Abstract
In order to cause endovascular infections and infective endocarditis, bacteria need to be able to adhere to the vessel wall while being exposed to the shear stress of flowing blood.
To identify the bacterial and host factors that contribute to vascular adhesion of microorganisms, appropriate models that study these interactions under physiological shear conditions are needed. Here, we describe an in vitro flow chamber model that allows to investigate bacterial adhesion to different components of the extracellular matrix or to endothelial cells, and an intravital microscopy model that was developed to directly visualize the initial adhesion of bacteria to the splanchnic circulation in vivo. These methods can be used to identify the bacterial and host factors required for the adhesion of bacteria under flow. We illustrate the relevance of shear stress and the role of von Willebrand factor for the adhesion of Staphylococcus aureus using both the in vitro and in vivo model.
Keywords: Immunology, Issue 100, Shear stress, Staphylococcus aureus, bacteria, adhesion, mesenteric circulation, von Willebrand factor, flow chamber, vascular infection, infective endocarditis, blood vessel, endothelium, subendothelial matrix
Introduction
To establish endovascular infections, pathogens require a mechanism to adhere to the endothelium, which lines the vessel wall and the inner surface of the heart, and to persist and establish an infection despite being exposed to the shear stress of rapidly flowing blood. The most frequent pathogen causing life-threatening endovascular infections and infective endocarditis is Staphylococcus aureus (S. aureus)1.
Various bacterial surface-bound adhesive molecules mediate adhesion to host tissue by interacting with extracellular matrix components. These MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) recognize molecules such as fibronectin, fibrinogen, collagen and von Willebrand factor (VWF). MSCRAMMs are important virulence factors of S. aureus and are implicated in the colonization and invasion of the host2. Most studies on these virulence factors have been performed in static conditions, and thus may not be representative for human infections where initial adhesion of the bacteria occurs in flowing blood.
In the case of bloodstream infections, bacteria need to overcome the shearing forces of flowing blood in order to attach to the vessel wall. Models that investigate the interaction between bacteria and endothelium or subendothelium under flow conditions are therefore of particular interest.
A recent study showed that the adhesion of S. aureus to blood vessels under shear stress is mediated by VWF3. VWF, a shear stress-operational protein, is released from endothelial cells upon activation. Circulating VWF binds to collagen fibers of the exposed subendothelial matrix. Our group reported that the von Willebrand factor-binding protein (vWbp) of S. aureus is crucial for shear-mediated adhesion to VWF4.
In this article, we present an in vitro flow chamber model where bacterial adhesion to different components of the extracellular matrix or to endothelial cells can be evaluated. To validate the findings from in vitro data, we have developed an in vivo model that visualizes and quantifies the direct interaction of bacteria with the vessel wall and the formation of bacteria-platelet thrombi in the mesenteric circulation of mice, using real-time intravital vascular microscopy.
Protocol
Animal experiments were approved by the Ethical Committee of the KU Leuven.
1. Preparing Bacteria for In Vitro Perfusions and In Vivo Experiments
We used S. aureus strain Newman for all experiments described in this manuscript. S. aureus Newman was stored in Brain Heart Infusion (BHI) with 10% glycerol at -80 °C.
Use a sterile loop to scrape the frozen bacteria off and inoculate in 5 ml Tryptic Soy Broth (TSB) O/N at 37 °C (OD600 >3).
Wash the bacteria by centrifugation (2,600 g, RT, 5 min) and resuspend the bacterial pellet in 5 ml PBS (phosphate buffered saline).
Prepare a 1 mg/ml solution of 5(6)-carboxy-fluorescein N-hydroxysuccinimidyl ester (carboxy-fluorescein) in ethanol. Dilute the 1 mg/ml carboxy-fluorescein solution to 150 µg/ml in laboratory grade water (e.g., MilliQ water). Protect the tubes from light with aluminum foil and store at -20 °C.
Centrifuge the bacteria (2,600 x g, RT, 5 min). Resuspend the bacterial pellet in 800 µl PBS and add 200 µl (final concentration of 30 µg/ml for perfusion experiments) or 400 µl (final concentration 50 µg/ml for in vivo experiments) of the 150 µg/ml carboxy-fluorescein solution. Protect the tubes from light with aluminum foil and incubate for 30 min at RT on a shaker.
After labeling, block with 6% bovine serum albumin (BSA) solution in PBS.
Dilute bacteria using optical densitometry (OD), an OD600 of 0.65 for in vitro experiments (corresponding to approximately 3 x 108 colony forming units (CFU)/ml for S. aureus) and an OD600 of 1.8 for in vivo experiments (corresponding to approximately 1 x 109 CFU/ml for S. aureus) in PBS. Protect the tubes from light with aluminum foil and leave on ice.
2. In Vitro Perfusion Experiments
- Coating of Glass Coverslips
- Dilute von Willebrand factor (VWF) (Haemate P, stock concentration 2,400 µg/ml) in laboratory grade water (deionized distilled) to a final concentration of 50 µg/ml.
- Dilute collagen in isotonic glucose solution (SKF solution, pH 2.7-2.9, as supplied by the manufacturer) to a final concentration of 160 µg/ml.
- Coat glass coverslips (24 × 50 mm) with VWF or collagen by dropping 200 µl of the coating on parafilm and place the coverslip on top of the droplet. The droplet will spread along the surface of the coverslip.
- Incubate the coverslip in a humidified container for 4 hr at RT. Carefully lift the coverslips from the parafilm with a blunt needle. Mount the coverslip in the bottom part of the flow chamber.
- Coating of Plastic Slips with Endothelial Cells
- Coat plastic slips (1-well PCA cell culture chamber, Sarstedt, Germany) with 1 ml of a 1% gelatin solution in PBS and incubate for 30 min at 37°C. Seed human umbilical vein endothelial cells (HUVECs) on the gelatin coated plastic slips and grow them to 70-80% confluency. Mount the plastic slip in the bottom part of the flow chamber.
- Perfusion Experiment NOTE: A schematic overview of the in vitro perfusion model is represented in Figure 1.
- Perform in vitro bacterial adhesion studies in a micro-parallel plate flow chamber at a laminar shear stress between 2.5 dyne/cm2 and 20 dyne/cm2 to simulate different physiological flow conditions.
- The flow chamber (in-house design) consists of a metal frame and a perfusion chamber made out of plexiglas (poly(methyl) methacrylate (PMMA)). By connecting it to a high-accuracy infusion pump (PHD 2000 Infusion, Harvard Apparatus, USA), we can generate flow rates between 0.0001 µl/min and 220.82 ml/min.
- Connect the tubing to the upper part of the flow chamber and inject medium in the tubing. Gently place the upper part of the flow chamber on top of the bottom part and assemble the flow chamber. Be careful to avoid air bubbles. Inject 1 ml of medium through the chamber to make sure that the chamber is not leaking and to remove excess coating solution. Avoid air bubbles.
- Place the mouse on a thermo-controlled heating pad at 37 °C on a microscope tray. Since this is a terminal procedure, there is no need for strict asceptic procedures. Make an incision near the jugular vein, gently remove the right side of the cervical muscle and isolate the jugular vein from the surrounding tissue.
- Set up the infusion pump and fluorescence microscope. Infusion pump settings depend on the syringe diameter and the desired flow rate (See section 2.4). From now on, work in a dark room.
- VWF Coating:
- Fill a syringe with fluorescently labeled bacteria and connect it to the inlet tube. Avoid air bubbles. Start the infusion pump for 10 min. The infusion time depends on the shear rate and the coating, bacteria and medium used and should represent the steady state of adhesion.
- After 10 min, wash away unbound bacteria by connecting a syringe with PBS to the inlet tube and starting the infusion pump.
- Take at least 15 images or movies at different locations after the wash process. Bacteria are small and potentially difficult to focus on. Prior to the in vitro flow experiment, the appropriate focal plane can be retrieved by placing a drop of fluorescently labeled bacteria on a coverslip and placing the coverslip in the flow chamber. Then, search for the appropriate focal plane and save the settings. NOTE: During the in vitro flow experiment, capturing the images during the washing step (± 5 min after start) ensures that only the signal for adherent bacteria is captured.
- Collagen Coating:
- Add 60 µg/ml VWF to the fluorescently labeled bacteria just before starting the perfusion. Fill a syringe with fluorescently labeled bacteria or fluorescently labeled bacteria supplemented with 60 µg/ml VWF and connect it to the inlet tube. Avoid air bubbles. Repeat steps 2.3.5.2 to 2.3.5.3
- Endothelial Cells:
- Activate the endothelial cells by perfusion with a 0.1 mM solution of the Ca2+-ionophore A23187 (stock solution 10 mM dissolved in dimethyl sulfoxide (DMSO)) in DMEM at the same shear rate as the bacterial perfusion for 10 min by perfusion with a 0.1 mM. Repeat steps 2.3.5.2 to 2.3.5.3.
Calculate shear rate and shear stress as follows. Shear rate = 6Q/wh2 Where: Q: flow rate in ml/min, w: width in cm, h: height in cm Shear stress (τ) = shear rate x viscosity (µ) Where µ: medium: 0.01 dynes x sec/cm2, whole blood: 0.04 dynes x sec/cm2
- Image analysis
- Obtain live images using an inverted fluorescence microscope with a black and white camera and develop using imaging software. Use exposure time of 1.5 sec. Take multiple snapshots (at least 15) randomly spread over the coated surface of the flow chamber and save them in the appropriate file format.
- Perform image analysis with ImageJ. Subtract the background to remove smooth continuous backgrounds from the image (Process – Substract Background) and define the threshold to set lower and upper threshold values, segmenting gray scale images into features of interest. Measure the area limited to the threshold.
- Compare bacterial adhesion, expressed as fluorescent area, e.g., using statistical analysis software. Compare the groups using one-way ANOVA or two-tailed Student’s t- test. Report all values as mean ± standard error of the mean (SEM). Consider a p-value of <0.05 significant (* p <0.05; ** p <0.01; *** p <0.001).
3. In Vivo Mesenteric Perfusion Model
- Preparation/surgery of the mouse
- Fast the mouse the night before the experiment in order to limit bowel movement.
- Give a 6-8 week old mouse (C57Bl/6) pre-operative analgesia by a subcutaneous injection of buprenorphine (0.1 mg/kg body weight (BW)) 20-30 min prior to the surgery.
- Anesthetize the mouse by intra-peritoneal injection of ketamine (125 mg/kg BW) and xylazine (12.5 mg/kg BW). Check by pedal reflex. Apply vet ointment to prevent dryness.
- Place the mouse on a thermo-controlled heating pad at 37 °C on a microscope tray. Since this is a terminal procedure, there is no need for strict asceptic procedures. Make an incision near the jugular vein, gently remove the right side of the cervical muscle and isolate the jugular vein from the surrounding tissue.
- Insert a 2 French intravenous catheter into the right jugular vein for infusion of fluorescently labeled bacteria or other solutions. Open the peritoneal cavity via a midline abdominal incision and use cotton swabs to spread the mesenterium and to visualize the mesenteric arteriolar and venular circulation.
- Place the mouse on the right side on a transparent plate and secure the cannula with tape. Use a hot pack to prevent hypothermia. To prevent dehydration of the tissue, drop 500 µl 0.9 % NaCl on the intestines.
- Fluorescence microscopy of bacterial adhesion to the mesenteric circulation
- Work in a dark room. Use cotton swabs to immobilize the vessels and visualize them under an inverted microscope.
- Topically apply 5 µl of a 10 mM solution of the Ca2+-ionophore A23187 dissolved in DMSO. After 10 sec, inject 100 µl labeled bacteria (see step 1) through the jugular catheter. Take time-lapse images. After the experiment is finished, euthanize the mouse according to institutional approved guidelines.
- Image Analysis
- Obtain live images using an inverted fluorescence microscope, captured using a black and white camera and developed using any imaging software. Apply automated exposure time and contrast optimization specific to the equipment used.
- Acquire time-lapse images using the ‘Acquisition’ tool in the toolbar (Multidimensional Acquisitions – Time) using 40 cycles of 1,000 images/sec. Save the images in an appropriate image file format.
- Process images using ImageJ analysis software to measure the area of fluorescent signal per image. Define the threshold to set lower and upper threshold values, segmenting gray scale images into features of interest. Identify the region of interest (blood vessel) and measure the area limited to the threshold and the region of interest. Compare bacterial adhesion, expressed as fluorescent area using any statistical or graphing software.
Representative Results
S. aureus adhesion to VWF, subendothelial matrix and endothelial cells is a shear stress dependent phenomenon
To emphasize the role of shear stress in the interaction between S. aureus and VWF, we performed perfusions over VWF coated coverslips at different shear rates (a schematic overview of the in vitro perfusion model is given in Figure 1. Adhesion of S. aureus to VWF increased with increasing shear rates from 250 sec-1 to 2,000 sec-1 (Figure 2), indicating that high shear forces do not inhibit but reinforce the adhesion of bacteria to VWF.
In order to investigate the contribution of VWF to bacterial adhesion to collagen, the main component of the subendothelial matrix, we perfused fluorescently labeled S. aureus over collagen in the presence or absence of VWF. In the absence of VWF, adhesion of S. aureus to collagen decreased with increasing shear rates. However, when VWF was present in the medium, the adhesion of S. aureus increased with increasing shear rates (Figure 3).
The in vitro flow model also allows us to examine the adhesion of bacteria to endothelial cells under flow. We perfused HUVECs with fluorescently labeled S. aureus at shear rates from 500 to 2,000 sec-1. Where indicated, HUVECs were activated with a Ca2+-ionophore, to cause release of VWF. Endothelial cell activation and the subsequent VWF release, increased adhesion of S. aureus (Figure 4A), which formed typical “string-like” patterns of fluorescently labeled bacterial clusters aligned in the direction of the shear force (Figure 4B), suggesting the binding of bacteria along a linear-stretched VWF molecule.
Initial in vivo bacterial adhesion in splanchnic veins is mediated by VWF
Since S. aureus is able to adhere to VWF, we used wildtype mice (Vwf+/+) and VWF-deficient mice (Vwf−/−) to investigate bacterial adhesion to the activated vessel wall in vivo. Real-time videomicroscopy of splanchnic veins allowed the in vivo visualization of circulating fluorescently labeled S. aureus (Schematic overview of the in vivo perfusion model is represented in Figure 5).
After pharmacological activation of the endothelium by the Ca2+-ionophore, we observed rapid local accumulation of individual bacteria and aggregates of bacteria to the vessel wall of WT mice (supplemental Videos 1 and 2). Almost no adhesion of bacteria was observed on the activated vessel wall of Vwf-deficient mice (supplemental Video 3) compared with adhesion in WT mice (Figure 6). The absence of VWF decreases the ability of S. aureus to adhere to the activated vessel wall.
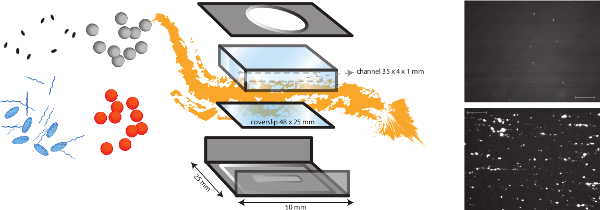 Figure 1. A schematic representation of the in vitro flow model. The in vitro flow model is a multifunctional model, which allows the study of different shear dependent mechanisms such as bacterial adhesion to the subendothelial matrix but also thrombus formation. The micro-parallel flow chamber is placed on a coverslip (plastic or glass) with different coatings of proteins and endothelial cells. The adhesion of different bacteria (orange and grey dots) can be analyzed, and the impact of the presence of plasma proteins, platelets and whole blood can be evaluated. Fluorescent markers for platelets (blue ovals) or fibrinogen (blue strings) can be used in combination with different inhibitors (black ovals) to distinguish bacterial and host factors. Representative images of bacterial adhesion of S. aureus to collagen coating in the presence (bottom) or absence (top) of VWF are shown (scale bar is 100 µm). Please click here to view a larger version of this figure.
Figure 1. A schematic representation of the in vitro flow model. The in vitro flow model is a multifunctional model, which allows the study of different shear dependent mechanisms such as bacterial adhesion to the subendothelial matrix but also thrombus formation. The micro-parallel flow chamber is placed on a coverslip (plastic or glass) with different coatings of proteins and endothelial cells. The adhesion of different bacteria (orange and grey dots) can be analyzed, and the impact of the presence of plasma proteins, platelets and whole blood can be evaluated. Fluorescent markers for platelets (blue ovals) or fibrinogen (blue strings) can be used in combination with different inhibitors (black ovals) to distinguish bacterial and host factors. Representative images of bacterial adhesion of S. aureus to collagen coating in the presence (bottom) or absence (top) of VWF are shown (scale bar is 100 µm). Please click here to view a larger version of this figure.
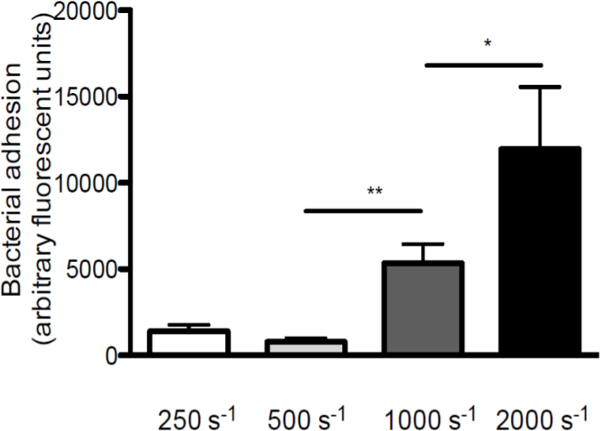 Figure 2. Adhesion of S. aureus to VWF increases with increasing shear rates. Micro-parallel flow chamber perfusion over coated VWF (50 µg/ml) with fluorescently labeled S. aureus Newman at shear rates of 250 to 2,000 sec-1 (sec-1) in medium (n >5). All results are expressed as mean ± SEM. *p <0.05, **p <0.01.
Figure 2. Adhesion of S. aureus to VWF increases with increasing shear rates. Micro-parallel flow chamber perfusion over coated VWF (50 µg/ml) with fluorescently labeled S. aureus Newman at shear rates of 250 to 2,000 sec-1 (sec-1) in medium (n >5). All results are expressed as mean ± SEM. *p <0.05, **p <0.01.
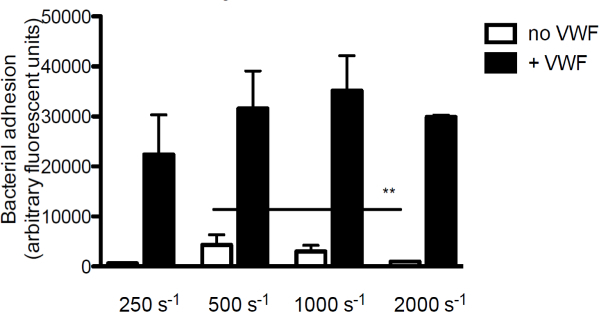 Figure 3. Adhesion of S. aureus to subendothelium is shear and VWF dependent. Micro-parallel flow chamber perfusion over coated collagen (160 µg/ml) with fluorescently labeled S. aureus Newman at shear rates of 250 to 2,000 sec-1 in medium (n >5). VWF (60 µg/ml) was present in the medium where indicated. All results are expressed as mean ± SEM. **p <0.01.
Figure 3. Adhesion of S. aureus to subendothelium is shear and VWF dependent. Micro-parallel flow chamber perfusion over coated collagen (160 µg/ml) with fluorescently labeled S. aureus Newman at shear rates of 250 to 2,000 sec-1 in medium (n >5). VWF (60 µg/ml) was present in the medium where indicated. All results are expressed as mean ± SEM. **p <0.01.
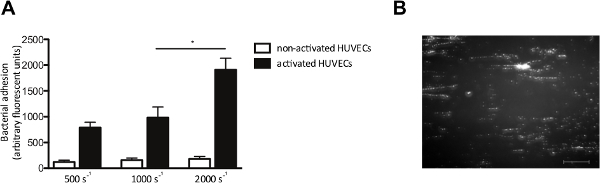 Figure 4. Adhesion of S. aureus to activated endothelial cells is shear dependent. Micro-parallel flow chamber perfusion over endothelial cells. (A) Human umbilical vein endothelial cells were activated with the Ca2+-ionophore A23187 (0.1 mM) followed by a 10 min perfusion of fluorescently labeled S. aureus Newman at shear rates of 500 to 2,000 sec-1 in medium (n >5). All results are expressed as mean ± SEM. *p <0.05. (B) Image of micro-parallel flow chamber perfusion over activated HUVECs with S. aureus at a shear rate of 1,000 sec-2. S. aureus forms strings of ± 200 microns length, suggesting adhesion to VWF multimers (scale bar is 100 µm). Please click here to view a larger version of this figure.
Figure 4. Adhesion of S. aureus to activated endothelial cells is shear dependent. Micro-parallel flow chamber perfusion over endothelial cells. (A) Human umbilical vein endothelial cells were activated with the Ca2+-ionophore A23187 (0.1 mM) followed by a 10 min perfusion of fluorescently labeled S. aureus Newman at shear rates of 500 to 2,000 sec-1 in medium (n >5). All results are expressed as mean ± SEM. *p <0.05. (B) Image of micro-parallel flow chamber perfusion over activated HUVECs with S. aureus at a shear rate of 1,000 sec-2. S. aureus forms strings of ± 200 microns length, suggesting adhesion to VWF multimers (scale bar is 100 µm). Please click here to view a larger version of this figure.
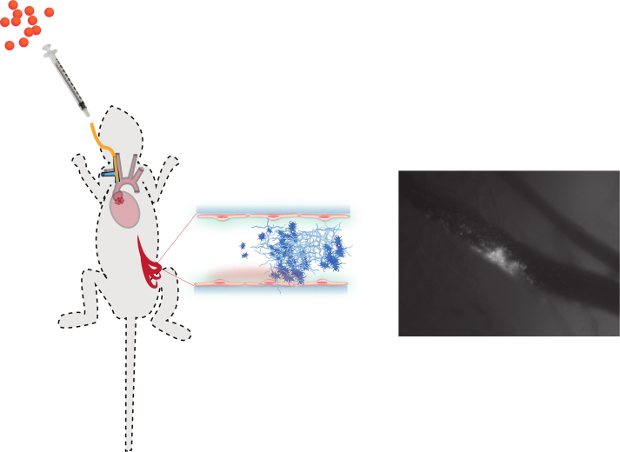 Figure 5. A schematic overview of the in vivo mesenteric perfusion model. A right jugular vein catheter (yellow line) is inserted for the administration of fluorescently labeled bacteria (orange dots), additional anesthetics or other components such as pharmaceutical inhibitors and antibodies. The peritoneal cavity is opened and the mesenterium is spread to visualize the blood vessels (venous and arterial) under a fluorescence microscope. After pharmacological activation of the endothelium by a Ca2+-ionophore, which induces the release of VWF, bacteria can be injected through the jugular vein catheter. Real-time intravascular video microscopy allows the in vivo visualization of circulating fluorescently labeled bacteria and the resulting formation of bacteria-platelet thrombi. Please click here to view a larger version of this figure.
Figure 5. A schematic overview of the in vivo mesenteric perfusion model. A right jugular vein catheter (yellow line) is inserted for the administration of fluorescently labeled bacteria (orange dots), additional anesthetics or other components such as pharmaceutical inhibitors and antibodies. The peritoneal cavity is opened and the mesenterium is spread to visualize the blood vessels (venous and arterial) under a fluorescence microscope. After pharmacological activation of the endothelium by a Ca2+-ionophore, which induces the release of VWF, bacteria can be injected through the jugular vein catheter. Real-time intravascular video microscopy allows the in vivo visualization of circulating fluorescently labeled bacteria and the resulting formation of bacteria-platelet thrombi. Please click here to view a larger version of this figure.
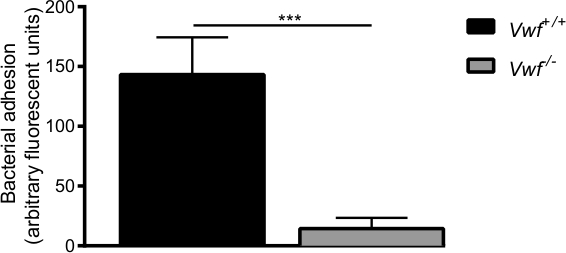 Figure 6. The initial adhesion of S. aureus to activated endothelium in vivo is mediated by VWF. In vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ and C57Bl/6-Vwf-/- mice. Adhesion of fluorescently labeled S. aureus to the locally activated vessel wall is significantly lower in Vwf-/- mice. All results are expressed as mean ± SEM. ***p <0.001, n >7.
Figure 6. The initial adhesion of S. aureus to activated endothelium in vivo is mediated by VWF. In vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ and C57Bl/6-Vwf-/- mice. Adhesion of fluorescently labeled S. aureus to the locally activated vessel wall is significantly lower in Vwf-/- mice. All results are expressed as mean ± SEM. ***p <0.001, n >7.
 Video 1: Real-time adhesion of
S. aureus
to activated vessel wall in
Vwf
+/+
mice.
Please click here to view this video.
Video 1: Real-time adhesion of
S. aureus
to activated vessel wall in
Vwf
+/+
mice.
Please click here to view this video.
 Video 2: Real-time aggregate formation and embolization of
S. aureus
in
Vwf
+/+
mice.
Please click here to view this video.
Video 2: Real-time aggregate formation and embolization of
S. aureus
in
Vwf
+/+
mice.
Please click here to view this video.
 Video 3: Real-time adhesion of S. aureus to activated vessel wall in Vwf-/- mice.In vivo mesenteric perfusion model with Vwf+/+ and Vwf-/- mice. Five µl of a Ca2+-ionophore (10 mM) was applied to the region of the visualized vascular bed. A suspension of carboxy-fluorescein-labeled S. aureus was injected through the jugular catheter. The mesenteric circulation was visualized under an inverted microscope. Please click here to view this video.
Video 3: Real-time adhesion of S. aureus to activated vessel wall in Vwf-/- mice.In vivo mesenteric perfusion model with Vwf+/+ and Vwf-/- mice. Five µl of a Ca2+-ionophore (10 mM) was applied to the region of the visualized vascular bed. A suspension of carboxy-fluorescein-labeled S. aureus was injected through the jugular catheter. The mesenteric circulation was visualized under an inverted microscope. Please click here to view this video.
Discussion
Shear stress is a crucial factor for the early bacterial adhesion to the vessel wall and for the subsequent generation of endovascular or endocardial vegetations and metastatic infections4,5. We described complementary in vitro and in vivo models to study the pathogenesis of endovascular infections under physiological shear stress. These models have allowed us to identify von Willebrand factor-binding protein (vWbp) as the major S. aureus protein to interact under flow with an injured vascular wall exposing VWF4.
Endovascular infections, and infective endocarditis in particular, are of concern not only because of sepsis-induced organ failure and death, but also because of local and distant (‘metastatic’) complications. To cause infective endocarditis and metastatic infections, bacteria have to adhere to the vessel wall and thus resist the shear stress of flowing blood. Most studies on bacteria virulence factors have been performed in static conditions. However, these established interactions might not withstand shear forces and studies under flow conditions can reveal new, previously unrecognized factors in bacteria-host interplay.
Using the micro-parallel flow chamber, we and others have shown the importance of VWF for vascular adhesion. Under shear stress, VWF progressively unfolds from its resting globular structure, and exposes the A1 domain that interacts with platelets via its GPIb receptor6. Flow chambers have been extensively used to study platelet function7.
Remarkably, also S. aureus adhesion under flow requires VWF, and in particular the A1 domain that is exposed upon shear. We identified vWbp to mediate VWF binding. vWbp is a coagulase that contributes to S. aureus pathophysiology by activating the host’s prothrombin. Staphylothrombin, the resulting complex of a bacterial coagulase and prothrombin, converts fibrinogen into insoluble fibrin8,9. Our studies have shown that vWbp does not only activate prothrombin, but triggers the formation of bacteria-fibrin-platelet aggregates, which enhance the adhesion to blood vessels under flow4,10,11.
The in vitro flow chamber model allows to study the different players in bacterial adhesion to cellular or matrix components. Bacterial virulence factors can be studied by using mutants or innocuous bacteria expressing specific surface proteins. Alternatively, pharmacologic inhibitors or blocking antibodies can be added to the medium in the flow chamber. The role of host factors such as different constituents of extracellular matrix can be studied by using coverslips with different coatings. The coverslips can also be covered with endothelial cells, of which the activation status can be modulated by adding specific stimulators. Apart from the vascular wall, the contribution of host blood cells and plasma proteins can be studied by adding these factors to the flowing medium. Thus, different conditions of increasing complexity can be studied under standardized conditions of laminar flow to unravel the interactions that allow bacteria to adhere to the vessel wall in vivo.
Interactions identified in the in vitro model are subsequently studied in an animal model to test their relevance in a complex organism. Other in vivo models to study dynamic interactions under flow have been described, such as the hamster dorsal skinfold chamber12 and the cremaster model13. In comparison, the mesenteric perfusion model described here offers several advantages because of its ease of use, the possibility to vary host genetic background of the mice and to evaluate pharmacological interventions.
In conclusion, the described models offer the possibility to study surface proteins not only of S. aureus, but of many other microorganisms in different host backgrounds, to better understand the pathogenesis of vascular infections.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen G0466.10, 11I0113N; “Eddy Merckx Research Grant” and the “Sporta research Grant” for Pediatric Cardiology, UZ Leuven, Belgium (J.C.); the Center for Molecular and Vascular Biology is supported by the Programmafinanciering KU Leuven (PF/10/014), by the “Geconcentreerde Onderzoeksacties” (GOA 2009/13) from the University of Leuven and a research grant from Boehringer-Ingelheim.
References
- Vanassche T, Peetermans WE, Herregods MC, Herijgers P, Verhamme P. Anti-thrombotic therapy in infective endocarditis. Expert Rev Cardiovasc Ther. 2011;9(9):1203–1219. doi: 10.1586/erc.11.100. [DOI] [PubMed] [Google Scholar]
- Heying R, van de Gevel J, Que YA, Moreillon P, Beekhuizen H. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: FnBPA is sufficient to activate human endothelial cells. Thromb Haemost. 2007;97(4):617–626. [PubMed] [Google Scholar]
- Pappelbaum KI, et al. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation. 2013;128(1):50–59. doi: 10.1161/CIRCULATIONAHA.113.002008. [DOI] [PubMed] [Google Scholar]
- Claes J, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor–binding protein. Blood. 2014;124(10):1669–1976. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006;15(5):256–263. doi: 10.1016/j.carpath.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Sixma JJ, Schiphorst ME, Verweij CL, Pannekoek H. Effect of deletion of the A1 domain of von Willebrand factor on its binding to heparin, collagen and platelets in the presence of ristocetin. Eur J Biochem/FEBS. 1991;196(2):369–375. doi: 10.1111/j.1432-1033.1991.tb15826.x. [DOI] [PubMed] [Google Scholar]
- Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF. Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood. 1999;94(8):2725–2734. [PubMed] [Google Scholar]
- Bjerketorp J, Jacobsson K, Frykberg L. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234(2):309–314. doi: 10.1016/j.femsle.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Friedrich R, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425(6957):535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- Vanassche T, et al. Fibrin formation by staphylothrombin facilitates Staphylococcus aureus-induced platelet aggregation. Thromb Haemost. 2012;107(6):1107–1121. doi: 10.1160/TH11-12-0891. [DOI] [PubMed] [Google Scholar]
- Vanassche T, et al. The role of staphylothrombin-mediated fibrin deposition in catheter-related Staphylococcus aureus infections. J Infect Dis. 2013;208(1):92–100. doi: 10.1093/infdis/jit130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkle MA, Lehrer S, Sohn HY, Conzen P, Pohl U, Krötz F. Selective inhibition of cyclooxygenase-2 enhances platelet adhesion in hamster arterioles in vivo. Circulation. 2004;110(14):2053–2059. doi: 10.1161/01.CIR.0000143234.51796.A9. [DOI] [PubMed] [Google Scholar]
- Kim KH, Barazia A, Cho J. Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus formation in live mice. J Vis Exp. 2013;2(74) doi: 10.3791/50329. [DOI] [PMC free article] [PubMed] [Google Scholar]


