Abstract
Almost all organisms ranging from single cell bacteria to humans exhibit a variety of behavioral, physiological, and biochemical rhythms. In mammals, circadian rhythms control the timing of many physiological processes over a 24-h period, including sleep-wake cycles, body temperature, feeding, and hormone production. This body of research has led to defined characteristics of circadian rhythms based on period length, phase, and amplitude. Underlying circadian behaviors is a molecular clock mechanism found in most, if not all, cell types including skeletal muscle. The mammalian molecular clock is a complex of multiple oscillating networks that are regulated through transcriptional mechanisms, timed protein turnover, and input from small molecules. At this time, very little is known about circadian aspects of skeletal muscle function/metabolism but some progress has been made on understanding the molecular clock in skeletal muscle. The goal of this chapter is to provide the basic terminology and concepts of circadian rhythms with a more detailed review of the current state of knowledge of the molecular clock, with reference to what is known in skeletal muscle. Research has demonstrated that the molecular clock is active in skeletal muscles and that the muscle-specific transcription factor, MyoD, is a direct target of the molecular clock. Skeletal muscle of clock-compromised mice, Bmal1−/− and ClockΔ19 mice, are weak and exhibit significant disruptions in expression of many genes required for adult muscle structure and metabolism. We suggest that the interaction between the molecular clock, MyoD, and metabolic factors, such as PGC-1, provide a potential system of feedback loops that may be critical for both maintenance and adaptation of skeletal muscle.
1. Introduction
The term Circadian comes from the Latin circa, “around,” and diem, “day,” meaning “about a day.” Almost all organisms ranging from single cell bacteria to humans exhibit a variety of behavioral, physiological, and biochemical circadian rhythms (Albrecht and Oster, 2001; Hastings et al., 2008; Merrow et al., 2005). The presence of a molecular clock within a cell and/or organism provides the necessary timekeeping for anticipation of daily changes in environmental/external conditions (Albrecht, 2002; Gekakis et al., 1998; Hastings et al., 2008; Holzberg and Albrecht, 2003; Schibler, 2009; Takahashi et al., 2008; Zhang and Kay, 2010). Synchronizing the molecular clock and intracellular physiology with external day–night cycles represents an evolutionary survival advantage for organisms (Albrecht and Oster, 2001; Holzberg and Albrecht, 2003; Oster et al., 2002). While much has been learned about circadian rhythms and the molecular clock, there is still very little known about its regulation and function in skeletal muscle (Almon et al., 2008; Andrews et al., 2010; McCarthy et al., 2007; Miller et al., 2007; Zhang et al., 2009). Thus, the goal of this chapter is twofold. First, we provide some fundamental background in circadian rhythms with introductions to terminology and concepts in circadian research. Second, we review what is known about the molecular clock, and when possible, incorporate research in skeletal muscle. At this stage, this is a very new and open field of research, so much is yet to be done to mechanistically link the function of the molecular clock in skeletal muscle to known biochemical and physiological outcomes. Additionally, much is still to be done to understand the mechanisms in place to coordinate and synchronize the clocks among the diverse groups of skeletal muscle throughout the body with the central clock and other peripheral tissues.
2. Characteristics of Oscillating Systems
The term “circadian” was first used in the late 1950s, to describe a rhythm with a period length of ~24 h by Franz Halberg. This was the start of the standardization of terms for the growing research in chronobiology (Halberg et al., 1959). Although the understanding and definition of many circadian terms have developed as discoveries have been made, the notion that organisms operate in a cyclic manner close to 24 h remains (Halberg, 1969). Many of the terms that will be discussed in this chapter are listed in Table 9.1.
Table 9.1.
Terms used in circadian rhythms research
| Term | Description |
|---|---|
| Amplitude | The difference between the mean of the rhythm and the peak or the trough |
| Circadian Time (CT) | Time as it relates to the endogenous rhythm of an organism measured under constant conditions. CT0 is defined as the onset of activity for a diurnal animal while CT12 is the onset of activity for nocturnal animals. |
| DD | Constant darkness |
| Dampening | Persistent decrease in the amplitude of a rhythm |
| Entrainment | When a stable phase relationship is established between an endogenous, self-sustained oscillator and an external time giver (“Zeitgeber”) |
| Free-running | A state when there are no capable entrainment cues; the self-sustained rhythm of the organism is observed |
| LD | Light cycle followed by a dark cycle; 12:12 LD-12 h of light then 12 h of dark repeating every 24 h |
| LL | Constant light |
| Masking | A change in the rhythm that does not reflect the true phase or period of the rhythm |
| Period | The time it takes to complete 1 cycle |
| Phase | The time of any point in a rhythm |
| Phase shift | A shift in the time of any point in a rhythm to earlier or later (phase delay, phase advance) |
| PRC | Phase response curve; A graphical representation of a stimuli’s temporal effect on the phase of a rhythm |
| Zeitgeber Time (ZT) | Time as it relates to an external Zeitgeber (“Zeit = time, geber = giver”). ZT0 refers to lights on for a diumal organism, such as humans, and ZT12 defines lights off for a nocturnal organism, such as rodents |
2.1. Tau
The term tau refers to the period of a rhythm and is the length, in time, of one complete cycle. One circadian cycle is classically found to be 20–28 h in length and is based on the photoperiod or exposure to light (Halberg et al., 1977). Period length can be measured for any variable that has a changing pattern, for example, oscillation or rhythm. The tau, or period, for a cycling variable is calculated by selecting one point in the cycle and measuring how long it takes to get to that same point in the next cycle. Period length is a principal measurement in chronobiology, as it illustrates a fundamental feature of time keeping, for example, how long is the cycle? Period can be measured in behaviors that cycle such as sleeping and wakefulness, locomotor activity, or eating/drinking. Many physiological, biochemical, and molecular variables are known to oscillate including body temperature, circulating cortisol levels, and tissue gene expression (mRNA/protein levels).
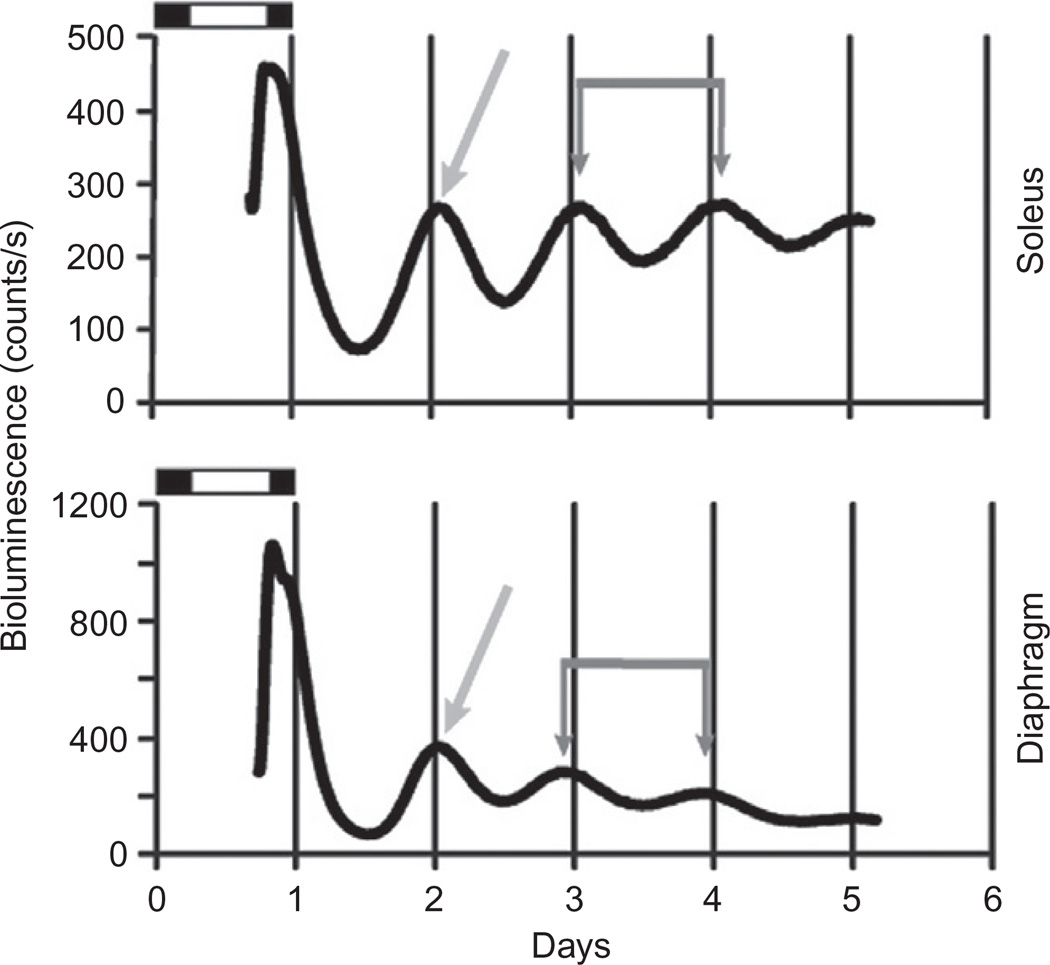
To identify different circadian rhythms across species of mammals, researchers commonly evaluate behaviors under constant conditions, such as total darkness. For example, strains of inbred mice demonstrate a shorter period of locomotor activity in darkness (<24 h), while humans have a longer tau (Czeisler et al., 1980). Rats and hamsters also display a period length close to 24 h that tends to decrease as the animals age (Rosenberg et al., 1991). While there are some variations in tau across animals, it is also clear that period length of cycling behaviors across many species are remarkably close to 24 h even after weeks without exposure to light, or in the case of blind animals, an entire lifetime (Lowe et al., 1967). As will be discussed in more detail in later sections, there is much research currently on understanding the tau of the molecular clock within single cells, coupled cells, or whole tissues. In these studies, period can be measured by rhythms generated by molecular oscillators. The PERIOD2::LUCIFERASE (Per2:: Luc) mouse is an animal model used to study endogenous molecular rhythms. mPer2, the mammalian Period2 gene is a core component of the molecular clock in mammals (see Section 5). Yoo et al. (2004) created a mouse in which the luciferase cDNA was knocked into the endogenous mPer2 locus (Yoo et al., 2004). The result is a chimeric protein that can be used to measure bioluminescence that is directly related to Period2 protein accumulation and degradation in the cell. An illustration of period measured using data from the diaphragm and soleus muscles taken from Per2::Luc mice is provided in Fig. 9.1. As can be seen in this figure, the tau is the time between two peaks in luminescence as noted by the two red arrows. As can be seen in Fig. 9.1, the molecular clock in two different skeletal muscles, diaphragm and soleus, exhibits period lengths that are very similar to 23.92 ± 0.01 h (mean ± sd dev). In normal, healthy conditions, the tau of the molecular clock will be the same, or very close to the tau for behavioral rhythms.
Figure 9.1.
Real-time bioluminescence data from soleus and diaphragm explants using PERIOD2::LUCIFERASE mice demonstrate that different muscles within the same animal are in phase. Soleus muscle (top) and diaphragm (bottom); bioluminescence on the y-axis measured in (photon) counts/second for 5 days (days on x-axis). Dark gray double arrows indicate peak bioluminescence on days 3 and 4 for each muscle; the time between these arrows represents the period length and is 23.92 ± 0.01 (avg ± Sd dev). The lighter gray single arrows point to the first peak following 24 h in culture; this peak reflects the phase of the molecular clock and for these samples = 24.10 ± 0.80 for the soleus muscle and 23.43 ± 0.65 for the diaphragm. (Wolff and Esser, unpublished data).
2.2. Phase
Another principal characteristic of circadian rhythms is phase. Phase describes the timing of a particular point within a rhythm. Most of the time, researchers use the peak (highest point), trough (lowest point), or another point in the cycle for reference when discussing phase. When using phase to describe a rhythm, it is necessary to identify the point of interest, such as the onset of light or dark or the peak cortisol level. The phase of a rhythm is often used to describe its relationship to another oscillator, such as the phase of the peak of gene expression relative to the phase of activity onset or light onset. The phase of gene expression (mRNA and protein) in molecular oscillators usually maintains a stable relationship, across tissues in an animal. For example, the peak in Per2 gene expression in the central clock in the suprachiasmatic nucleus (SCN) occurs about 4–6 h prior to the peak in Per2 gene expression in skeletal muscles and other peripheral tissues (Yamazaki et al., 2000; Yoo et al., 2004). In Fig. 9.1, the phase of luminescence (blue arrow) was measured at the first peak after 24 h in culture. We have also found that the phase between different skeletal muscles is very similar, with the phase of the soleus being 24.10 ± 0.80 h versus 23.43 ± 0.65 in the diaphragm. This means that the first peak of Per2::Luc bioluminescence occurs right around midnight in both muscles, ~5–6 h after the peak in the SCN. The fact that the molecular clocks between different muscles are in phase is not surprising as the molecular clocks across diverse tissues are synchronized under normal conditions. However, this is the first report comparing the phase between two different muscles of different function and developmental origin. The synchronization of this phase relationship will be discussed later (Sections 3.2. and 4).
2.3. Amplitude
Amplitude is a third principal descriptor of circadian rhythms. Amplitude is defined as a measure of the difference between the calculated mean value of a rhythm and the peak or trough (highest and lowest points, respectively). Amplitude is often used to describe the robustness of the oscillator or rhythm. Within the field of chronobiology, a robust rhythm is sometimes viewed as more responsive or more stable and this is in contrast to a weak rhythm or one that is dampened (Aschoff, 1998). Changes in amplitude have been observed in aged animals, where many investigators have reported alterations in the timing and responsiveness of endogenous and behavioral rhythms (Turek et al., 1995; Wise et al., 1988; Wyse and Coogan, 2010). One piece of evidence for a destabilized clock in aged animals was demonstrated by Davidson et al. (2006) when they used a jet-lag experimental paradigm to demonstrate that persistent advances in the onset of light could produce accelerated mortality in aged animals compared with a younger cohort (Davidson et al., 2006). There are no data available on the amplitude of circadian rhythms in skeletal muscle at this time. Discussions of jet-lag and phase shifting will be provided in Section 3.3.
3. Environmental Influence on Endogenous Oscillators
The ability to synchronize an endogenous rhythm with an environmental time cue provides the animal with a biological advantage when performing daily activities (Feillet et al., 2006; Halberg and Cornelissen, 1993). Environmental stimuli can affect how long the clock is running (period), what time the clock is set to in relation to other clocks (phase), and the stability of the clock (amplitude). Although it has been demonstrated that clock-driven rhythms continue to run in the experimentally controlled absence of cues from the environment (Aschoff, 1990; Gibbs, 1976; Wilson et al., 1976), exogenous stimuli can profoundly impact the rhythm of endogenous oscillators, and can be used to understand how the organisms maintain a synchronous relationship with their surroundings.
3.1. Entrainment
An animal is considered entrained when its rhythm is aligned with a known external cue, such as light (Aschoff et al., 1975). The rhythm can be behavioral or molecular; it is endogenous and self-sustained (Aschoff, 1967; Aschoff and Wever, 1976). Behavioral measurements such as loco-motor activity or drinking activity can be used to monitor entrainment (Aschoff, 1978). In an entrained animal, the period length (average time between activity onset from one day to the next) will match the period of the environmental cue or zeitgeber (“time giver,” zeitgebers will be discussed in Section 3.2). For example, under experimental conditions an animal may be housed within a 24-h light–dark (LD) cycle with 12 h of light and 12 h of darkness (LD 12:12); the lights come on every 24 h. In an entrained state, the period length of the animal’s behavior will also be 24 h. The phase of the behavior will also be synchronized with the LD cycle. Phase is often measured in hours or sometimes degrees. Under entraining conditions, the phase relationship between the animal’s rhythm and the LD cycle is considered stable. That means that there is no shift in the timing of the rhythm relative to that of the entraining cycle, so there are 0 h (or 0°) of difference between the phase of the rhythm and the entraining cue (Aschoff, 1960; Mrosovsky et al., 1992). In the presence of a zeitgeber, such as light, the time is measured relative to the zeitgeber, thus zeitgeber time (ZT). ZT is usually measured in 24:00 h with ZT12 designated as the time of lights-off for a nocturnal animal, such as a mouse. In contrast to the entrained state, free running is defined as rhythmic behavior of an animal in the absence of a competent zeitgeber (Aschoff, 1978, 1998, 1999). Free running describes an animal’s activity when the endogenous self-sustaining rhythm is in control of behavior and physiology. As in the example above of a 24-h LD cycle, if the same animal is released into constant darkness, the endogenous rhythm of the animal will persist and the period for that animal will likely not measure exactly 24 h, as described earlier in Section 2.1 (Schwartz and Zimmerman, 1990). Endogenous period length differs among species and strains (Holmes and Mistlberger, 2000; Mistlberger, 1991; Mrosovsky, 1999; Stephan, 1983). When animals are housed under conditions with constant light or darkness, the “time” is often measured relative to the endogenous circadian rhythm. Under these conditions, researchers cannot use ZT to define time since there is no set time cue for reference so they developed the terminology circadian time (CT). CT denotes the time relative to a point during the animal’s behavioral rhythm. The most common point of reference for using CT is the onset of animal activity. In these conditions, activity onset is defined as CT12 for a nocturnal animal and times before or after activity onset are reported relative to CT12.
3.2. Zeitgebers
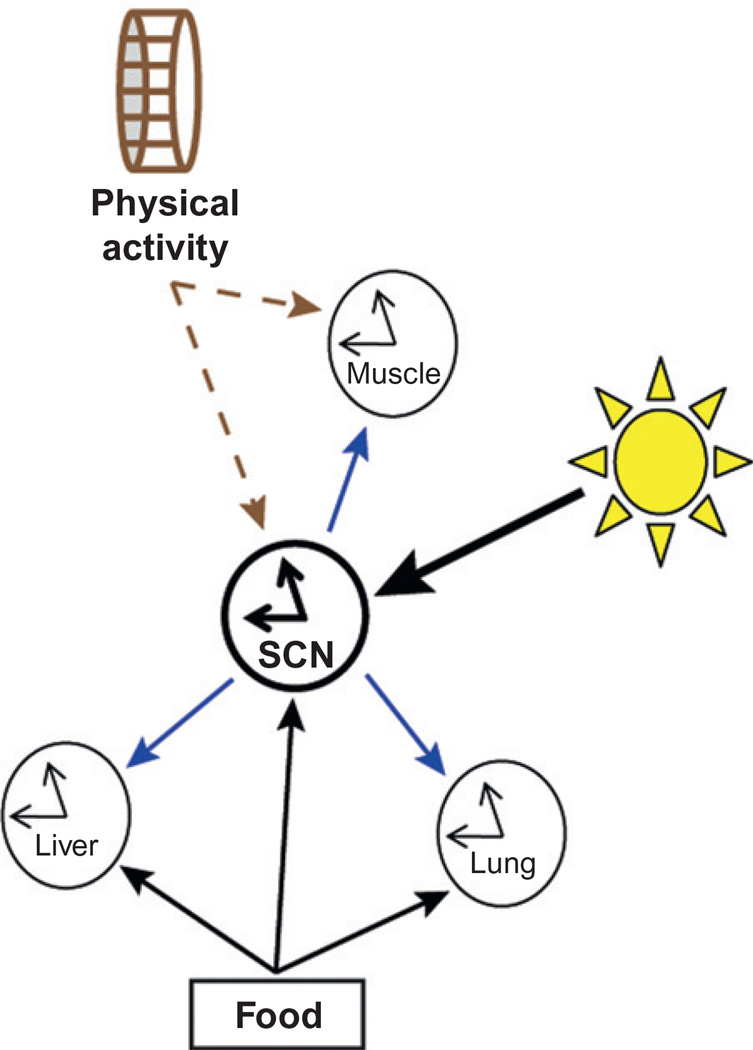
Environmental stimuli that are able to shift the timing of behavioral and molecular rhythms are critically important to understanding how oscillators can be synchronized to the environment and to each other. Figure 9.2 illustrates some of the major input and output pathways within the circadian system and the relationships that have been established or that are being evaluated by ongoing research. By far, the most comprehensively studied circadian zeitgeber is the photic time cue, light. Synchronization of the molecular clock in the SCN with the LD cycle via the retinohypothalamic tract (RHT) has been well established (Johnson et al., 1988). This major input pathway (light to SCN) synchronizes behavioral rhythms as well as molecular rhythms in peripheral oscillators as demonstrated in Fig. 9.2 by the thick arrows going from the light to SCN and on to the peripheral tissues (Guo et al., 2005). While the LD cycle has been studied vigorously in many species (Devlin and Kay, 2001) nonphotic zeitgebers, such as feeding and physical activity/exercise, have been shown to influence behavior and molecular rhythms (Edgar and Dement, 1991; Mistlberger, 1991; Mrosovsky, 1996).
Figure 9.2.
Molecular clocks within the central and peripheral tissues respond to both photic and nonphotic timing cues. This cartoon depicts the proposed interplay among time cues, the central clock, SCN, and peripheral clocks (liver/muscle/lung). The central clock, SCN, is synchronized by light (Takahashi et al., 1984), and the peripheral tissues (primarily liver) have been shown to be synchronized by time of feeding (Escobar et al., 1998; Honma et al., 1983). The SCN is also possibly affected by time of feeding (Angeles-Castellanos et al., 2007). Additionally, we suggest that physical activity, in the form of a running, may also be a time cue that acts to synchronize clocks in skeletal muscle (Yamanaka et al., 2008).
As depicted by the thin solid lines in Fig. 9.2, changing the time of food presentation has measurable effects on the molecular clock in the liver and lung and there is evidence that specific brain regions are involved when access to food is restricted (Damiola et al., 2000; Honma et al., 1983; Mieda et al., 2006). Several groups have demonstrated that the presentation of food at almost any time of day, for as little as 4 h or as many as 12 h, alters the rhythm of animal behavior and the molecular clock in tissues (Davidson et al., 2002; Diaz-Munoz et al., 2000; Escobar et al., 1998; Satoh et al., 2006; Stokkan et al., 2001). When the animal is presented with food for a restricted time, locomotor activity levels in the hours preceding the meal are greatly increased. This activity is termed food-anticipatory activity or FAA (Mistlberger, 1994; Stephan et al., 1979). Evidence of FAA in the absence of the SCN suggests the existence of a central oscillator located in another region of the brain outside the SCN (Angeles-Castellanos et al. 2005; Hara et al., 2001). The likelihood that the brain could harbor such a structure has made it a target of ongoing research with respect to restricted feeding.
Feeding is the only nonphotic time cue known in mammals but there are other nonphotic cues, such as scheduled activity and repeated treatment with methamphetamine, that have been shown to alter behavioral and molecular rhythms in animals. Scheduled bouts of activity can entrain behavioral rhythms in rodents and enhance reentrainment following phase shifts in rodents and humans (Edgar and Dement, 1991; Mrosovsky, 1996; Mrosovsky et al., 1992; Yagita et al., 2010; Yamanaka et al., 2008). The results of some studies suggest that the molecular clock in skeletal muscle is responsive to exercise and may be entrained by scheduled bouts of physical activity; in Fig. 9.2, this is represented by the dashed line between physical activity and muscle (Yamanaka et al., 2008; Zambon et al., 2003). In 2008, Yamanaka et al. advanced the time of lights on by 8 h for 4 consecutive days while providing running wheels to a subset of mice. The mice that received the wheel synchronized their behavior with the new LD cycle more quickly than the control group. Additionally, the molecular clock in skeletal muscle was rapidly shifted to the new LD cycle (Yamanaka et al., 2008). This result suggests that the molecular clock in skeletal muscle could be synchronized by a cue(s) from physical activity. The effect of repeated methamphetamine treatment on circadian rhythms is also an area of ongoing research. In this work, the most profound interaction between methamphetamines and circadian behavior was demonstrated by synchronization of locomotor rhythms in an arrhythmic animal treated with methamphetamines (Honma et al., 1987; Iijima et al., 2002). Animals with genetic mutations as well as SCN lesion have a similar behavioral response to methamphetamine introduction via drinking water (Hiroshige et al., 1991; Masubuchi et al., 2001). They show consolidated running behavior with a period length greater than 24 h (Mohawk et al., 2009). Still, a methamphetamine-sensitive oscillator has not been identified, nor the method by which the drug synchronizes rhythms.
3.3. Phase response curves, phase shifts, and jet-lag
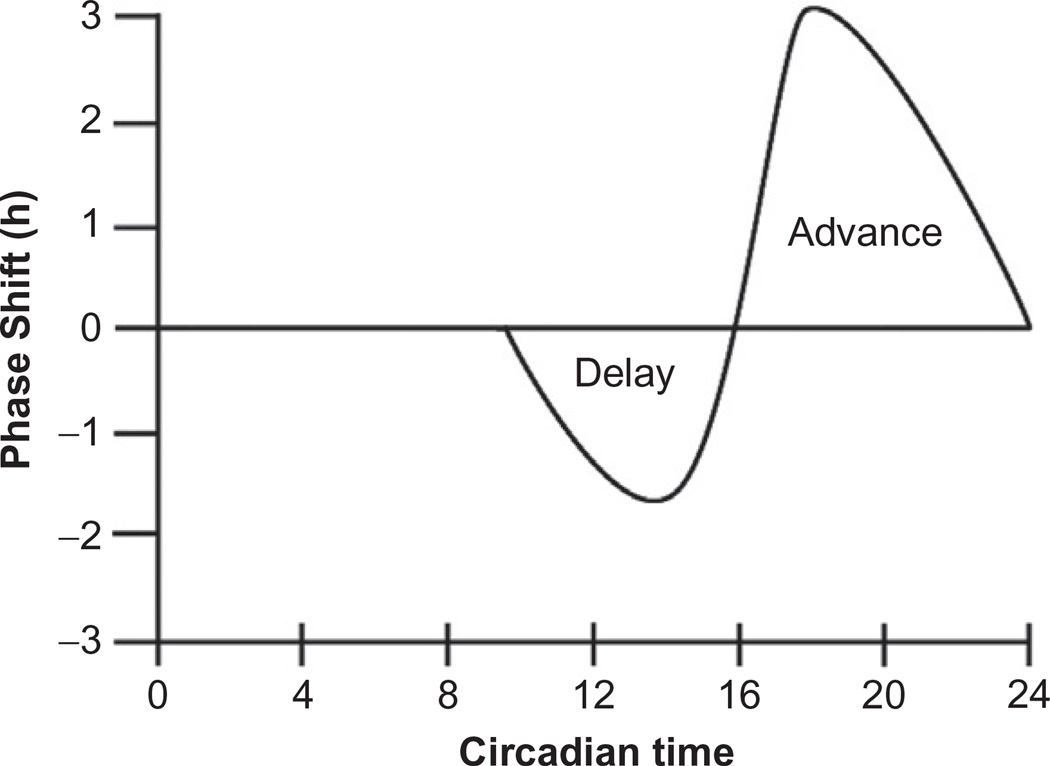
The early studies on phase were focused on the relationship between external zeitgebers (time cues) and their effect on endogenous rhythms, because if the clock (timing) is important, then understanding what synchronizes it is essential (Aschoff, 1967; Haus and Halberg, 1959). Phase shifting occurs when a particular point in the rhythm is advanced or delayed. The phase of the rhythm often refers to the time of the peak in a rhythm or the onset of a behavioral rhythm. A phase advance is a shift in the timing of the phase to occur earlier. A phase delay is a shift to a later time. In order to study the effect of different environmental stimuli on phase, the phase response curve (PRC) has been used to illustrate the behavioral response of an animal to an external time cue (Mrosovsky, 1996; Mrosovsky et al., 1992). Many studies have shown that the response of the circadian system to an external cue is very complex. For example, light cues given at different times and/or durations can have alternate effects on phase of the rhythmic activity. One example of a PRC that could reflect the effect of light pulses on circadian behavior is drawn in Fig. 9.3. In this graph, time of day is along the x-axis and the amount of the phase shift, either an advance (+) or delay (−), in hours are along the y-axis. A PRC will have one peak and one nadir within a 24-h period.
Figure 9.3.
Phase response curve for a nocturnal animal in response to light pulses given at different times of day. This is an example of a phase response curve, where CT0–CT12 indicates the subjective day (light) for a nocturnal animal and CT12–CT24 is subjective night (dark). During the first 12 h (CT0–CT12) administering light pulses have no effect on phase of locomotor behavior in a nocturnal animal. In contrast, a light pulse given in the early subjective night (CT12–CT16) will produce phase delays in the onset of locomotor activity. Whereas, the same light pulse given between CT16–CT22 induces phase advances in the onset of locomotor activity. This example just provides repeatable behavioral evidence in mice demonstrating a time of day effect for the response to a defined light cue.
PRCs, such as the example in Fig. 9.3, are created when the animal is in constant conditions (DD) so that CT0–CT12 is the subjective day and CT12–CT24 is the subjective night; CT12 is the time of activity onset for a nocturnal animal. As illustrated in Fig. 9.3, light pulses have no effect on the phase of activity onset during the subjective day (through CT12), which is the time when the animal would normally be exposed to daylight. However, light pulses during the subjective night cause phase delays early on (CT12–CT16) and phase advances during the latter portion of the subjective night (CT16–CT22). The subjective night is when the nocturnal animal would normally be in darkness and active. Unlike the PRC created by photic stimuli, exposure to a running wheel (nonphotic cue) at any time during the subjective day produces a significant phase advance while the same wheel has no effect on phase during the subjective night. While there is a lot of complexity when evaluating the PRCs from different animals with different stimuli, what is clear is that a single environmental stimulus can cause either a phase advance or a phase delay depending on the time at which the animal was exposed to the stimulus. This time of day effect is a fundamental observation in circadian biology and illustrates the dynamic nature of the molecular system that underlies the behavioral biology.
It has been established that light pulses do shift phase and continual shifting produces a phenomenon known as jet-lag. When an animal’s environmental cues are shifting chronically, the clock has difficulty catching up and studies have shown that over time, or under compromised conditions, the consequences of continued shifting of the clock are significant (Davidson et al., 2006; Filipski et al., 2002). In an article in Current Biology, Davidson et al. (2006) demonstrated that aged mice exposed to chronic phase advances of 6 h every week for 8 weeks had increased mortality compared with nonshifted controls. The effects of jet-lag are also significant when examining the clinical problems associated with working a shifted schedule (Harrington et al. 1990; Knutsson, 2003). Therefore, the discussion is growing around what sets behavioral and molecular rhythms and the presence of nonphotic timing cues that may be able to offset the negative effects of shift work (Angeles-Castellanos et al., 2005; Mieda et al., 2006).
4. Organization of the Circadian System
The presence of circadian rhythms that persist in constant conditions were the observations that led to the suggestion that circadian behaviors are driven by an organized endogenous timing system within the animal (Aschoff, 1960, 1967; Halberg et al., 1965). The SCN of the hypothalamus was first suggested as the circadian pacemaker when its surgical ablation caused arrhymic behavior in rats (Stephan and Zucker, 1972). A second demonstration that the SCN was responsible for behavioral rhythms was revealed when transplantation of cells from the SCN of intact animals restored rhythms to arrhythmic SCN-lesioned animals. Further, the endogenous period length of the donor was restored in the recipient, suggesting that period length is an intrinsic property of the SCN (DeCoursey and Buggy, 1989; Lehman et al., 1987; Ralph et al., 1990). Additionally, it was discovered that the SCN was directly linked to the RHT and was thereby receiving timing information in the form of light, from the LD cycle (Johnson et al., 1988).
A major focus of circadian research has been on the SCN and neurological control of behavioral rhythms. However, the introduction of the Luciferase reporter system in mice has provided scientists with the tools to ask more questions about peripheral oscillators and how the network of tissues/organs is synchronized in the animal. Firefly luciferase has been widely used in animals, mainly rodents, to measure rhythms in real-time from explanted tissues (Davidson et al., 2002; Yamazaki and Takahashi, 2005; Yamazaki et al., 2000; Yoo et al., 2004). Using these tools has allowed for the discovery of oscillators at the cellular level in nonneuronal tissue (Izumo et al., 2003; Welsh et al., 2004). Once the existence of self-sustaining oscillators in peripheral tissues was established, the hypothesis rose that somehow these oscillators must be synchronized to the LD cycle through the SCN or perhaps another pacemaker. In 2005, Guo et al. utilized the SCN-lesioned mice with a parabiosis approach to ask whether there is some blood-borne factor that facilitates the synchronization of peripheral tissues to the pacemaker in the SCN. SCN-lesioned mice were surgically united with SCN intact mice and the shared circulation provided for a humoral exchange between the two mice. If the SCN was controlling the secretion of a synchronizing agent, the molecular rhythms in the arrhythmic mice would be restored. They found that some peripheral tissues (liver, kidney) were resynchronized in the SCN-lesioned animals, but others were not (heart, skeletal muscle; Guo et al., 2005). This data suggests that there must be other nonhumoral pathways, such as innervation, by which the peripheral oscillators are synchronized within the animal to the environmental time cues.
The presence of circadian oscillators in all cell types within the body has lead to a divergence of interesting work within the field. The reality that SCN lesions produce behavioral and molecular arrhythmicity, suggested its role as dominant pacemaker and has maintained the current hierarchical view of a master/slave relationship between the pacemaker in the SCN and the peripheral oscillators which it controls. However, there is growing evidence that peripheral clocks can be synchronized independently of the SCN and that nonphotic timing cues may alternatively entrain peripheral clocks through pathways unknown at this time (Castillo et al., 2004; Hara et al., 2001; Tahara et al. 2010).
5. The Organization of the Mammalian Molecular Clock
The molecular circadian clock is a genetically based mechanism inherent to each mammalian cell type, including skeletal muscle cells. The circadian clock generates cell-autonomous and self-sustaining rhythms, which prepare the cell to anticipate and adapt to exogenous stimuli (Grundschober et al., 2001; Panda et al., 2002; Storch et al., 2002; Yamazaki et al., 2000; Zambon et al., 2003). The generation of circadian rhythms is driven by a series of interconnected positive and negative transcriptional/translational feedback loops, sometimes referred to as TTFL (Zhang and Kay). Interwoven with these autoregulatory feedback loops are posttranslational modifications of core clock proteins, which govern protein stability and degradation and modulate the length of the circadian period (tau; Lee et al., 2001). The circadian clock is a stable and redundant network; multiple loops work in concert to generate rhythms; within each loop, core clock members have functional homologues that complement each other; and several pathways of transcriptional, translational, and posttranslational control are in place to compensate for each other and ensure the stability and accuracy of the molecular clock (Lowrey and Takahashi, 2004; Shearman et al., 2000). While individual components are different, the molecular clock mechanism is evolutionary conserved from cyanobacteria to humans, confirming its importance in normal physiology and behavior (Grundschober et al., 2001; Harmer et al., 2001; Panda et al., 2002; Rosbash, 2009; Storch et al., 2002).
5.1. The core molecular clock
Using a forward genetics approach, the Clock gene (Circadian Locomotor Output Cycles Kaput) was the first component of the molecular clock to be identified in mammals. Mice homozygous for the Clock mutation (ClockΔ19) have significantly longer circadian periods (tau ~27.3 h) and become behaviorally arrhythmic when housed in constant dark conditions (Lee et al., 2001; Vitaterna et al., 1994). The CLOCK protein plays a fundamental role in circadian rhythm generation; it defines not only tau but also rhythm persistence under constant conditions. The CLOCKΔ19 protein lacks the transactivation domain and acts in a dominant negative fashion, as the circadian phenotype of the heterozygous mutant is less severe than that of a hemizygous (one mutated copy of Clock, one null allele; King et al., 1997).
The ClockΔ19 phenotype was rescued by transgenic expression of a bacterial artificial chromosome harboring the wild-type Clock gene, further supporting a functional role of Clock as an integral component of the molecular clock. In addition, CLOCK was found to be a member of the bHLH-PAS (basic Helix Loop Helix-Period Arnt Single-minded) family of transcription factors. These factors work as dimers through binding via their HLH-PAS domains, suggesting that CLOCK binds to another bHLH-PAS protein to perform its function as a regulator of circadian rhythms (Antoch et al., 1997; Kewley et al., 2004).
The bHLH-PAS protein BMAL1 (Brain and Muscle ARNT Like protein 1) was identified in a yeast two hybrid screen as a binding partner for CLOCK. The Bmal1 transcript is coexpressed with Clock in the SCN and retina, implicating Bmal1 as another component of the molecular clock (Gekakis et al., 1998). The knockout of Bmal1 was generated in mice by targeted deletion of the bHLH domain. The Bmal1−/− mice were arrhythmic in behavior and these findings confirmed that BMAL1 is a central and nonredundant component of the molecular clock (Bunger et al., 2000).
Dimerization of CLOCK to BMAL1 via their HLH-PAS domains results in translocation of the heterodimer to the nucleus, where it binds to an E-box sequence (CACGTG) in the promoter regions of the other components of the core clock, such as Period (Per1 and Per2) and Crypto-chrome (Cry 1, Cry 2, and Cry 3) genes. CLOCK and BMAL1 activate transcription of Per and Cry and are referred to as the positive arm of the molecular clock (Gekakis et al., 1998; Hogenesch et al., 1998; Kume et al., 1999).
BMAL1 availability is the rate-limiting factor for the CLOCK:BMAL1 complex formation, nuclear translocation, and transcriptional activation. The BMAL1 protein accumulates with a circadian profile in both the SCN and peripheral tissues (Lee et al., 2001). Although total CLOCK protein levels do not oscillate in the SCN (Gekakis et al., 1998), there is a clear circadian oscillation in nuclear to cytoplasmic distribution of CLOCK both in vitro and in vivo. This is disrupted in Bmal1−/− mice, suggesting that BMAL1 is necessary for rhythmic accumulation of the CLOCK protein in the nucleus and hence the rhythmicity of CLOCK:BMAL1-dependent transcriptional activity (Kondratov et al., 2003).
CLOCK:BMAL1-mediated transcription of Per and Cry leads to their protein accumulation in the cytoplasm. PER and CRY proteins form multimeric complexes with each other and with casein kinase 1ε and translocate to the nucleus to inhibit the transcriptional activity of the CLOCK:BMAL1 heterodimer (Eide et al., 2002; Kume et al., 1999; Lee et al., 2001; Sangoram et al., 1998). PER is responsible for regulation of nuclear entry of the complex, as shown by lack of nuclear CRY accumulation in Per1−/− Per2−/− mutant mice (Lee et al., 2001). CRY is primarily responsible for the inhibitory actions of the complex. CRY inhibits the histone acetyltransferase p300, leading to a decrease in CLOCK:BMAL1-mediated transcription (Etchegaray et al., 2003). The CRY:PER-mediated repression of the CLOCK:BMAL1 activity constitutes the negative feedback component of the molecular clock loop.
The CLOCK:BMAL1-CRY:PER loop is referred to as the central autoregulatory feedback loop due to the strong inhibition that PER and CRY impose on their own transcription. Additional transcriptional/translational feedback loops exist to ensure the robustness and fidelity of the molecular clock. One such loop involves members of the retinoic acid-related orphan nuclear receptor family of transcription factors, RevErbα and RORα. Transcription of RevErbα and RORα is enhanced by CLOCK: BMAL1-binding at E-box sequences in their promoter regions (Preitner et al., 2002; Sato et al., 2004). In turn, accumulation of RevErbα protein inhibits the expression of Bmal1 (Preitner et al., 2002). RORα on the other hand, activates the expression of Bmal1 (Sato et al., 2004). Both RevErbα and RORα exert their function by binding to retinoic acid-related orphan nuclear receptor response elements (RORE sequences) in the promoter region of Bmal1. Other members of the ROR and RevErb families have been found to have similar functions in the molecular clock (Guillaumond et al., 2005).
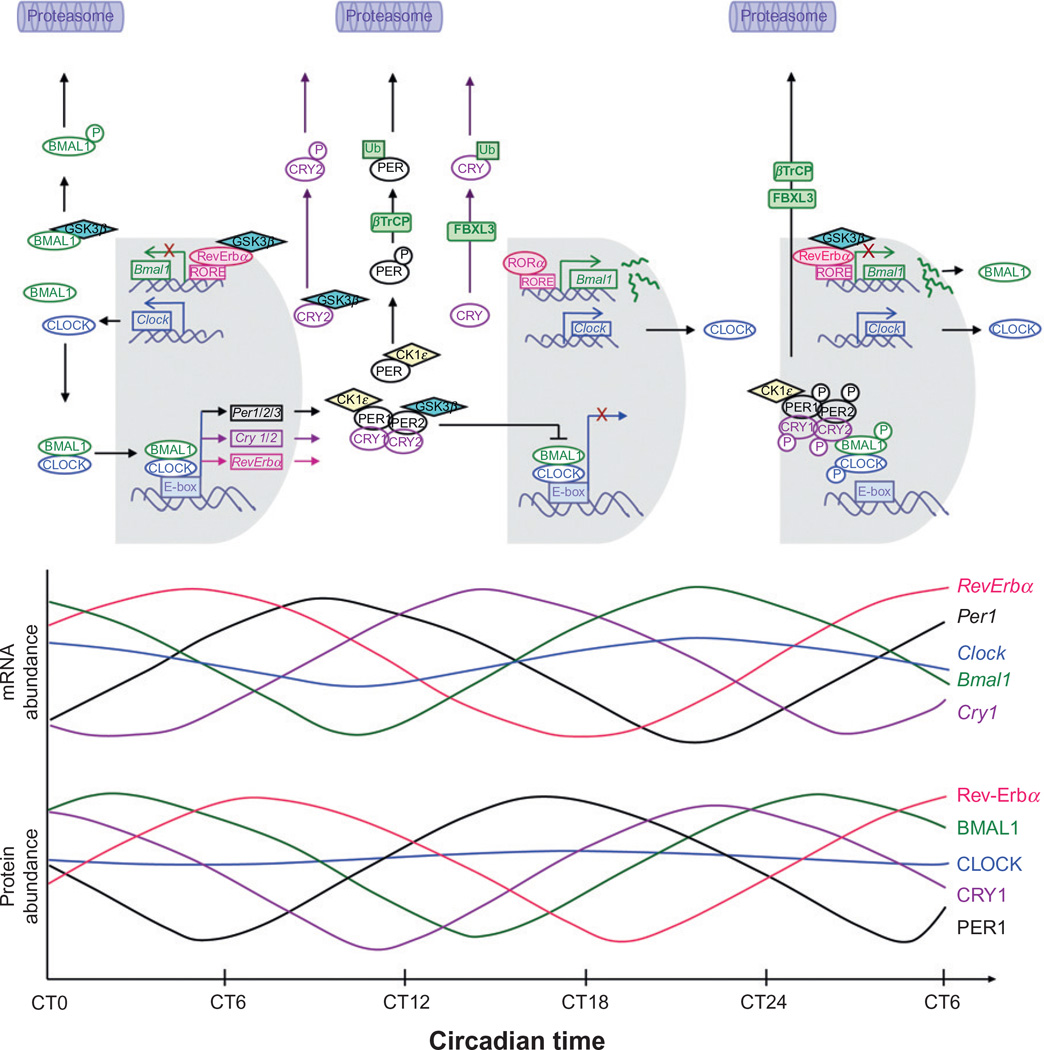
These autoregulatory transcriptional/translational feedback loops drive the rhythmic expression of core clock components, with the positive regulators oscillating antiphase to the negative regulators. Figure 9.4 shows a simplified version of the temporal events of circadian rhythm generation in rodent peripheral tissues. There is a 0–6-h delay between the mRNA expression and protein abundance rhythms, therefore transcription and translation alone cannot account for the generation of 24-h circadian rhythms. Delays are imposed on the system by posttranslational modifications controlling protein–protein interactions, nuclear localization, and protein degradation, as discussed in the following section (Harmer et al., 2001; Lee et al., 2001; Lowrey and Takahashi, 2004; Panda et al., 2002; Shearman et al., 2000).
Figure 9.4.
Mammalian molecular clock and oscillation of mRNA and protein in peripheral tissues. The molecular clock is regulated by interacting transcription–translation feedback loops. As depicted in this figure, the expression of components of the molecular clock are determined by the balance of synthetic versus degradative processes. Specifically, the circadian cycle starts with the accumulation of the BMAL1 protein in the cytoplasm (CT15–CT3). As BMAL1 accumulates to sufficient levels in the cytoplasm, it forms heterodimers with CLOCK, which is constitutively present over the circadian cycle. Nondimerized BMAL1 is targeted by the kinase GSK3β, for phosphorylation and subsequent degradation. Upon dimerization, the CLOCK: BMAL1 heterodimer translocates to the nucleus where it binds to E-box sequences in the promoter region of the negative regulator genes (Per, Cry, RevErba) and induces their transcription. This leads to peaks in the mRNA levels of Per1, Per2, Per3, Cry1, Cry2, and RevErba between CT2 and CT17 followed by peaks in their protein levels 4–6 h later. In the cytoplasm, the negative regulators form multimeric complexes that translocate to the nucleus to inhibit the transcriptional activity of the CLOCK:BMAL1 heterodimer. The kinetics of this interaction depends on the availability of the negative regulators. Free CRY and PER are targeted by GSK3β and CK1ε respectively for phosphorylation and subsequent degradation via the proteasomal pathway. At around CT15–CT20, RORα binds to RORE elements in the promoter region of Bmal1 and induces Bmal1 transcription. This allows for the accumulation of Bmal1 mRNA with a peak at around CT21. Meanwhile, CK1ε phosphorylates all members of the PER/CRY complex as well as CLOCK and BMAL1. This hyperphosphorylated complex is shuttled to the proteasome for degradation between CT21 and CT24, freeing the E-box site for another round of CLOCK:BMAL1 binding. The delay imposed by transcription, translation, and posttranslational modifications is what defines the period of circadian rhythms (Lee et al., 2001; Lowrey and Takahashi, 2004; Sahar et al., 2010; Shearman et al., 2000; Ueda et al., 2002).
5.2. Posttranslational modifications as modulators of the circadian period
Transcription and translation of core clock components plays a critical role in rhythm generation, whereas delays imposed by posttranslational modifications (PTMs) are important for determining the period (tau) of circadian rhythms (Dey et al., 2005; Godinho et al., 2007; Iitaka et al., 2005; Lee et al., 2001; Lowrey et al., 2000; Nakahata et al., 2008; Sanada et al., 2004; Siepka et al., 2007; Vanselow et al., 2006). PTMs play essential roles in regulating nuclear localization, protein stability, and protein degradation (Eide et al., 2002, 2005; Lowrey et al., 2000; Sahar et al., 2010; Shirogane et al., 2005; Vielhaber et al., 2000; Yin et al., 2010). PTMs are carried out by enzymes that not only respond to environmental stimuli such as nutrient availability but also control a wide range of cellular processes outside the molecular clock (Etchegaray et al., 2003; Lee et al., 2001; Nakahata et al., 2008; Rayasam et al., 2009; Sanada et al., 2002). On one hand, this indicates that the molecular clock tau can potentially be influenced by the environment, as modulated in part by the actions of PTM enzymes. On the other hand, while the environment might modulate tau, the significant overlap in the system of PTMs, is an indicator that several mechanisms are operating to insure the fidelity of the molecular clock and that multiple signals are needed to disturb molecular clock function. In this section, we will briefly discuss what is currently known about three types of posttranslational modifications imposed on core clock proteins: phosphorylation, acetylation, and ubiquitination and their effect on the molecular clock function.
5.2.1. Phosphorylation of clock proteins
Several kinases work together to phosphorylate molecular clock components. Phosphorylation has different effects on the circadian period depending on the time of day, the kinase involved, and the proteins being phosphorylated. Phosphorylation modulates the period of circadian rhythms by controlling the start, duration, and termination of both the activating and repressing phases of the molecular clock mechanism. During the early subjective day in peripheral tissues, phosphorylation of the positive components leads to their degradation via the 26S proteasomal pathway, delaying the onset of positive transcriptional activation (Sahar et al., 2010; Spengler et al., 2009; Yin et al., 2006). Toward the end of the subjective day, phosphorylation of the negative regulators inhibits their nuclear entry and primes them for degradation. This keeps the negative regulators out of the nucleus and unable to perform their repressive function, imposing a delay in the molecular clock (Akashi et al., 2002; Eide et al., 2005; Harada et al., 2005; Iitaka et al., 2005; Shirogane et al., 2005; Vielhaber et al., 2000). Accumulation of the kinases in the nucleus at the beginning of the subjective night phosphorylates and enhances the repressor function of the inhibitory complex (Eide et al., 2002; Sanada et al., 2002, 2004). Hyperphosphorylation of the inhibitory complex at the end of the circadian night leads to degradation of the complex and termination of the repressive state (Lee et al., 2001). Here, we will review the major effects of three important kinases on the molecular clock: casein kinase 1 epsilon, glycogen synthase kinase 3 beta, and mitogen-activating protein kinase.
5.2.1.1. Casein kinase 1 epsilon
Casein kinase 1 epsilon (CK1ε) is a member of the serine/threonine family of protein kinases, known to phosphorylate a broad range of substrates (Chergui et al., 2005; Klimowski et al., 2006). CK1ε was first implicated in circadian rhythms when a point mutation (Arg178Cys) in its catalytic region was found to be the basis of the tau mutation in the short circadian period hamster (Lowrey et al., 2000; Ralph and Menaker, 1988).
CK1ε does not show circadian rhythms in abundance or activity in vivo (Lee et al., 2001). CK1ε targets members of both the positive and negative limbs of the molecular clock and primarily controls the duration of the repressor state. At the end of the circadian day, CK1ε binds to and phosphorylates PER1, inducing a conformational change hindering the nuclear localization signal. This inhibits nuclear entry of PER1 and leads to its cytoplasmic accumulation and subsequent degradation. By keeping PER1 out of the nucleus, CK1ε delays the PER1-mediated repression of the CLOCK:BMAL1-dependent transcription. CK1ε controls PER1 availability in the cytoplasm, which is a rate-limiting step for inhibitory complex formation (Vielhaber et al., 2000). In addition, CK1ε phosphorylates the other members of the PER family (PER2 and PER3) and primes them for ubiquitination and degradation (Akashi et al., 2002; Eide et al., 2005; Shirogane et al., 2005). At the beginning of the circadian night, CRY1 protein levels begin to rise; CRY1 binds to the PER:CK1ε complex in the cytoplasm, it promotes nuclear entry of the CRY1: PER:CK1ε complex and increases nuclear localization of CK1ε (Eide et al., 2002). Once in the nucleus, the CRY1: PER:CK1ε complex binds to and inhibits the transcriptional activity of the CLOCK:BMAL1 heterodimer. Following the negative regulation, the CLOCK:BMAL1: PER:CRY complex becomes hyperphosphorylated by CK1ε and the entire complex is shuttled to degradation pathways at the end of the circadian night. This ends the repressive state, opens up the E-box for another round of CLOCK: BMAL1-binding, and signals the beginning of a new circadian cycle (Lee et al., 2001). Through this series of events, CK1ε controls the beginning, duration, and termination of the repressor state of the molecular clock.
Further, nuclear CK1ε increases BMAL1 phosphorylation, which in turn enhances CLOCK:BMAL1-mediated transcription in vitro. Phosphorylation of BMAL1 by CK1ε is not dependent on the presence of the PER protein, suggesting that CK1ε phosphorylates BMAL1 at a time when the inhibitory complex is not bound to the CLOCK:BMAL1 heterodimer (Eide et al., 2002). Hence, CK1ε also controls the length of the transcriptional activation state of the molecular clock.
Phosphorylation of core clock proteins by CK1ε controls their stability, subcellular localization, and protein turnover and has an effect on the period of circadian rhythms. This is supported by studies in both hamster and humans in which the mutations of either CK1ε or PER2 affect period. As mentioned above, in the tau mutant hamster, CK1ε is unable to phosphorylate the PER proteins. Hypophosphorylated PER is translocated to the nucleus, where its accumulation leads to earlier repression of the CLOCK: BMAL1-mediated transcription, shortening the circadian period to 20 h (Lowrey et al., 2000). Although the circadian expression profile of Per1 and Per2 is not altered in the SCN of tau mutants, the nuclear PER protein levels decline more rapidly. The authors suggest that the repressive phase of the cycle is shorter, leading to a shorter circadian cycle (Dey et al., 2005). In humans, familial advance sleep phase syndrome (FASPS) is a condition in which people go to sleep earlier and earlier each day. Studies of these individuals determined that they have a very short endogenous period of ~20 h versus >24 h in most humans. This is due to a mutation in PER2 that affects a phosphorylation site (target of CK1ε). This leads to a decrease in PER2 protein stability, premature nuclear clearance of PER2 containing complexes, early termination of transcriptional repression, and a subsequent shortening of the circadian period (Vanselow et al., 2006).
5.2.1.2. Glycogen synthase kinase 3 beta
Glycogen synthase kinase 3 beta (GSK-3β), is another member of the serine/threonine family of protein kinases, known to phosphorylate components of a wide variety of processes, such as glycogen synthesis, cell proliferation, embryogenesis, axon growth, and cardiomyocyte hypertrophy (Rayasam et al., 2009).
GSK-3β phosphorylation (which is indirectly correlated to its activity level) oscillates with a circadian profile in both SCN and liver. In the liver, GSK-3β is active throughout the circadian cycle, with peak activity at the end of circadian night and beginning of circadian day. During these times, GSK-3β is responsible for phosphorylation of the positive loop members, determining the onset of transcriptional activation (Sahar et al., 2010; Yin et al., 2006). During the late circadian day and early circadian night, GSK-3β phosphorylates members of the negative loop and modulates the start of transcriptional repression (Harada et al., 2005; Iitaka et al., 2005).
During the early circadian day, GSK-3β targets BMAL1 for phosphorylation, followed by subsequent ubiquitination and degradation via the proteasomal pathway. A mutated GSK-3β increases BMAL1 protein levels and dampens BMAL1 protein oscillations. This suggests that GSK-3β plays a critical role in the regulation of cytoplasmic BMAL1 abundance, which is a rate-limiting step in the CLOCK:BMAL1 heterodimer formation, nuclear translocation, and transcriptional activation (Sahar et al., 2010). Further, GSK-3β phosphorylates CLOCK and primes it for degradation. GSK-3β can only phosphorylate CLOCK after a BMAL1-dependent priming phosphorylation event at a different residue on CLOCK. A CLOCK mutant lacking the phosphorylation site reduces CLOCK degradation and enhances the CLOCK:BMAL1 transcriptional activity, leading to a delay in the peak of Per1 transcript and a lengthening of the circadian period (Spengler et al., 2009). At the same time, GSK3β phosphorylates and stabilizes the RevErbα protein by preventing its degradation by the proteasome. Stabilized RevErbα inhibits Bmal1 transcription at a time when BMAL1 protein is accumulating in the cytoplasm (Yin et al., 2006). GSK-3β imposes a necessary delay step in the clock, between BMAL1 protein accumulation at the end of the circadian night and CLOCK:BMAL1 transcriptional activation during early-mid circadian day.
During the late circadian day–early circadian night, GSK-3β phosphorylates members of the negative limb, but has opposing effects on their activity, depending on the member involved. GSK-3β interacts with PER2 in the PER:CRY:CK1ε complex, enhances its nuclear entry, and triggers the start of transcriptional repression. This is supported by the finding that GSK-3β inhibition through pharmacological agents leads to the cytoplasmic accumulation of PER2, a delay in the start of the transcriptional repression state and hence a lengthening of the circadian period in vitro (Iitaka et al., 2005). In addition, GSK-3β phosphorylates CRY2 and targets it for proteasomal degradation in vivo. Phosphorylation of CRY2 by GSK-3β requires prior phosphorylation by MAPK at a different serine residue showing that both kinases must work in concert to mediate CRY degradation (Harada et al., 2005; Sanada et al., 2004). By mediating CRY2 degradation, GSK-3β delays inhibitory complex formation and the start of the repressive state, lengthening the circadian period. This finding might explain the shortening of the circadian period length observed in vitro when GSK-3β is inhibited by siRNA interference or by small molecules (Hirota et al., 2008). Depending on the availability of PER2 and CRY2, GSK-3β can either delay or speed up the molecular clock by regulating the time of onset of transcriptional repression. By regulating the onset of both transcriptional activation and transcriptional repression, GSK-3β can modulate the period length of circadian rhythms.
5.2.1.3. Mitogen activated protein kinase
Mitogen-activated protein kinase (MAPK) is the largest subfamily of the serine/threonine family of protein kinases. They participate in many signal transduction pathways with roles in protein turnover, cell growth, transcription factor activation, chromatin modification, and gene expression (Cuadrado and Nebreda, 2010).
MAPK exhibits circadian rhythms of activity in vivo (Obrietan et al., 1998) and has been shown to phosphorylate members of both the positive and negative loops of the molecular clock. This activity contributes to regulation of the termination of transcriptional activation as well as the duration of transcriptional repression. MAPK binds to and phosphorylates BMAL1 in the nucleus and negatively regulates CLOCK:BMAL1-mediated transcription (Sanada et al., 2002). This leads to the termination of the CLOCK:BMAL1-mediated transcriptional activation phase during the late circadian day–early circadian night.
During the circadian night, MAPK phosphorylates both CRY1 and CRY2 and enhances their repression of the CLOCK:BMAL1 heterodimer, lengthening the period of circadian rhythms. This is supported by the finding that mutations in theMAPK phosphorylation sites of CRY lead to a decrease in the CRY repressor function on the CLOCK:BMAL1-mediated transcription. This could be due to the reduced affinity of CRY for the CLOCK: BMAL1 dimer. The authors propose that MAPK functions to lengthen the period of negative regulation during the subjective night. This is a time when protein levels of the negative regulators are declining, yet their mRNA levels are still low. By regulating both the positive and negative arms of the molecular clock, it is suggested that MAPK imposes the appropriate time lag for the generation of 24-h rhythms (Sanada et al., 2002, 2004).
5.2.2. Acetylation/deacetylation of clock proteins
Acetylation and deacetylation are important enzymatic reactions that control gene expression via chromatin remodeling. Acetylation of histones unfolds chromatin to expose promoter regions to the transcription machinery and is associated with activation of gene expression, whereas deacetylation leads to silencing of gene expression (Etchegaray et al., 2003; Nakahata et al., 2008). In addition to histones, histone acetyltransferases (HAT) and histone deacetylases (HDAC) target members of the molecular clock, modulate their expression, and have an effect on the circadian period length (Asher et al., 2008; Doi et al., 2006; Etchegaray et al., 2003; Nakahata et al., 2008).
The histone acetyltransferase, p300, associates with CLOCK in a circadian manner, suggesting that p300 is a member of the transcription activating complex. Consistent with this, H3 histone acetylation at the promoter region of Per1 and Per2 shows robust circadian rhythms in phase with the Per1/Per2 mRNA and p300: CLOCK complex formation. Further, p300 is inhibited by the CRY protein leading to a decrease in CLOCK:BMAL1-mediated transcription at the Per1 promoter, suggesting that the repressor action of CRY is mediated in part by its actions on chromatin structure (Etchegaray et al., 2003). The histone deacetylase SirtT1 targets histone H3, leading to chromatin condensation, hindrance of the promoter sites, and gene silencing. SirtT1 deacetylase activity shows a robust circadian rhythm antiphase to the rhythm of histone H3 acetylation (Nakahata et al., 2008). This antiphase oscillation of HAT and HDAC activity implies that acetylation/deacetylation are important mechanisms in controlling clock gene expression and that they have roles in the initiation, duration, and termination of both the activating and repressing phases of the circadian cycle.
In addition to its role as a transcription factor, it has been shown that CLOCK protein can function as a histone acetylase (Doi et al., 2006) capable of acetylating its binding partner, BMAL1. Dimerization of CLOCK to BMAL1 is critical for the acetylation of BMAL1 at a specific lysine residue in the carboxy terminal. BMAL1 acetylation by CLOCK is rhythmic over the circadian cycle in mouse liver and increases CRY binding to and repression of the CLOCK:BMAL1 heterodimer (Hirayama et al., 2007). Thus acetylation is important for determining the time of transcriptional repression initiation. SirT1 also binds to the CLOCK:BMAL1 dimer in the promoter regions of circadian genes and deacetylates BMAL1, inducing gene expression silencing. This is consistent with the finding that SirT1−/− mice show increases in the amplitude and length of circadian gene rhythms in the liver. Further, SirT1 deacetylates PER2 protein in the PER:CLOCK:BMAL1 complex and contributes to its degradation (Asher et al., 2008; Nakahata et al., 2008).
Recent work has placed SirT1 in the crossroad between the molecular clock and cellular metabolism. This role of SirT1 will be explored further in Section 6.2. The interplay between acetylases and deacetylases is of critical importance, assuring that molecular clock components are activated/silenced appropriately in a time of day-dependent manner.
5.2.3. Ubiquitination of clock proteins
As mentioned above, phosphorylation of most clock components primes them for degradation via the 26S proteasomal pathway. An intermediate and necessary step for degradation via the proteasome is the labeling of proteins with ubiquitins, mediated by the action of ubiquitin ligases. Ubiquitin ligases preferentially target members of the negative limb of the molecular clock and control their protein turnover by promoting their degradation (Busino et al., 2007; Eide et al., 2005; Yin et al., 2010). Ubiquitin ligases increase the transcriptional activity of the CLOCK: BMAL1 heterodimer and regulate the initiation and termination of both the transcriptional activation and transcriptional repression states and hence the period of circadian rhythms (Godinho et al., 2007; Shirogane et al., 2005; Siepka et al., 2007). Here, we will briefly discuss what is currently known about the roles of four main ubiquitin ligases in the circadian system: β-TrCP, Fbxl3, Arf-bp1, and Pam.
β-TrCP is a ubiquitin ligase adapter protein, member of the SCF (Skp1/cullin/F-box) E3 ubiquitin ligase complex. β-TrCP recognizes the phosphorylated form of PER2. Phosphorylation of PER2 by CK1ε exposes a binding site for β-TrCP on PER2. This is followed by polyubiquitination and subsequent degradation (Eide et al., 2005). The two isoforms of β-TrCP (β-TrCP1 and β-TrCP2) bind to phosphorylated PER1 on a region different from the CK1ε binding site and lead to its degradation. Knockdown of β-TrCP leads to stabilization of the PER1 protein and to a decrease in CLOCK:BMAL1-mediated transcription (Shirogane et al., 2005).
The orphan F-box protein, Fbxl3, is another member of the SCF E3 ubiquitin ligases, which is found to bind to CRY1 and CRY2 and decrease their half-life. Knocking down Fbxl3 or silencing it by small hairpin RNAs, stabilizes the CRY proteins and increases their half-lives. This leads to repression of CLOCK:BMAL1 dependent transcription as shown by a decrease in oscillation and expression of Per1, Per2, and Cry1. Fbxl3 poly-ubiquitinates the CRY proteins and shuttles them to the proteasome (Busino et al., 2007). Consistent with this, a mutation in Fbxl3 which decreases CRY2 binding increases the circadian period to 27 h (Godinho et al., 2007). A second Fbxl3 mutation leading to its loss of function in a murine model is associated with decreased CRY1 degradation, increased CRY1 protein stability, longer repression of CLOCK:BMAL1-dependent gene transcription, and a longer circadian period of over 26 h (Siepka et al., 2007). Fbxl3 thus plays a role in regulating circadian period length by controlling the start and end times of both transcriptional activation and transcriptional repression.
Two additional members of the E3 ligase family, Arf-bp1 and Pam, were recently found to associate with RevErbα and mediate its ubiqitination and degradation. Inhibiting Arf-bp1 and Pam via small interfering RNAs leads to stabilization of RevErbα protein. Knocking down Arf-bp1 and Pam altered the amplitude of RevErbα oscillations and suppressed both Bmal1 and CLOCK:BMAL1-dependent gene expression. This suggests that Arfbp1 and Pam can modulate the molecular clock by regulating RevErbα protein stability and thus the duration of the inhibitory actions of RevErbα on Bmal1 transcription (Yin et al., 2010).
Ubiquitin ligases control the protein stability of the negative regulators of the molecular clock. By promoting PER and CRY degradation at the end of the circadian day, ubiquitin ligases lengthen the transcriptional activation state and delay the start of the transcriptional repression state. Ubiquitination of PER and CRY at the end of the circadian night facilitates inhibitory complex degradation and signals the termination of the repressing state, allowing CLOCK:BMAL1 to start a new transcription cycle (Busino et al., 2007; Eide et al., 2005; Yin et al., 2010). Thus, ubiquitin ligases serve as a balance between the activating and repressing states of the molecular clock oscillator.
In summary, posttranslational modifications of core clock proteins are important for introducing necessary delays in the clock mechanism to ensure 24-h oscillations. PTMs control protein stability, nuclear localization, and protein degradation, which in turn affect protein activity and the timing of molecular clock transcriptional activation and transcriptional repression. PTMs contribute to the fidelity of the molecular clock and provide fine tuning of the core clock mechanism.
6. The Molecular Clock in Skeletal Muscle
Several studies have documented that the molecular clock is present and functional in skeletal muscle, however, not much is known about its function in skeletal muscle physiology and pathology (Yamazaki et al., 2000; Zambon et al., 2003; Zylka et al., 1998). Expression profiling determined that ~7% of skeletal muscle transcriptome is expressed in a circadian manner, including genes involved in protein metabolism, transcription, cytoskeletal organization, and signaling (McCarthy et al., 2007). This suggests that the molecular clock is involved in some aspects of skeletal muscle physiology and is supported by the finding that the ClockΔ19 skeletal muscle shows a significant loss or phase shifting in the circadian rhythms of genes involved in structural organization, contractile performance, and metabolism (McCarthy et al., 2007).
In this section, we will discuss what is currently known about the role of the molecular clock in skeletal muscle. The molecular clock regulates the rhythmic expression of skeletal muscle-specific clock control genes, it has functions in skeletal muscle metabolism, and when aberrant, it leads to skeletal muscle pathologies (Andrews et al., 2010; Bae et al., 2006; Fulco et al., 2003; Kennaway et al., 2007; Kondratov et al., 2006; Liu et al., 2007; McCarthy et al., 2007; McDearmon et al., 2006; Vieira et al., 2008).
6.1. MyoD as a clock-controlled gene in skeletal muscle
Clock-controlled genes are genes outside the core clock, whose rhythmic expression is driven by the activity of the CLOCK:BMAL1 heterodimer in their promoter regions (Lowrey and Takahashi, 2004). Recent work from our laboratory has identified MyoD (myogenic determination factor 1) as a skeletal muscle-specific clock controlled gene. The CLOCK:BMAL1 heterodimer binds to core enhancer (CE) sequences in the promoter region of MyoD and drives its transcription (Andrews et al., 2010). This is consistent with expression profiling data showing that MyoD mRNA oscillates with a circadian profile in rodent skeletal muscle (McCarthy et al., 2007).
MyoD is a skeletal muscle-specific bHLH transcription factor, which is activated early during myogenesis and commits undifferentiated cells to the muscle lineage. MyoD is known as a master regulator of the muscle-specific transcription program by controlling the expression of many structural, functional, and metabolic skeletal muscle-specific genes (Bergstrom et al., 2002; Molkentin and Olson, 1996; Rudnicki et al., 1993; Tapscott, 2005). The circadian oscillation of MyoD mRNA is abolished in the skeletal muscle of both Bmal1−/− and CLOCKΔ19 mice. This is associated with a downregulation of many MyoD controlled genes, with functions ranging from skeletal muscle structure to metabolism. Further, skeletal muscle from both Bmal1−/− and CLOCKΔ19 mice show disturbances in both force production and myofilament organization (discussed in more detail in Section 6.3), suggesting that the molecular clock regulates skeletal muscle structure and function by controlling MyoD expression (Andrews et al., 2010).
6.2. The clock and metabolism in skeletal muscle
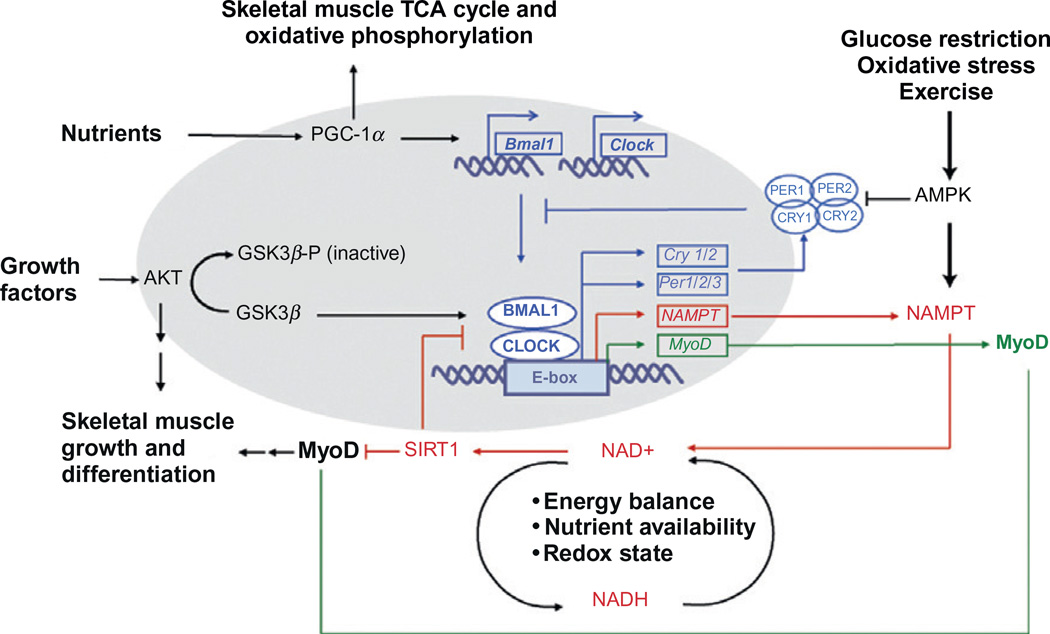
Recent work has shown that the molecular clock in skeletal muscle can be modulated by molecules that are part of the metabolic sensing machinery. These metabolic sensors respond to extracellular environmental changes, such as nutrient availability, contractile activity, energy balance, and redox status and modulate skeletal muscle physiological processes (Fulco et al., 2003; Lira et al., 2010; Muoio and Koves, 2007; Puigserver and Spiegelman, 2003; Vinciguerra et al., 2010; Witczak et al., 2008). At the same time, metabolic sensors introduce posttranslational modifications in the core molecular clock components and modulate molecular clock rhythms (Asher et al., 2008; Lamia et al., 2009; Liu et al., 2007; Nakahata et al., 2008). Not surprisingly, these metabolic sensors exhibit circadian rhythms in their activity levels, suggesting that a mutually dependent link exists between cell metabolism and circadian rhythms (Andrews et al., 2010; Lamia et al., 2009; Liu et al., 2007; McCarthy et al., 2007). In this chapter, we will focus on the actions of AMPK, PGC1α, and SirT1and their roles as metabolic sensors and molecular clock regulators.
AMPK (adenosine monophosphate-activated protein kinase) is a wellrecognized mediator of metabolic signals. In skeletal muscle, AMPK is activated by processes that increase the AMP:ATP ratio, such as exercise, oxidative stress, glucose restriction, and intracellular Ca2+ concentrations. In response to these stimuli, activated AMPK participates in the regulation of protein, carbohydrate, and lipid metabolic pathways in skeletal muscle (Witczak et al., 2008). Transgenic mice lacking the AMPKγ subunit in skeletal muscle specifically show increased glycogen synthesis post exercise. In addition, they exhibit an increase in lipid oxidation as shown by the lack of triglyceride accumulation in skeletal muscle (Vieira et al., 2008).
Activation of AMPK by low glucose in mouse embryonic fibroblasts increases the period of circadian rhythms and dampens the amplitude of Bmal1 oscillations. AMPK phosphorylates CRY1 and primes it for degradation. Decreasing CRY1 stability leads to reduction of input from the negative feedback loop and the subsequent lengthening of the circadian period. Therefore, AMPK acts as a sensor of metabolic activity and relays nutrient/metabolic signals to circadian clocks. Recently, studies have found that AMPK itself exhibits circadian rhythms in expression levels, nuclear localization, and phosphorylation (a measure of activity) providing more evidence for the interrelationship between AMPK and the molecular clock (Lamia et al., 2009). Under conditions of oxidative stress, energy depletion (exercise), or low nutrient availability, activated AMPK in skeletal muscle may be able to impose a delay in the molecular clock, by increasing the duration of transcriptional activation.
PGC1α/β (peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1 alpha/beta) is a well-known transcriptional coactivator that senses the energy balance of the organism. In skeletal muscle, PGC1 responds to nutrient stimuli and exercise and controls rate-limiting enzymes in the tricarboxylic acid cycle and oxidative phosphorylation pathways. PGC1α interacts with MEF2 (myocyte enhancer factor 2) and increases GLUT4 levels leading to increase in glucose uptake in skeletal muscle. PGC1α/β stimulates mitochondrial biogenesis, regulates fatty acid metabolism, mediates fiber type switching, and enhances exercise tolerance (Lira et al., 2010; Muoio and Koves, 2007; Puigserver and Spiegelman, 2003).
PGC1α/β mRNA and protein levels in skeletal muscle oscillate with a circadian period (Andrews et al., 2010; Liu et al., 2007; McCarthy et al., 2007). PGC1α enhances Bmal1 transcription by activating RORα and RORγ. This interaction is mediated by the action of the p300 histone acetyltransferase recruited to the Bmal1 promoter by PGC1α and is dependent on the levels of RevErbα and RevErbβ. This is consistent with the finding that PGC1α protein peaks at a time when Bmal1 transcription is highest. The Bmal1 transcription is high at a time when the CLOCK: BMAL1 dependent transcription is repressed. By controlling the time of Bmal1 transcription, PGC1α regulates the length of circadian rhythms (Liu et al., 2007).
The importance of PGC1 as a communicator between the core clock and cell metabolism is further supported by the finding that circadian rhythms of locomotor activity were disrupted in PGC1α-null mice, associated with a disruption in either amplitude or period in several circadian clock genes in skeletal muscle and liver. In addition, rate-limiting enzymes in oxidative phosphorylation and tricarboxylic acid (TCA) cycle had lost their diurnal pattern of expression in the PGC1α-null skeletal muscle and liver (Liu et al., 2007). Unlike AMPK, PGC1α puts the clock in a transcriptional repressive state by promoting Bmal1 transcription. It remains to be established, but it is possible that certain skeletal muscle processes, such as oxidative phosphorylation and the TCA cycle might preferentially occur during specific CTs.
Sirtuin 1 (SirT1), a member of the NAD+-dependent protein deacetylase family, is a mediator of metabolism in response to nutritional availability and exercise. The activity of SirT1 is influenced by the NAD+: NADH ratio, which reflects cellular energy homeostasis and redox status. The majority of the NAD+ is synthesized by the conversion of nicotinamide to nicotinamide mononucleotide utilizing the rate-limiting enzyme NAMPT (nicotinamide phosphoribisyl transferase; Fulco et al., 2003; Ramsey et al., 2009). In vitro, SirT1 overexpression in skeletal muscle cells blocks myocyte proliferation and differentiation by deacetylating and inhibiting MyoD (Fulco et al., 2003). Blockage of differentiation also occurs when cultured myoblasts are grown in a medium with restricted glucose. This is due to activation of AMPK, which activates NAMPT, leading to NAD+ accumulation and SirT1 activation (Vinciguerra et al., 2010).
SirT1 exhibits circadian rhythms in both protein levels and histone deacetylase activity. As discussed in Section 5.2.2, SirT1 deacetylates and degrades PER2 and affects the amplitude of circadian clock gene expression, including Bmal1, Per2, and Cry1. In addition, SirT1 deacetylates and silences Bmal1 and forms a complex with CLOCK:BMAL1 in the promoter regions of clock control genes (Asher et al., 2008; Nakahata et al., 2008). In turn, the CLOCK:BMAL1 dimer binds to E box sequences in the promoter region of NAMPT and upregulates NAMPT transcription, leading to circadian oscillations in NAMPT mRNA levels (Ramsey et al., 2009). SirT1 activity puts the clock in a transcriptional repressive state and simultaneously inhibits MyoD, a skeletal muscle-specific transcription factor that regulates skeletal muscle growth and differentiation. That skeletal muscle physiological and metabolic processes exhibit circadian rhythms, has been documented by several groups and reviewed recently (Zhang et al., 2009). SirT1 then serves as a sensor of metabolism and responds by regulating the time of the molecular clock, so that processes involved in skeletal muscle growth, differentiation, and maintenance can occur at appropriate CTs.
At this time, it is clear that there are links between the molecular clock and metabolism in all cells/organisms studied. From this work, we propose that metabolic signals will act as both inputs and outputs of the molecular clock in muscle. The circadian and metabolic oscillators can modulate each other through the interlocking actions of key players such as AMPK, PGC1α, and SirT1. A summary of the interplay between metabolism and the molecular clock is shown in Fig. 9.5.
Figure 9.5.
Interplay between the circadian clock and metabolism in skeletal muscle. There is a significant overlap between the factors that modulate the molecular clock and the factors critical for metabolism in skeletal muscle. Metabolic sensors, such as AMPK, SirT1, and PGC1α respond to nutrient availability, contractile activity, energy balance, and redox status, and modulate both skeletal muscle metabolism and the molecular clock. In turn, the molecular clock controls the rhythmic expression of these metabolic sensors, suggesting that there is an interdependent link between skeletal muscle metabolism and the molecular clock.
6.3. Skeletal muscle pathologies in circadian mutants
Although expression profiling data have identified key processes in skeletal muscle physiology that may be affected or regulated by the molecular clock, not much is known regarding the role of the molecular clock in normal skeletal muscle physiology. However, certain lessons can be learned from the work with various circadian mutants that exhibit, among other things, pathologies in skeletal muscle (summarized in Table 9.2).
Table 9.2.
Skeletal muscle abnormalities in circadian mutants
| Mutation | Circadian phenotype | Skeletal muscle pathology | Reference |
|---|---|---|---|
| Bmal1−/− | Arrhythmic behavior and expression of clock genes in both SCN and peripheral tissues | Sarcopenia age-associated decrease in muscle fiber number and fiber diameter Reduced lifespan |
Kondratov et al. (2006) |
| Muscle-rescued Bmal1−/− |
Arrhythmic wheel running behavior | Normal body weight Normal activity levels Improved longevity |
McDearmon et al. (2006) |
|
Bmal1−/− ClockΔ19 |
Arrhythmic wheel running behavior | Decreased maximal force production Myofilament disarrangement Mitochondrial pathology |
Andrews et al. (2010) |
| ClockΔ19 + Mel | Liver and skeletal muscle-specific arrhythmic expression of clock genes | Decreased GLUT4 expression Possible skeletal muscle insulin resistance |
Kennaway et al. (2007) |
| Per2−/− | Short circadian period (tau = 22 h) Arrhythmic in constant darkness |
Reduced forced locomotor performance without alteration in skeletal muscle contractile function | Bae et al. (2006) |
| RORb−/− | Slightly longer circadian period, (tau = 24.3 h) | Muscle weakness when young, gain strength with age “Duck Like” gait Locomotor difficulties |
Andre et al. (1998) |
The Bmal1−/− mice exhibit an arrhythmic behavior and demonstrate decreased activity, decreased body weight, increased sleep fragmentation, arthropathy, age-associated pathologies, and a subsequent decrease in lifespan (Bunger et al., 2005; Kondratov et al., 2006; Laposky et al., 2005). Skeletal muscle pathologies include sarcopenia, or a decrease in muscle mass. Muscle mass loss is very pronounced in the Bmal1−/− mice and is only surpassed by fat mass loss. Sarcopenia is associated with a decrease in muscle fiber number and in the diameter of the remaining fibers. Moreover, this pathology starts developing later in life, as muscles from young Bmal1−/− animals (10 weeks of age) are indistinguishable from controls (Kondratov et al., 2006).Rescuing Bmal1 only in skeletal muscle was not sufficient to rescue the rhythmic behavior, but it did result in increased locomotor activity levels, restored body weight, and increased lifespan (McDearmon et al., 2006). These findings suggest that an intact molecular clock within skeletal muscle is sufficient for maintenance of skeletal muscle. In addition, these findings are intriguing as they indicate that (1) the health of the skeletal muscle can influence voluntary locomotor activity levels and (2) increased muscle mass can decrease mortality.
Recent work from our laboratory is the first evidence of direct evaluation of skeletal muscle function in two phenotypically different circadian mutant mice. We studied skeletal muscle from the Bmal1−/− mice (arrhythimc) and ClockΔ19 mice (long period length). Surprisingly, we found that specific tension was significantly reduced in skeletal muscle of both Bmal1−/− and ClockΔ19 mice. This reduction in force was associated with a disorganization of myofilament arrangement and decreased expression of key structural genes, such as actin, myosin, and titin. Further, skeletal muscles of Bmal1−/− and ClockΔ19 mice exhibit signs of a mitochondrial pathology, characterized by a significant decrease in mitochondrial volume and compromised function (reduced respiratory capacity ratio and increased respiration uncoupling) of the remaining mitochondria. The presence of a similar skeletal muscle phenotype in two different circadian mutants confirms the importance of the circadian clock in the maintenance of skeletal muscle structural and functional integrity (Andrews et al., 2010).
Using an approach opposite to McDearmon et al. (2006) Kennaway and colleagues used the “CLOCKΔ19 + Mel” mutant as a model of intact central rhythmicity, but aberrant rhythmicity in liver and skeletal muscle only. In addition to impaired glucose tolerance and reduced insulin secretion, with loss of rhythmicity in rate-limiting enzymes of glucose metabolism in the liver, the CLOCKΔ19 + Mel mutant has reduced expression levels of Glut4 in skeletal muscle. The authors proposed that a reduction in Glut4 could lead to decreased insulin-mediated glucose uptake, suggesting that skeletal muscle could be insulin-resistant in the CLOCKΔ19 + Mel mutant mouse (Kennaway et al., 2007). This suggests that the peripheral clock in skeletal muscle contributes to the regulation of metabolic pathways in skeletal muscle and that this alone is sufficient to affect systemic metabolic health.
Per2−/− mice have a shorter period length (tau ~22 h) and become arrhythmic in constant dark conditions (Zheng et al., 1999). The running endurance of Per2−/− mice is significantly lower when compared to wildtype mice, as measured by forced running distance. This is not due to changes in muscle contractile parameters or changes in contractile proteins. Glycolytic enzymes are upregulated in the Per2−/− skeletal muscle, suggesting that Per2−/− skeletal muscle depends more on glycolysis for energy production (Bae et al., 2006). Components of the clock are thus involved in the regulation of both skeletal muscle performance and the shift in metabolic pathways of energy production.
RORβ−/− mice show a significant increase in the circadian period length, implicating RORβ as a component of the molecular clock. RORβ−/− mice show muscle weakness and smaller body size when young, but this ameliorates with age. The muscle weakness is assessed as an inability to walk and frequent falls sideways while attempting to support and balance body weight. As adults, RORβ−/− mice show a “duck-like” gait. These symptoms might be part of a broader syndrome, the vacillans phenotype. These symptoms could be a result of central nervous system problems, or could reflect intrinsic problems with the muscles (Andre et al., 1998).
While there are very few studies on direct skeletal muscle evaluation in circadian mutants, disruption of the molecular clock network via genetic ablation of core clock components, has provided several lines of evidence for the importance of circadian rhythms in normal skeletal muscle physiology. The molecular clock is needed for skeletal muscle growth and maintenance, contractile performance, structural organization, glucose metabolism, and energy production. Whether these processes require central or skeletal muscle-specific molecular clock function is currently under investigation and has not been fully elucidated.
6.4. Exercise as a potential zeitgeber for the skeletal muscle molecular clock
As described in Section 3.2, exercise may be a potential zeitgeber for the skeletal muscle molecular clock. Resistance exercise has been shown to phase shift the circadian expression of clock genes in skeletal muscle in humans (Zambon et al., 2003). Although this has not been demonstrated in skeletal muscle cells, work with neonatal cardiomyocytes shows that contractile activity can modulate the molecular clock through the actions of the CLOCK protein. CLOCK localizes to the Z-disk in neonatal cardiomyocytes. In response to contractile activity, CLOCK translocates to the nucleus to influence gene expression. The stretch sensing machinery of the sarcomere is composed of a multitude of structural and signaling molecules located in the Z-disk region. Localization of CLOCK in proximity to the Z-disk puts CLOCK in the right position to sense energy expenditure associated with contractile activity. Thus, contraction of the myocardium may influence the molecular clock and the molecular clock in turn can influence the contractile activity of cardiac muscle (Qi and Boateng, 2006).
7. Summary
Circadian biology has been studied across plant and animal species for many decades with much of the early work focused on animal/organism behavior. This body of research has led to defined characteristics of circadian rhythms based on period length, phase, and amplitude. These criteria can be applied at the whole body level to circadian behavior through to the molecular behavior of components of the clock. The goal of this chapter was to provide the basic terminology and concepts of circadian rhythms with a more detailed review of the current state of knowledge of the molecular clock with reference to what is known in skeletal muscle. The study of the circadian behavior and the molecular clock in skeletal muscle is in the very early stages. Research has demonstrated that the molecular clock is active in skeletal muscles and that the muscle-specific transcription factor, MyoD, is a direct target of the molecular clock. Skeletal muscle of clock-compromised mice, Bmal1−/− and ClockΔ19 mice, exhibit significant disruption in normal expression of many genes required for adult muscle structure and metabolism. We suggest that the interaction between the molecular clock, MyoD, and metabolic factors, such as PGC-1 provide an interesting system of feedback loops that are critical for both maintenance and adaptation of skeletal muscle.
REFERENCES
- Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell Biol. 2002;22:1693–1703. doi: 10.1128/MCB.22.6.1693-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U. Invited review: regulation of mammalian circadian clock genes. J. Appl. Physiol. 2002;92:1348–1355. doi: 10.1152/japplphysiol.00759.2001. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Oster H. The circadian clock and behavior. Behav. Brain Res. 2001;125:89–91. doi: 10.1016/s0166-4328(01)00288-1. [DOI] [PubMed] [Google Scholar]
- Almon RR, Yang E, Lai W, Androulakis IP, Ghimbovschi S, Hoffman EP, Jusko WJ, Dubois DC. Relationships between circadian rhythms and modulation of gene expression by glucocorticoids in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1031–R1047. doi: 10.1152/ajpregu.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-Andre M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA. 2010;107:19090–19095. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Diaz-Munoz M, Escobar C. Food entrainment modifies the c-Fos expression pattern in brain stem nuclei of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R678–R684. doi: 10.1152/ajpregu.00590.2004. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Human circadian rhythms in activity, body temperature and other functions. Life Sci. Space Res. 1967;5:159–173. [PubMed] [Google Scholar]
- Aschoff J. Features of circadian rhythms relevant for the design of shift schedules. Ergonomics. 1978;21:739–754. doi: 10.1080/00140137808931777. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Interdependence between locomotor activity and duration of wakefulness in humans during isolation. Experientia. 1990;46:870–871. doi: 10.1007/BF01935542. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Circadian parameters as individual characteristics. J. Biol. Rhythms. 1998;13:123–131. doi: 10.1177/074873098128999970. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Masking and parametric effects of high-frequency light-dark cycles. Jpn. J. Physiol. 1999;49:11–18. doi: 10.2170/jjphysiol.49.11. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the Zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Aschoff J, Wever R. Human circadian rhythms: a multioscillatory system. Fed. Proc. 1976;35:236–232. [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Bae K, Lee K, Seo Y, Lee H, Kim D, Choi I. Differential effects of two period genes on the physiology and proteomic profiles of mouse anterior tibialis muscles. Mol. Cells. 2006;22:275–284. [PubMed] [Google Scholar]
- Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell. 2002;9:587–600. doi: 10.1016/s1097-2765(02)00481-1. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- Castillo MR, Hochstetler KJ, Tavernier RJ, Jr, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R551–R555. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P. Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J. Neurosci. 2005;25:6601–6609. doi: 10.1523/JNEUROSCI.1082-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Stokkan KA, Yamazaki S, Menaker M. Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol. Behav. 2002;76:21–26. doi: 10.1016/s0031-9384(02)00680-7. [DOI] [PubMed] [Google Scholar]
- DeCoursey PJ, Buggy J. Circadian rhythmicity after neural transplant to hamster third ventricle: specificity of suprachiasmatic nuclei. Brain Res. 1989;500:263–275. doi: 10.1016/0006-8993(89)90322-3. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Circadian photoperception. Annu. Rev. Physiol. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- Dey J, Carr AJ, Cagampang FR, Semikhodskii AS, Loudon AS, Hastings MH, Maywood ES. The tau mutation in the Syrian hamster differentially reprograms the circadian clock in the SCN and peripheral tissues. J. Biol. Rhythms. 2005;20:99–110. doi: 10.1177/0748730404274264. [DOI] [PubMed] [Google Scholar]
- Diaz-Munoz M, Vazquez-Martinez O, Aguilar-Roblero R, Escobar C. Anticipatory changes in liver metabolism and entrainment of insulin, glucagon, and corticosterone in food-restricted rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R2048–R2056. doi: 10.1152/ajpregu.2000.279.6.R2048. [DOI] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC. Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am. J. Physiol. 1991;261:R928–R933. doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J. Biol. Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol. Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Diaz-Munoz M, Encinas F, Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am. J. Physiol. 1998;274:R1309–R1316. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Albrecht U, Challet E. “Feeding time” for the brain: a matter of clocks. J. Physiol. Paris. 2006;100:252–260. doi: 10.1016/j.jphysparis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, Claustrat B, Hastings MH, Levi F. Host circadian clock as a control point in tumor progression. J. Natl. Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gibbs FP. Correlation of plasma corticosterone levels with running activity in the blinded rat. Am. J. Physiol. 1976;231:817–821. doi: 10.1152/ajplegacy.1976.231.3.817. [DOI] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O’Neill J, Chesham JE, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- Grundschober C, Delaunay F, Puhlhofer A, Triqueneaux G, Laudet V, Bartfai T, Nef P. Circadian regulation of diverse gene products revealed by mRNA expression profiling of synchronized fibroblasts. J. Biol. Chem. 2001;276:46751–46758. doi: 10.1074/jbc.M107499200. [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F. Chronobiology. Annu. Rev. Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- Halberg F, Carandente F, Cornelissen G, Katinas GS. Glossary of chronobiology (author’s transl) Chronobiologia. 1977;4(Suppl 1):1–189. [PubMed] [Google Scholar]
- Halberg F, Cornelissen G. Rhythms and blood pressure. Ann. Ist Super Sanita. 1993;29:647–665. [PubMed] [Google Scholar]
- Halberg F, Engeli M, Hamburger C, Hillman D. Spectral resolution of low-frequency, small-amplitude rhythms in excreted 17-ketosteroids; probable androgen-induced circaseptan desynchronization. Acta Endocrinol. (Copenh) 1965;50(Suppl. 103):101–154. doi: 10.1530/acta.0.050s0005. [DOI] [PubMed] [Google Scholar]
- Halberg F, Peterson RE, Silber RH. Phase relations of 24-hour periodicities in blood corticosterone, mitoses in cortical adrenal parenchyma, and total body activity. Endocrinology. 1959;64:222–230. doi: 10.1210/endo-64-2-222. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J. Biol. Chem. 2005;280:31714–31721. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Eskes GA, Dickson P, Rusak B. Lesions dorsal to the suprachiasmatic nuclei abolish split activity rhythms of hamsters. Brain Res. Bull. 1990;24:593–597. doi: 10.1016/0361-9230(90)90164-u. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J. Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- Haus E, Halberg F. 24-Hour rhythm in susceptibility of C mice to a toxic dose of ethanol. J. Appl. Physiol. 1959;14:878–880. doi: 10.1152/jappl.1959.14.6.878. [DOI] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- Hiroshige T, Honma K, Honma S. SCN-independent circadian oscillators in the rat. Brain Res. Bull. 1991;27:441–445. doi: 10.1016/0361-9230(91)90139-b. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc. Natl. Acad. Sci. USA. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Mistlberger RE. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol. Behav. 2000;68:655–666. doi: 10.1016/s0031-9384(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Holzberg D, Albrecht U. The circadian clock: a manager of biochemical processes within the organism. J. Neuroendocrinol. 2003;15:339–343. doi: 10.1046/j.1365-2826.2003.00992.x. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol. Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- Honma K, von Goetz C, Aschoff J. Effects of restricted daily feeding on freerunning circadian rhythms in rats. Physiol. Behav. 1983;30:905–913. doi: 10.1016/0031-9384(83)90256-1. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur. J. Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J. Biol. Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc. Natl. Acad. Sci. USA. 2003;100:16089–16094. doi: 10.1073/pnas.2536313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Owens JA, Voultsios A, Boden MJ, Varcoe TJ. Metabolic homeostasis in mice with disrupted Clock gene expression in peripheral tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1528–R1537. doi: 10.1152/ajpregu.00018.2007. [DOI] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, Turek FW, Takahashi JS. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics. 1997;146:1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM. Site-specific casein kinase 1epsilon-dependent phosphorylation of Dishevelled modulates beta-catenin signaling. FEBS J. 2006;273:4594–4602. doi: 10.1111/j.1742-4658.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occup. Med. (Lond.) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Lowe CH, Hinds DS, Lardner PJ, Justice KE. Natural free-running period in vertebrate animal populations. Science. 1967;156:531–534. doi: 10.1126/science.156.3774.531. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Nakamura W, Honma K. Circadian activity rhythm in methamphetamine-treated Clock mutant mice. Eur. J. Neurosci. 2001;14:1177–1180. doi: 10.1046/j.0953-816x.2001.01749.x. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science. 2006;314:1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M, Spoelstra K, Roenneberg T. The circadian cycle: daily rhythms from behaviour to genes. EMBO Rep. 2005;6:930–935. doi: 10.1038/sj.embor.7400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Effects of daily schedules of forced activity on free-running rhythms in the rat. J. Biol. Rhythms. 1991;6:71–80. doi: 10.1177/074873049100600108. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Baer ML, Menaker M. The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes. Proc. Natl. Acad. Sci. USA. 2009;106:3519–3524. doi: 10.1073/pnas.0813366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Further experiments on the relationship between the period of circadian rhythms and locomotor activity levels in hamsters. Physiol. Behav. 1999;66:797–801. doi: 10.1016/s0031-9384(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Salmon PA, Menaker M, Ralph MR. Nonphotic phase shifting in hamster clock mutants. J. Biol. Rhythms. 1992;7:41–49. doi: 10.1177/074873049200700104. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl. Physiol. Nutr. Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Oster H, Maronde E, Albrecht U. The circadian clock as a molecular calendar. Chronobiol. Int. 2002;19:507–516. doi: 10.1081/cbi-120004210. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Qi L, Boateng SY. The circadian protein Clock localizes to the sarcomeric Z-disk and is a sensor of myofilament cross-bridge activity in cardiac myocytes. Biochem. Biophys. Res. Commun. 2006;351:1054–1059. doi: 10.1016/j.bbrc.2006.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7:e62. doi: 10.1371/journal.pbio.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RS, Zee PC, Turek FW. Phase response curves to light in young and old hamsters. Am. J. Physiol. 1991;261:R491–R495. doi: 10.1152/ajpregu.1991.261.2.R491. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Harada Y, Sakai M, Todo T, Fukada Y. Serine phosphorylation of mCRY1 and mCRY2 by mitogen-activated protein kinase. Genes Cells. 2004;9:697–708. doi: 10.1111/j.1356-9597.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- Sanada K, Okano T, Fukada Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J. Biol. Chem. 2002;277:267–271. doi: 10.1074/jbc.M107850200. [DOI] [PubMed] [Google Scholar]
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, et al. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Kawai H, Kudo N, Kawashima Y, Mitsumoto A. Time-restricted feeding entrains daily rhythms of energy metabolism in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1276–R1283. doi: 10.1152/ajpregu.00775.2005. [DOI] [PubMed] [Google Scholar]
- Schibler U. The 2008 Pittendrigh/Aschoff lecture: peripheral phase coordination in the mammalian circadian timing system. J. Biol. Rhythms. 2009;24:3–15. doi: 10.1177/0748730408329383. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Shirogane T, Jin J, Ang XL, Harper JW. SCFbeta-TRCP controls clockdependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J. Biol. Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle. 2009;8:4138–4146. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. Circadian rhythms in the rat: constant darkness, entrainment to T cycles and to skeleton photoperiods. Physiol. Behav. 1983;30:451–462. doi: 10.1016/0031-9384(83)90152-x. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav. Neural. Biol. 1979;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Hirao A, Moriya T, Kudo T, Shibata S. Effects of medial hypothalamic lesions on feeding-induced entrainment of locomotor activity and liver Per2 expression in Per2::luc mice. J. Biol. Rhythms. 25:9–18. doi: 10.1177/0748730409352782. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Turek FW, Penev P, Zhang Y, van Reeth O, Zee P. Effects of age on the circadian system. Neurosci. Biobehav. Rev. 1995;19:53–58. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E, Nilsson EC, Nerstedt A, Ormestad M, Long YC, Garcia-Roves PM, Zierath JR, Mahlapuu M. Relationship between AMPK and the transcriptional balance of clock-related genes in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1032–E1037. doi: 10.1152/ajpendo.90510.2008. [DOI] [PubMed] [Google Scholar]
- Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol. Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M, Fulco M, Ladurner A, Sartorelli V, Rosenthal N. SirT1 in muscle physiology and disease: lessons from mouse models. Dis. Model Mech. 2010;3:298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MM, Rice RW, Critchlow V. Evidence for a free-running circadian rhythm in pituitary-adrenal function in blinded adult female rats. Neuroendocrinology. 1976;20:289–295. doi: 10.1159/000122495. [DOI] [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED. Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA. 1988;85:5305–5309. doi: 10.1073/pnas.85.14.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol. Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- Yagita K, Horie K, Koinuma S, Nakamura W, Yamanaka I, Urasaki A, Shigeyoshi Y, Kawakami K, Shimada S, Takeda J, Uchiyama Y. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc. Natl. Acad. Sci. USA. 107:3846–3851. doi: 10.1073/pnas.0913256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Honma S, Honma K. Scheduled exposures to a novel environment with a running-wheel differentially accelerate re-entrainment of mice peripheral clocks to new light-dark cycles. Genes Cells. 2008;13:497–507. doi: 10.1111/j.1365-2443.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Joshi S, Wu N, Tong X, Lazar MA. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erb alpha. Proc. Natl. Acad. Sci. USA. 2010;107:11614–11619. doi: 10.1073/pnas.1000438107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003;4:R61. doi: 10.1186/gb-2003-4-10-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dube TJ, Esser KA. Working around the clock: circadian rhythms and skeletal muscle. J. Appl. Physiol. 2009;107:1647–1654. doi: 10.1152/japplphysiol.00725.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]