Abstract
Plant and fungal lipoxygenases catalyze the oxidation of polyunsaturated fatty acids, creating fatty acid hydroperoxides (oxylipins). Fungal oxylipins are required for normal fungal development and secondary metabolism, and plant host-derived oxylipins interfere with these processes in fungi, presumably by signal mimicry. The maize lipoxygenase gene ZmLOX3 has been implicated previously in seed-Aspergillus interactions, so we tested the interactions of a mutant maize line (lox3–4, in which ZmLOX3 is disrupted) with the mycotoxigenic seed-infecting fungi Aspergillus flavus and Aspergillus nidulans. The lox3–4 mutant was more susceptible than wild type maize to both Aspergillus species. All strains of A. flavus and A. nidulans produced more conidia and aflatoxin (or the precursor sterigmatocystin) on lox3–4 kernels than on wild type kernels, in vitro and under field conditions. Although oxylipins did not differ detectably between A. flavus-infected kernels of the lox3–4 and WT maize, oxylipin precursors (free fatty acids) and a downstream metabolite (jasmonic acid) accumulated to greater levels in lox3–4 than in WT kernels. The increased resistance of the lox3–4 mutant to other fungal pathogens (Fusarium, Colletotrichum, Cochliobolus, and Exserohilum) is in sharp contrast with results described herein for Aspergillus, suggesting that outcomes of lipoxygenase-governed host-pathogen interactions are pathogen-specific.
Additional keywords: 9S-HPODE, oxylipin, aflatoxin, sterigmatocystin, lipoxygenase, lipid signal, plant-microbe interaction
INTRODUCTION
One of the most economically important problems of cereal production worldwide is the contamination of seed by mycotoxins produced by seed infecting fungi. These include the genera Aspergillus and Fusarium, which are serious pathogens of developing seeds as well as stored grain. These fungi not only reduce grain integrity, but they also contaminate seed with mycotoxins, such as aflatoxin (AF) produced by Aspergillus flavus and A. parasiticusx, and fumonisin by Fusarium verticillioides. Arguably, the crop most seriously afflicted by mycotoxigenic fungi is maize, which is frequently contaminated with both of these toxins.
Aspergilli primarily colonize and contaminate the lipid rich tissues of the host seed, such as the embryo and aleurone (Brown et al. 1993; Keller et al. 1994). At least two mechanisms may contribute to the lipid/aflatoxin connection. First, some plant fatty acids may be metabolized through mitochondrial and peroxisomal β-oxidation in the fungus, providing acyl-CoA precursors necessary for AF production (Maggio-Hall et al. 2005). In particular, oleic acid (a C18 monounsaturated fatty acid) induces proliferation of peroxisomes, thus increasing β-oxidation and subsequent AF production. Another hypothesis for the increased AF contamination of these seed tissues is that host derived, oxidized lipids function as signal molecules that stimulate AF production in the fungus (Brodhagen and Keller 2006). C18- and C16- polyunsaturated fatty acids (PUFAs), particularly linolenic (C18:3) and linoleic (C18:2) fatty acids, can be oxidized by plant lipoxygenases (LOXs) either at position 9 or 13 of their carbon chains (Feussner and Wasternack 2002). Because most distinct LOX isoforms preferentially catalyze either one or the other reaction, they are referred to as 9-LOXs or 13-LOXs. LOX primary products (fatty acid hydroperoxides) are rapidly converted into a large class of oxygenated polyenoic fatty acids collectively called oxylipins, via at least six separate biosynthetic enzymatic pathways (Feussner and Wasternack 2002; Porta and Rocha-Sosa 2002). Accumulating evidence suggests diverse physiological functions of oxylipins in plants, such as growth and development, resistance to insects and pathogens, and tolerance to salt, drought, and cold (Howe and Schilmiller 2002). For example, jasmonic acid (JA) and other 13-LOX-derived hydroperoxide products function as signals that regulate expression of defense-related and developmental genes (Turner et al. 2002).
Besides their role as endogenous signals in plant defense and development, host oxylipins have recently emerged as important signals in cross-kingdom communication between plants and pathogenic fungi (Brodhagen et al. 2008; Gao and Kolomiets 2008; Tsitsigiannis and Keller 2007). The original evidence for this idea was obtained from bioassays showing that the primary products of plant 13- and 9-LOXs (13S-hydroperoxyoctadecadienoic acid and 9S-hydroperoxyoctadecadienoic acid, or 13S-HPODE and 9S-HPODE, respectively) modulate fungal developmental processes including vegetative growth and sporulation. Moreover, 13S-HPODE and 9S-HPODE altered the production of AF and other secondary metabolites by Aspergillus spp. (Burow et al. 1997; Calvo et al. 1999).
The physiological effects of plant-derived oxylipins on Aspergillus spp. are often attributed to mimicry by 13S-HPODE and 9S-HPODE of structurally similar fungal oxylipins (Tsitsigiannis and Keller 2006). A. nidulans produces a group of oxylipins collectively named psi factor (for precocious sexual inducer), via the enzymatic action of the dioxygenases PpoA, PpoB and PpoC (Champe et al. 1987; Champe and El-Zayat, 1989; Mazur et al. 1990; Mazur et al. 1991; Tsitsigiannis et al., 2004a and 2004b). Specific oxylipins that comprise psi factor have differing (and sometimes synergistic) effects on the ratio of sexual to asexual spore production (Champe et al., 1987; Champe and El-Zayat, 1989; Tsitsigiannis et al., 2004a; 2004b; Tsitsigiannis et al., 2005), the ability of the fungus to colonize host seed and the production of secondary metabolites (Tsitsigiannis and Keller 2006). Among the secondary metabolites affected is sterigmatocystin (ST), the penultimate precursor to AF in A. flavus and A. parasiticus, but the final product of the biosynthetic pathway in A. nidulans. Treatment of Aspergillus cultures with the pure plant oxylipin 9S-HPODE resulted in increased conidiation (Calvo et al. 1999) and AF production as well as accumulation of a ST biosynthetic gene transcript (Burrow et al. 1997). We therefore hypothesized that 9S-HPODE, produced by 9-LOX enzymes, might be a susceptibility factor in maize for AF contamination.
9S-HPODE may potentially be produced in maize tissues by six different putative maize 9-LOXs (M. Kolomiets, unpublished results). Expression of the gene for one of these, ZmLOX3, occurs in the developing embryos (Gao et al. 2007), and is further upregulated during kernel infection by both A. flavus and F. verticillioides (Wilson et al., 2001). Therefore, we predicted that disruption of ZmLOX3 might decrease sporulation and AF production. We previously generated a mutated ZmLOX3 allele, called lox3–4, by insertion of the transposable element Mutator into the ZmLOX3 coding sequence. The lox3–4 line was more resistant than the WT to F. verticillioides: infected lox3–4 kernels yielded 2-fold fewer conidia than infected near-isogenic WT kernels (Gao et al. 2007). Furthermore, F. verticillioides produced 100- o 200-fold less fumonisin B1 in the lox3–4 kernels than the WT kernels. In addition to F. verticillioides, lox3–4 mutant was more resistant to two foliar pathogens, Colletotrichum graminicola and Cochliobolus heterostrophus (Gao et al. 2007), as well as to the root rotting pathogen Exserohilum pedicellatum (Isakeit et al. 2007). Collectively, these data provided strong evidence that a single genetic element, the functional ZmLOX3 gene, contributes to the susceptibility of maize to diverse fungal pathogens.
The present study examined the role of ZmLOX3 in maize interactions with AF-producing A. flavus and ST-producing A. nidulans. In sharp contrast to the results obtained with the other fungal pathogens, conidiation and toxin production were increased during colonization of the lox3–4 mutant in both laboratory and field conditions. Analysis of the lipids in the infected seed suggested that changes in fatty acid levels, rather than changes in levels of oxylipins themselves, could underlie the increased growth and toxin biosynthesis of these Aspergillus species on the lox3–4 mutant.
RESULTS
Conidia and aflatoxin production by A. flavus are increased on lox3–4 mutant
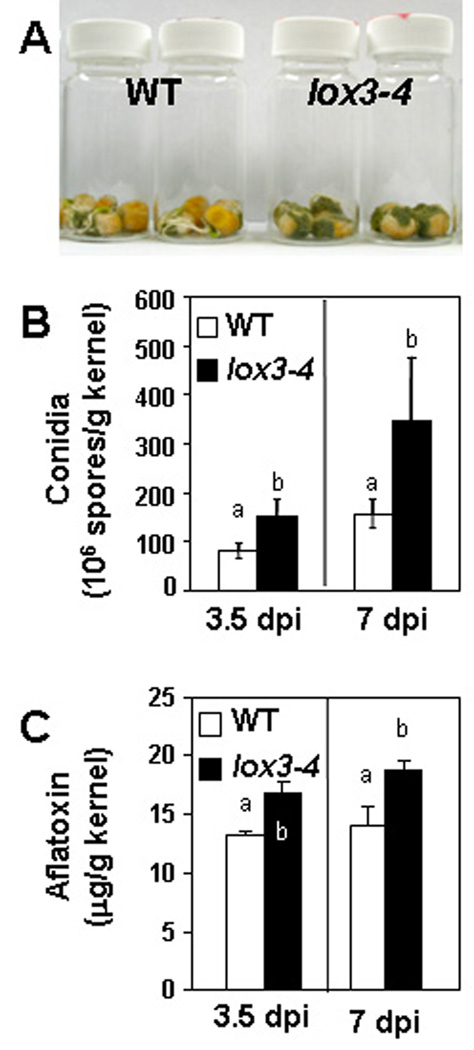
To test our hypothesis that disruption of ZmLOX3 might decrease sporulation and AF production, we performed kernel assays on the knockout lox3–4 mutant and near-isogenic WT maize. As Figure 1A illustrates, lox3–4 mutant kernels were colonized by A. flavus NRRL 3357 to visibly greater levels compared to the WT. Similar results were obtained when we infected kernels with A. flavus 12S (data not shown). Consistent with the increase in fungal growth, the lox3–4 mutant kernels supported production of significantly more conidia at 3.5 and seven days post infection (dpi). We observed similar results whether we infected with A. flavus NRRL 3357 (Figure 1B) or A. flavus 12S (data not shown). Although attempts to measure AF from A. flavus 12S-infected kernels were unsuccessful, we observed that maize lox3–4 kernels infected with A. flavus NRRL 3357 produced significantly more AF than WT kernels at both 3.5 and seven dpi (Figure 1C).
Figure 1.
The lox3–4 mutant permits greater colonization, conidiation and AF production by A. flavus than the WT in kernel assays. (A) Comparison of visible colonization by A. flavus NRRL 3357-infected kernels. WT, A. flavus-infected wild type kernels; lox3–4, A. flavus-infected lox3–4 kernels. The pictures were taken at seven days post inoculation (dpi). Results were similar for kernels infected with A. flavus 12S (data not shown). (B) Conidiation by A. flavus NRRL 3357 following infection of lox3–4 mutant and WT maize kernels. Similar results were obtained when kernels were infected with A. flavus 12S (data not shown). (C) AF production by A. flavus NRRL 3357 on lox3–4 mutant and WT maize kernels. Error bars denote one standard error. Different letters above bars denote significantly different means (P < 0.05, ANOVA) between WT and mutant within same time point. dpi, days post infection.
Aflatoxin accumulation and Aspergillus ear rot on the lox3–4 mutant in the field
Next we tested whether the lox3–4 mutant would support greater fungal colonization and AF production under field conditions as in kernel assays. In spring of 2005 and 2006, seeds from the NILs were planted in two separate Texas locations, Weslaco and College Station. In addition to any naturally existing disease pressure, the corn ears of both genotypes were inoculated ten days after mid-silking with conidia from the A. flavus NRRL 3357 strain. AF production and ear rot disease severity were examined after harvest.
AF production
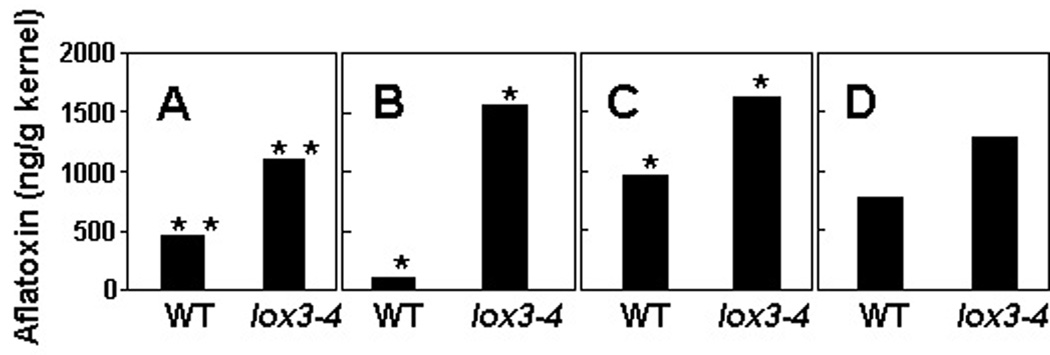
AF accumulation was greater in the lox3–4 mutant than in the WT in both years and at both locations (Figure 2). The differences were significantly greater in inoculated ears in 2005 in College Station (P < 0.05) and Weslaco (P < 0.01), and in 2006 in Weslaco (P < 0.05). In 2006 in College Station, the lox3–4 mutant accumulated about 1.5 times more AF than the WT, but the difference was not statistically significant. Even in the non-inoculated control ears (which were naturally infected by indigenous Aspergillus) the lox3–4 mutant accumulated up to ten-fold more AF than the WT NIL; at the Weslaco site, these differences were statistically significant in both 2005 and 2006 (e.g. at Weslaco in 2006, there was 460 ppb AF on lox3–4 kernels vs. 46 ppb AF on WT kernels, P < 0.05). At the College Station site, the differences in AF accumulation from incidental Aspergillus infection on uninoculated lox3–4 and WT ears were not significant (data not shown).
Figure 2.
The lox3–4 mutant accumulated significantly higher levels of AF in field. (A) Weslaco 2005; (B) College Station 2005; (C) Weslaco 2006; (D) College Station 2006. Values are the means of four replicates with 8~12 cobs each. Asterisks above the columns indicate significant differences between genotypes (* denotes P < 0.05, ** denotes P < 0.01; ANOVA). Log-transformed values were used for the analysis. Untransformed means are presented.
Ear rot incidence and disease severity
At College Station in both 2005 and 2006, AF contamination was significantly correlated with Aspergillus ear rot severity and incidence (r = 0.857, P < 0.001 and r = 0.533, P < 0.05, respectively, in 2005; and r = 0.517, P < 0.05 and r = 0.808, P < 0.001, respectively, in 2006). In 2005 at College Station, the difference in incidence of ear rot caused by A. flavus between the two genotypes (39% for the lox3–4 mutant and 24% for the WT) was not statistically significant (P > 0.05), but the severity of ear rot was significantly greater (P < 0.05) for the lox3–4 mutant (9.3%) than for the WT (1.2%). In 2006 at College Station, the incidence of ear rot was 54% for the lox3–4 mutant and 27% for the WT; however, this difference was not statistically significant (P > 0.05). In 2006, the severity of ear rot caused by A. flavus in both locations was 1% or less, and there were no evident differences between the genotypes at either location. There was no correlation of AF with ear rot in Weslaco in either year. In 2005 and 2006, the incidence of ear rot in Weslaco was less than 1% for both genotypes and there were no evident differences between the genotypes (P > 0.05).
Lipid profiling in A. flavus-and F. verticillioides-infected lox3–4 mutant and WT kernels
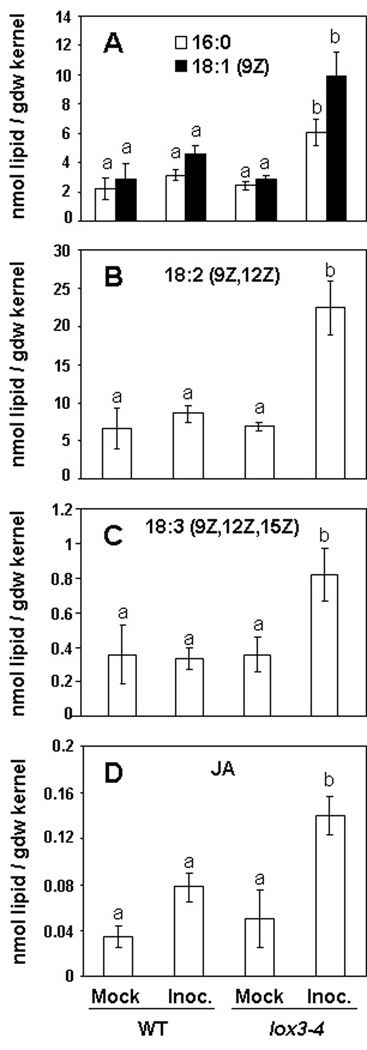
To investigate whether specific oxylipins or their fatty acid precursors were associated with the increased susceptibility of the mutant to A. flavus, we measured levels of 42 different oxylipins and free fatty acids in the lox3–4 mutant and WT kernels after infection with A. flavus 12S. Of these, four free fatty acid oxylipin precursors, palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3) were significantly increased in the lox3–4 mutant but not WT kernels infected with A. flavus at 72 hpi (P < 0.05; Figure 3A–3C). Among the 9- or 13-LOX-derived oxylipins measured in this study, a difference between concentrations in the mutant and the WT was observed only for jasmonic acid (JA), one of the biologically active products of the 13-LOX-derived octadecanoid biosynthetic pathway. As Figure 3D shows, JA concentrations increased approximately three-fold (P < 0.05) in response to A. flavus infection in the lox3–4 mutant kernels. Although WT kernels responded to the infection by increased production of JA, this increase was not statistically significant.
Figure 3.
Contents of selected free fatty acids and jasmonic acid (JA) in A. flavus 12S-infected lox3–4 and WT kernels. Differences were observed in (A) palmitic (16:0) and oleic (18:1), (B) linoleic (18:2), and (C) linolenic (18:3) acid contents, as well as in (D) JA contents. At least four replicates comprising at least four inoculated kernels each were measured 72 hours post-infection. Error bars denote one standard error. Letters above bars denote significant differences (P < 0.05, ANOVA) between treatments within each genotype.
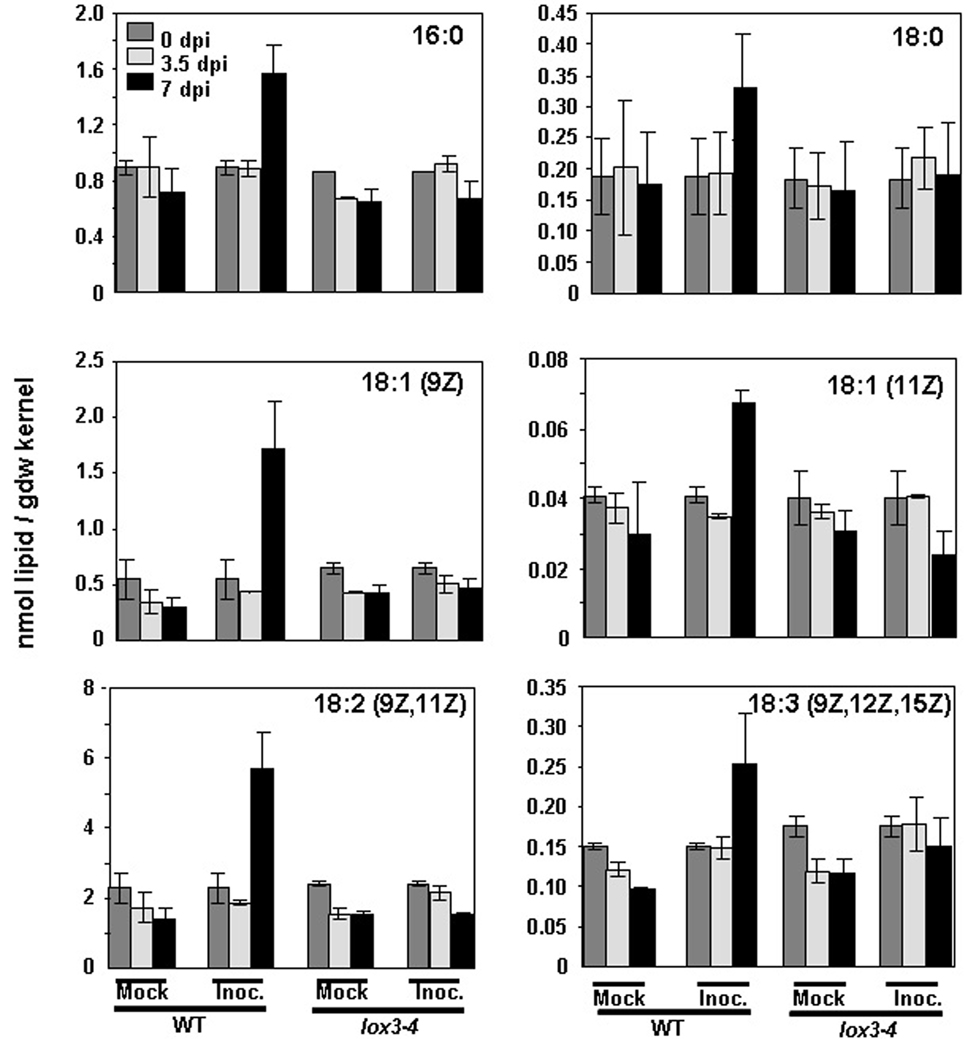
Because our previous study showed that the lox3–4 mutant was more resistant to contamination with fumonisin B1 and supported production of fewer F. verticillioides conidia (Gao et al. 2007), we tested whether the fatty acid content of F. verticillioides-infected kernels differed from that observed in A. flavus-infected kernels. In contrast to the observations described above for A. flavus-infected kernels, it was WT F. verticillioides-infected kernels, not the lox3–4 mutant kernels, that exhibited increased fatty acids. Specifically, C16:0, C18:0, C18:1 (including both 9Z and 11Z isomers), C18:2 and C18:3 fatty acids were increased following infection of WT but not lox3–4 mutant kernels with F. verticillioides. This increase in certain fatty acids could not be detected by 3.5 dpi, but was observed later, at 7 dpi (Figure 4). Compared to noninfected seed (0 dpi), the levels of induction were the strongest for three of the most abundant seed fatty acids, C16:0, C18:1 (9Z) and C18:2 (with induction levels averaging 1.8X, 3.1X and 2.5X, respectively). Importantly, no difference in the fatty acid levels was observed between the mutant and WT in the non-infected, dry seed (0 dpi), suggesting that ZmLOX3 exerts its effect on the fatty acid composition and seed-pathogen interactions only in response to infection. These results can be explained by the fact that ZmLOX3 expression is not detectable in dry, non-germinating seed but is induced highly by pathogen infection (Wilson et al. 2001). Unlike in the A. flavus-infected kernels, JA levels did not differ between the mutant and WT at any time points after F. verticillioides infection (data not shown).
Figure 4.
Contents of selected free fatty acids in Fusarium verticilliodes-infected lox3–4 and WT kernels. 16:0, palmitic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; 18:3, linolenic acid. The data are expressed as means ± SD (n=2). dpi, days post infection.
Sporogenesis and mycotoxin production by Aspergillus nidulans oxylipin mutants
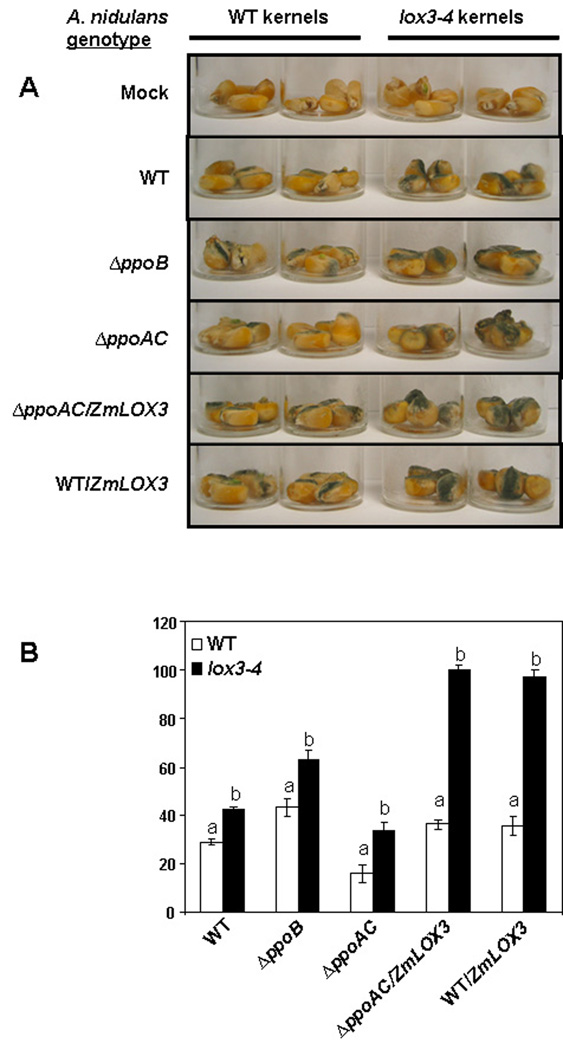
Because an earlier study showed that A. nidulans oxylipin mutants were aberrant in seed colonization of WT maize and peanut (Tsitsigiannis and Keller 2006), we next investigated whether these fungal oxylipin mutants were affected in their ability to colonize seed, or to produce spores and mycotoxin, when grown on the lox3–4 mutant. Specifically, we compared the spore and ST production on maize WT and lox3–4 kernels of five A. nidulans strains, including WT, ΔppoB, and ΔppoAC, and two strains heterologously expressing the ZmLOX3 gene (Table 1). Regardless of the specific A. nidulans oxylipin genotype, the lox3–4 kernels always supported more fungal growth than did WT kernels (Figure 5A). This was reflected in the increased number of conidia produced on the lox3–4 mutant compared to WT kernels by all fungal strains (Figure 5B).
Table 1.
A. flavus and A. nidulans strains used in this study
| Strains | Genotype | Genotype description | Source |
|---|---|---|---|
| A. flavus 12S | WT | Wild type | Calvo et al. (1999) |
| A. flavus NRRL 3357 | WT | Wild type | Wicklow et al. (1998) |
| RDIT9.32 | WT | A. nidulans veA (wild type) | Tsitsigiannis et al. (2004b) |
| RDIT59.1 | ΔppoB | A. nidulans ΔppoB∷pyroA pyroA4 veA | Tsitsigiannis et al. (2005) |
| RDIT54.7 | ΔppoAC |
A. nidulans ΔppoA∷metG ΔppoC∷trpC metG1 trpC801 veA |
Tsitsigiannis et al. (2004a) |
| RDIT106.5 | ΔppoAC+ZmLOX3 |
A. nidulans ΔppoA∷metG ΔppoC∷trpC gpdA(p)∷ZmLOX3∷pyroA veA |
Brodhagen et al. (2008) |
| RDIT98.5 | WT+ZmLOX3 | A. nidulans gpdA(p)∷ZmLOX3∷pyroA veA | Brodhagen et al. (2008) |
Figure 5.
Phenotypes of A. nidulans oxylipin mutants growing on maize WT and lox3–4 mutant kernels. Comparisons were made between WT and lox3–4 mutant kernels inoculated with various A. nidulans strains, regarding (A) visual differences in fungal growth and (B) production of asexual spores. Conidia were counted and pictures were taken at seven days post-infection. Five replicates of four kernels each were compared. Error bars represent one standard error. Letters above bars denote significant differences (P < 0.05, ANOVA) between WT and lox3–4 mutant kernels infected with the same A. nidulans strain.
As previously observed in culture and on a different line of WT maize (Table 2), the ΔppoB mutant produced more conidia, and the double ΔppoAC mutant fewer conidia, than the WT A. nidulans when grown on WT kernels. This trend was conserved on the lox3–4 mutant kernels. Addition of the ZmLOX3 gene into A. nidulans permitted still greater conidiation on lox3–4 seeds (P < 0.05). Finally, ΔppoAC mutants, which have been shown previously to produce more sexual spores than WT fungus when grown on synthetic medium (Table 2), also produced more sexual spores on the lox3–4 mutant than on WT kernels (data not shown).
Table 2.
Overview of phenotypes of A. nidulans WT and oxylipin mutants. Proportions are relative within, but not across, columns.
| Genotype | Asexual spores | Sexual spores | Sterigmatocystin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Agara | Peanutb | Maize | Agara | Peanutb | Maize | Agarc | Peanutc | Maize | |
| WT | ++ | +++ | +++ | ++ | (25)+d | ND | +++ | + | ++ |
| ΔppoA | +++ | +++++ | ND | + | (25)+ | ND | + | ++ | ND |
| ΔppoB | ++++++ | ++++++ | ++++ | + | (25)+ | ND | ++++++ | ++ | +++++ |
| ΔppoC | + | ++ | ND | +++ | (12)+ | ND | ++++ | ++ | ND |
| ΔppoAC | + | + | ++ | ++++++ | + | ND | − | − | + |
| ΔppoABC | + | + | ND | (12)+ | + | ND | − | − | − |
| WT + ZmLOX3 | +++e | ND | +++ | ++e | ND | ND | (9)+ | ND | (8)+ |
| ΔppoAC + ZmLOX3 | ++e | ND | +++ | ++e | ND | ND | ++++ | ND | (8)+ |
Except where noted, data adapted from Tsitsigiannis and associates (2005); Table 4.
Except where noted, proportions derived from Tsitsigiannis and Keller (2006); Figure 5
Except where noted, proportions estimated visually from Tsitsigiannis and Keller (2006); Figure 1 and Figure 2
Indicates number of ‘+’ symbols
Proportions derived from Brodhagen and associates (2008)
ND = not determined
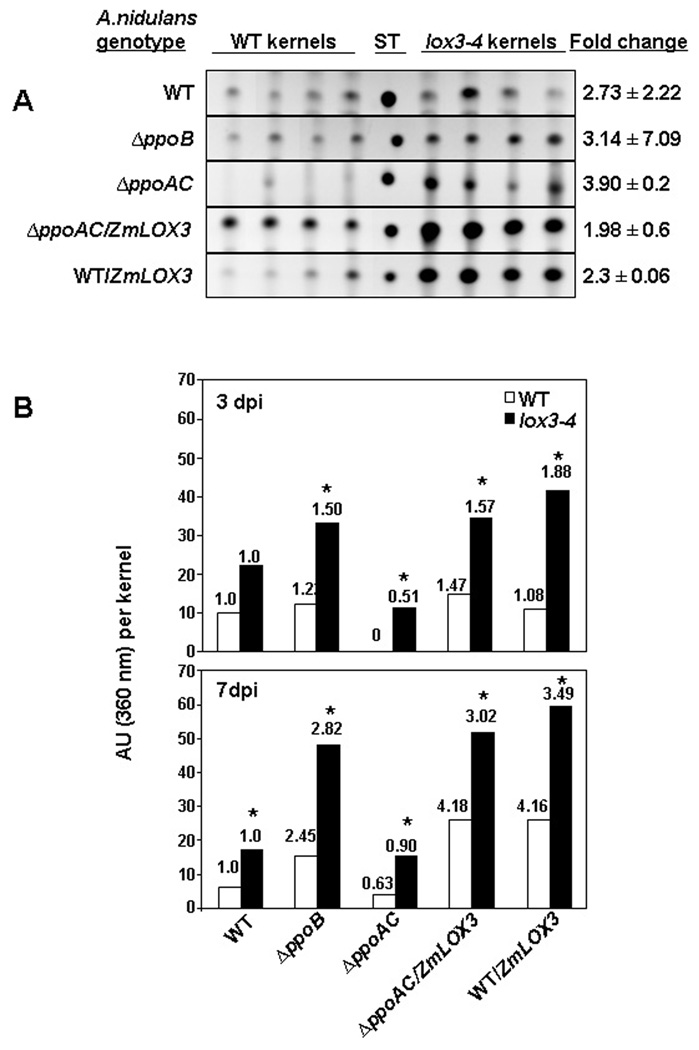
ST production was also higher on the lox3–4 kernels than on the WT, regardless of A. nidulans strain (Figure 6A). However, the ST production levels varied among fungal strains. ST production on the lox3–4 mutant kernels was greatest in both the ZmLOX3-expressing A. nidulans strains (Figure 6A and Figure 6B). This correlated with conidial production (Figure 5A). The greatest enhancement in ST production on the lox3–4 kernels was by ΔppoAC, a strain barely able to synthesize ST on WT kernels (Figure 6A and 6B; Tsitsigiannis and Keller 2006).
Figure 6.
ST production by A. nidulans WT and Δppo mutants after inoculation onto lox3–4 and WT kernels at 3.5 and seven dpi. ST was separated from crude extracts by thin layer chromatography and quantified by densitometry. (A) Effect of maize ZmLOX3 deletion: sections from thin layer chromatograms, demonstrating differences in ST production by A. nidulans strains grown on WT or lox3–4 mutant kernels at seven dpi. The numbers on the right denote ratios (lox3–4:WT) of ST production in infected kernels as measured by densitometry. Values shown are the means of four replicates ± one standard deviation. (B) Effect of A. nidulans Δppo deletions: ST production at three and seven dpi by WT A. nidulans and Δppo mutants grown on maize WT and lox3–4 kernels. Numbers above bars denote the ratios of ST production (Δppo mutant:WT) of A. nidulans within each maize genotype. Values shown are the means of four replicates of five kernels each. Asterisks above bars represent significant differences in ST production for each fungal genotype between WT and lox3–4 mutant kernels (P < 0.05). ST, sterigmatocystin standard. dpi, days post infection.
DISCUSSION
A general role for a maize 9-LOX in susceptibility to fungal pathogens appeared to be supported by two recent studies showing that loss of the 9-LOX gene, ZmLOX3 resulted in decreased fumonisin production by the mycotoxigenic maize pathogen F. verticillioides (Gao et al. 2007) and that expression of this same gene remediated ST production in an A. nidulans oxylipin mutant (Brodhagen et al. 2008). However, the current study shows that the lox3–4 mutant maize is more susceptible to Aspergillus growth, and to AF and ST contamination, under both field and kernel assay conditions. Because one unexpected result was the lack of a difference in 9S-HPODE between A. flavus-infected WT and lox3–4 kernels, possible insights and caveats of the lipid profiles are first discussed.
Our previous studies involving the lox3–4 mutant suggest that disruption of ZmLOX3 resulted in altered oxylipins profiles only in the tissues or treatments in which the WT ZmLOX3 gene would normally be expressed either constitutively or in an inducible manner. For instance, when oxylipins in healthy (uninfected) four-day-old germinating embryos of WT and lox3–4 mutant kernels were profiled, 9-LOX-derived oxylipins were lower in the lox3–4 mutant seeds (Gao et al. 2007). Four-day-old germinating kernels were used in that study to quantify oxylipins because at this developmental stage ZmLOX3 transcripts accumulated to the highest levels (Gao et al. 2007; Wilson et al. 2001). Inactivation of ZmLOX3 also affected oxylipin profiles of the non-seed tissues that express this gene constitutively: the levels of 9-LOX-derived oxylipins were lower but JA levels were higher in healthy roots of the lox3–4 mutant than in the WT (Gao et al. 2008). No difference was observed in the oxylipins levels between the untreated mutant and WT leaves, a tissue in which ZmLOX3 transcripts are not detectable. ZmLOX3 was also not expressed in either untreated dry seed or in control mock-inoculated but non-germinating seed. However, ZmLOX3 was induced in A. flavus infected kernels (Wilson et al. 2001). Thus, 9-LOX derived oxylipins were not expected to change in non-germinating kernels but were expected to be lower in lox3–4 mutant than WT kernels after A. flavus attack. However, no significant differences in 9-LOX derived oxylipins between WT and lox3–4 kernels were observed even after A. flavus infection (data not shown). The only difference in oxylipins was observed in kernels infected with A. flavus, where JA, a 13-LOX derivative, was higher in the lox3–4 mutant than in the WT (Figure 3D). These observations underscore the importance of kernel phenology on the overall influence of ZmLOX3 on the oxylipin profile, as well as the role of pathogen attack. Because Aspergillus infection is known to alter expression of several LOX genes in maize kernels (Wilson et al., 2001), it is possible that any differences in oxylipin profiles between WT and lox3–4 kernels infected with A. flavus may have been overshadowed by upregulation of LOX genes other than ZmLOX3 during the defense response. Because maize harbors several 9-LOX genes, it is possible that the expression of other 9-LOX genes were upregulated in ungerminated seeds to compensate for the missing ZmLOX3. For example, in maize root tissues, expression of ZmLOX5 (a 9-LOX) and ZmLOX10 (a 13-LOX) are normally not constitutive, but inducible (e.g. during nematode attack). However, in the lox3–4 mutant roots, both ZmLOX5 and ZmLOX10 were constitutively expressed (Gao et al. 2008). Similar compensatory mechanisms were shown for A. nidulans, where loss of individual ppo genes causes altered expression of the remaining ppo genes (Tsitsigiannis et al., 2005). Finally, our lipid profiles crudely averaged overall kernel lipid composition, and would not have detected localized differences within a seed where oxylipin production might be very low, or very high (e.g. in cells responding to pathogen attack). Thus, oxylipin levels may indeed vary in the embryonic maize tissues of infected lox3–4 seed at a finer level of resolution detected in this study, and such differences might be locally important to a germinating fungal spore or intruding hyphae.
Although our methods did not reveal any detectable differences in levels of 9S-and 13S-HPODE, there were significant differences in free fatty acid levels in the lox3–4 kernels versus WT kernels. A. flavus-infected lox3–4 kernels were increased in palmitic (C16:0), oleic (C18:1), linoleic (C18:2), and linolenic (C18:3) acid content (Figure 3A – 3C). Like 9S-HPODE, oleic and linoleic acid have been shown to induce Aspergillus conidiation (Calvo et al. 1999) and AF and ST biosynthesis (Burow et al. 1997, Maggio Hall et al. 2005). Oleic acid in particular can be a powerful stimulant of ST and AF production, presumably due to an indirect effect on ST and AF precursor abundance. Oleic acid enhances the proliferation of peroxisomes, one of the organellar locations where β-oxidation of fatty acids occurs in Aspergillus (Maggio Hall et al. 2005). β-oxidation metabolizes fatty acids into abundant pools of acyl-CoA, which is required for AF/ST polyketide biosynthesis in the Aspergilli. β-oxidation mutants of A. nidulans were defective in ST production in oleic acid-amended culture medium as well as on maize seed (Maggio-Hall et al. 2005). Furthermore, field studies have shown that peanut seeds with high oleic acid content are more heavily contaminated with AF (Xue et al. 2003). Thus, it is possible that the increased oleic acid content of the infected lox3–4 kernels contributed to the higher ST/AF production by the fungi.
To examine the interplay of maize and Aspergillus oxylipin signaling, WT and lox3–4 seeds were inoculated with A. nidulans WT and known oxylipin mutants. The phenotype of each A. nidulans strain reflects its unique oxylipin profile (with which the host oxylipin profile presumably blends). Notably, a ΔppoAC mutant of A. nidulans, which produces no detectable ST in culture and little on seed, produced near-WT levels of the mycotoxin on lox3–4 mutant maize kernels. This observation suggests that an unknown factor(s) in the mutant kernels compensates for the missing fungal oxylipins (for example, the increased oleic acid levels of Aspergillus-infected lox3–4 kernels). In seeming contradiction, expression of ZmLOX3 in the ΔppoAC mutant also restores ST production (Brodhagen et al. 2008). This suggests that separate regulatory mechanisms connect ZmLOX3 to ST production when the gene is expressed in maize versus fungal tissues. Because the lox3–4 kernels supported greater conidiation, regardless of Aspergillus ppo genotype, than did WT kernels, addition of the ZmLOX3 gene back into the Aspergillus-seed pathosystem (via expression in the fungus) was expected to complement the effect of the lox3–4 mutation by lowering conidial production Instead, A. nidulans expressing ZmLOX3 produced the most conidia of any genotype on lox3–4 seeds. The latter result may reflect a synergy between high oleic acid in the lox3–4 seeds and ZmLOX3 in the fungus, both of which by themselves have a positive effect on ST production (Maggio-Hall et al. 2005; Brodhagen et al. 2008). Alternatively, ZmLOX3 expressed in the fungus may simply reflect a simpler signal in the fungus (a single 9-LOX and its product) than in the plant, where expression or loss of ZmLOX3 occurs in the context of many synergistic, opposing, or compensatory LOXs, and a complex array of downstream derivatives from the oxylipin biosynthetic pathways. The solo ZmLOX3 expressed in the fungus reflects the bioassays in which pure 9S-HPODE was added to fungal cultures; the phenotype resulting from lox3–4 deletion in host kernels is apparently, and predictably, less straightforward.
This work reveals that A. flavus and F. verticillioides, two prevalent maize seed pathogens, respond oppositely to the deletion of a maize 9-LOX gene. Multiple lines of evidence from previous experiments pointed to 9-LOX activity as a susceptibility factor in the maize/Aspergillus interaction (reviewed in Tsitsigiannis and Keller, 2006). Inactivation of ZmLOX3 in maize revealed that the relationship is more complex than we hypothesized, as lox3–4 mutants are more susceptible to Aspergillus colonization and AF accumulation. By contrast, although F. verticillioides is able to infect the lox3–4 mutant kernels similarly to the WT, fumonisin and microconidia production in the mutant seed is dramatically decreased (Gao et al. 2007). One possible explanation for the different phenotypes of the two fungi on lox3–4 mutant maize kernels is their ability to induce fatty acid accumulation either in the mutant or WT. In contrast to the increased fatty acid content in A. flavus-infected lox3–4 mutant kernels, it was the WT seed and not the mutant that responded to F. verticillioides infection by increased production of all fatty acids tested. Clearly, additional experimentation will be required to elucidate biological relevance of the fatty acids on the differential behavior of the two fungi on the lox3–4 mutant seed.
An opposite ability to suppress either Fusarium or Aspergillus (but not both) also has been observed on maize bearing mutations in other LOX genes (data not shown), suggesting that, while individual LOX genes contribute uniquely to pathogen susceptibility, the outcomes of oxylipin-governed host-pathogen interactions are pathogen-specific. This phenomenon is critical from a practical standpoint, because Aspergillus and Fusarium coexist naturally in maize fields, where engineered resistance to one pathogen at the expense of susceptibility to the other is useless.
MATERIALS AND METHODS
Plant materials and fungal strains
The generation of maize lox3–4 mutants by Mutator transposable element-insertional mutagenesis was described previously (Gao et al. 2007). The mutant and WT are near-isogenic lines (NILs) at the BC4F5 genetic stage in the B73 background.
Aspergillus flavus and A. nidulans strains used in this study are listed in Table 1. A. flavus strains were cultured on potato dextrose agar (Difco Laboratories, Detroit, MI) at 29°C and A. nidulans strains were cultured at 37°C on glucose minimal medium (GMM; Calvo et al. 2001). Cultures were grown inside cardboard boxes to achieve continuous dark.
Fusarium verticilliodes strain 7600 (M3125; Fungal Genetics Stock Center, University of Missouri, Kansas City, KS, U.S.A.) was grown on potato dextrose agar (B&D, Sparks, MD, U.S.A.) at 28°C.
Kernel infection assays and spore enumeration
To elucidate the effect of the ZmLOX3 gene disruption on the production of conidia, we used A. flavus strain NRRL 3357 and five A. nidulans strains (Table 1) in a kernel assay. The lox3–4 and wild-type kernels were surface-disinfested with 0.6% sodium hypochlorite for ten min and rinsed five times with distilled H2O. To provide an infection court, the kernels were wounded by cutting the embryo side with razor blade with the cut depth of about 0.5 mm. Seeds were blotted dry, placed in a 20 ml glass scintillation vial (Wheaton Science, Millville, NJ), and inoculated with 1×106 conidia suspended in 200 µL of 0.001% Tween 20. Control seeds received an equal amount of 0.001% Tween 20. Four or six inoculated or mock-inoculated kernels were used per biological replicate, with six replicates per experiment. The vials were kept in a box with wetted filter paper to form humidity chambers and incubated at 29 °C with twelve h light/dark cycles. Sterile distilled H2O was added as needed, to keep the filter paper base of the humidity chambers moist. Kernels were harvested at 3.5 and seven days post inoculation (dpi) for spore counting (as described above) and quantification of AF or ST. For ST measurement, six infected kernels per replicate were frozen in liquid nitrogen until analysis. Five and six replicates were used for spore counting and ST measurement, respectively. The conidiation experiments were repeated at least four times with consistent results.
To determine the effect of the ZmLOX3 gene disruption on the lipid composition of infected kernels and the production of conidia, we inoculated kernels of A. flavus strain 12S as described by Wilson and associates (2001). Three biological replicates each containing six kernels of either the lox3–4 or WT kernels were inoculated by overlaying wounded embryos with five µL of 0.001% (v/v in water) Tween 80 containing an estimated 5,000 conidia of A. flavus 12S. Control seeds were inoculated with pure 0.001% Tween 80. Kernels were harvested at 48 and 72 hours post infection (hpi), and vortexed for one min in 0.001% Tween 80 to dislodge spores. The spore suspension was decanted, and spores were enumerated using a hemacytometer. Our isolate of A. flavus 12S did not produce AF to reliably enough for accurate comparisons, so instead, A. flavus NRRL 3357 (Wicklow et al. 1998; Windham et al. 2003) was examined for AF production as below. Spore enumeration was also performed on WT and lox3–4 kernels infected as described below with A. flavus NRRL 3357, with results similar to those for A. flavus 12S (data not shown).
Aflatoxin and sterigmatocystin quantification
A. flavus NRRL 3357 infected seed were examined for AF content using monoclonal affinity columns and fluorescence determination with the Vicam Aflatest (Vicam, Watertown, MA), according to the USDA-FGIS protocol (USDA Aflatoxin Handbook 1997), with the following modification. Kernels of a known weight (0.5 – 1.3 g) were ground and extracted with 20 ml 80% (v/v in water) methanol using a blender. Each treatment consisted of four biological replicates with four kernels per replicate.
For the ST analysis in planta, corn kernels were collected in 50 ml Falcon tubes and vortexed for one min in 0.001% Tween 80 to dislodge spores. This suspension (still containing kernels) was extracted sequentially in acetone and then in chloroform, for a final ratio of 1:1:1 (v/v/v) acetone:chloroform:0.001% Tween 80. Following each solvent addition, samples were shaken for ten min at 150 rpm, allowed to stand for five min, and centrifuged for ten min at 3000 rpm. The organic lower phase was collected and the solvent was evaporated in a fume hood. The presence of abundant seed lipids in the samples hampered the clear observation of ST on TLC plates at this stage of purification, so a second extraction was carried out as follows. Samples were resuspended in five ml of 0.1 M NaCl methanol:water [55:45 (v/v)] and 2.5 ml of hexane, and vortexed for one min. Samples were centrifuged at 2000 rpm for five min. The hexane layer was collected and the interphase layer (containing fatty acids) was discarded. The remaining aqueous phase was washed with an additional 2.5 ml hexane. The hexane extracts were combined and allowed to evaporate. The residue was then resuspended in 500 µl of hexane, 30 µl of which was spotted onto silica gel TLC plates and separated in a hexane:ethyl acetate [40:10(v/v)] solvent system. Quantification of ST spot intensity (detected at 245 nm) on the TLC plates was accomplished using a CAMAG II densitometer (Muttenz, Switzerland) equipped with CATS TLC software (version 3.20). Experiments were performed with four replications per treatment. All procedures were carried out at room temperature.
Profiling of oxylipins and fatty acids
Quantification of oxylipins and fatty acids was conducted as described in Stumpe et al. (2005). For measurement of oxylipins and fatty acids, six kernels of lox3–4 and near-isogenic WT were inoculated with conidia of A. flavus 12S and incubated for 72 h at 29°C in darkness, as described above. Four and six replicates were used for mock- and A. flavus-inoculated lox3–4 or NIL WT with four kernels each, respectively. For oxylipin and fatty acid profiling of Fusarium verticilliodes-inoculated kernels, the kernel assay was conducted similarly to that of A. flavus. Briefly, the lox3–4 and WT kernels were inoculated with 1×106 conidia suspended in 200 µL of 0.001% Tween 20. Control seeds received an equal amount of 0.001% Tween 20. Six inoculated or mock-inoculated kernels in glass vials were kept at room temperature for 3.5 and 7 d, respectively. Upon harvesting, the kernels were immediately frozen in liquid nitrogen, ground to fine powder, lypholized and used for measurement of oxylipins and fatty aicds.
Field evaluation of resistance to aflatoxin contamination
Field experiments were conducted over the course of two years at two Texas Agricultural Experiment Stations: Weslaco (WES), where the soil is a Harlingen clay, and College Station (Burleson County) (CS), where the soil is a Miller-Norwood-Pledger clay loam. Corn was planted at Weslaco on March 17, 2005 and Feb. 15, 2006, and at College Station on April 7, 2005 and March 30, 2006. Each replicate consisted of a single 7.3 m row, with 76 cm row spacing and 30 cm plant spacing, and an average of 20 plants per row. There were four replicates per genotype arranged in a randomized complete block design.
Primary ears were inoculated ten days after mean silking date with three ml of an aqueous suspension containing 3 × 107 conidia of A. flavus NRRL 3357 (Wicklow et al. 1998; Windham et al. 2003), using the non-wounding silk channel method (Zummo and Scott 1992). The conidial inoculum was prepared as described previously (Betrán and Isakeit 2004). Half of the plants within each replicate were inoculated and the rest remained uninoculated.
Ears were hand-harvested when kernel moisture was below 15%. Ears were husked and ear rot was assessed by quantifying the proportion of kernels with visible fungal colonization (expressed as percent of infected kernels). For aflatoxin quantification, ears were shelled, and kernels were bulked and then ground using a Romer mill (Romer Labs, Union, MO). AF was quantified from 50 g subsamples using the VICAM AflaTest® fluorometer (VICAM, Watertown, MA) according to the USDA-FGIS protocol as described above.
Statistical analysis
Kernel assays
For conidial production in kernel assays, sample means were compared using ANOVA (α = 0.05; Tukey-Kramer means comparisons). For oxylipins and AF concentrations from kernel assays, means were compared via ANOVA (α = 0.05), and Fisher’s protected LSD test was used to separate means. For ST concentrations from kernel assays, differences between infected WT and lox3–4 mutant kernels were assessed using Student’s t test.
Field samples
AF concentrations in ng/g were log-transformed to equalize variance. Means were compared via ANOVA (α = 0.05), and Fisher’s protected LSD test was used to separate means.
Statistical tests were done using the SPSS statistical package (SPSS Inc, Chicago, IL) or JMP, version 3 (SAS Institute, Cary, NC).
Distribution of materials
Novel materials described in this publication may be available for non-commercial research purposes upon acceptance and signing of a material transfer agreement. In some cases such materials may contain or be derived from materials obtained from a third party. In such cases, distribution of material will be subject to the requisite permission from any third-party owners, licensors or controllers of all or parts of the material. Obtaining any permission will be the sole responsibility of the requestor. Plant germplasm will not be made available except at the discretion of the owner and then only in accordance with all applicable governmental regulations.
ACKNOWLEDGEMENTS
This research was funded by a NIEHS NRSA training grant through the Molecular and Environmental Toxicology Center at the University of Wisconsin to M.B, BARD No. FI-384-2006 to S.H.B., and NSF IOB-0544428 to M.V.K.and N.P.K.
LITERATURE CITED
- Betrán FJ, Isakeit T. Aflatoxin accumulation in maize hybrids of different maturities. Agron. J. 2004;96:565–570. [Google Scholar]
- Brodhagen M, Keller NP. Signalling pathways connecting mycotoxin production and sporulation. Mol. Plant Pathol. 2006;7:285–301. doi: 10.1111/j.1364-3703.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- Brodhagen M, Tsitsigiannis DI, Hornung E, Goebel C, Feussner I, Keller NP. Reciprocal oxylipin-mediated cross-talk in the Aspergillus-seed pathosystem. Mol. Microbiol. 2008;67:378–391. doi: 10.1111/j.1365-2958.2007.06045.x. [DOI] [PubMed] [Google Scholar]
- Brown RL, Cotty PJ, Cleveland TE, Widstrom NW. Living maize embryo influences accumulation of aflatoxin in maize kernels. J. Food Prot. 1993;56:967–971. doi: 10.4315/0362-028X-56.11.967. [DOI] [PubMed] [Google Scholar]
- Burow GB, Nesbitt TC, Dunlap J, Keller NP. Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Mol. Plant-Microbe Interact. 1997;10:380–387. [Google Scholar]
- Calvo AM, Gardner HW, Keller NP. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 2001;276:25766–25774. doi: 10.1074/jbc.M100732200. [DOI] [PubMed] [Google Scholar]
- Calvo AM, Hinze LL, Gardner HW, Keller NP. Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 1999;65:3668–3673. doi: 10.1128/aem.65.8.3668-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champe SP, El-Zayat AAE. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J. Bacteriol. 1989;171:3982–3988. doi: 10.1128/jb.171.7.3982-3988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champe SP, Rao P, Chang A. An endogenous inducer of sexual development in Aspergillus nidulans. J. Gen. Microbiol. 1987;133:1383–1387. doi: 10.1099/00221287-133-5-1383. [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Ann. Rev. Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Gao X, Shim WB, Göbel C, Kunze S, Feussner I, Meeley R, Balint-Kurti P, Kolomiets M. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with the mycotoxin fumonisin. Mol. Plant Microbe Interact. 2007;20:922–933. doi: 10.1094/MPMI-20-8-0922. [DOI] [PubMed] [Google Scholar]
- Gao XQ, Kolomiets M. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by mycotoxigenic fungi. Toxin Reviews. 2008 (in press) [Google Scholar]
- Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Curr. Opinion Plant Biol. 2002;5:230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- Isakeit T, Gao X, Kolomiets M. Increased resistance of a maize mutant lacking the 9-lipoxygenase gene, ZmLOX3, to root rot caused by Exserohilum pedicellatum. J. Phytopathol. 2007;155:758–760. [Google Scholar]
- Keller NP, Butchko RAE, Sarr B, Phillips TD. A visual pattern of mycotoxin production in maize kernels by Aspergillus spp. Phytopathol. 1994;84:483–488. [Google Scholar]
- Maggio-Hall LA, Wilson RA, Keller NP. Fundamental contribution of β-oxidation to polyketide mycotoxin production in planta. Mol. Plant-Microbe Interact. 2005;18:783–793. doi: 10.1094/MPMI-18-0783. [DOI] [PubMed] [Google Scholar]
- Mazur P, Meyers HV, Nakanishi K. Structural elucidation of sporogenic fatty acid metabolites from Aspergillus nidulans. Tetrahedron Lett. 1990;31:3837–3840. [Google Scholar]
- Mazur P, Nakanishi K, El-Zayat AAE, Champe SP. Structure and synthesis of sporogenic psi factors from Aspergillus nidulans. J. Chem. Soc. Chem. Commun. 1991;20:1486–1487. [Google Scholar]
- Porta H, Rocha-Sosa M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002;130:15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpe M, Carsjens JG, Stenzel I, Gobel C, Lang I, Pawlowski K, Hause B, Feussner I. Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochem. 2005;66:781–791. doi: 10.1016/j.phytochem.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP. Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol. Microbiol. 2006;59:882–892. doi: 10.1111/j.1365-2958.2005.05000.x. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell. 2004a;3:1398–1411. doi: 10.1128/EC.3.6.1398-1411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Kowieski TM, Zarnowski R, Keller NP. Three putative oxylipin biosynthetic genes integrate sexual and asexual development in Aspergillus nidulans. Microbiology. 2005;151:1809–1821. doi: 10.1099/mic.0.27880-0. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Zarnowski R, Keller NP. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 2004b;279:11344–11353. doi: 10.1074/jbc.M310840200. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007;15:109–118. doi: 10.1016/j.tim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:S153–S164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RA, Gardner HW, Keller NP. Cultivar-dependent expression of a maize lipoxygenase responsive to seed infesting fungi. Mol. Plant-Microbe Interact. 2001;14:980–987. doi: 10.1094/MPMI.2001.14.8.980. [DOI] [PubMed] [Google Scholar]
- Wicklow DT, Norton RA, McAlpin CE. Beta-carotene inhibition of aflatoxin biosynthesis among Aspergillus flavus genotypes from Illinois corn. Mycoscience. 1998;39:167–172. [Google Scholar]
- Windham GL, Williams WP, Buckley PM, Abbas HK. Inoculation techniques used to quantify aflatoxin resistance in corn. J. Toxicol. Toxin Rev. 2003;22:313–325. [Google Scholar]
- United States Department of Agriculture. Grain Inspection, Packers and Stockyards Administration, Federal Grain Inspection Service. Washington, D.C: Aflatoxin Handbook; 1997. pp. 8-1–8-27. Access online at: http://www.gipsa.usda.gov/GIPSA/webapp?area=home&subject=lr&topic=hb-afl. [Google Scholar]
- Xue HQ, Isleib TG, Payne GA, Wilson RF, Novitzky WP, O'Brian G. Comparison of aflatoxin production in normal- and high-oleic backcross-derived peanut lines. Plant Dis. 2003;87:1360–1365. doi: 10.1094/PDIS.2003.87.11.1360. [DOI] [PubMed] [Google Scholar]
- Zummo N, Scott GE. Interaction of Fusarium moniliforme and Aspergillus flavus on kernel infection and aflatoxin contamination in maize ears. Plant Dis. 1992;76:771–773. [Google Scholar]