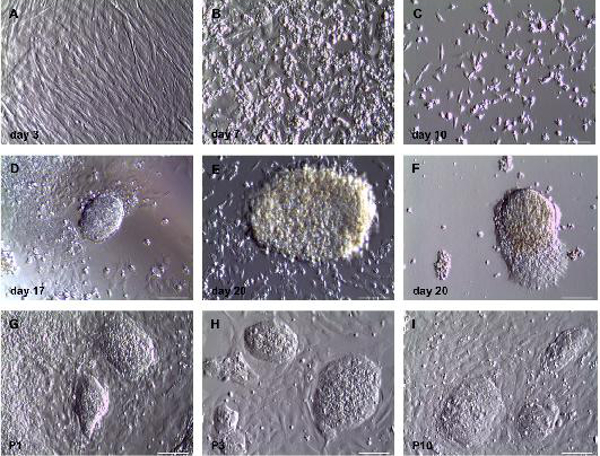 Figure 2. Morphological analyses of direct conversion of human fibroblasts into induced neural progenitor cells using phase contrast microscopy.(A) Human fibroblasts three days after infection with OKSM – Sendai virus and two days after addition of neuroinduction medium. Cells show typical fibroblast-like morphology. (B) Seven days post infection cells with changed morphology show up. Cells are split to reduce confluency on the same day. (C) Ten days post infection only cells with changed morphology are present. (D) First small neuroepithelial-like colonies can be found 17 days after infection. These colonies increase in size (E), and outgrowing cells can be found (F) three days later. Cells are picked on day 21 post infection and expandable cell lines can be generated from them (G-I). Different clones are shown in passage 1 (G), passage 3 (H) and passage 10 (I) after picking, respectively. Scale bar = 200 µm. Please click here to view a larger version of this figure.
Figure 2. Morphological analyses of direct conversion of human fibroblasts into induced neural progenitor cells using phase contrast microscopy.(A) Human fibroblasts three days after infection with OKSM – Sendai virus and two days after addition of neuroinduction medium. Cells show typical fibroblast-like morphology. (B) Seven days post infection cells with changed morphology show up. Cells are split to reduce confluency on the same day. (C) Ten days post infection only cells with changed morphology are present. (D) First small neuroepithelial-like colonies can be found 17 days after infection. These colonies increase in size (E), and outgrowing cells can be found (F) three days later. Cells are picked on day 21 post infection and expandable cell lines can be generated from them (G-I). Different clones are shown in passage 1 (G), passage 3 (H) and passage 10 (I) after picking, respectively. Scale bar = 200 µm. Please click here to view a larger version of this figure.
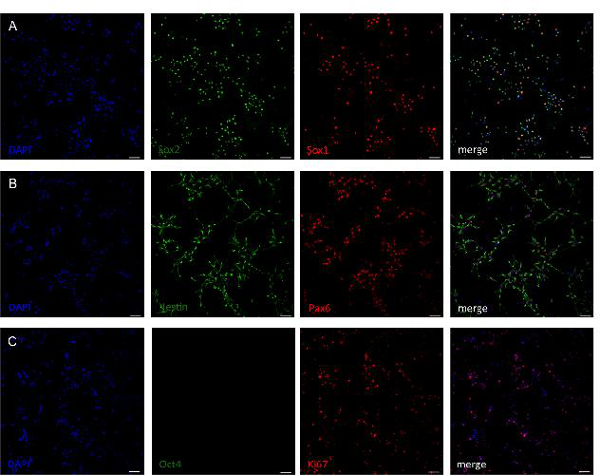 Figure 3. Analysis of neural stem cell marker gene expression of generated neural progenitor cell lines. Antibody staining of cell lines after 10 passages revealed that cells express the neural stem cell markers Sox1, Sox2 (A) as well as Nestin and Pax6 (B). Cells are negative for pluripotency marker Oct4 indicating absence of pluripotency (C). The majority of cells stain positive for marker Ki67, which shows a high proliferative state (C). Scale bar = 50 µm. Please click here to view a larger version of this figure.
Figure 3. Analysis of neural stem cell marker gene expression of generated neural progenitor cell lines. Antibody staining of cell lines after 10 passages revealed that cells express the neural stem cell markers Sox1, Sox2 (A) as well as Nestin and Pax6 (B). Cells are negative for pluripotency marker Oct4 indicating absence of pluripotency (C). The majority of cells stain positive for marker Ki67, which shows a high proliferative state (C). Scale bar = 50 µm. Please click here to view a larger version of this figure.
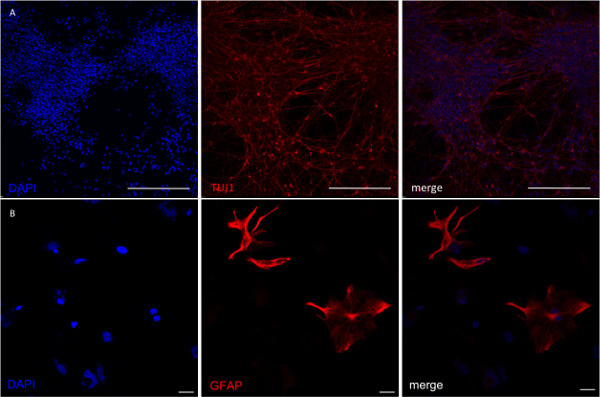 Figure 4. Analysis of NPCs after directed differentiation into neuronal and glial lineages. (A) To achieve neuronal specification iNPCs were differentiated for seven days in the presence of BDNF, GDNF, Vitamin C and cAMP. (B) Astrocyte differentiation was performed after predifferentiation of cells with EGF and FGF. After 2 weeks rare GFAP-positive cells were present in the culture. Their number continuously increased with further cultivation. Scale bar in A = 200 µm, scale bar in B = 50 µm. Please click here to view a larger version of this figure.
Figure 4. Analysis of NPCs after directed differentiation into neuronal and glial lineages. (A) To achieve neuronal specification iNPCs were differentiated for seven days in the presence of BDNF, GDNF, Vitamin C and cAMP. (B) Astrocyte differentiation was performed after predifferentiation of cells with EGF and FGF. After 2 weeks rare GFAP-positive cells were present in the culture. Their number continuously increased with further cultivation. Scale bar in A = 200 µm, scale bar in B = 50 µm. Please click here to view a larger version of this figure.
Discussion
Here we show the isolation and direct conversion of human fibroblasts into expandable transgene-free neural progenitor cells and their differentiated progeny as a putative basis for cell-replacement therapy or application in drug screening analyses. Direct lineage conversion of somatic cells into NPCs has been achieved by forced expression of lineage-specific transcription factors 26,33,34. Nevertheless, in many cases fetal fibroblasts were used for transdifferentiation experiments33,34. For biomedical applications the usage of these cells is inexpedient as this cell source precludes the clinical use for autologous cell replacement therapy. Recently, studies were published that describe the generation of neural precursor cells from more easily accessible tissues, such as hematopoietic cells43,44. The successful generation of tripotent neural stem cell lines could be shown for murine blood-derived B cells by the inducible overexpresion of eight transgenes43. Moreover, Castano et al. published an approach for induction of neuronal progenitors from human hematopoietic cells using Sox2- and c-myc-Sendai virus44. The protocol described enables fast and efficient generation of neural cells from CD133+ cord blood cells. However, when applied for conversion of adult peripheral blood mononuclear cells the protocol yielded low efficiency and the expansion capacity of the generated cell lines was very limited44. Thus, the only study published with peripheral blood cells in the human system to date44 clearly shows the limitations of this particular cell type in direct conversion experiments. Since the availability of patient-specific cells is crucial for autologous cell replacement, the preferred cell type still represent adult fibroblasts, which can be easily derived via a skin punch biopsy as described in our study.
The overexpression of transgenes needed for transdifferentiation has mostly been achieved by integrative viral vectors26,33,34. This hinders the applicability of the derived cell lines for biomedical applications since the integration of the viruses into the host genome might lead to insertional mutagenesis or unwanted reactivation of the transgenes7,8. Transdifferentiation into NPCs without transgene overexpression but based on chemical treatment has been described for human fibroblasts45. However, in this study these precursor cells were only present in a transient state as the aim of the study was the generation of mature Schwann cells45. Recently, other transgene-free approaches based on application of Sendai virus have been described for the direct conversion of human fibroblasts into NPCs23,44 and seem to be the preferred strategy to date. To derive expandable NPCs that can be subsequently differentiated into neuronal and glial lineages, we apply overexpression of all Yamanaka-factors by Sendai virus together with heat inactivation of virus23 as well as neural induction by addition of small molecules to the culture media22,31 with important modifications as described in the following.
Although, in our hands, the protocol is efficiently working with many fibroblast lines tested, there is an apparent tendency that the direct conversion of fibroblast cells from old donors exhibiting a poor proliferative potential result in a low efficiency of iNPC generation. We observed colony forming efficiencies of up to 0.2% after a viral transduction efficiency of more than 90% as judged by infection of cells with Oct4 only and subsequent staining. This efficiency is about 3 times higher than previously published23.
The precisely adjusted application of an optimized viral MOI is critical. Batch to batch variability in Sendai virus titer has to be taken into account. Since the virus is usually acquired commercially, respective information can be found on the manufacturer’s homepage. We suggest to use a relatively low MOI of 3 for transdifferentiation experiments.
Addition of human LIF in our hands is crucial for the self renewal of the established iNPC lines as has been described for the differentiation of human pluripotent cells into NPCs31. Without addition of LIF, cells start to differentiate as early as two days after withdrawal in a non-reversible manner.
Cell culture after infection is performed at 39 °C and not using standard 37 °C cell culture conditions. This rise in temperature starts already one day after infection and leads to a heat-inactivation of the Sendai virus. The culture at 39 °C until day 14 after infection is essential for generation of neuroepithelial colonies.
As a matter of fact, sometimes iNPC colonies come up later than described in the protocol section. In our experience, as long as the cells remain proliferative they can be polyclonally passaged and isolated by manual picking. The established cell lines from those colonies show similar properties as described for the early occurring colonies.
During splitting of established cell lines it is important not to seed a too small number of cells as they require cell-to-cell contacts or paracrine signals to proliferate well. If cells are seeded in too low density proliferation will cease. In this case, cells may be rescued by replating on a cell culture dish with a smaller surface to re-initiate proliferation.
For glial differentiation of iNPCs it is very important to seed the cells in a high density and to split them when the plate is almost confluent. If cells are split too early, predominantly neuronal differentiation will be observed.
The process described in this manuscript harbors the potential to be applied in a semi- or fully-automated manner, which would be necessary to achieve the desired biomedical potential, including disease modeling and cell replacement therapy. Automation would also enable cost-efficient scale-up and parallelization of the generation of neural progenitor cells.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We would like to thank all members of the Stem Cell and Regenerative Medicine Group of the University of Würzburg for helpful suggestions and Martina Gebhardt as well as Heike Arthen for excellent technical support. This work was supported by grants from the Deutsche Forschungsgemeinschaft DFG (ED79/1-2), the German Ministry of Education and Research BMBF (01 GN 0813), the Bavarian Research Network Induced Pluripotent Stem Cells “forIPS” and the “Stiftung Sibylle Assmus”. Figure 1 was produced using Servier Medical Art, available from www.servier.com.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19(7):1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee YH, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121(6):2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Soldner F, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27(3):543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat N, et al. Simple generation of human induced pluripotent stem cells using poly-beta-amino esters as the non-viral gene delivery system. J Biol Chem. 2011;286(14):12417–12428. doi: 10.1074/jbc.M110.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Nu Y, Wang J, Guo X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci Rep. 2012;2:657. doi: 10.1038/srep00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnali M, Edenhofer F. Generation of transducible factors. Biol Chem. 2008;389(7):851–861. doi: 10.1515/BC.2008.106. [DOI] [PubMed] [Google Scholar]
- Zhou H, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awe JP, et al. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade state. Stem Cell Res Ther. 2013;4(4):87. doi: 10.1186/scrt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadari A, et al. Excision of viral reprogramming cassettes by Cre protein transduction enables rapid, robust and efficient derivation of transgene-free human induced pluripotent stem cells. Stem Cell Res Ther. 2014;5(2):47. doi: 10.1186/scrt435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Pang ZP, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9(6):575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- Han DW, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10(4):465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10(4):473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Generation of integration-free and region-specific neural progenitors from primate fibroblasts. Cell Rep. 2013;3(5):1580–1591. doi: 10.1016/j.celrep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Südhof TC, Werning M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA. 2012;109(7):2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, et al. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12(2):126–137. doi: 10.2174/156652412798889018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, et al. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24(1):126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer K, et al. Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Reports. 2014;3(3):423–431. doi: 10.1016/j.stemcr.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3(9):e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy , Socci G, Tabar ND, Studer V, L Human ES cell-derived rosettes reveal a functional distinct early neural stem cell stage. Genes Dev. 2008;22(2):152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. A rosette-type self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA. 2009;106(9):3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA. 2011;108(20):8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P, et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One. 2013;8(3):c59252. doi: 10.1371/journal.pone.0059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, et al. Direct conversion of human fibroblasts into neuronal restricted progenitors. J Biol Chem. 2014;289(8):5250–5260. doi: 10.1074/jbc.M113.516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursor in an animal model of multiple sclerosis. PLoS One. 2008;3(9):e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, et al. Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis. Stem Cells. 2012;30(12):2810–2829. doi: 10.1002/stem.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, Pundt LL, Blount JP, Conrad JA, Low WC. Transplantation of human fetal tissue from spontaneous abortions to a rodent model of Parkinson’s disease. Cell Transplant. 1996;5(1):69–75. doi: 10.1177/096368979600500112. [DOI] [PubMed] [Google Scholar]
- Takagi Y, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115(1):102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, et al. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinson’s Dis. 2011;1(4):395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1(6):703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Dezawa M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, et al. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34(3):310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Cassady JP, et al. Direct lineage conversion of adult mouse liver cells and B lymphocytes to neural stem cells. Stem Cell Rep. 2014;3(6):948–956. doi: 10.1016/j.stemcr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano J, et al. Fast and efficient neural conversion of human hematopoietic cells. Stem Cell Rep. 2014;3(6):1118–1131. doi: 10.1016/j.stemcr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma EC, et al. Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Rep. 2014;3(4):539–547. doi: 10.1016/j.stemcr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


