Abstract
We report a large multicenter genome-wide association study of Plasmodium falciparum resistance to artemisinin, the frontline antimalarial drug. Across 15 locations in Southeast Asia, we identified at least 20 mutations in kelch13 (PF3D7_1343700) affecting the encoded propeller and BTB/POZ domains, which were associated with a slow parasite clearance rate after treatment with artemisinin derivatives. Nonsynonymous polymorphisms in fd (ferredoxin), arps10 (apicoplast ribosomal protein S10), mdr2 (multidrug resistance protein 2) and crt (chloroquine resistance transporter) also showed strong associations with artemisinin resistance. Analysis of the fine structure of the parasite population showed that the fd, arps10, mdr2 and crt polymorphisms are markers of a genetic background on which kelch13 mutations are particularly likely to arise and that they correlate with the contemporary geographical boundaries and population frequencies of artemisinin resistance. These findings indicate that the risk of new resistance-causing mutations emerging is determined by specific predisposing genetic factors in the underlying parasite population.
Preventing the spread of P. falciparum resistance to artemisinin derivatives, the frontline drugs for severe and uncomplicated malaria, is an urgent priority for global health1. Although artemisinin derivatives remain effective, the rate at which they clear malaria parasites from the blood has progressively declined in Southeast Asia for at least 5 years, threatening control strategies based on the overall efficacy of artemisinin combination therapies (ACT)2–6. This is of serious concern, as the spread across Asia to Africa of resistance to previous frontline drugs, chloroquine and sulfadoxine-pyrimethamine (Fansidar), originated in exactly the same region7. It is particularly important to prevent the development of high-level artemisinin resistance (loss of artemisinin efficacy) and the spread of resistance to Africa, where most malaria deaths occur.
To develop an effective strategy to combat drug resistance and contain it within Southeast Asia, it is imperative to understand the genetic factors that determine how it emerges and spreads. Artemisinin resistance in P. falciparum was recently associated with multiple SNPs in a gene on chromosome 13, referred to here as kelch13, mapping to the β-propeller domain of the encoded kelch-like protein, PF3D7_ 1343700 (ref. 8), but many questions remain. What other genes are involved? Is the spread of resistance due mainly to parasite migration or to the emergence of new mutations? Why do new resistance-conferring mutations emerge in some places more often than others? How does this observation relate to the underlying genetic structure of the parasite population?
Here we report new insights into the genetic architecture of the artemisinin-resistant P. falciparum parasites that are spreading throughout Southeast Asia, based on analysis of 1,612 samples from 15 locations in Cambodia, Vietnam, Laos, Thailand, Myanmar and Bangladesh. P. falciparum genome sequencing and genotype calling at >600,000 SNP positions was performed on all samples. We first conducted a genome-wide association study (GWAS) with clinical data on 928 individuals with malaria recruited during 2011–2013 by the Tracking Resistance to Artemisinin Collaboration (TRAC) and 135 affected individuals recruited during 2009-2010 by the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (Table 1). We then performed a population genetics analysis, incorporating additional samples collected by multiple projects in the same region (Supplementary Table 1) to translate our GWAS findings into an understanding of genetic architecture.
Table 1. Geographical distribution of the samples used in the GWAs.
| Contributor | Years | Country (region) | Code | Location | Samples |
|---|---|---|---|---|---|
| TRAC | 2011–2013 | Bangladesh | BD | Ramu | 50 |
| Myanmar | MM | Bago division | 59 | ||
| Thailand (western) | WTH | Mae Sot | 103 | ||
| Thailand (southern) | STH | Ranong | 20 | ||
| Thailand (eastern) | ETH | Sisakhet | 21 | ||
| Vietnam | VN | Binh Phuoc | 97 | ||
| Laos | LA | Attapeu | 77 | ||
| Cambodia (western) | WKH | Pursat, Pailin | 185 | ||
| Cambodia (northern) | NKH | Preah Vihear | 106 | ||
| Cambodia (northeastern) | NEKH | Ratanakiri | 95 | ||
| Democratic Republic of the Congo | CD | Kinshasa | 112 | ||
| Nigeria | NG | Ilorin | 3 | ||
| NIAID/NIH | 2009–2010 | Cambodia (western) | WKH | Pursat | 89 |
| Cambodia (northeastern) | NEKH | Ratanakiri | 46 | ||
| Total | Cambodia (northeastern) | NEKH | Ratanakiri | 1,063 |
This table includes samples for which both phenotype (PC t1/2) and genotype data were available. Abbreviations used in this paper to indicate the respective geographical regions are shown in the “Code” column. NIAID/NIH, National Institute of Allergy and Infectious Diseases of the US National Institutes of Health.
Results
Genome-wide association study
We analyzed data on 1,063 individuals receiving artemisinin derivative treatment for P. falciparum malaria at 13 locations in Cambodia, Vietnam, Laos, Thailand, Myanmar, Bangladesh, Democratic Republic of the Congo and Nigeria. Parasite response to the drug was evaluated by determining parasite densities in blood samples collected every 6 h after admission (Online Methods). Clinical phenotype was expressed as the parasite clearance half-life (PC t1/2), representing the time taken for artesunate to reduce parasite density by half during the log-linear decline in parasite densities; the findings at each location have been reported elsewhere6,9.
We performed whole-genome sequencing on pretreatment blood samples, after enrichment of parasite DNA by leukocyte depletion10, using an Illumina sequencing platform. Sequence reads were aligned against the P. falciparum 3D7 reference genome and combined with a collection of worldwide samples to discover variants and perform quality control based on genome coverage and other metrics (Online Methods)11. This process identified 681,587 high-quality exonic SNPs in the global data set, from which we obtained a data set of 18,322 SNPs with minor allele frequency (MAF) > 0.01 that were well covered in a set of 1,063 samples used for GWAS analysis. The association between SNP genotypes and PC t1/2 (treated as a continuous dependent variable) was analyzed using a linear regression mixed-model algorithm, implemented in FaST-LMM12. This algorithm corrected for the confounding effect of population structure by treating genetic similarity as a random effect, reducing the genomic inflation factor λGC from 14.24 to 1.003 (Supplementary Fig. 1). At each SNP, samples for which the genotype was missing (for example, owing to low coverage) or heterozygous (for example, owing to mixed infection) were excluded from the test.
The GWAS identified strong signals of association (P < 1 × 10−7) at nine independent loci (Fig. 1 and Table 2). The significance threshold chosen is conservative if Bonferroni correction is applied to <20,000 SNP tests; Supplementary Table 2 shows the results with a threshold of P < 1 × 10−5. The strongest signal of association (P = 4 × 10−26) was for a nonsynonymous SNP (referred to as k13-C580Y here) in kelch13 producing a p.Cys580Tyr substitution in the encoded propeller domain. This gene is within a genomic region identified by previous association studies13,14, and the SNP corresponds exactly to the most common kelch13 variant found to be associated with artemisinin resistance8,15.
Figure 1.
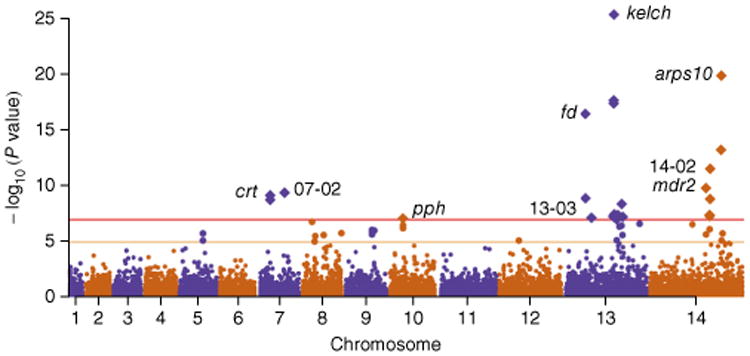
Manhattan plot showing the significance of SNP association in the GWAS. Each point represents 1 of the 18,322 SNPs with MAF > 0.01 in a set of 1,063 samples, colored according to chromosome. The x axis represents genomic location, and the y axis represents the P value for the SNP's association calculated using a linear mixed model (Online Methods). SNPs with P ≤ 1 × 10−7 after Bonferroni correction (n = 24; above the horizontal red line) are represented by diamond symbols. The nine loci containing these SNPs are identified in the plot and listed in Table 2. Polymorphisms with association P ≤ 1 × 10−5 (n = 24) are shown in a larger size between the horizontal orange and red lines and are listed in supplementary table 2.
Table 2. Genomic loci most strongly associated with Pc t1/2 identified in the GWAs.
| Top SNP | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Locus | Chr. | Position | Gene ID | Gene description | N/S | Alteration | P value |
| 13-01 (kelch) | 13 | 1,725,259 | PF3D7_1343700 | Kelch protein, putative | N | p.Cys580Tyr | 4 × 10−26 |
| 14-01 (arps10) | 14 | 2,481,070 | PF3D7_1460900.1 | Apicoplast ribosomal protein S10 precursor, putative | N | p.Val127Met | 1 × 10−20 |
| 13-02 (fd) | 13 | 748,395 | PF3D7_1318100 | Ferredoxin, putative | N | p.Asp193Tyr | 3 × 10−17 |
| 14-02 | 14 | 2,098,642 | PF3D7_1451200 | Conserved Plasmodium protein, unknown function | S | p.71Asn | 3 × 10−12 |
| 14-03 (mdr2) | 14 | 1,956,225 | PF3D7_1447900 | Multidrug resistance protein 2+ (heavy metal transport family) (MDR2) | N | p.Thr484Ile | 2 × 10−10 |
| 07-02 | 7 | 896,660 | PF3D7_0720700 | Phosphoinositide-binding protein, putative | N | p.Cys1484Phe | 4 × 10−10 |
| 07-01 (crt) | 7 | 405,600 | PF3D7_0709000 | Chloroquine resistance transporter (CRT) | N | p.Ile356Thr | 7 × 10−10 |
| 13-03 | 13 | 958,469 | PF3D7_1322700 | Conserved Plasmodium protein, unknown function | N | p.Thr236Ile | 7 × 10−8 |
| 10-01 (pph) | 10 | 490,720 | PF3D7_1012700 | Protein phosphatase, putative | N | p.Val1157Leu | 8 × 10−8 |
This table shows nine loci identifed across the genome that contained one or more SNPs signifcant with a Bonferroni-corrected threshold (P ≤ 1 × 10−7), ordered by increasing P value. For each locus, the SNP within the locus exhibiting the strongest signal is listed; for information about other results within these loci, refer to Supplementary Table 2. For each SNP, we show locus name; chromosome number (Chr.); nucleotide position; gene ID and description; whether the SNP is nonsynonymous (N) or synonymous (S); encoded alteration; and association P value. For ease of reference, some loci are named after their highest scoring gene.
There were strong signals of association with other SNPs causing nonsynonymous changes, including in arps10 encoding a p. Val127Met substitution in apicoplast ribosomal protein S10 (P = 1 × 10−20; referred to as arps10-V127M here); in fd encoding a p.Asp193Tyr substitution in ferredoxin (P = 3 × 10−17; fd-D193Y); in mdr2 encoding a p.Thr484Ile substitution in multidrug resistance protein 2 (P = 2 × 10−10; mdr2-T484I); in pib7 encoding a p.Cys1484Phe substitution in putative phosphoinositide-binding protein (P = 4 × 10−10; pib7- C1484F); in crt encoding p.Ile356Thr and p. Asn326Ser substitutions in chloroquine resistance transporter (P = 7 × 10−10; crt-I356T and crt-N326S, respectively); and in pph encoding a p.Val1157Leu substitution in protein phosphatase (P = 8 × 10−8; pph-V1157L). Although previous GWAS did not identify these additional loci, two genes in close proximity to arps10 were reported to be mutated along with kelch13 during in vitro selection for artemisinin resistance8. In the following sections, we examine these loci and their interrelationships in more detail.
Diversity and phenotypic effect of resistance alleles
Previous work has associated artemisinin resistance with multiple SNPs in kelch13 corresponding to the propeller domain. Only the k13-C580Y variant showed significant association in our analysis, but GWAS are poorly powered to detect associations with low-frequency variants, particularly when there is allelic heterogeneity. Detailed analysis of sequence variation identified 33 nonsynonymous SNPs in kelch13 for which at least one sample had a homozygous call (Supplementary Table 3). When these SNPs were tested individually for association with parasite clearance, a clear pattern emerged: at least 20 of the 25 nonsynonymous changes in kelch13 affecting the highly conserved BTB/POZ and propeller domains8 were associated with prolonged PC t1/2, whereas none of the variants in the upstream P. falciparum–specific portion of the gene were (Supplementary Fig. 2 and Supplementary Table 3). Thirteen of these 20 mutations were previously observed to circulate in Cambodia8. On the basis of these findings, we classified samples into those with and without kelch13 resistance alleles, defined here as nonsynonymous changes affecting the BTB/POZ and propeller domains with respect to the P. falciparum reference genome. The median PC t1/2 was 6.5 h (interquartile range (IQR) = 5.4−7.8 h) for samples homozygous for a kelch13 resistance allele and 2.6 h (2.0−3.3 h) for those without a kelch13 resistance allele. No samples carried more than one resistance allele in the gene, apart from samples with clear evidence of mixed infection. The median PC t1/2 was 5.9 h (4.4−7.1 h) for samples from Southeast Asia containing a mixture of parasites with and without kelch13 resistance alleles. We found no homozygous kelch13 mutations affecting the propeller domain in the African samples. Eight samples from Democratic Republic of the Congo carried different kelch13 propeller domain– affecting mutations in the heterozygous state, but none of these was observed in more than three samples, and they were not associated with elevated PC t1/2 (median = 1.9 h, range = 1.0−4.6 h).
We repeated the GWAS analysis treating kelch13 resistance alleles collectively as a covariate. This adjustment resulted in a major reduction in the GWAS signals at other loci, but it did not abolish them completely. After correcting for the effect of kelch13 variants, the estimated prolongation of PC t1/2 was 0.54 h for mdr2-T484I (P = 5 × 10−5), 0.58 h for arps10-V127M (P = 4 × 10−5), 0.53 h for fd-D193Y (P = 5 × 10−4), 0.52 h for pph-V1157L (P = 9 × 10−5) and 0.47 h for crt-I356T (P = 5 × 10−4; Supplementary Table 4). A further iteration, in which the mdr2-T484I allele was added as a covariate along with kelch13 variants, showed only a small residual effect for arps10-V127M and the other loci, implying that the phenotypic effects of these loci are not mutually independent. In summary, we find that multiple mutations in kelch13 affecting the BTB/POZ and propeller domains are the strongest predictors of prolonged PC t1/2 across the genome. Other genomic loci are associated with PC t1/2, largely owing to their population genetics relationship to kelch13 resistance alleles.
Analysis of founder populations
To investigate the relationship between kelch13 and other loci, we began by examining the founder effects previously observed in Cambodia, which appear to be due to the recent population expansion of multiple strains of artemisinin-resistant P. falciparum.16 An iterative approach (Online Methods) was used to identify founder populations, defined here as distinct outlier clusters of samples showing loss of diversity and numerous polymorphisms with high FST values in comparison to the general population, consistent with founding events and recent population expansions. We identified seven founder populations strongly associated with artemisinin resistance: five in Cambodia and two in Vietnam (Supplementary Figs. 3-5 and Supplementary Tables 5-8).
We identified SNP markers common to all artemisinin-resistant founder populations by performing a genome-wide analysis of the FST value between each artemisinin-resistant founder population and the artemisinin-sensitive core population in the same country and then combining the results across all founder populations (Supplementary Table 9). This analysis showed that fd-D193Y was the SNP most strongly associated with resistant founder populations and that other strong associations included crt-I356T, crt-N326S, arps10-V127M and mdr2-T484I (Supplementary Table 9). These findings coincide closely with the top signals of association in the GWAS (Table 2), with the notable exception of kelch13 variants. We repeated this analysis to screen for genes containing multiple SNPs where each was a marker for a specific founder population (Online Methods). The kelch13 locus ranked at the top, having five SNPs with close to 100% frequency in specific resistant founder populations and close to 0% frequency in the artemisinin-sensitive core population (Supplementary Fig. 6, and Supplementary Tables 10 and 11). All seven founder populations were associated with a mutant kelch13 allele: three carried the common k13-C580Y variant, and the remaining four had the k13-R539T, k13-Y493H, k13-I542T and k13-P553L variants (encoding p.Arg539Thr, p.Tyr493His, p.Ile542Thr and p.Pro553Leu substitutions, respectively).
In summary, each artemisinin-resistant founder population was strongly associated with a specific kelch13 resistance allele. In addition, most founder populations in Cambodia and Vietnam shared the same alleles at fd, crt, mdr2, aprs10 and other loci identified by conventional GWAS analysis (Table 3). By analogy with cancer genetics, these findings suggest a model in which kelch13 mutations act as driver mutations for the emergence of the artemisinin-resistant parasite strains that have recently undergone population expansion in Cambodia and Vietnam. Independent kelch13 mutations often but not invariably arise in combination with specific alleles at other positions in the genome, which we refer to here as ‘background’ alleles.
Table 3. Allele frequencies for SNPs that are highly associated with artemisinin resistance.
| Locus | Chr. | Position | Gene ID | Alteration | GH | CD | BD | MM | TH | LA | VN-C | KH-C | VN-F01 | VN-F04 | WKH-F01 | WKH-F02 | WKH-F03 | WKH-F04 | NKH-F02 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kelch | 13 | 1725259 | PF3D7_1343700 | p.Cys580Tyr | 0.00 | 0.00 | 0.00 | 0.11 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.96 | 0.00 | 1.00 | 0.00 | 0.98 |
| kelch | 13 | 1725340 | PF3D7_1343700 | p.Prp553Leu | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.03 | 0.00 | 0.00 | 0.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| kelch | 13 | 1725370 | PF3D7_1343700 | p.Ile543Thr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| kelch | 13 | 1725382 | PF3D7_1343700 | p.Arg539Thr | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 |
| kelch | 13 | 1725521 | PF3D7_1343700 | p.Tyr493His | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| fd | 13 | 748395 | PF3D7_1318100 | p.Asp193Tyr | 0.00 | 0.01 | 0.02 | 0.65 | 0.90 | 0.02 | 0.06 | 0.02 | 1.00 | 0.82 | 0.98 | 1.00 | 1.00 | 1.00 | 0.98 |
| mdr2 | 14 | 1956225 | PF3D7_1447900 | p.Thr484Ile | 0.00 | 0.00 | 0.06 | 0.79 | 0.79 | 0.22 | 0.28 | 0.22 | 1.00 | 0.89 | 1.00 | 1.00 | 1.00 | 1.00 | 0.97 |
| arps10 | 14 | 2481070 | PF3D7_1460900.1 | p.Val127Met | 0.00 | 0.00 | 0.00 | 0.49 | 0.70 | 0.12 | 0.13 | 0.08 | 1.00 | 0.07 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 |
| crt | 7 | 405362 | PF3D7_0709000 | p.Asn326Ser | 0.01 | 0.00 | 0.31 | 1.00 | 1.00 | 0.12 | 0.14 | 0.06 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 |
| crt | 7 | 405600 | PF3D7_0709000 | p.Ile356Thr | 0.02 | 0.29 | 0.84 | 0.99 | 0.99 | 0.13 | 0.15 | 0.05 | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 |
| pph | 10 | 490720 | PF3D7_1012700 | p.Val1157Leu | 0.00 | 0.00 | 0.01 | 0.32 | 0.22 | 0.04 | 0.31 | 0.09 | 1.00 | 0.92 | 1.00 | 1.00 | 1.00 | 0.80 | 0.99 |
Allele frequencies in the seven artemisinin-resistant founder populations (columns on the right) are compared to those in the core populations of Vietnam (VN-C) and Cambodia (KH-C) and in other populations (GH, Ghana; CD, Democratic Republic of the Congo; BD, Bangladesh; MM, Myanmar; TH, Thailand; LA, Laos). For each SNP, we show locus name; chromosome number (Chr.); nucleotide position; gene ID; encoded alteration; and frequency of the mutant or non-ancestral allele in various populations (bold indicates that the mutant allele is the majority allele).
Geographical and genetic compartments of emerging resistance
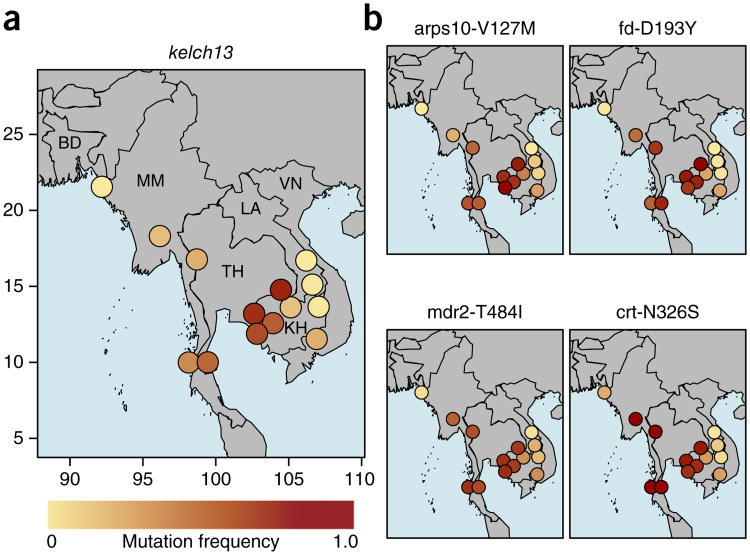
To understand how artemisinin resistance is spreading across Southeast Asia, we constructed a map of kelch13 resistance allele frequencies across all 15 sampling locations (Fig. 2 and Supplementary Table 12). The overall picture is suggestive of diffusion from a central hotspot of high-frequency resistance in the area of western Cambodia, with intermediate-frequency resistance in Vietnam, Thailand and Myanmar and very low-frequency resistance in Laos and Bangladesh. However, there are areas of sharp discontinuity in the frequency of kelch1 3 resistance alleles, for example, at the junction between Cambodia, Thailand and Laos, raising the possibility that the spread of resistance is compartmentalized.
Figure 2.
Distributions of genetic background mutations associated with artemisinin resistance across Southeast Asian sites. Mutant allele frequencies at each site are represented by colored circles, where deeper shades of red denote higher allele frequencies. Country codes correspond to those listed in Table 1. The two Bangladeshi sites, which are relatively close to each other, are combined because of the low sample sizes. (a) Distribution of kelch13 resistance mutations (defined as any nonsynonymous mutation in kelch13 affecting the BTB/POZ or propeller domains). (b) Distribution of four mutations identified as potential background mutations in artemisinin-resistant parasites: arps10-V127M, fd-D193Y, mdr2-T484I and crt-N326S. Representative geographical coordinates are included on the axes in a.
To investigate how the geographical distribution of resistance alleles relates to the genetic structure of the parasite population, we constructed a neighbor-joining tree grouping samples according to genome-wide genetic similarity (Fig. 3a). The tree showed three major compartments of population structure, corresponding to the western (WSEA) and eastern (ESEA) parts of Southeast Asia and to Bangladesh (BD). WSEA comprised Myanmar and western Thailand, and ESEA comprised Cambodia, Vietnam, Laos and eastern Thailand; these two major compartments of parasite population structure were separated by a malaria-free corridor running through the center of Thailand (Fig. 3b). In ESEA, there was considerable variation in the frequency of kelch13 resistance alleles, with an area of high resistance comprising three locations in western Cambodia and eastern Thailand and an area of low resistance comprising three sites in Laos and northeastern Cambodia. Between the high-resistance and low-resistance areas were two locations in northern Cambodia and southern Vietnam with intermediate levels of resistance. Parasites from the high-resistance and low-resistance areas clearly separated in the neighbor-joining tree (Fig. 3a), whereas parasites from the intermediate-resistance area fell into two groups, one aligned with high resistance and the other with low resistance.
Figure 3.
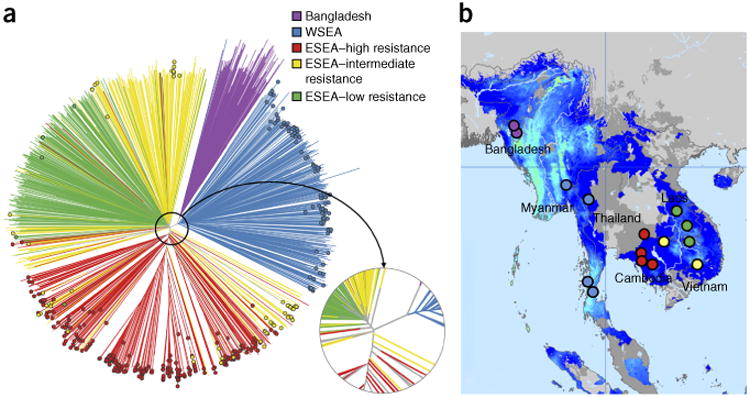
Population structure and distribution of kelch13 mutants in Southeast Asia. (a) Neighbor-joining tree showing population structure across the 15 Asian sampling sites. Branches with colored tip symbols indicate that the samples are kelch13 mutants, whereas those without tip symbols are wild type for kelch13. The circular subpanel shows a magnified view of the major branching points near the tree root. Mixed-infection samples (with a mixture of wild-type and mutant parasites) were omitted. (b) Map of the 15 sites showing the geographical location of samples in the tree. Bangladeshi (purple) and Thailand-Myanmar border region (WSEA; blue) samples form separate branches, whereas the lower Mekong region (ESEA) samples divide into two major groups, separating samples in high-resistance areas (red) from those in low-resistance areas (green); parasites in intermediate-resistance areas (yellow) are split between these two groups. Map colors indicate the endemicity of P. falciparum in 2010: light blue, high; darker blue, low; gray, absent (the map was adapted from http://www.map.ox.ac.uk/)34.
These findings raise a key question: what are the differences between the parasite populations residing on either side of the geographical boundary between high and low resistance? This data set provided two examples for such a comparison—the boundaries between WSEA and BD and between the high-resistance and low-resistance areas of ESEA. We performed a genome-wide screen for SNPs showing high FST between resistant and non-resistant parasite population compartments (Fig. 4 and Supplementary Table 13). In the WSEA-BD comparison, the strongest signals of differentiation included fd-D193Y (FST = 0.64), mdr2-T484I (FST = 0.53), crt-N326S (FST = 0.53) and arps10-V127M (FST = 0.44). In ESEA, when comparing areas of high- and low-resistance, the strongest signals of differentiation included fd-D193Y (FST = 0.85), crt-N326S (FST = 0.71), crt-I356T (FST = 0.7), arps10-V127M (FST = 0.64), pph-Y1133N (FST = 0.5) and mdr2-T484I (FST = 0.44). Combining these findings, the SNPs most clearly marking the geographical boundary of resistance were fd-D193Y, mdr2-T484I, crt-N326S and arps10-V127M. Allele frequency maps showed that the patterns of geographical variation in these alleles were remarkably similar to that for kelch13 resistance alleles (Fig. 2b). We found that the arps10-V127M, fd-D193Y and mdr2-T484I alleles were rare or absent in 2 African populations (113 parasites from the Democratic Republic of the Congo and 475 parasites from Ghana), suggesting that they are the product of evolutionary selection within Southeast Asia (Table 3).
Figure 4.
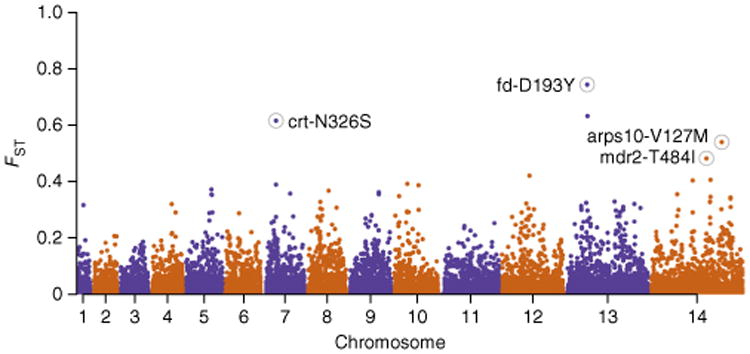
Genome-wide analysis of SNP differentiation between resistant and non-resistant geographical compartments. We selected two pairs of geographical compartments, each consisting of a resistant and a proximal non-resistant compartment: Bangladesh versus WSEA and high-resistance versus low-resistance sites in ESEA. For each pair, we estimated FST at every SNP across the genome and computed the mean of the two SNP estimates. In this Manhattan plot, mean FST is plotted at the corresponding genomic position. Polymorphisms at four loci associated with the artemisinin resistance genetic background are among the highest scorers and are highlighted by labeled circles.
The WSEA and ESEA parasite populations were clearly distinct, both geographically and genetically, and they displayed some differences in the genetic features of artemisinin resistance. PC t1/2 was somewhat longer in WSEA than in ESEA for parasites without resistance alleles (P = 2 × 10−5) and shorter for parasites with resistance alleles (P = 4 × 10−3), such that kelch13 resistance alleles prolonged PC t1/2 by a median of 3.2 h in WSEA in comparison to the time of 3.9 h in ESEA. In ESEA, where there was extreme heterogeneity in allele frequency with marked founder effects, background alleles were strongly associated with the presence of resistance alleles in individual samples, whereas in WSEA, where there was less evidence of founder effects, background alleles were present at high frequency but were weakly associated with resistance alleles in individual samples (Supplementary Table 14). A possible contributory factor is the higher level of malaria transmission in WSEA, which tends to increase the likelihood of recombination between parasites of different genetic types and thus to decouple resistance alleles from the genetic background on which they originated.
Spread of resistance between population compartments
To what extent is the spread of artemisinin resistance across Southeast Asia due to the geographical migration of resistant parasites as opposed to multiple origins of resistance in different locations? Multiple origins are clearly an important factor, as we observed a wide repertoire of kelch13 resistance alleles, most of which appeared to be localized in their geographical distribution (Supplementary Table 15). A notable exception was the k13-C580Y allele, present in 16% of the 1,612 samples in this study and observed at 3 locations in WSEA and 7 locations in ESEA. A simple reconstruction of haplotypes in the genomic flanking regions extending 100 kb on either side of the kelch13 sequence encoding the propeller region showed a wide variety of haplotypic backgrounds surrounding the different resistance alleles (Fig. 5a). In our data set, most resistance alleles were associated with one or more unique haplotypes, suggesting that they have originated from separate mutational events. In some cases, the same kelch13 allele was accompanied by different haplotypes, which may indicate that some mutations might have emerged independently multiple times, as recently suggested by another analysis17.
Figure 5.
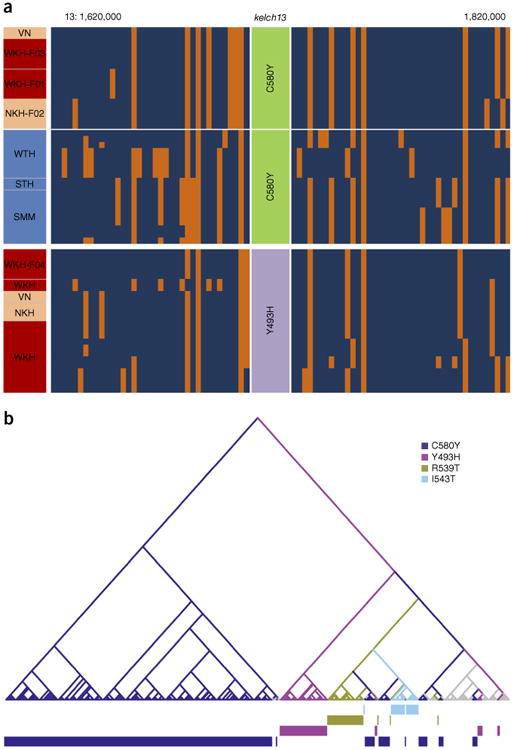
Analyses of the haplotypes surrounding kelch13 in resistant mutants. (a) Haplotype diagram. Each vertical column represents a SNP (MAF ≥ 0.1 in our data set; within 100 kb on either side of the kelch13 gene), and each horizontal line represents a sample; at the intersection, the color represents the genotyped allele in the sample: blue, reference; orange, alternative. Haplotypes are shown for a selection of samples carrying the mutations k13-C580Y (top) and k13-Y493H (bottom), organized by geographical compartment, as shown in the left-hand column (blue, WSEA; red, ESEA high resistance; pink, ESEA intermediate resistance). The samples' regions or founder populations are indicated. (b) Tree based on the LCHLs for samples containing kelch13 mutations: samples with long shared haplotypes cluster closely. Tips represent samples and have been colored by the alteration present in the sample. Internal branches have been colored by the most frequent alteration present in the subtrees to which they lead. The bars at the bottom offer visual aid for tracking how different mutations cluster and segregate across the tree. Branches have been colored by the most frequent mutation present in the subtree they subtend. Colored bars at the bottom show which alteration corresponds to the tree tip directly above. Details of the method are discussed in the Online Methods.
To assess whether parasite migration has had a major role alongside independent emergence, we performed a more detailed demographic analysis. For each pair of kelch13-mutant samples, we estimated the longest common haplotype length (LCHL), that is, the nucleotide distance on either side of the kelch13 gene over which the samples' haplotypes were identical. These estimates were then used to cluster samples that were likely to share the same recent demographic history (Fig. 5b). Samples carrying less common kelch13 mutations tended to cluster by allele, as expected if each of these clusters originated from a different recent evolutionary event. In contrast, the majority of samples carrying the most common allele (k13-C580Y) formed a large branch comprising several clusters, consistent with a common origin of the k13-C580Y alleles shared by different subpopulations in Cambodia. However, we observed two separate clusters of Cambodian k13-C580Y mutants, whose flanking haplotypes were similar to those of parasites carrying other kelch13 mutations (k13-R529T and k13-I543H, encoding p.Arg529Thr and p.Ile543His substitutions, respectively), suggesting that this allele might have emerged independently multiple times in ESEA. We also found that k13-C580Y mutants in western Thailand occupied a separate branch from those in ESEA and shared core haplotypes with other WSEA mutants, suggestive of an independent mutational event, a conclusion supported by the short length of the haplotype shared by ESEA and WSEA parasites (Supplementary Fig. 7). For a number of the most common alleles (k13-C580Y, k13-I543T, k13-Y493H and k13-R539T), we found clusters containing ESEA parasites from more than one country (Cambodia, Vietnam and eastern Thailand), indicating that mutants have crossed international borders, at least within this region (Supplementary Fig. 8). However, we found no evidence that k13-C580Y mutants from ESEA might have migrated to the WSEA region or vice versa, consistent with the observation that k13-C580Y mutants are genetically more similar to samples from their own geographical region than to k13-C580Y mutants in other regions (Supplementary Fig. 9).
Discussion
This large multicenter GWAS shows that the major genomic locus controlling P. falciparum resistance to artemisinin in Southeast Asia at the present time is kelch13. We identify at least 20 distinct resistance alleles—alleles associated with artemisinin resistance—arising from multiple independent mutations in the kelch13 sequences encoding the propeller and BTB/POZ domains. Most resistance alleles appear to be localized to a relatively small geographical area, and we find that the most widespread resistance allele, k13-C580Y, has originated independently in multiple locations. We conclude that the spread of artemisinin resistance across several countries in Southeast Asia is primarily due to the proliferation of newly emerging mutations in the kelch13 sequences encoding the propeller and BTB/POZ domains.
Understanding the factors that lead to the emergence of new kelch13 mutations is therefore central to the problem of containing and controlling artemisinin resistance. Geographical location is clearly a major risk factor: kelch13 resistance alleles are well established in Cambodia, Vietnam, Thailand and Myanmar but are absent or found at much lower frequency in Laos and Bangladesh. Mutations in kelch13 affecting the propeller and BTB/POZ domains were also found to be rare in African samples. Thus, the key question is what differentiates the P. falciparum in regions in Southeast Asia from those in neighboring countries and other parts of the world. A wide range of epidemiological factors could potentially be involved, including the intensity of transmission, the vector species and antimalarial drug usage, but the fact that the problem has emerged independently in several countries with different levels of malaria intensity and different treatment policies makes it somewhat unlikely that such factors are the sole cause.
We find that mutations in kelch13 mapping to the propeller and BTB/POZ domains are mostly likely to arise on a particular genetic background that is common in parts of Southeast Asia, and we identify the strongest markers of this genetic background to be nonsynonymous variants of arps10 on chromosome 14 and fd on chromosome 13. Other background markers include nonsynonymous mutations in mdr2 on chromosome 14 and crt on chromosome 7. The association between background markers and kelch13 mutations operates at multiple levels. Local variations in the proportion of samples carrying kelch13 resistance alleles are correlated with background marker frequency. Background markers show high levels of genetic differentiation at the geographical boundary between areas of high and low resistance and are absent or at much lower frequency in other parts of the world. Different artemisinin-resistant founder populations with independent kelch13 driver mutations tend to share the same background alleles. Background markers are strongly associated with slow parasite clearance rates after correcting for population structure using a linear mixed-model GWAS analysis, and these associations are greatly attenuated when kelch13 resistance alleles are collectively treated as a covariate. Thus, the background markers can be regarded as markers of the risk that an artemisinin-sensitive parasite in Southeast Asia will acquire a kelch13 mutation that makes it artemisinin resistant.
There are several ways in which genetic background could influence risk of the emergence of a new resistance-conferring mutation. The polypeptide sequences of kelch propeller domains are highly conserved across Plasmodium species, raising the possibility that kelch13 mutations carry a biological fitness cost, as is the case for many drug resistance mutations. Genetic background might reduce the fitness cost through compensatory mutations elsewhere in the genome. Alternatively, these background mutations might boost the selective advantage of kelch13 mutations by enhancing their phenotypic effects on artemisinin resistance. It is certainly plausible that different components of the background have different roles: the observation that mutations in the close neighborhood of arps10 emerged alongside kelch13 mutations during the in vitro development of artemisinin resistance8 points to a possible interaction between arps10 and kelch13, whereas we estimated a marginal contribution to PC t1/2 by the fd mutation. We also considered the hypothesis that the genetic background might have been selected for conferring resistance to an ACT partner drug, but, given that it is present across a region where at least three different partner drugs (piperaquine, mefloquine and amodiaquine) have been used, this seems unlikely. A further possibility is that the genetic background is simply a marker for a particular evolutionary niche in which the biological fitness costs and benefits are altered from the norm, for example, where most parasites already have high levels of multidrug resistance. This raises the questions of why the background markers have grown to high frequency in Southeast Asia and what their functional role is. It is clear that they emerged considerably before kelch13 mutations and that they have at best a small effect on artemisinin resistance.
Two prominent background markers affect the apicoplast proteins encoded in the nuclear genome, apicoplast ribosomal protein S10 and ferredoxin. The apicoplast is a relict plastid with a range of metabolic functions, and the apicoplast ribosomal protein complex is the target of clindamycin and tetracycline, which have both been used as anti-malarial drugs18. Ferredoxin is a key component of the apicoplast electron transport chain19 and might therefore affect the parasite's ability to withstand the oxidant stress created by artemisinin. The ferredoxin pathway has been implicated in the mode of action of primaquine20, and halofantrine resistance has been associated with a SNP in the fd locus, PF3D7_1318300 (ref. 21). Two other background markers affect transporter genes localized in the digestive vacuole of the parasite, the multidrug resistance transporter 2 and chloroquine resistance transporter22,23. The role of mdr2 in antimalarial drug resistance remains unclear, but it has been implicated in resistance to antifolates24,25 and tolerance to heavy metals26. In crt, there are two closely linked background markers, both nonsynonymous variants affecting the same part of the membrane-spanning structure, some distance from the p.Lys76Thr variant that is the major determinant of chloroquine resistance27.
In the past 60 years, the lower Mekong region has been the epicenter of drug resistance. Repeatedly, resistance has emerged here before spreading to the rest of the world, successively undermining the effectiveness of chloroquine, sulfadoxine-pyrimethamine and mefloquine28. The vast majority of parasites in this region are multidrug resistant, possessing both crt mutations encoding p.Lys76Thr and dhfr mutations encoding p.Ser108Asn, the critical determinants of chloroquine and pyrimethamine resistance, respectively. Several factors have been proposed to explain such a propensity for drug resistance, such as low transmission rates promoting high rates of parasite inbreeding16, a checkered history of public health interventions29 and the widespread availability of poor-quality antimalarials30. It has also been hypothesized that P. falciparum in Southeast Asia may have a genetic predisposition to develop resistance-causing mutations31; thus far, this hypothesis has been backed by inference from experimental systems32,33. Here we describe the first clear epidemiological evidence, to our knowledge, for such an effect. Scientifically, these data provide a foundation for investigating the complex multistage processes by which antimalarial drug resistance evolves in natural parasite populations. Practically, these findings raise concern that, unless efforts to eliminate malaria from the greater Mekong subregion are rapidly and successfully implemented, further mutations may lead to high-level resistance both to artemisinin and to ACT partner drugs. Although artemisinin resistance appears to have made little progress in other parts of the world, the situation might change as the phenotype continues to evolve, and understanding the multiple genetic factors involved in this process may provide vital clues about how to prevent its spread.
URLs
MalariaGEN Plasmodium falciparum Community Project, http://www.malariagen.net/projects/parasite/pf; European Nucleotide Archive (ENA), http://www.ebi.ac.uk/ena/; P. falciparum 3D7 reference sequence V3, ftp://ftp.sanger.ac.uk/pub/pathogens/Plasmodium/falciparum/3D7/3D7.latest_version/version3/; Parasite Clearance Estimator, Worldwide Antimalarial Resistance Network (WWARN), https://www.wwarn.org/toolkit/data-management/parasite-clearance-estimator; R language, http://www.r-project.org/; R language ape package, http://ape-package.ird.fr/; R language stats package, http://stat.ethz.ch/R-manual/R-patched/library/stats/html/00Index.html; ADMIXTURE program, http://www.genetics.ucla.edu/software/admixture/; PLINK toolset, http://pngu.mgh.harvard.edu/∼purcell/plink/.
Online Methods
Ethics statement
All samples in this study were derived from blood samples obtained from patients with P. falciparum malaria, collected with informed consent from the patient or a parent or guardian. At each location, sample collection was approved by the appropriate local ethics committee: Ethical Committee, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam; Ethics Committee for Biomedical Research of the Ministry of Health, Institute of Malariology-Parasitology-Entomology, Ho Chi Minh City, Vietnam; National Ethics Committee for Health Research, Ministry of Health, Phnom Penh, Cambodia; Ethics Committee, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; Tak Province Community Ethics Advisory Board (T-CAB), Tak, Thailand; Government of the Republic of the Union of Myanmar, Ministry of Health, Department of Medical Research (lower Myanmar); National Ethics Committee for Health Research, Ministry of Health, Lao Peoples' Democratic Republic; National Research Ethics Committee, Bangladesh Medical Research Council; Comité d'Ethique, Ecole de Santé Publique, Université de Kinshasa, Ministère de l'Enseignement Superieur, Universitaire et Recherche Scientifique, Democratic Republic of the Congo; Ethical Review Committee, University of Ilorin Teaching Hospital, Ilorin, Nigeria; Navrongo Health Research Centre Institutional Review Board, Navrongo, Ghana; Institutional Review Board, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA; Ethics Review Committee, World Health Organization, Geneva, Switzerland; and Oxford Tropical Research Ethics Committee (OxTREC), Oxford, UK.
Phenotype estimation
Full details of clinical studies that contributed pheno-types for the GWAS analysis, including treatment regimens and clearance rate estimation, can be obtained from the trial registrations at ClinicalTrials.gov: NCT01350856 for the TRAC study and NCT00341003 and NCT01240603 for the US National Institutes of Health study. Clinical study reports also detail in depth the methods used in these studies6,9.
During treatment, parasite densities were estimated by counting parasitized erythrocytes in blood smears from peripheral blood samples taken at 0, 4, 6, 8 and 12 h after patient admission and then every 6 h until two consecutive counts were negative. PCt1/2 estimates were computed from these parasite counts, by fitting a statistical model35 using the Parasite Clearance Estimator developed by WWARN.
Sample preparation, sequencing and genotyping
DNA was extracted directly from blood samples taken from patients at admission, after leukocyte depletion to minimize contamination from human DNA. Leukocyte depletion was achieved by CF11 filtration for most samples10 or alternatively by Lymphoprep density gradient centrifugation (Axis-Shield) followed by Plasmodipur filtration (Euro-Diagnostica)36 or by Plasmodipur filtration alone. Genomic DNA was extracted using the QIAamp DNA Blood Midi or Maxi kit (Qiagen), and the quantities of human and Plasmodium DNA were determined by fluorescence analysis using a Qubit instrument (Invitrogen) and multispecies quantitative PCR(qPCR) using the Roche LightCycler 480 II system, as described previously11. Samples with >50 ng of DNA and <80% human DNA contamination were selected for sequencing on the Illumina HiSeq platform following the manufacturer's standard protocols37. Paired-end sequencing reads of 200–300bp in length were obtained, generating approximately 1Gb of read data per sample.
Polymorphism discovery, quality control and sample genotyping followed a process described elsewhere11. Short sequence reads from 3,281 P. falciparum samples included in the MalariaGEN Plasmodium falciparum Community Project were aligned against the P. falciparum 3D7 reference sequence V3 using the bwa program38 as previously described11, to identify an initial global set of 3,373,632 potential SNPs. This list was then used to guide stringent realignment using the SNP-o-matic algorithm39, to reduce misalignment errors. Stringent alignments were then examined by a series of quality filters, with the aim of removing alignment artifacts and their sources. In particular, the following were removed: (i) noncoding SNPs; (ii) SNPs where polymorphisms had extremely low support (<10 reads in 1 sample); (iii) SNPs with more than 2 alleles, with the exception of loci known to be important for drug resistance, which were manually verified to not have artifacts; (iv) SNPs where coverage across samples was lower than the 25th percentile or higher than the 95th percentile of coverage in coding SNPs (these thresholds were determined from an analysis of artifact incidence); (v) SNPs located in regions of relatively low uniqueness11; (vi) SNPs where heterozygosity levels were found to be inconsistent with the heterozygosity distribution at the SNP's allele frequency; and (vii) SNPs where the genotype could not be established in at least 70% of samples. These analyses produced a final list of 681,587 high-quality SNPs in the 14 chromosomes of the nuclear genome, whose genotypes were used for analysis in this study.
All samples were genotyped at each high-quality SNP by a single allele, on the basis of the number of reads observed for the two alleles at that position in the sample. At positions with fewer than five reads, the genotype was set to undetermined (no call was made). At all other positions, the sample was determined to be heterozygous if both alleles were each observed in more than two reads; otherwise, the sample was called as homozygous for the allele observed in the majority of reads. For the purposes of estimating allele frequencies and genetic distances, a within-sample allele frequency (fw) was also assigned to each valid call. For heterozygous calls, fw was estimated as the ratio of the non-reference read count to the reference read count; homozygous calls were assigned fw = 0 when called with the reference allele and fw = 1 when called with the non-reference allele.
For specific analyses that required no genotype missingness in our data set, we produced a set of genotypes where missing calls (with coverage <5 reads) were assigned a genotype by simple imputation. First, we considered missing calls where the two flanking positions (on each side) had valid genotypes, imputing with the allele that most frequently appeared at the same position between the same flanking alleles in the full sample set. Finally, remaining samples with missing genotypes were assigned with the most common allele at that position in their population.
Genotype-phenotype association
Genotype-phenotype association analysis (GWAS) and correction for population structure was performed using a linear mixed model, implemented in FaST-LMM v2.06. We tested 17,395 SNPs with MAF > 0.01 where genotype was encoded as the number of non-reference alleles (0 or 1). At each SNP, heterozygous calls were excluded from the test, to minimize the confounding effect of mixed infections. PCt1/2, estimated as described above, was used as the continuous dependent variable. A relationship matrix was calculated using a subset of 11,785 unlinked SNPs with MAF > 0.01, extracted using PLINK v1.07 (options: –indep-pairwise 100 10 0.3 –maf 0.01). In estimating the relationship matrix, we found that the exclusion of proximal SNPs (either within 10 kb or 100 kb of the tested variant) had no significant effect on the results (data not shown). Given the number of independent SNPs used, we applied Bonferroni correction to define for all GWAS analyses a significance threshold of P ≤ 1 × 10−7. In addition, we defined a ‘suggestive’ threshold at P ≤ 1 × 10−5 to help define high-ranking loci.
In a later analysis aimed at disentangling the residual effect of high-ranking loci after discounting the effect of kelch13 mutations, we performed a conditional analysis, including the kelch13 allele genotype as a fixed effect, as implemented in FaST-LMM.
Population genetics analysis
For a given population P, we estimated the non-reference allele frequency (NRAF) at a given SNP as the mean of the within-sample allele frequency (fw) for all samples in P that had a valid genotype at that SNP. The MAF was computed as min(NRAF, (1 – NRAF)).
As input to population structure analyses, we computed an N × N pairwise distance matrix, where N was the number of samples. Each cell of the matrix contained an estimate of the genetic distance between the relevant pair of samples, obtained by summing the pairwise distance at each SNP, estimated from within-sample allele frequency (fw). When comparing a pair of samples sA and sB at a single SNP i where a genotype could be called in each sample, with within-sample allele frequencies fA and fB, respectively, the distance dAB was estimated as dAB = fA(1 − fB) + fB(1 − fA). The genome-wide distance DAB between the two samples was then calculated as:
where nAB is the number of SNPs where both samples could be genotyped, wi is a linkage disequilibrium (LD) weighting factor and α is a scaling constant, equal to 70% of the number of coding positions in the genome (included because our genotyping covers approximately 70% of the coding genome). The exact value of α did not influence the analyses conducted in this study. The LD weighting factor, which corrects for the cumulative contribution of physically linked polymorphisms, was computed at each SNP i with MAF ≥ 0.1 in our sample set by considering a window of m SNPs (j = 0, 1, …, m) centered at i. For each value of j, we computed the squared correlation coefficient between SNPs i and j. Ignoring positions j where , the weighting factor wi was computed by:
Principal-coordinate analysis (PCoA) of pairwise distance matrices was performed using the classical multidimensional scaling (MDS) method40. PCoA is a computationally efficient variant of principal-component analysis (PCA) in which a pairwise distance matrix is used as input, rather than a table of genotypes. For each PCoA of a subset of N samples, we used an N × N pairwise distance matrix. The matrix was supplied as input to the MDS algorithm, using the cmdscale function in the R language stats package. The same pairwise distance matrix was also used to produce a neighbor-joining tree41 using the nj implementation in the R ape package.
To estimate the FST value between two populations at a given SNP, we used FST = 1 − (π̂s / π̂t), where π̂s is the expected average probability that two samples chosen at random from the same population carry a different allele at the SNP and π̂t is the expected average probability that two samples chosen at random from the joint population carry different alleles. Estimates for FST were obtained by using the NRAF values for the two populations (p1 and p2) to compute:
and
Heteroallelic association analysis
We considered only samples from the two core populations (namely, KH-C and VN-C) and the seven artemisinin-resistant founder populations (WKH-F01, WKH-F02, WKH-F03, WKH-F04, NKH-F02, VN-F01 and VN-F04). Each founder population was compared to the respective core population (KH-C for WKH-F01, WKH-F02, WKH-F03, WKH-F04 and NKH-F02; VN-C for VN-F01 and VN-F04) to calculate the pairwise FST of all SNPs. Only SNPs with FST ≥ 0.3 in at least one comparison were then considered (8,699 of 681,546). For each gene i containing at least one SNP meeting the above criterion (n = 3,482), we calculated for each pair-wise comparison j (1 ≤ j ≤ 7) a score Sij equal to the maximum FST across all qualifying SNPs. The score Si for the gene was then calculated as the arithmetic mean of Sij across the seven comparisons.
kelch13 allele genotyping
In analyses that required samples to be assigned a kelch13 genotype, this was derived from the read counts at nonsynonymous SNPs in kelch13, using a procedure aimed at minimizing missing calls. At each position, the sample was assigned the reference allele if supported by ≥1 read for the reference allele and the alternative allele if supported by ≥2 reads for the alternative allele (≤3 reads where coverage exceeded 50 reads). Positions with no assigned allele were classified as missing, and those with both alleles were defined as heterozygous. Samples with ≥ 1 missing position were labeled with a missing genotype; samples with ≥ 1 heterozygous position were labeled as heterozygous; samples in which a single position carried exclusively the alternative allele were classified as single mutant and labeled with the mutation name; and the remainder of the samples were labeled as wildtype. No multiple mutants were identified in this data set. After the initial assessment of mutation phenotype (Fig. 2), we repeated genotyping using the same procedure but only considering nonsynonymous SNPs in kelch13 encoding the resistance domains (the BTB/POZ and propeller domains); samples carrying mutations only mapping outside these domains were labeled as wild type.
Identification of populations and classification of samples
To identify core and founder populations, we used the following multistep method, aimed at a conservative classification of samples, applied separately to different geographical regions (Supplementary Figs. 10–13 and Supplementary Note). To minimize ascertainment biases, we only used samples that were includedin the SNP discovery phase. We applied the ADMIXTURE V1.23 program to estimate ancestry proportions for the selected samples, applying a model-based approach42 that used majority-allele, imputed genotypes as input. All SNPs with extremely low MAF (MAF ≤ 0.01) were discarded owing to their low informative value in the inference process. Because the ADMIXTURE model requires low LD between SNPs, we also excluded SNPs belonging to highly linked pairs, selected by the PLINK toolset by scanning each chromosome in turn with a sliding window of 100 SNPs in size and removing any SNP with a correlation coefficient of ≥ 0.02 with any other SNP within the window.
For each run of ADMIXTURE, a hypothetical number K of ancestral populations was chosen, and each sample was assigned a fraction for each ancestral population. We ran the algorithm for multiple values of K ≥ 2. To avoid fitting to local minima, for each K we ran the algorithm 50 times with different random seeds and assessed the distributions of cross-validation errors and loglikelihood for all runs. For our data sets, the cross-validation error distributions presented large plateaus where solutions showed only marginal improvement as K increased (Supplementary Fig. 14), accompanied by an increase in variance, making it problematic to choose an optimum value of K. Therefore, we followed a conservative iterative process for robustly assigning samples to populations. First, we used a published method for identifying a value of K that showed the uppermost level of structure43. Starting with this value, we gradually increased K to capture structure at a finer resolution. For each K, we chose the solution with the lowest cross-validation error. On the basis of the proportions estimated in this solution, we assigned each sample to one of K groups corresponding to the putative ancestral populations. A sample was assigned to a group if the proportion estimated for the corresponding ancestor was >0.5 and at least four times higher than the second highest proportion. Samples not meeting these criteria were assigned to an ‘unclassified’ group. We then used group labels to identify clusters of samples that consistently grouped together at different values of K. One classification mismatch at most (where the group assignmentwas different for one value of K) was allowed for cluster assignment. The value of K was incremented until newly identified clusters were deemed to be too small (n < 5) or unstable (where the cluster separated as an independent group at one value of K and then merged with a different group at a higher value of K). Samples assigned to the unclassified group for more than a single value of K were not assigned to clusters.
Core populations were identified by genetic similarity to populations previously found to be sensitive and representative of wild-type genotypes16. Putative founder clusters were characterized by a pairwise count of highly differentiated SNPs (FST ≥ 0.5) with respect to a reference core population, expected to be much higher in a founder population than in a core population. We also performed PCoA to visualize the clustering of the putative founders and their separation along at least one component from the core populations and from each other. Populations with insufficient support for founder effects or with low numbers (n < 5) were discarded (samples were assigned to the unclassified group).
Demography of kelch13 mutations
We based our analysis on the length of the longest haplotypes shared by a pair of samples. In both strand directions, starting from the mutation of interest, we found the closest positions where the two sequences differed (breakpoints). The distance, in base pairs, between two breakpoints was the LCHL for the pair. To account for heteroallelism, we collapsed all kelch13 mutations into a single one and used position 580 as the notional locus of the mutation of interest. To minimize the influence of possible artifacts, we defined a breakpoint only if the mismatching allele had a frequency of >5% among samples carrying the same kelch13 mutation or among samples wildtype for kelch13. Variants arising from the same recent evolutionary event will be embedded within identical haplotypes, whereas mutations originating separately will share shorter haplotypes; LCHL is also expected to decrease with age, owing to the effects of recombination. Hence, clustering by LCHL is expected to group together samples with common recent demographic history. Accordingly, we constructed a pairwise matrix of the inverse of LCHL, from which a tree was constructed using a standard hierarchical clustering method (hclust) implemented in the R stats package using the Ward's minimum variance criterion.
Supplementary Material
Acknowledgments
We thank the following colleagues for their efforts in support of this work: P. Vauterin, G. Band and Q.S. Le; J. Anderson, D. Dek, S. Duong, R. Gwadz, S. Mao, V. Ou, B. Sam, C. Sopha, V. Try and T. Wellems; the personnel at Phuoc Long Hospital, Bu Gia Map Health Station, Malaria Control Center of Binh Phuoc Province, Vietnam; M. Phommasansack, B. Phimphalat and C. Vilayhong; and A.K. Tshefu. Special thanks are given to V. Cornelius and K. Johnson for their continual support of the analysis group. The sequencing for this study was funded by the Wellcome Trust through core funding of the Wellcome Trust Sanger Institute (098051). The Wellcome Trust also supports the Wellcome Trust Centre for Human Genetics (090532/Z/09/Z), the Resource Centre for Genomic Epidemiology of Malaria (090770/Z/09/Z) and the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme. The Centre for Genomics and Global Health is supported by the UK Medical Research Council (G0600718). This work was funded in part by the Bill and Melinda Gates Foundation (OPP1040463), the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health and the Department for International Development (PO5408). P.R. is a staff member of the World Health Organization. The views expressed in this publication are those of the authors and do not necessarily reflect the positions, decisions, policies or views of their employers or of the funding organizations.
Footnotes
Accession codes. A document containing lists of ENA accession codes for all samples used in the present study is available from http://www.malariagen.net/data/pf-sample-info.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
Author Contributions: C.A., P.L., M.D., M.I., L.A.-E., T.-N.N.T., H.T.T., D.B., F.N., A.P.P., S.P., K.C., C.M.C., C.N., S. Suon, S. Sreng, P.N.N., M. Mayxay, M.K., B.H., Y.H., K.T.H., M.P.K., M.A.F., C.I.F., M.O. and O.A.M. carried out field and laboratory work to obtain P. falciparum samples for sequencing. E.A.A., C.A., P.L., T.-N.N.T., H.T.T., D.B., F.N., A.P.P., S.P., K.C., C.M.C., C.N., P.N.N., M. Mayxay, M.K., B.H., Y.H., K.T.H., M.P.K., M.A.F., C.I.F., M.O. and O.A.M. carried out clinical studies to obtain parasite clearance data. D.M., S.O.O., E.D., S.C. and B.M. developed and implemented methods for sample processing and sequencing library preparation. J.S. and M. Manske managed data production pipelines. E.A.A., C.W., P.R., C.G.J., S.T.-H., C.V.P., N.P.D., A.M.D., R.M.F., N.J.W., O.M., B.M. and D.P.K. contributed to study design and management. O.M., R.A., J.A.-G., C.C.A.S., G.M. and D.P.K. performed data analyses. O.M., R.A., J.A.-G., C.C.A.S., G.M. and D.P.K. performed data analyses. O.M., R.A. and D.P.K. drafted the manuscript, which was reviewed by all authors.
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Dondorp AM, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011;365:1073–1075. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phyo AP, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hien TT, et al. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J. 2012;11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyaw MP, et al. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS ONE. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amaratunga C, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mita T, Tanabe K, Kita K. Spread and evolution of Plasmodium falciparum drug resistance. Parasitol Int. 2009;58:201–209. doi: 10.1016/j.parint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley EA, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesan M, et al. Using CF11 cellulose columns to inexpensively and effectively remove human DNA from Plasmodium falciparum–infected whole blood samples. Malar J. 2012;11:41. doi: 10.1186/1475-2875-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manske M, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Listgarten J, et al. Improved linear mixed models for genome-wide association studies. Nat Methods. 2012;9:525–526. doi: 10.1038/nmeth.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takala-Harrison S, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci USA. 2013;110:240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheeseman IH, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley EA, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miotto O, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takala-Harrison S, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2014 Sep 1; doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl EL, Rosenthal PJ. Apicoplast translation, transcription and genome replication: targets for antimalarial antibiotics. Trends Parasitol. 2008;24:279–284. doi: 10.1016/j.pt.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Kimata-Ariga Y, Saitoh T, Ikegami T, Horii T, Hase T. Molecular interaction of ferredoxin and ferredoxin–NADP+ reductase from human malaria parasite. J Biochem. 2007;142:715–720. doi: 10.1093/jb/mvm184. [DOI] [PubMed] [Google Scholar]
- 20.Vásquez-Vivar J, Augusto O. Hydroxylated metabolites of the antimalarial drug primaquine. Oxidation and redox cycling. J Biol Chem. 1992;267:6848–6854. [PubMed] [Google Scholar]
- 21.Van Tyne D, et al. Identifcation and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genet. 2011;7:e1001383. doi: 10.1371/journal.pgen.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidock DA, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalis MG, Wilson CM, Zhang Y, Wirth DF. Characterization of the pfmdr2 gene for Plasmodium falciparum. Mol Biochem Parasitol. 1993;62:83–92. doi: 10.1016/0166-6851(93)90180-6. [DOI] [PubMed] [Google Scholar]
- 24.Martinelli A, Henriques G, Cravo P, Hunt P. Whole genome re-sequencing identifies a mutation in an ABC transporter (mdr2) in a Plasmodium chabaudi clone with altered susceptibility to antifolate drugs. Int J Parasitol. 2011;41:165–171. doi: 10.1016/j.ijpara.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briolant S, et al. The F423Y mutation in the pfmdr2 gene and mutations N51I, C59R, and S108N in the pfdhfr gene are independently associated with pyrimethamine resistance in Plasmodium falciparum isolates. Antimicrob Agents Chemother. 2012;56:2750–2752. doi: 10.1128/AAC.05618-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg E, et al. pfmdr2 confers heavy metal resistance to Plasmodium falciparum. J Biol Chem. 2006;281:27039–27045. doi: 10.1074/jbc.M601686200. [DOI] [PubMed] [Google Scholar]
- 27.Millet J, et al. Polymorphism in Plasmodium falciparum drug transporter proteins and reversal of in vitro chloroquine resistance by a 9,10-dihydroethanoanthracene derivative. Antimicrob Agents Chemother. 2004;48:4869–4872. doi: 10.1128/AAC.48.12.4869-4872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 29.Payne D. Did medicated salt hasten the spread of chloroquine resistance in Plasmodium falciparum? Parasitol Today. 1988;4:112–115. doi: 10.1016/0169-4758(88)90042-7. [DOI] [PubMed] [Google Scholar]
- 30.Tabernero P, Fernandez FM, Green M, Guerin PJ, Newton PN. Mind the gaps—the epidemiology of poor-quality anti-malarials in the malarious world— analysis of the WorldWide Antimalarial Resistance Network database. Malar J. 2014;13:139. doi: 10.1186/1475-2875-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beez D, Sanchez CP, Stein WD, Lanzer M. Genetic predisposition favors the acquisition of stable artemisinin resistance in malaria parasites. Antimicrob Agents Chemother. 2011;55:50–55. doi: 10.1128/AAC.00916-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen S, Ferdig M. QTL analysis for discovery of genes involved in drug responses. Curr Drug Targets Infect Disord. 2004;4:53–63. doi: 10.2174/1568005043480916. [DOI] [PubMed] [Google Scholar]
- 34.Gething PW, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auburn S, et al. An effective method to purify Plasmodium falciparum DNA directly from clinical blood samples for whole genome high-throughput sequencing. PLoS ONE. 2011;6:e22213. doi: 10.1371/journal.pone.0022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentley DR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manske HM, Kwiatkowski DP. SNP-o-matic. Bioinformatics. 2009;25:2434–2435. doi: 10.1093/bioinformatics/btp403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gower JC. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika. 1966;53:325–328. [Google Scholar]
- 41.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 42.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.