Abstract
Sublethal ischemia protects tissues against subsequent, more severe ischemia through the upregulation of endogenous mechanisms in the affected tissue. Sublethal ischemia has also been shown to upregulate protective mechanisms in remote tissues. A brief period of ischemia (5-10 min) in the hind limb of mammals induces self-protective responses in the brain, lung, heart and retina. The effect is known as remote ischemic preconditioning (RIP). It is a therapeutically promising way of protecting vital organs, and is already under clinical trials for heart and brain injuries. This publication demonstrates a controlled, minimally invasive method of making a limb – specifically the hind limb of a rat – ischemic. A blood pressure cuff developed for use in human neonates is connected to a manual sphygmomanometer and used to apply 160 mmHg pressure around the upper part of the hind limb. A probe designed to detect skin temperature is used to verify the ischemia, by recording the drop in skin temperature caused by pressure-induced occlusion of the leg arteries, and the rise in temperature which follows release of the cuff. This method of RIP affords protection to the rat retina against bright light-induced damage and degeneration.
Keywords: Medicine, Issue 100, remote ischemic preconditioning, ischemic conditioning, ischemic tolerance, light injury, neuroprotection, mediated neuroprotection, stroke, retina, electroretinogram, rat
Introduction
The survival of most, perhaps all, tissues in the face of metabolic stress can be improved by prior conditioning with a period of sublethal ischemia1,2. Ischemic preconditioning (IP) in practical terms is the exposure of tissue to sublethal ischemia, before the tissue experiences more severe stressors, such as a subsequent ischemic insult. In animal models, IP provides striking protection to the brain, retina, heart and lungs3-6. Correspondingly, observations in stroke patients showed a link between previous transient ischemic attacks and better clinical outcomes7,8. IP also protects retinal photoreceptors from non-ischemic injuries9.
The effectiveness of IP in diverse tissues and injuries suggests that it is activating an innate mechanism of cell survival present in all tissue. Ischemic preconditioning of the myocardium has been suggested to have protective effects through the upregulation of hypoxia inducible factor (HIF), known to regulate many metabolic pathways through the release of adenosine or through the opening of mitochondrial ATP potassium channels10,11. Adenosine release and ATP potassium channels are implicated in cerebral ischemia but, investigations into the neuroprotective mechanisms of ischemic conditioning to date have been focused on modifications to anti-excitotoxicity, anti-apoptotic and anti-inflammatory pathways12,13. Overall, understanding of the molecular process of ischemic conditioning for protecting neurons is limited.
Remote ischemic preconditioning attempts to condition distant critically important organs (heart, brain, lung) by generating ischemia in less critical tissues. Remote ischemic preconditioning (RIP) using the hind limb has been demonstrated to be neuroprotective in rodent models of stroke14-17. The method described by us provides a simple, reliable and non-invasive protocol for inducing RIP.
The vast majority of RIP protocols involve the hind limb, presumably because the femoral artery located in the upper hind limb can be easily identified and accessed for surgical clamping and tourniquet application. In invasive limb ischemic studies for the study of brain and skin protection, ischemia is induced by separating the femoral artery from the groin ligaments and clamping the femoral artery2,15,18.
The ischemia resulting from either limb cuffing or femoral artery clamping has been confirmed by changes at the limb including a loss of pulse, decrease in oxygenation and a drop in skin temperature. Remote ischemia can be confirmed by the loss of pulse by using laser Doppler or ultrasound Doppler17-19. Skin temperature can be used as alternative to Doppler although the relationship is non-linear20,21. Accurate temperature recordings are commonplace in laboratories and can be easily incorporated into remote ischemic studies.
An alternative to femoral clamping surgery is the induction of ischemia using a tourniquet. Tourniquet application produces comparable ischemia to that achieved with vessel clamping; Kutchner et al. compared invasive femoral artery clamping to a non-invasive tourniquet and found both methods halted blood flow to the limb and reduced skin damage in a plastic surgery model of skin flap ischemia18. Cuffing either the leg or arm and raising the cuff pressure to above systolic blood pressure has been found to be protective against ischemic damage in pigs and humans17,19,22.
Different tourniquet approaches to inducing remote ischemic include the use of a blood pressure cuff or an elastic band17,22,23. However, the use of an elastic band to induce ischemia is an unsafe method, potentially giving rise to an unregulated amount of pressure in the limb, with pressure rises above 500 mmHg being recorded in humans24. Further, limb ischemia using an elastic band leads to muscle damage in rats following the removal of the band23, as assessed by Evans Blue Dye, an in vivo marker of myofiber permeability25. In contrast, delivery of a controlled pressure to the tourniquet can be achieved using a blood pressure cuff connected to a sphygmomanometer17,19,22,26.
In this study, a light injury model of photoreceptor degeneration was used to demonstrate the neuroprotective efficacy of remote ischemic preconditioning. Remote ischemia was induced immediately before light injury, and prevented subsequent photoreceptor degeneration as confirmed by retinal function testing. The accompanying video will demonstrate the application of non-invasive remote ischemia.
Protocol
Ethics statement: The protocol follows the animal care guidelines of University of Sydney, AEC #5657. Anaesthesia was approved by animal ethics committee (University of Sydney, AEC #5657).
1. Equipment Preparation
Use real time skin temperature tracking. Switch on computer, and data acquisition hardware.
Open temperature recording software and adjust temperature setting to between 30-35 °C and the frequency of sampling to every 100 msec.
Optional: Insert rectal thermometer to ensure core temperature remains stable at 37.5 °C.
2. Calibration of the Manual Sphygmomanometer
Connect neonatal arm cuff to sphygmomanometer.Use a size 2 cuff for a 250-550 g rat. An adaptor may be necessary to connect the cuff tubing to the sphygmomanometer.
Deflate the cuff by either loosening the air release valve or disconnecting the cuff tubing from the adaptor. Ensure no pressure remains in the tubing and the manometer needle rests at zero inside the oval/rectangle.
Check the pressure between tubing, manometer and cuff. Inflate the cuff with gentle pumps of the inflation bulb until it reads 100 mmHg on the manometer. Ensure the pressure remains constant. Deflate the cuff by slowly opening the air release value.
3. Animal Preparation
Note: Animals that are to undergo light injury require dark adaption the night before remote ischemia. Animals undergoing light damage require dark rearing (12 hr light:dark cycle (5 lux))
Perform remote ischemia in either awake or anesthetised rodents. Ensure that animals have a healthy muscle tone. Ensure this by pinching the upper hind limb to confirm there is adequate muscle present. RIP-induced protection against light injury has been tested in sedentary rats up to 6 months of age.
- Anesthetised preparation for RIP
- Inject rats with an intraperitoneal injection of 60 mg/kg ketamine and 5 mg/kg xylazine. Check the depth of anaesthesia by extending the leg and pinching the skin on the underside of the foot. The animal has no reflex if it is deeply anesthetised. Apply artificial tears to avoid corneal dryness whilst under anaesthesia.
- Place rats on either a heat pad or circulating water heater tubing to maintain a constant body temperature of 37.5 °C. Position the rat in the prone position with the lower limbs’ foot pads facing up. Either the right or left limb may undergo remote ischemia.
- Awake preparation for RIPNote: Awake animal experimentation requires two people. One person restrains the animal and the second person operates the manual sphygmomanometer. The experimenters must be confident to perform the procedure as restraint increases the risk of injury to handlers. The rats undergoing remote ischemia must be conditioned to manual restraint. Depending on intuitional guidelines manual restraint should be progress from 30 sEC to a maximum of 5 min over a number of weeks. Timid animals that fail to acclimatise to manual restraint should be excluded from awake experimentation. Lastly, manual restraint is likely to cause stress (and potentially introduce confounds to the study) to animals and a sham cohort (placement of cuff without inflammation) must be used to accurately interpret RIP study results.
- Cut a towel into a 15 cm x 30-50 cm piece and place the short edge perpendicular to the rat’s spine, covering the head to the top of the hind limbs.
- Tuck the short edge under the rat’s torso tightly and begin to wrap the rat with the remaining long edge of towel. Secure the wrapped animal under the arm in a supine position. If the rat is held under the left arm, free the rat’s right limb from the towel.
4. Application of Skin Temperature Probe
Extend the leg of the rat which is to undergo ischemia and place the skin probe on the footpad. Position the skin probe to maximise contact between the temperature probe and the skin. Firmly push the probe into the footpad and affix the probe with paper tape.
Check the skin probe placement by tracking the temperature on temperature recording software. Ensure that the skin temperature is between 30-34 °C and remains stable. Track the skin temperature for 1-2 min. Adjust the skin probe if the temperature is unstable or below 30 °C.
5. Remote Ischemia
Deflate the cuff and ensure the air pressure valve is closed. Extend the leg and loosely encircle the cuff on the upper hind limb. Use the forefinger and thumb to extend the leg and the lower digits to keep the loosened cuff in position.
Raise the anesthetised animal’s cuff pressure to 160 mmHg, and in awake animals increase the cuff pressure to 180 mmHg. Note: The anesthetised blood pressure of rats ranges from 120-140 mmHg and rises to 160 mmHg when conscious. Commence the timer and foot temperature recordings once the correct pressure is reached. Note: The foot temperature should drop by 2 °C after 5 min of constant pressure.
Maintain the position of the cuff above the animal’s “knee” throughout the ischemia. Cuff pressure will begin to drop after a few minutes, or if the rat’s limb is moving.
Repeatedly pump the inflation bulb in short bursts to maintain the desired cuff pressure repetitive short burst of pumping
Remote ischemia can be delivered continuously for between 5 and 15 min. The ischemia reperfusion protocol includes 2 periods of 5 min ischemia with an intervening 5 min reperfusion.
Deflate the cuff pressure by loosening the air pressure valve. Check the temperature change over the course of the ischemia protocol. Release the cuff.
Continue with injury experimentation. Animals under the effect of anaesthesia will need to be placed on a heat pad. Continue to monitor animals until ambulatory. Animals cannot be returned to housing until walking.
6. Light Injury - Retinal Degeneration Model
Dark adapt the animals overnight (12-15 hr). Immediately following remote ischemia conditioning or sham remote ischemia (animal restraint) place animals in Perspex housing with food and water.
Switch on fluorescent lighting (1,000 lux) located above Perspex housing at 9 am for 24 hr. Following light exposure, return the animals to dim cyclic lighting for 7 days.
7. Post-remote Ischemia Procedures
- Vision assessment with Electroretinogram (ERG): Note: The ERG set-up and flash protocol followed Brandli and Stone26.
- Dark adapt animals overnight (12-15 hr). Under dim red illumination anesthetize the animals by intraperitoneal injection of ketamine and xylazine (60 mg/kg and 5 mg/kg, respectively). Mydriatic (atropine sulphate 1.0%), corneal anaesthetic (proxymetacaine 0.5%).
- Apply corneal hydration (Carbomer polymer) eye drops immediately to the cornea. Apply eye gel at 20 min intervals to maintain corneal hydration.
- Draw a loosely tied thread around the eyeball to aid stable ERG recordings. Monitor temperature using a rectal probe and maintain animal’s body temperature at 37-37.5 °C.
- Position the head inside a Ganzfeld integrating sphere. Note: The Ganzfeld is a fully programmable light stimulus that delivers uniform whit flashes from LEDs to the eye.
- Record the electroretinogram using a custom-made 4 mm platinum positive electrode lightly touching the cornea and a 2 mm diameter Ag/AgCl pellet electrode inserted into the mouth. Reference both electrodes to a stainless steel needle inserted subcutaneously into the rump.
- Record signals with band-pass setting of 0.3-1,000 Hz (-3 dB), with a 2 kHz acquisition rate (AD Instruments). After a stable ERG recording is established subject the animal to a 10 min dark adaptation before commencing recordings.
- Follow the flash protocol as previously described by Brandli and Stone26.
- Program the duration of the flash (we used flashes 1-2 msec in duration), and set its intensity to -4.4 to 2.0 log scot cd.s.m-2. Use bright flashes (2.0 log scot cd.s.m-2, 1 ms) to measure the retinal function. In this study it is the comparison between control, light injury and light injury with RIP.
8. TUNEL Assay
Euthanize animals by intraperitoneal injection of phenobarbital overdose (100 mg/kg). Enucleate the eyes and fix in 4% paraformaldehyde.
Wash the eyes in PBS before cyroprotecting eyes overnight in sucrose 30% (w/v). Embed the eyes in OCT compound and cut into 20 µm sagittal sections using a cryostat.
Perform the TUNEL assay on retinal sections with DAPI staining following the protocol of Maslim et al.27
Use fluorescence microscopy for TUNEL counts of the retina. TUNEL cells were recorded from the outer nuclear layer (ONL); the outermost layer of the retina which contains photoreceptor nuclei. In this study, TUNEL counts were made in triplicate for each eye, with 5 eyes for each treatment group.
Use one-way ANOVA to statistical compare group means of control, light injury, and light injury + RIP rats.
Representative Results
A blood pressure cuff elevated to above 160 mmHg halts blood flow to the hind limb as seen clearly in Figure 1B. The lack of tissue oxygenation resulted in a reducing the animal’s foot temperature for an ischemia-reperfusion protocol (Figure 2). The foot temperature (33 °C) was lower than core temperature and reliably reduced during cuff pressure elevation (31 °C) rising when the cuff was deflated (32 °C). A single 1,000 lux light injury was delivered to dim raised albino rats with or without remote ischemic preconditioning. Retinal function was recorded and assessed using the electroretinogram (ERG).
The ERG is the summation of electrical responses originating from the inner and outer neurons of the retina to light stimulation as shown in Figure 3. The ERG waveform has an early negative peak arising from phototransduction (minimum approximately 10 msec after light flash) termed the a-wave and a large positive peak from the inner retina (maximum approximately 80 msec after light flash) termed the b-wave. The dark-adapted ERG from a normal dim raised rat showed a large photoreceptor and inner retinal response to a bright 2.0 log cd.s.m-2 flash (Figure 3A). One week following light injury the ERG recordings had a severe reduction in amplitude relative to controls, reflecting the loss of photoreceptors; see Figure 3B. Preconditioning the hind limb with ischemia using a reperfusion protocol of 2 x 5 min immediately before ischemia protected the photoreceptors from light injury. The RIP ERG amplitudes were greater than light injury alone, with a slight reduction to the a-wave see (Figure 3C). Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay on cryopreserved sections of the retina confirmed a reduction of apoptotic cells in light damaged animals receiving RIP relative to sham-treated light injury animals (Figure 4).
The induction of ischemia to the hind limb relies on the correct placement of the cuff, as seen in Figure 1. A cuff placed below the “knee” does not protect photoreceptors from light injury as reflected in the reduced ERG amplitudes, see Figure 3D.
In conclusion, when administered correctly hind limb ischemia was able to protect retinal neurons from light injury.
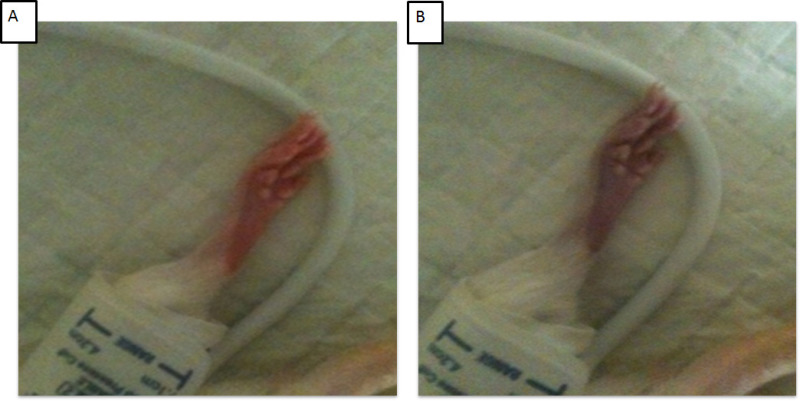 Figure 1: Cuff placement and effect of cuff pressure above 160 mmHg. (A) Shows the hind limb and foot before cuff pressure elevation. (B) Shows the foot during elevation of cuff pressure above 160 mmHg. Note the position of the cuff above the “knee”. Please click here to view a larger version of this figure.
Figure 1: Cuff placement and effect of cuff pressure above 160 mmHg. (A) Shows the hind limb and foot before cuff pressure elevation. (B) Shows the foot during elevation of cuff pressure above 160 mmHg. Note the position of the cuff above the “knee”. Please click here to view a larger version of this figure.
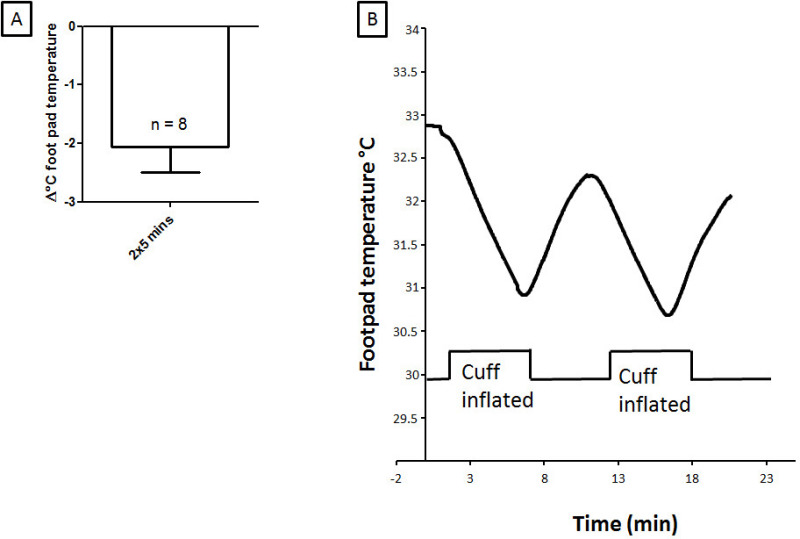 Figure 2: Foot temperature is reduced during cuff inflation. Inflation of the cuff on the hind limb for 2 x 5 min at 160 mmHg reduced skin temperature during ischemia. (A) Shows the group average for 2 x 5 min RIP foot temperature changes. (B) Shows a representative foot temperature (°C) tracing for 2 x 5 min ischemia. Please click here to view a larger version of this figure.
Figure 2: Foot temperature is reduced during cuff inflation. Inflation of the cuff on the hind limb for 2 x 5 min at 160 mmHg reduced skin temperature during ischemia. (A) Shows the group average for 2 x 5 min RIP foot temperature changes. (B) Shows a representative foot temperature (°C) tracing for 2 x 5 min ischemia. Please click here to view a larger version of this figure.
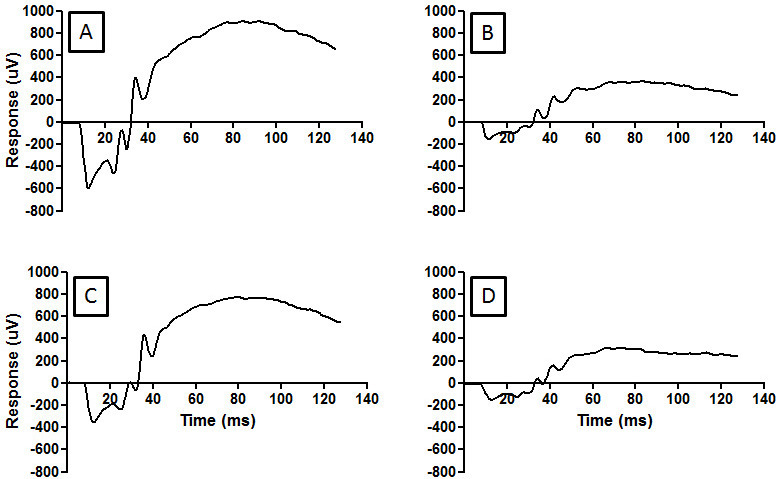 Figure 3: RIP preserves retinal function as demonstrated in the ERG compared to light damage rats. Exposure to bright light for 24 hr damages the photoreceptors of the retina. The ERG measures the health of inner and outer retina as an electrical response (microvolts [µV]) to light stimulation. The normal retinal response to 2.0 log cd.s.m-2 light stimulation seen in (A). Photoreceptor damage from bright light results in a smaller ERG amplitude (B). RIP was able to rescue the photoreceptors following light injury (C). Incorrect cuff placement during RIP does not protect photoreceptors from injury (D). Please click here to view a larger version of this figure.
Figure 3: RIP preserves retinal function as demonstrated in the ERG compared to light damage rats. Exposure to bright light for 24 hr damages the photoreceptors of the retina. The ERG measures the health of inner and outer retina as an electrical response (microvolts [µV]) to light stimulation. The normal retinal response to 2.0 log cd.s.m-2 light stimulation seen in (A). Photoreceptor damage from bright light results in a smaller ERG amplitude (B). RIP was able to rescue the photoreceptors following light injury (C). Incorrect cuff placement during RIP does not protect photoreceptors from injury (D). Please click here to view a larger version of this figure.
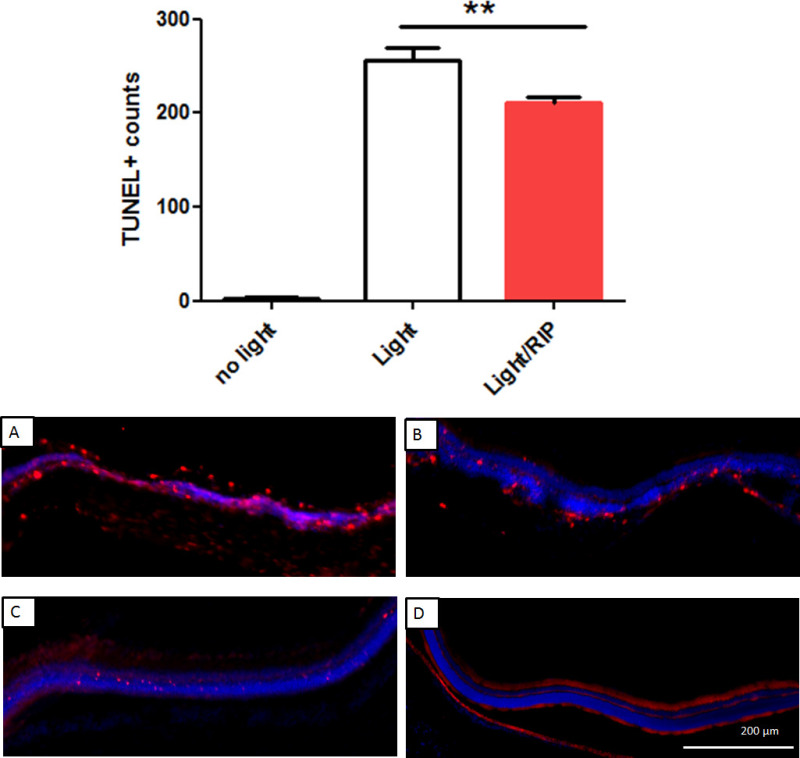 Figure 4: TUNEL+ cell counts. A bar graph comparing group result for light injury, injury + RIP demonstrate the reduction in apoptosis with RIP. TUNEL+ cell were counted across the entire span of the retina (8,000 µm). Top panel: group average of TUNEL+ cells were lower for RIP treated rats (210 ± 4.9, n = 5) compared to light injury alone (255 ± 10, n = 5), p <0.01, one-way ANOVA. Undamaged retinas (no light injury) had very low (3.0 ± 1.4, n = 5) apoptotic cells. (A) Representative image of superior light injured retina. (B) Representative image of superior RIP-light injured retina. (C) Representative image of inferior light injured retina. (D) Representative image of inferior RIP-light injured retina. Please click here to view a larger version of this figure.
Figure 4: TUNEL+ cell counts. A bar graph comparing group result for light injury, injury + RIP demonstrate the reduction in apoptosis with RIP. TUNEL+ cell were counted across the entire span of the retina (8,000 µm). Top panel: group average of TUNEL+ cells were lower for RIP treated rats (210 ± 4.9, n = 5) compared to light injury alone (255 ± 10, n = 5), p <0.01, one-way ANOVA. Undamaged retinas (no light injury) had very low (3.0 ± 1.4, n = 5) apoptotic cells. (A) Representative image of superior light injured retina. (B) Representative image of superior RIP-light injured retina. (C) Representative image of inferior light injured retina. (D) Representative image of inferior RIP-light injured retina. Please click here to view a larger version of this figure.
Discussion
Rodent hind limb ischemia was successfully induced with a manual sphygmomanometer and cuff delivering neuroprotection to the photoreceptors of the retina. A finding consistent with ischemic conditioning induced protection of photoreceptors from light injury9,28.
Essentially, remote ischemia causes brief oxygen deprivation to tissues. Hence, remote ischemic preconditioning has many similarities to ischemic conditioning or alternatively termed ischemic tolerance, hypoxic preconditioning, and to some extent, anaerobic exercise. Cells respond to ischemic challenge by releasing a large variety of proteins, nucleoside and transcription factors that either offer neuroprotection directly or affect cells to become tolerant of subsequent metabolic stress13.
In the literature, remote ischemia protocols have included a range of durations and frequencies.Our lab has tested 5, 10, and 2 x 5 min ischemia protocols on normal retina function26. Of these protocols 2 x 5 min produced the greatest ERG amplitude change in normal rats and was selected to test in a model of light injury. Short and repeated 5 min ischemic events have also been shown to be preventive in recurring strokes in humans and to reduce infarct size in experimental stroke in pigs17,22. However, the most appropriate duration of ischemic preconditioning is likely to be dependent on the animal model used. For example, a reduction in infarct size against focal ischemia has been observed in longer 2 x 15 min and 3 x 15 min protocols, but not in 3 x 5 min protocols in rats15.
The time between IP and ischemic injury also needs to be considered for effective neuroprotection. Two time windows have been classified for cardioprotection induced by IP. These are the “classic conditioning” window, which occurs 0-12 hr after IP and the “second window”, which occurs 3-4 days after IP29. In a focal stroke model, RIP was found to be protective at multiple time points, including those outside the classic and second windows15. However, there have been few studies which have compared the time periods of neuroprotection in RIP and IP.
A further consideration for remote ischemia protection is the timing of the conditioning, including whether it is applied before injury (preconditioning) or after injury (postconditioning). The majority of remote ischemia testing has used preconditioning despite postconditioning studies having recently been found to be protective of both retinal and cerebral neurons30,31.
In summary, the induction of neuroprotection in hind limb ischemic conditioning is specific to the disease model, animal species, the duration of ischemia, and the timing of ischemia. A review by Kaniora et al. provides further details about the variety of remote ischemia protocols, including the species, RIP protocols, RIP sites, injury models, injury outcomes and proposed protection mechanisms32.
The minimally invasive cuff on the hind limb allows for RIP in both awake and animals provided the body temperature is maintained. In anesthetized experimentation, the animal’s body temperature must be maintained to prevent hypothermia. Internal temperature monitoring will prevent the animal undergoing hypothermia or hyperthermia. Hypothermia and hyperthermia are well-known pre-conditioning stimuli in both stroke models and light injury33-36. The JoVE method presented can be performed in awake animals thereby preventing body temperature confounds.
Anesthetics may introduce a different set of confounds in RIP experimentation. Isoflurane can participate in myocardium protection via opening of ATP-sensitive potassium channels, a similar protective mechanism reported in ischemic conditioning37. Although the size of the infarct in stroke models remains large in sham-treated animals given isoflurane, the molecular mechanism underlying remote ischemic conditioning may be masked by the effects of anesthetics. Ketamine, an NMDA antagonist, has a multitude of protective effects in vivo38, including the potential to prevent excitotoxicity to neurons, activate the mTOR pathway and release BDNF into the serum39-41. Ketamine has been reported to enhance neuronal survival following brain trauma in humans and reduce light injury in rodent photoreceptors42,43. Investigations into the mechanism of remote ischemic conditioning with awake blood pressure cuffing will avoid anesthetic confounds.
Effective hind limb ischemia relies on the correct placement of the cuff, consistency of the cuff pressure and cuff pressure elevation above systolic blood pressure as seen in Figure 1. A cuff placed below the “knee” does not protect photoreceptors from light injury as reflected in the reduced electroretinogram (ERG) amplitudes. The difference in conditioning based on the position of the cuff is likely due to differences in muscle mass and proximity to the femoral artery. In addition, animals should be standardized for age, weight, body temperature and gender.
In summary, remote ischemia can be induced by a non-invasive blood pressure cuff, which avoids muscle injury and has the flexibility for awake or anesthetized experimentation. Remote ischemic preconditioning is an emerging neuroprotective strategy and this protocol will enable further studies into its mechanisms and applications.
Disclosures
The author has nothing to disclose.
Acknowledgments
The author is grateful for the assistance of Mrs. Sharon Spana (University of Sydney) in rodent monitoring, handling and experimentation. The authorwould like to thank Prof. Jonathan Stone and Dr. Dan Johnstone for the assistance in the preparation of this manuscript. PhD funding support has been provided by University of Sydney and Australian Center for Excellence in Vision.
References
- Meller R, Simon RP. Tolerance to Ischemia-an Increasingly Complex Biology. Translational Stroke Research. 2013;4(1):40–50. doi: 10.1007/s12975-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, et al. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K-ATP channels in a rat model of focal cerebral ischemic reperfusion injury. Journal of Cerebral Blood Flow and Metabolism. 2012;32(5):851–859. doi: 10.1038/jcbfm.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin DW, D'Sa A, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia-reperfusion protects against acute lung injury. Journal of Vascular Surgery. 2002;35(6):1264–1273. doi: 10.1067/mva.2002.121981. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia -A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Barone FC, et al. Ischemic preconditioning and brain tolerance - Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29(9):1937–1950. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- Roth S, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Investigative Ophthalmology & Visual Science. 1998;39(5):777–785. [PubMed] [Google Scholar]
- Wegener S, et al. Transient ischemic attacks before ischemic stroke: Preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Weih M, et al. Attenuated stroke severity after prodromal TIA - A role for ischemic tolerance in the brain. Stroke. 1999;30(9):1851–1854. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- Casson RJ, Wood JPM, Melena J, Chidlow G, Osborne NN. The effect of ischemic preconditioning on light-induced photoreceptor injury. Investigative Ophthalmology & Visual Science. 2003;44(3):1348–1354. doi: 10.1167/iovs.02-0368. [DOI] [PubMed] [Google Scholar]
- Ettaiche M, et al. ATP-sensitive potassium channels (K-ATP) in retina: a key role for delayed ischemic tolerance. Brain Research. 2001;890(1):118–129. doi: 10.1016/s0006-8993(00)03152-8. [DOI] [PubMed] [Google Scholar]
- Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovascular Research. 2006;70(2):212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine-A1-receptors, and ATP-senstive K+ channels in cerebral ischemic preconditioning. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends in Neurosciences. 2003;26(5):248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Ren C, et al. Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res. 2011;33(5):514–519. doi: 10.1179/016164111X13007856084241. [DOI] [PubMed] [Google Scholar]
- Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151(4):1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Research. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Jensen HA, et al. Remote Ischemic Preconditioning Protects the Brain Against Injury After Hypothermic Circulatory Arrest. Circulation. 2011;123(7):714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- Kuntscher MV, et al. Ischemic preconditioning by brief extremity ischemia before flap ischemia in a rat model. Plastic and Reconstructive Surgery. 2002;109(7):2398–2404. doi: 10.1097/00006534-200206000-00034. [DOI] [PubMed] [Google Scholar]
- Kharbanda RK, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106(23):2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- Perl W, Cucinell SA. LOCAL BLOOD FLOW IN HUMAN LEG MUSCLE MEASURED BY A TRANSIENT RESPONSE THERMOELECTRIC METHOD. Biophysical Journal. 1965;5(2):211–230. doi: 10.1016/s0006-3495(65)86712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuksanovic V, Sheppard LW, Stefanovska A. Nonlinear relationship between level of blood flow and skin temperature for different dynamics of temperature change. Biophysical Journal. 2008;94(10):L78–L80. doi: 10.1529/biophysj.107.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Souza MVP, et al. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Brazilian Journal of Medical and Biological Research. 2009;42(10):921–929. doi: 10.1590/s0100-879x2009005000025. [DOI] [PubMed] [Google Scholar]
- Hixson FP, Shafiroff BB, Werner FW, Palmer AK. DIGITAL TOURNIQUETS - A PRESSURE STUDY WITH CLINICAL RELEVANCE. Journal of Hand Surgery-American. 1986;11A(6):865–868. doi: 10.1016/s0363-5023(86)80239-8. [DOI] [PubMed] [Google Scholar]
- Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. Journal of Anatomy. 2002;200(1):69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandli A, Stone J. Remote ischemia influences the responsiveness of the retina: observations in the rat. Invest Ophthal Vis Sci. 2014;55(4) doi: 10.1167/iovs.13-13525. [DOI] [PubMed] [Google Scholar]
- Maslim J, Valter K, Egensperger R, Hollander H, Stone J. Tissue oxygen during a critical developmental period controls the death and survival of photoreceptors. Investigative Ophthalmology & Visual Science. 1997;38(9):1667–1677. [PubMed] [Google Scholar]
- Grimm C, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nature Medicine. 2002;8(7):718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Vander Heide R. Clinically Useful Cardioprotection: Ischemic Preconditioning Then and Now. Journal of Cardiovascular Pharmacology and Therapeutics. 2011;16(3-4):251–254. doi: 10.1177/1074248411407070. [DOI] [PubMed] [Google Scholar]
- Zhou YL, et al. Remote Limb Ischemic Postconditioning Protects Against Neonatal Hypoxic-Ischemic Brain Injury in Rat Pups by the Opioid Receptor/Akt Pathway. Stroke. 2011;42(2):439–444. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron. 2006;50(1):63–74. doi: 10.1016/j.neuron.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Kanoria S, Jalan R, Seifalian AM, Williams R, Davidson BR. Protocols and mechanisms for remote ischemic preconditioning: A novel method for reducing ischemia reperfusion injury. Transplantation. 2007;84(4):445–458. doi: 10.1097/01.tp.0000228235.55419.e8. [DOI] [PubMed] [Google Scholar]
- Maier CM, et al. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia - Effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29(10):2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Reith J, et al. Body temperature in acute stroke: Relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347(8999):422–425. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Tytell M, Gower DJ, Welch WJ. HYPERTHERMIA PROTECTS AGAINST LIGHT DAMAGE IN THE RAT RETINA. Science. 1988;241(4874):1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- Wang XD, et al. Neuronal degradation in mouse retina after a transient ischemia and protective effect of hypothermia. Neurological Research. 2002;24(7):730–735. doi: 10.1179/016164102101200663. [DOI] [PubMed] [Google Scholar]
- Tonkovic-Capin M, et al. Delayed cardioprotection by isoflurane: role of K(ATP) channels. American Journal of Physiology-Heart and Circulatory Physiology. 2002;283(1):H61–H68. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- Pfenninger E, Himmelseher S. Neuroprotective effects of ketamine on a cellular level. Anaesthesist. 1997;46:S47–S54. doi: 10.1007/pl00002465. [DOI] [PubMed] [Google Scholar]
- Hirose K, Chan PH. BLOCKADE OF GLUTAMATE EXCITOTOXICITY AND ITS CLINICAL-APPLICATIONS. Neurochemical Research. 1993;18(4):479–483. doi: 10.1007/BF00967252. [DOI] [PubMed] [Google Scholar]
- Welberg L. Psychiatric disorders: Ketamine modifies mood through mTOR. Nature reviews. Neuroscience. 2010;11(10):666. doi: 10.1038/nrn2916. [DOI] [PubMed] [Google Scholar]
- Garcia LSB, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32(1):140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nature Reviews Neuroscience. 2004;5(10):747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Hertle DN, et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain. 2012;135:2390–2398. doi: 10.1093/brain/aws152. [DOI] [PubMed] [Google Scholar]


