Abstract
In the normal intestinal epithelium transforming growth factor β-1 (TGFβ-1) acts as a growth inhibitor, but in malignant cells it may act as a tumor promoter. However, only limited information is available on genetic variation in the TGFB1 gene and its relationship to circulating levels and risk of colorectal cancer. To characterize associations of genetic variation [tagging single-nucleotide polymorphisms (tagSNP) and haplotypes with frequency >0.05] at the TGFB1 locus with circulating TGFβ-1 and risk of colorectal neoplasia, we conducted two case-control studies (including 271 colorectal adenoma cases and 544 controls, and 535 colorectal adenocarcinoma cases and 656 controls) among Japanese Americans, Caucasians, and Native Hawaiians in Hawaii. Serum TGFβ-1 was measured by sandwich ELISA among the subjects of the first study. The variant A allele for tagSNP rs6957 was associated with higher serum TGFβ-1 [means (in ng/mL) and 95% confidence interval (95% CI) for AA or AG, 32.6 (30.6–34.7); GG, 29.0 (25.1–32.9); Pdifference = 0.05] after adjusting for age and other factors. Homozygous carriers of the variant G allele for tagSNP rs11466345 had a statistically significantly lower risk of adenocarcinoma [AG versus AA: odds ratio (OR), 0.9 (95% CI, 0.7–1.2); GG versus AA: OR, 0.4 (95% CI, 0.2–0.7); Ptrend = 0.01]. The haplotype carrying both variants was also statistically significantly associated with a reduced risk of adenocarcinoma (OR, 0.3; 95% CI, 0.1–0.8). Although not statistically significant, the direction and magnitude of the corresponding ORs were similar for adenoma. These results suggest that a haplotype containing SNP rs11466345 at the 3′ end of TGFB1 is associated with genetic susceptibility to colorectal neoplasia.
Introduction
Transforming growth factor β (TGFβ) and its signaling pathway play a role in many critical cellular processes, ranging from embryogenesis to homeostasis, extracellular matrix formation, angiogenesis, hematopoiesis, and immune function (1). In normal epithelium, including the intestinal epithelium, TGFβ acts as a growth inhibitor (2). However, during the carcinogenic process, transformed cells may become resistant to TGFβ signaling (3) and TGFβ then acts as a tumor promoter by enhancing tumor cell motility and invasiveness. Among the isoforms described, TGFβ-1 is the one most ubiquitously expressed (4) and most frequently up-regulated in tumor cells (3).
Certain inherited variants in the promoter region of the TGFB1 gene (G—800A and C—509T) and in exon 1 (L10P and R25P) and exon 5 (T2631) have been associated with higher circulating concentrations in a small number of studies (5–7) and, thus, could possibly affect cancer risk (8, 9). The T263I single-nucleotide polymorphism (SNP) is located in the pro-protein portion of TGFβ-1 that is cleaved from the active part of the protein. It has been hypothesized that activation of the latent TGFβ-1 protein by cleavage of pro-TGFβ-1 is an important event in the signaling cascade leading to growth control (10, 11). Similarly, the G—800A SNP is located in a consensus cyclic AMP response element binding protein (CREB) half site and may cause reduced affinity for CREB transcription factors (5) whose binding is important for transcription control.
No comprehensive study of the genetic variation in the TGFB1 gene and its relationship to circulating levels or colorectal cancer risk has been reported. The only data available are from three recent reports, one on L10P with colorectal polyps (12), one on C—509T and colorectal cancer (13), and the third on the four previously mentioned SNPs and advanced colorectal adenoma (14). In the first study (12), no association was found with L10P and adenoma but a lower risk of hyperplastic polyps was suggested for P allele carriers who were current or past smokers (P trend = 0.05). In the second study, no association was found between C—509T and colorectal cancer. In the third study (14), —509TT and 10PP genotypes were associated with advanced colorectal adenoma {odds ratio (OR), 1.89 [95% confidence interval (95% CI), 1.16–3.09] and 1.37 (95% CI, 1.02–1.86), respectively}. The —509TT association was greater for the subsets of participants with multiple adenomas and those with rectal adenomas.
The goal of this study was to characterize the genetic variation in TGFB1 and use the data from two case-control studies to clarify the associations of SNPs and haplotypes at this locus with circulating levels of TGFβ-1 and risk of colorectal adenoma and adenocarcinoma.
Materials and Methods
Adenoma Subjects
Two flexible-sigmoidoscopy screening clinics on the island of Oahu, Hawaii were used to recruit participants in an ongoing colorectal adenoma matched case-control study as previously described (15). Thirty-six percent of the interviewed adenoma cases were identified as part of the baseline screening exam at the Hawaii site of the Prostate Lung Colorectal and Ovarian (PLCO) screening trial between July 1996 and February 2000. The remaining cases were identified at the Kaiser Permanente-Hawaii Gastroenterology Screening Clinic between January 1995 and June 2002. Cases were screenees with histologically confirmed first-time adenoma(s) of the colorectum and were of Japanese, Caucasian, or Hawaiian ethnicity. Controls were selected from screenees with a normal sigmoid colon and rectum. Controls were individually matched to the cases on age, sex, ethnicity, and screening date (±3 months) and clinic. The participation rates were 66.1% for cases (n = 294) and 68.0% for controls (n = 622). The main reasons for nonparticipation for cases were subject refusal (21.4%), ineligible race (3.4%), physician refusal (2.3%), and inability to locate (2.3%); the main reasons for control nonparticipation were subject refusal (20.8%), physician refusal (2.2%), and inability to locate (2.0%). Two hundred seventy-three (92.5%) of interviewed case patients and 554 (89.2%) of interviewed control patients agreed to provide blood, and 271 cases and 544 controls had sufficient serum remaining for analysis in this study. Participants who agreed to provide a blood sample were similar to those who did not with respect to age, sex, energy intake, lifetime ethanol intake, and lifetime recreational physical activity. However, those who agreed to participate were less likely to be Native Hawaiian, more likely to be Japanese or Caucasian, and had fewer pack-years of smoking compared with those who did not agree to participate.
Adenocarcinoma Subjects
The second study has previously been described in detail (16). Cases diagnosed with a first adenocarcinoma of the colon or rectum before age 85 years were identified through the Hawaii Tumor Registry, a member of the U.S. National Cancer Institute Surveillance, Epidemiology and End Results program, between July 1994 and December 1999. Cases were eligible if they were Japanese, Caucasian, or Native Hawaiian residents of the island of Oahu. Controls were selected from an ongoing health survey conducted by the Hawaii State Department of Health. This survey, modeled after the National Health Information Survey, takes an annual random 2% sample of households within the state. An additional source of controls ≥65 years of age was Medicare participants on Oahu. One control was individually matched to each case by sex, ethnicity, and age (±2 years). Personal interviews were obtained from 768 matched case-control pairs. The participation rate was 58.2% for cases and 53.2% for controls. The main reason for nonparticipation in both cases and controls was refusal (22.5% and 34.8%, respectively). Other reasons included death, severe diseases, and inability to locate (15). Twelve percent of the cases were in the proximal colon, 45% in the distal colon, and 43% in the rectum. DNA samples were available from 535 (70%) of interviewed cases and 656 (85%) of interviewed controls. DNA samples were unavailable for the remainder of participants due to refusal or sample depletion by previous analyses. Cases and controls who donated blood were similar to those who did not with regard to age, sex, ethnicity, as well as other factors associated with colorectal cancer risk in this study. All participants with available samples were used in analyses regardless of matched set completion status.
Data Collection
In both studies, exposure information was collected via virtually identical interview-administered questionnaires designed to obtain demographic and lifestyle information, including lifetime histories of physical activity, tobacco smoking, and alcohol drinking; medical history; family cancer history; and, for females, reproductive and hormone use history. The interview also included a validated food frequency questionnaire with 268 food items (17, 18), and a detailed history of vitamin and mineral supplement use was also taken. This research was approved by the University of Hawaii Committee on Human Studies, Straub Clinic and Hospital Review Board, Kaiser Permanente Hawaii Institutional Review Board, and the Johns Hopkins Bloomberg School of Public Health Committee on Human Research.
Blood Collection
Blood samples in the adenoma study were collected in the morning after a 10-h fast and stored on ice until processed. Processing was completed within 2 h of collection and specimens were stored at −80°C. Serum samples of cases and controls were analyzed together in the same analytic batch giving priority to matched sets and in situations where serum was not available for all members of a matched set (due to either refusal to give blood or depleted serum), participants of the same ethnicity, sex, and of similar age.
Serum TGFβ-1 Assays
Serum TGFβ-1 was determined in the adenoma study using a quantitative sandwich enzyme immunoassay technique with a Quantikine human TGFβ-1 kit (R&D Systems) following the manufacturer’s instructions. The detection limit of the assay is 7 pg/mL and has <1% cross-reactivity with isoforms TGFβ-2 and TGFβ-3. We analyzed 36 paired quality control samples (1 paired sample from 10 individuals and 2 sets of paired samples from 13 individuals), yielding an intrabatch coefficient of variation of 4.6% for the assay and an interbatch coefficient of variation of 16.5%.
SNP Selection
The TGFB1 gene, located on chromosome 19, was surveyed for variation from 20 kb upstream to 10 kb downstream from the coding region. SNPs were identified from public databases, including the National Center for Biotechnology Information dbSNP database7 build 124 and the HapMap Project8 public release 14 (19), and were selected approximately every 1 to 2 kb to provide dense coverage of the gene. Preference for selection as potential tagSNPs was given first to SNPs that have previously been reported as potentially functional (resulting in either differences in expression in vitro or differences in circulating concentrations of TGFβ-1 by allele), nonsynonymous SNPs, or SNPs previously associated with cancer risk. Other considerations for selection included heterozygosity with a minor allele frequency of at least 5% and being in evolutionary conserved regions.
The selected SNPs were then genotyped on a multiethnic panel of Japanese (n = 70), Caucasian (n = 70), Native Hawaiian (n = 69), African American (n = 70), and Latino (n = 70) samples from the Multiethnic Cohort study (20, 21), plus an additional 31 (−10%) duplicate samples inserted throughout the panel. These quality control pairs showed 100% agreement for all SNPs. A sample size of 70 per ethnic group was selected so that any haplotype with frequency of ≥5% would be represented among the 140 chromosomes with a probability of >99%. These data were used to select haplotype-tagging SNPs (see below). The selected haplotype-tagging SNPs were then genotyped in the cases and controls of both studies.
Tests of Hardy-Weinberg equilibrium were carried out among controls from each study separately for all SNPs, after stratification by ethnicity. This test is a χ2 test of (observed − expected)2/expected with 1 degree of freedom. In the case of low allele frequencies, an exact test was used based on an exact calculation of the probability of observing H heterozygotes conditional on the number of copies of the minor SNP allele (22). Pairwise linkage disequilibrium was calculated using the r2 statistic between all pairs of SNPs.
Linkage Disequilibrium Block Determination and Tagging SNP Selection
The program Haploview (23) was used to determine blocks of linkage disequilibrium (LD) determined in each ethnic group using the criteria of Gabriel et al. (24). Separately by ethnicity, common haplotypes were predicted from the multiethnic panel genotype data within LD blocks using the methods described by Stram et al. (25) and implemented in their statistical package TagSNPsv2. We used the statistical criteria and selected additional tagSNPs in regions not represented in the haplotype blocks by the methods of de Bakker et al. (26) with the program Tagger (available in Haploview), which prioritizes tagSNPs to serve as a proxy for others at a given r2, either singly or by a group of two to three SNPs. We selected two additional tagSNPs in that way for a total of 16 tagSNPs.
DNA Extraction and Genotyping Assays
DNA was purified from blood lymphocytes using QIAamp 96 DNA Blood Kits (Qiagen). Genotyping was done by the 5′ nuclease TaqMan allelic discrimination assay [Applied Biosystems (ABI)]. If a genotyping assay failed manufacture or in-house quality control at ABI, another SNP was selected in its place to maintain the dense coverage of the gene. In two cases, one where a missense SNP failed design at ABI and another where no other SNPs existed nearby, MGB Eclipse assays were ordered from Epoch Biosciences and processed in a manner similar to the TaqMan assays. Sixty-five duplicate QC pairs were genotyped on the adenoma study plates with 100% agreement for all but one SNP (rs11466345), which had 99% agreement. Ninety-three duplicate QC pairs were genotyped on the adenocarcinoma study plates with 100% agreement for all but two SNPs (rs11466345 and 1800471), which had 97% and 99% agreement, respectively. The call rate was >99% for all assays. All ethnic-specific genotype frequencies in the controls met the criteria for Hardy-Weinberg equilibrium (P > 0.05) except for three SNPs in Caucasians [rs10416269 in the adenoma (P = 0.04) and adenocarcinoma (P = 0.01) studies, rs1982073 in the adenoma study (P = 0.001), and rs1549934 in the adenocarcinoma study (P = 0.04)]. Among these comparisons with Hardy-Weinberg expectations, 2.6 were expected to be significant by chance alone.
Statistical Analysis
Haplotype estimation
Haplotypes were inferred for cases and controls separately by ethnicity using the statistical software PHASE version 2.1 (27–29), which uses a Bayesian approach to evaluate the unknown haplotype frequencies in light of the known genotype data using Gibbs sampling, a type of Markov Chain-Monte Carlo algorithm. The output of this program includes a “best” reconstruction haplotype for each individual. For analyses, we assumed a haplotypes model comparing one or two copies of a specific haplotypes versus all other haplotypes.
Genetic variation as a predictor of serum concentration
We carried out a cross-sectional analysis of the adenoma study participants to examine genetic variation in TGFB1 as a predictor of serum concentration. All analyses were first stratified by case-control status to rule out the possibility that adenoma or recent polypectomy was associated with altered levels of serum TGFβ-1. Case-control status was not associated with serum level. Additionally, all blood was collected well after the removal of the cases’ adenomas, and there is evidence (30) that serum levels are affected by colorectal cancer but return to baseline once tumors are removed. Therefore, all subjects were combined for the analyses presented in this article. For each SNP, the variant allele was considered to be the one with the lower frequency, based on the reference sequence in dbSNP. The distributions of continuous variables were checked for normality and transformations were carried out as indicated. T tests and ANOVA were used to test for differences in mean serum TGFβ-1 concentration by various categories. Least-squares means of serum TGFβ-1 by genotype and haplotype were then calculated to adjust for additional factors. The P value for trend for increasing number of variant alleles was obtained via an F test with 1 degree of freedom. Additionally, all least-squared means were adjusted for age (as a continuous variable), sex, race, and screening clinic. We further adjusted models for pack-years of smoking and lifetime alcohol drinking, two variables associated with serum TGFβ-1 in our study. However, they did not alter the means so the models presented are not adjusted for these variables. We also considered the effect of length of storage of blood samples by adjusting models for time between date of blood draw and serum TGFβ-1 assay and found that time of storage did not alter the results.
Adenoma and adenocarcinoma risks
Case-control analyses were carried out separately but in the same manner for both studies. We considered dominant, recessive, and codominant modes of inheritance by treating each genotype successively as an indicator variable of type “any variant” versus “no variant allele,” as an indicator of two variants versus less than two variants, or as a dosage variable of 0, 1, or 2 for increasing number of variants, respectively. Using the method of Stephens et al. (27), we first carried out a permutation test as a global test for difference in haplotype frequency in each LD block. Disease risk was estimated with the ORs and 95% CIs from unconditional logistic regression adjusting for the study matching criteria. ORs were estimated for each haplotype using all other haplotypes as the reference category. Adjustment was made for known risk factors including pack-years of smoking, energy intake, drink-years of alcohol, and intakes of red meat, sweets, total daily fluids, dietary fiber, and total fruit for the adenoma study, and for factors regular aspirin use, pack-years of smoking, lifetime recreational physical activity, body mass index (BMI), and intake of energy, calcium, non-starch polysaccharides from vegetables, and folate for the adenocarcinoma study. These further adjustments did not materially alter the ORs for the SNPs or haplotypes studied and, therefore, results are presented adjusted only for the study matching criteria.
Results
Genetic variation at the TGFB1 locus
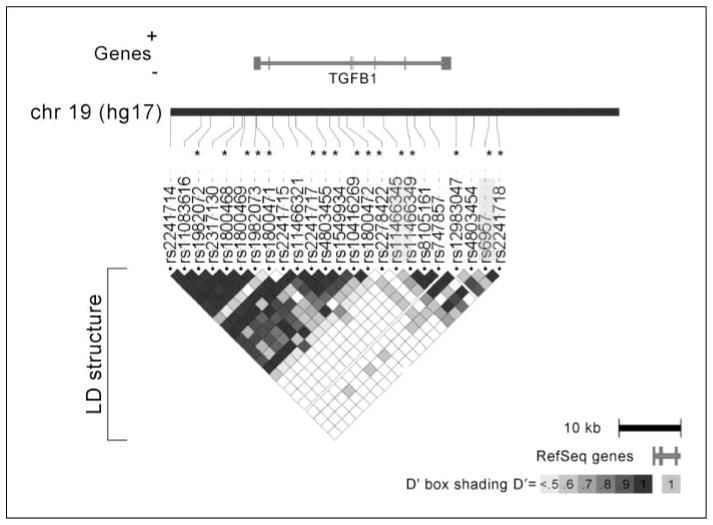
Table 1 lists the 26 SNPs, along with their frequencies, that were genotyped on the multiethnic panel, spanning 39.8 kb of the TGFB1 locus, from 9.6 kb upstream to 7.0 kb downstream from the coding region and with an average coverage of 1 SNP per 1.6 kb. SNPs rs11673525 and rs1989457 showed no variation and, therefore, did not contribute to the selection of tagSNPs. There were two main blocks of LD in the ethnic groups represented (Japanese Americans, Caucasians, and Native Hawaiians) in the case-control studies (which did not differ appreciably across these ethnic groups) as shown in Fig. 1. The first block spans 20.5 kb from the upstream/promoter region through the first three exons of the gene (16 SNPs), and the second block spans 13.9 kb from exon 4 into the downstream region of the gene (8 SNPs). The distance between the two blocks is 5.4 kb. Common haplotypes (>5%) were inferred within each of these two blocks of LD. The numbers of tagSNPs selected separately by ethnicity for Native Hawaiians, Japanese American, Caucasian, African American, and Latino groups were 11, 7, 9, 14, and 15, respectively, or 15 total for the former three groups included in the case control studies. (See Supplementary Table S1 for ethnic-specific genotype and haplotype frequencies in the two studies.) The common haplotypes within each ethnic group of the case-control studies (Japanese, Caucasian, and Native Hawaiian) accounted for 70% to 96% of all the chromosomes. The varied from 0.83 to 1.00 across the five ethnic groups.
Table 1.
Twenty-six SNPs selected for study in the TGFB1 gene locus
| SNP | Alias | Gene location | Base change | Minor allele | Chromosome position | Minor allele frequency, %
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| H* | J | C | A | L | ||||||
| LD block 1 | ||||||||||
| rs2241714 | Promoter | G/A | A | 46561232 | 42.8 | 48.5 | 27.9 | 18.8 | 39.7 | |
| rs11673525 | Promoter | C/T | T | 46559623 | No variation | |||||
| rs11083616 | Promoter | T/C | C | 46557483 | 44.2 | 49.3 | 37.9 | 42.6 | 42.6 | |
| rs1982072† | Promoter | A/T | T | 46556349 | 42.8 | 48.6 | 27.1 | 24.3 | 39.1 | |
| rs2317130 | Promoter | C/T | T | 46553514 | 42.8 | 48.6 | 27.5 | 32.6 | 39.9 | |
| rs1800468† | G–800A | Promoter | G/A | A | 46552427 | 2.2 | 0.0 | 10.7 | 1.4 | 5.8 |
| rs1800469† | C–509T | Promoter | C/T | T | 46552136 | 42.8 | 47.9 | 27.1 | 25.4 | 39.1 |
| rs1982073† | L10P,T869C | Exon 1 | T/C | C | 46550761 | 44.2 | 48.6 | 37.9 | 45.7 | 45.0 |
| rs1800471† | R25P, G915C | Exon 1 | G/C | C | 46550716 | 1.5 | 0.0 | 19.1 | 7.7 | 5.1 |
| rs2241715 | Intron 1 | G/T | T | 46548726 | 42.8 | 47.9 | 26.8 | 32.9 | 40.6 | |
| rs11466321 | Intron 1 | T/C | C | 46546756 | 0.7 | 0.0 | 10.7 | 5.7 | 2.9 | |
| rs2241717† | Intron 2 | T/G | G | 46545892 | 59.4 | 52.1 | 40.7 | 27.1 | 52.9 | |
| rs1989457 | Intron 2 | G/A | A | 46543955 | No variation | |||||
| rs4803455† | Intron 2 | C/T | T | 46543349 | 37.5 | 46.4 | 47.9 | 49.3 | 42.6 | |
| rs1549934† | Intron 3 | C/T | T | 46541798 | 39.1 | 47.9 | 62.0 | 75.4 | 47.8 | |
| rs10416269† | Intron 3 | T/C | C | 46540688 | 43.5 | 47.9 | 64.5 | 76.4 | 42.9 | |
| Between LD blocks | ||||||||||
| rs1800472† | T263I | Exon 5 | C/T | T | 46539700 | 0.7 | 0.7 | 2.1 | 2.1 | 3.6 |
| rs2278422† | Intron 5 | G/C | C | 46537598 | 68.8 | 93.6 | 42.9 | 32.1 | 63.6 | |
| LD block 2 | ||||||||||
| rs11466345† | Intron 5 | A/G | G | 46535301 | 27.2 | 17.9 | 8.6 | 13.6 | 7.1 | |
| rs11466349† | Intron 5 | C/G | G | 46532409 | 6.5 | 10.9 | 0.0 | 12.7 | 6.4 | |
| rs8105161 | Intron 5 | A/G | G | 46531471 | 32.4 | 30.0 | 16.4 | 20.3 | 15.7 | |
| rs747857 | Intron 6 | C/T | T | 46529587 | 6.5 | 10.7 | 0.0 | 13.8 | 6.5 | |
| rs12983047† | 3′-UTR | T/C | C | 46526339 | 36.0 | 30.0 | 17.1 | 22.1 | 15.0 | |
| rs4803454 | 3′-UTR | C/A | A | 46524236 | 0.0 | 0.0 | 0.0 | 0.7 | 2.9 | |
| rs6957† | 3′-UTR | A/G | G | 46522446 | 31.2 | 30.0 | 19.3 | 23.6 | 17.1 | |
| rs2241718† | 3′-UTR | G/A | A | 46521446 | 24.6 | 18.1 | 18.6 | 8.6 | 10.3 | |
Abbreviation: UTR, untranslated region.
H, Hawaiian; J, Japanese; C, Caucasian; A, African American; L, Latino.
Selected tagSNPs.
Figure 1.
Genetic variation in the TGFB1 locus. There are two main blocks of LD in the ethnic groups represented in the case-control studies (Japanese Americans, Caucasians and Native Hawaiians). The first block spans 20.5 kb from the upstream/promoter region through the first three exons of the gene (16 SNPs), and the second block spans 13.9 kb from exon 4 into the downstream region of the gene (8 SNPs). The distance between the two blocks is 5.4 kb. TagSNPs are indicated by asterisks and SNPs statistically significantly associated with serum level or risk are highlighted in light grey.
SNPs, haplotypes, and mean serum TGFβ-1
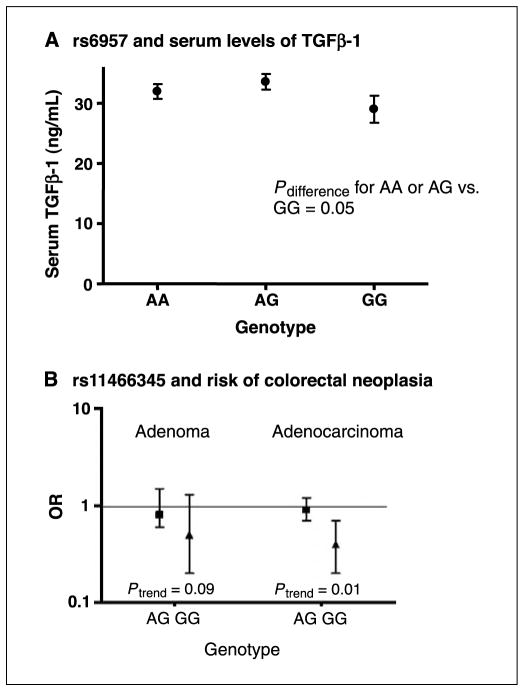
Serum TGFβ-1 levels ranged from 2.1 to 78.2 ng/mL for cases, with a mean of 30.8 ng/mL and a median of 29.9 ng/mL, and from 1.9 to 69.9 ng/mL for controls, with a mean of 31.2 ng/mL and a median of 30.6 ng/mL. Because these means did not vary by case-control status (P = 0.65), cases and controls were combined for the genotype-serum levels analysis. In the analysis of the SNP data (Supplementary Table S2), we observed a statistically significantly higher mean serum TGFβ-1 for the A allele of rs6957 [Fig. 2A; AA or AG, 32.6 ng/mL (30.6–34.7 ng/mL); GG, 29.0 ng/mL (25.1–32.9 ng/mL); Pdifference = 0.05]. When a codominant model was considered, the P trend was 0.03. The codominant association with rs6957 was also suggested in Japanese Americans (P trend = 0.05 for fully adjusted model) but not in Caucasians or Hawaiians. There was also a borderline significant association for SNP rs12983047 (Ptrend = 0.08). The haplotype analysis (Supplementary Table S3) did not show any significant differences in mean serum level by number of haplotypes including haplotype 2C, which contains alleles C and G of SNPs rs12983047 and rs6957, respectively.
Figure 2.
A, mean serum TGFβ-1 levels and 95% CIs by genotype for rs6957 adjusted for age, sex, ethnicity, and recruitment clinic. B, ORs and 95% CIs for adenoma and adenocarcinoma by genotype for rs11466345 adjusted for age, sex, ethnicity, and recruitment clinic.
Case-control analyses
In the adenoma study, the distribution of cases and controls by Japanese, Caucasian, and Hawaiian ethnicities was 40% and 37%, 42% and 46%, and 18% and 17%, respectively. For the adenocarcinoma study, the distributions were 59% and 60%, 28% and 26%, and 13% and 14%, respectively. Compared with controls, adenoma cases smoked more cigarettes and consumed more energy, red meat, and less dietary fiber. With respect to cancer compared with controls, adenocarcinoma cases were less educated, less likely to have used aspirin regularly, had smoked more cigarettes, participated in less lifetime recreational physical activity, were heavier, and consumed more energy, less calcium, less non-starch polysaccharides from vegetables, and less folate (15, 16).
For each study, we first carried out a global test for case-control difference in haplotype frequencies for each LD block, overall and stratified by ethnicity. For block 1, there were no statistically significant differences in haplotype frequencies in any ethnic group in either study. For block 2, there were statistically significant differences in haplotype frequencies between adenocarcinoma cases and controls among Japanese (P = 0.05) and Native Hawaiians (P = 0.03), but not in Caucasians (P = 0.66). This test was also statistically significant with all subjects combined (P = 0.01). For adenoma cases and controls, the difference in block 2 haplotype frequencies was of borderline significance (P = 0.09).
The risk estimates for the SNPs are presented by study for all subjects combined in Table 2 and for each ethnic/racial group in Supplementary Table S4. The G allele of rs11466345 (Table 2) was inversely associated with adenocarcinoma risk in a codominant model for all races combined [AG versus AA genotype: OR, 0.9 (95% CI, 0.7–1.2); GG versus AA: OR, 0.4 (95% CI, 0.2–0.7); P trend = 0.01]. The corresponding ORs for adenoma were of similar magnitude but did not reach statistical significance (P trend = 0.09). For adenocarcinoma, we also observed a Ptrend of borderline significance for SNP rs11466349 for all races (P = 0.05), although the ORs were not statistically significant [CG versus CC: OR, 1.4 (95% CI, 0.9–2.0); GG versus CC: OR, 2.7 (95% CI, 0.5–15.1)]. The risk estimates for these two SNPs were similar across the three ethnic/racial groups but did not reach statistical significance (Supplementary Table S4).
Table 2.
ORs and 95% CIs for TGFB1 SNPs and risk of colorectal adenoma and adenocarcinoma
| SNP | Genotype | Adenoma
|
Adenocarcinoma
|
||
|---|---|---|---|---|---|
| Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | ||
| LD block 1 | |||||
| rs1982072 | AA | 102/185 | 1.0 | 182/205 | 1.0 |
| AT | 113/234 | 0.8 (0.6–1.1) | 220/276 | 0.9 (0.7–1.2) | |
| TT | 52/114 | 0.8 (0.5–1.2) | 92/138 | 0.8 (0.5–1.1) | |
| P trend* | 0.17 | 0.13 | |||
| rs1800468 (G–800A) | GG | 251/496 | 1.0 | 467/587 | 1.0 |
| GA | 18/40 | 1.0 (0.5–1.7) | 27/31 | 1.0 (0.6–1.8) | |
| AA | 0/0 | 1/2 | 0.6 (0.05–6.4) | ||
| P difference | 0.90 | 0.93 | |||
| rs1800469 (C–509T) | CC | 103/188 | 1.0 | 178/200 | 1.0 |
| CT | 113/233 | 0.8 (0.6–1.2) | 218/274 | 0.9 (0.7–1.2) | |
| TT | 52/113 | 0.8 (0.5–1.2) | 91/134 | 0.8 (0.6–1.1) | |
| P trend | 0.21 | 0.17 | |||
| rs1982073 (L10P, T869C) | TT | 58/120 | 1.0 | 96/133 | 1.0 |
| TC | 113/211 | 1.1 (0.8–1.6) | 222/273 | 1.1 (0.9–1.5) | |
| CC | 90/162 | 1.2 (0.8–1.8) | 154/165 | 1.3 (0.9–1.8) | |
| P trend | 0.43 | 0.15 | |||
| rs1800471 (R25P, G915C) | GG | 250/507 | 1.0 | 470/583 | 1.0 |
| GC | 10/12 | 1.9 (0.8–4.6) | 8/6 | 1.5 (0.5–4.4) | |
| CC | 2/1 | 4.5 (0.4–51.0) | 5/11 | 0.5 (0.2–1.4) | |
| P trend | 0.06 | 0.35 | |||
| rs2241717 | TT | 77/134 | 1.0 | 135/155 | 1.0 |
| TG | 121/246 | 0.8 (0.6–1.2) | 227/286 | 0.9 (0.7–1.2) | |
| GG | 70/154 | 0.8 (0.5–1.1) | 132/172 | 0.9 (0.6–1.2) | |
| P trend | 0.17 | 0.48 | |||
| rs4803455 | GG | 81/171 | 1.0 | 146/197 | 1.0 |
| GT | 121/233 | 1.1 (0.8–1.6) | 225/281 | 1.1 (0.8–1.4) | |
| TT | 60/120 | 1.1 (0.7–1.6) | 116/135 | 1.2 (0.8–1.6) | |
| P trend | 0.72 | 0.37 | |||
| rs1549934 | CC | 76/135 | 1.0 | 136/160 | 1.0 |
| CT | 124/246 | 0.9 (0.6–1.2) | 225/285 | 0.9 (0.7–1.3) | |
| TT | 69/155 | 0.8 (0.5–1.1) | 133/173 | 0.9 (0.7–1.3) | |
| P trend | 0.17 | 0.59 | |||
| rs10416269 | TT | 82/149 | 1.0 | 131/158 | 1.0 |
| TC | 123/231 | 0.9 (0.6–1.3) | 223/274 | 1.0 (0.7–1.3) | |
| CC | 63/150 | 0.7 (0.5–1.1) | 123/161 | 0.9 (0.7–1.3) | |
| P trend | 0.10 | 0.71 | |||
| Between LD blocks | |||||
| rs1800472 (T263I) | CC | 254/518 | 1.0 | 487/608 | 1.0 |
| CT | 13/17 | 1.8 (0.8–3.7) | 6/8 | 0.8 (0.3–2.5) | |
| TT | 0/10 | 0/0 | |||
| P difference | 0.14 | 0.75 | |||
| rs2278422 | GG | 28/80 | 1.0 | 53/63 | 1.0 |
| GC | 77/166 | 0.9 (0.6–1.5) | 111/136 | 1.0 (0.6–1.6) | |
| CC | 154/289 | 1.0 (0.6–1.7) | 331/419 | 1.1 (0.7–1.8) | |
| P trend | 0.97 | 0.64 | |||
| LD block 2 | |||||
| rs11466345 | AA | 204/366 | 1.0 | 350/397 | 1.0 |
| AG | 57/120 | 0.8 (0.6–1.5) | 127/161 | 0.9 (0.7–1.2) | |
| GG | 6/20 | 0.5 (0.2–1.3) | 13/37 | 0.4 (0.2–0.7) | |
| P trend | 0.09 | 0.01 | |||
| rs11466349 | CC | 244/486 | 1.0 | 422/550 | 1.0 |
| CG | 25/49 | 1.0 (0.6–1.6) | 67/66 | 1.4 (0.9–2.0) | |
| GG | 0/0 | 4/2 | 2.7 (0.5–15.1) | ||
| P trend | 0.84 | 0.05 | |||
| rs12983047 | TT | 168/320 | 1.0 | 269/357 | 1.0 |
| TC | 85/185 | 0.9 (0.6–1.2) | 196/219 | 1.2 (0.9–1.5) | |
| CC | 15/30 | 0.9 (0.5–1.8) | 29/42 | 0.9 (0.5–1.5) | |
| P trend | 0.41 | 0.51 | |||
| rs6957 | AA | 164/311 | 1.0 | 262/344 | 1.0 |
| AG | 86/183 | 0.9 (0.6–1.2) | 198/222 | 1.1 (0.9–1.5) | |
| GG | 16/28 | 1.1 (0.6–2.0) | 27/38 | 0.9 (0.6–1.6) | |
| P trend | 0.68 | 0.49 | |||
| rs2241718 | GG | 184/348 | 1.0 | 326/400 | 1.0 |
| GA | 72/159 | 0.9 (0.6–1.2) | 151/189 | 1.0 (0.8–1.3) | |
| AA | 10/18 | 1.1 (0.5–2.3) | 13/20 | 0.8 (0.4–1.6) | |
| P trend | 0.56 | 0.62 | |||
NOTE: ORs were adjusted for age, sex, ethnicity, and recruitment clinic.
P trend for increasing number of variant alleles.
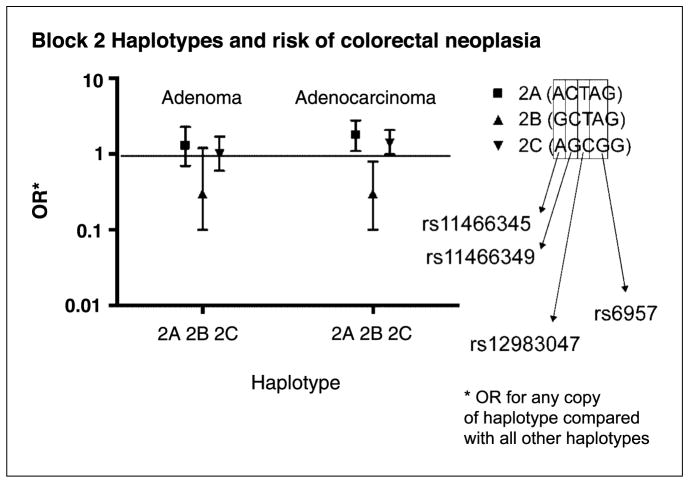
The results of the case-control analyses for haplotypes are presented in Table 3. Consistent with the findings for the SNP analyses, haplotype 2B, containing the G allele of SNP rs11466345 and the C allele of SNP rs11466349, was inversely associated with colorectal cancer risk (comparing individuals with one or two copies of this haplotype to those with no copy: OR, 0.3; 95% CI, 0.1–0.8). Haplotypes 2A and 2C, which contain the A allele for rs11466345, were directly associated with risk [haplotype 2A: OR, 1.8 (95% CI, 1.1–2.8); haplotype 2C: OR, 1.4 (95% CI, 1.0–2.1)], which is also consistent with the SNP analysis. In the ethnic-specific analyses, the relationship with haplotype 2A was observed in Caucasians (OR, 12.2; 95% CI, 1.6–27.6) and suggested in Native Hawaiians (OR, 2.4; 95% CI, 0.9–5.9). The relationships with haplotypes 2B and 2C were also observed in Japanese [haplotype 2B: OR, 0.5 (95% CI, 0.1–0.5); haplotype 2C: OR, 1.6 (95% CI, 1.0–2.4)]. The patterns of association for block 2 and the risk of adenoma were consistent with those for adenocarcinoma, especially for haplotype 2B, but were not statistically significant (haplotype 2B: OR, 0.3; 95% CI, 0.1–1.2; Table 3).
Table 3.
ORs and 95% CIs for risk of adenoma and adenocarcinoma based on specific haplotypes versus all other haplotypes, Honolulu, Hawaii 1994–2002
| Haplotype | All races
|
||
|---|---|---|---|
| Cases (n) | Controls (n) | OR (95% CI) | |
| Block 1 | |||
| Adenoma | |||
| 1A (TGCTGGGTT) | 35 | 77 | 0.9 (0.6–1.4) |
| 1B (AGTCGGGTT) | 162 | 349 | 0.8 (0.6–1.1) |
| 1C (TACTGTTCC) | 17 | 40 | 0.9 (0.5–1.6) |
| 1D (TGCTGTTCC) | 179 | 339 | 1.2 (0.9–1.7) |
| 1E (TGCCCTGCC) | 18 | 26 | 1.7 (0.9–3.2) |
| Adenocarcinoma | |||
| 1A (TGCTGGGTT) | 82 | 80 | 1.3 (0.9–1.8) |
| 1B (AGTCGGGTT) | 327 | 404 | 0.9 (0.7–1.2) |
| 1C (TACTGTTCC) | 30 | 30 | 1.1 (0.6–1.9) |
| 1D (TGCTGTTCC) | 245 | 391 | 1.1 (0.9–1.4) |
| 1E (TGCCCTGCC) | 21 | 33 | 0.7 (0.4–1.2) |
| Block 2 | |||
| Adenoma | |||
| 2A (ACTAG) | 254 | 504 | 1.3 (0.7–2.3) |
| 2B (GCTAG) | 2 | 13 | 0.3 (0.1–1.2) |
| 2C (AGCGG) | 25 | 48 | 1.0 (0.6–1.7) |
| Adenocarcinoma | |||
| 2A (ACTAG) | 491 | 557 | 1.8 (1.1–2.8) |
| 2B (GCTAG) | 8 | 27 | 0.3 (0.1–0.8) |
| 2C (AGCGG) | 71 | 67 | 1.4 (1.0–2.1) |
NOTE: ORs comparing one or two copies of a haplotype to all other haplotypes and adjusted for age, sex, and ethnicity.
Results did not differ by colon versus rectum or by stage for single SNPs or for haplotypes (data not shown). We also did not detect any interactions between number of variant alleles and body mass index, alcohol use, or smoking status (the three variables found to be associated with serum TGFβ-1 level in the adenoma study), or with regular aspirin use.
Discussion
We used two case-control studies, a screening-based study of colorectal adenoma and a population-based case control study of colorectal adenocarcinoma, to study in a comprehensive manner the genetic variation at the TGFB1 locus and its relationship with serum TGFβ-1 levels and risk of colorectal neoplasia. Consistent with previous studies (6, 7, 30), we observed a large interindividual variation in serum TGFβ-1 level among the subjects tested. The individual levels we observed were also similar to those in previous studies (6, 7, 30). We found that the variant A allele for tagSNP rs6957 was associated with an increased serum level of TGFβ-1 (Pdifference = 0.05; Fig. 2A). This association remained after adjustment for variables that were associated with serum TGFβ-1 levels in our study (age, ethnicity, smoking, and alcohol). We also found that the G allele of SNP rs11466345 (Fig. 2B) and the haplotype on which this allele and allele A of rs6957 fall (2B, GCTAG; Fig. 3) were inversely associated with risk of colorectal neoplasia. This association was consistent across the three ethnic groups studied. The G allele of the adjacent tagSNP rs11466349 (not found on haplotypes 2B) was also directly yet marginally associated with adenocarcinoma (P trend = 0.05). However, haplotype 2A, which was associated with an increased risk of adenocarcinoma in our study, also contained the A allele of rs6957 but contained the A allele of rs11466345. Thus, the combination of both the G allele for rs11466345 and the A allele for rs6957 seems to be important in risk reduction. Interestingly, none of the SNPs previously suggested to be functional, including G—800A, C—509T, L10P, R25P, and T263I, were found to be statistically significantly associated with serum level or adenoma or adenocarcinoma risk. For C—509T, the trend for point estimates of mean serum concentration seemed to increase with number of variant alleles as expected based on previous studies (5–7). Berndt et al. (14) reported recessive model associations between —509TT and 10PP genotypes and advanced adenomas that were larger with multiple and rectal adenomas in a 94% Caucasian population. We did not have the power to stratify Caucasians (or the other ethnicities) by adenoma substage or subsite, and this could account for our null results.
Figure 3.
ORs and 95% CIs for adenoma and adenocarcinoma risk by haplotypes in LD block #2 (A–C). The ORs are for carriers of at least one copy of a particular haplotype, compared with all other subjects adjusted for age, sex, and ethnicity.
The two most commonly studied among these SNPs, L10P (rs1982073) and C—509T (rs1800469), have yielded mixed results in relation to cancer risk in past studies. A direct association between L10P and breast cancer has been observed in some studies (31–33), but not in others (34–36). Another study saw no evidence for an association with L10P and advanced prostate cancer (37). Dunning et al. (31) reported a direct association between C—509T and invasive breast cancer, as did Ewart-Toland et al. (37) with late-stage prostate cancer. Recently, no association was found between any of the five previously identified SNPs and prostate cancer in Caucasians and African Americans in the PLCO (38). With regard to colorectal neoplasia, Sparks et al. (12) found no association between L10P and adenoma, but did find a suggestive inverse association of the P allele with hyperplastic polyps among ever smokers (P trend = 0.05). Macarthur et al. (13) found no association between C—509T genotype and colorectal cancer. Berndt et al. (14) found a statistically significant increased risk of advanced adenoma for the 10PP genotype and the —509TT genotype within the PLCO population. The magnitude of the latter association was higher in those with multiple adenomas and in the subset of rectal adenomas.
The SNP associated with risk in our study (rs11466345) is intronic. However, the haplotype block in which it lies also covers the last two exons and the 3′ untranslated region of the gene. Thus, it is possible that this SNP is in linkage disequilibrium with a causal SNP that may either result in a change in amino acid or affect a possible splice variant or the stability of the mRNA. In addition, we found allele A of SNP rs6957, also on the same haplotype, to be associated with higher serum TGFβ-1 levels (Ptrend = 0.04), the direction proposed to be protective against colorectal neoplasia. None of the SNPs we measured either in the multiethnic panel or the adenoma and adenocarcinoma cases and controls were in exons 6 and 7 because of the absence of SNPs with frequency >0.05 in the public SNP databases.
The strengths of our study include a comprehensive approach toward characterizing the TGFB1 gene by leveraging the linkage disequilibrium that exists at this locus, as well as carefully adjusting for potential confounders. The consistency of the results observed across ethnic/racial groups suggests that population stratification is an unlikely explanation for the observed association with risk. Finally, we studied two end points representing early and late stages of colorectal neoplasia. Although, for breast cancer, it has been proposed that TGFβ-1 may be protective at early stages and detrimental effect at later stages (35, 39–42), our results do not suggest such a dual role in colorectal cancer.
One limitation of our study is that circulating TGFβ-1 was only measured at one time point and it is unclear how well a single measure may reflect long-term levels. Thus, intraindividual variation may have reduced our ability to detect a difference in serum level by case-control status. It is possible that one serum measure may not be enough to show the association with disease but is enough to show relationship with genotype. Survival bias may have been an issue in our case-control analysis of colorectal cancer. However, this is unlikely because the median time from identification of case to interview was reasonably short (4.5 months) and we did not find any differences in results by stage for adenocarcinoma. For the genetic analysis, we did not sequence the gene and instead used public SNP databases; thus, some variation could have been missed due to incompleteness of these databases. Controls in the adenoma study only received a flexible sigmoidoscopy. Thus, any adenoma located in the proximal colon may have been missed. Assuming that proximal and distal adenomas have the same relationships with the SNPs under study, as suggested by the adenocarcinoma data, the failure to identify proximal adenomas would result in nondifferential misclassification and attenuation of the risk estimates, but would not create a spurious association. Additionally, the overall statistical power for the adenoma study was lower than that of the adenocarcinoma study. There was also a lack of statistical power to detect associations in stratified (site or stage) and interaction analyses (BMI, alcohol, etc.) in both studies. Whereas a strength of our study is the inclusion of several ethnicities, our reduced sample sizes resulted in low statistical power for detecting ethnic differences. Finally, although we carried out a relatively large number of tests in this study, the likelihood that our finding for block 2 was due to chance is not supported by the global test, which was statistically significant (P = 0.01).
In summary, our data suggest that the A allele of SNP rs6957 may be associated with increased serum TGFβ-1 levels, and that allele G of SNP rs11466345 and its underlying haplotype (which also harbors allele A of rs6957) are inversely associated with colorectal cancer and, possibly, adenoma. If these findings are reproduced, future studies should examine SNPs in the last 2 exons, last intron, and the 3′ end of the gene for disease association with an even greater resolution than in the current study.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute research grants CA113066 and CA72520 and training grant T32 CA009314 (B.S. Saltzman).
We thank the Hawaii Tumor Registry, Castle Medical Center, Kaiser-Permanente Medical Center, Kuakini Medical Center, Queen’s Medical Center, the Pacific Health Research Institute, Straub Clinic and Hospital, St. Francis Medical Center, and Wahiawa General Hospital for their collaboration. We thank our interviewing and phlebotomy staff for data collection; Annette Lum-Jones, Ann Seifried, and Michelle Ault for genotyping; Geoffrey K. Maiyoh for TGFβ-1 serum analysis; and Maj Earle, Lucy Shen, and Anne Tome for data support; and Anthony J. Alberg and Elizabeth A. Platz for their insight and helpful comments. We also thank the two anonymous reviewers for their constructive comments.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Schuster N, Krieglstein K. Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 2.Roman C, Saha D, Beauchamp R. TGF-β and colorectal carcinogenesis. Microsc Res Tech. 2001;52:450–7. doi: 10.1002/1097-0029(20010215)52:4<450::AID-JEMT1030>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 4.Elliott RL, Blobe GC. Role of transforming growth factor β in human cancer. J Clin Oncol. 2005;23:2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Grainger DJ, Heathcote K, Chiano M, et al. Genetic control of the circulating concentration of transforming growth factor type β1. Hum Mol Genet. 1999;8:93–7. doi: 10.1093/hmg/8.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Miyauchi A, Takagi Y, Tanaka M, Mizuno M, Harada A. Association of the C-509→T polymorphism, alone of in combination with the T869→C polymorphism, of the transforming growth factor-β1 gene with bone mineral density and genetic susceptibility to osteoporosis in Japanese women. J Mol Med. 2001;79:149–56. doi: 10.1007/s001090100190. [DOI] [PubMed] [Google Scholar]
- 7.Yokota M, Ichihara S, Lin TL, Nakashima N, Yamada Y. Association of a T29→C polymorphism of the transforming growth factor-β1 gene with genetic suscep tibility to myocardial infarction in Japanese. Circulation. 2000;101:2783–7. doi: 10.1161/01.cir.101.24.2783. [DOI] [PubMed] [Google Scholar]
- 8.Hauck EW, Hauptmann A, Schmelz HU, Bein G, Weidner W, Hackstein H. Prospective analysis of single nucleotide polymorphisms of the transforming growth factor β-1 gene in Peyronie’s disease. J Urol. 2003;169:369–72. doi: 10.1016/S0022-5347(05)64129-8. [DOI] [PubMed] [Google Scholar]
- 9.Syrris P, Carter ND, Metcalfe JC, et al. Transforming growth factor-β1 gene polymorphisms and coronary artery disease. Clin Sci Lond. 1998;95:659–67. doi: 10.1042/cs0950659. [DOI] [PubMed] [Google Scholar]
- 10.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J Cell Biol. 1990;110:1361–7. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cambien F, Ricard S, Troesch A, et al. Polymorphisms of the transforming growth factor-1 gene in relation to myocardial infarction and blood pressure: the Etude Cas-Temoin de l’Infarctus du Myocarde (ECTIM) Study. Hypertension. 1996;28:881–7. doi: 10.1161/01.hyp.28.5.881. [DOI] [PubMed] [Google Scholar]
- 12.Sparks R, Bigler J, Sibert JG, Potter JD, Yasui Y, Ulrich CM. TGFβ1 polymorphism (L10P) and risk of colorectal adenomatous and hyperplastic polyps. Int J Epidemiol. 2004;33:955–61. doi: 10.1093/ije/dyh102. [DOI] [PubMed] [Google Scholar]
- 13.Macarthur M, Sharp L, Hold GL, Little J, El Omar EM. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiol Biomarkers Prev. 2005;14:1613–8. doi: 10.1158/1055-9965.EPI-04-0878. [DOI] [PubMed] [Google Scholar]
- 14.Berndt SI, Huang W, Chatterjee N, et al. Transforming growth factor β1 (TGFB1) gene polymorphisms and risk of advanced colorectal adenoma. Carcinogenesis. 2007;28:1965–70. doi: 10.1093/carcin/bgm155. [DOI] [PubMed] [Google Scholar]
- 15.Le Marchand L, Donlon T, Seifried A, Kaaks R, Rinaldi S, Wilkens LR. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J Natl Cancer Inst. 2002;94:454–60. doi: 10.1093/jnci/94.6.454. [DOI] [PubMed] [Google Scholar]
- 16.Le Marchand L, Hankin JH, Wilkens LR, et al. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1259–66. [PubMed] [Google Scholar]
- 17.Hankin JH, Yoshizawa CN, Kolonel LN. Reproducibility of a diet history in older men in Hawaii. Nutr Cancer. 1990;13:129–40. doi: 10.1080/01635589009514054. [DOI] [PubMed] [Google Scholar]
- 18.Hankin JH, Wilkens LR, Kolonel LN, Yoshizawa CN. Validation of a quantitative diet history method in Hawaii. Am J Epidemiol. 1991;133:616–28. doi: 10.1093/oxfordjournals.aje.a115934. [DOI] [PubMed] [Google Scholar]
- 19.The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 20.Haiman CA, Stram DO, Pike MC, et al. A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Hum Mol Genet. 2003;12:2679–92. doi: 10.1093/hmg/ddg294. [DOI] [PubMed] [Google Scholar]
- 21.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4:519–27. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 22.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–93. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 25.Stram DO, Haiman CA, Hirschhorn JN, et al. Choosing haplotype-tagging SNPS based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered. 2003;55:27–36. doi: 10.1159/000071807. [DOI] [PubMed] [Google Scholar]
- 26.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 27.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005;76:449–62. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-β1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–61. doi: 10.1002/(sici)1097-0142(19990201)85:3<554::aid-cncr6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Dunning AM, Ellis PD, McBride S, et al. A transforming growth factor β1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res. 2003;63:2610–5. [PubMed] [Google Scholar]
- 32.Ziv E, Cauley J, Morin PA, Saiz R, Browner WS. Association between the T29→C polymorphism in the transforming growth factor β1 gene and breast cancer among elderly white women: The Study of Osteoporotic Fractures. JAMA. 2001;285:2859–63. doi: 10.1001/jama.285.22.2859. [DOI] [PubMed] [Google Scholar]
- 33.Kaklamani VG, Baddi L, Liu J, et al. Combined genetic assessment of transforming growth factor-β signaling pathway variants may predict breast cancer risk. Cancer Res. 2005;65:3454–61. doi: 10.1158/0008-5472.CAN-04-2961. [DOI] [PubMed] [Google Scholar]
- 34.Krippl P, Langsenlehner U, Renner W, et al. The L10P polymorphism of the transforming growth factor-β1 gene is not associated with breast cancer risk. Cancer Lett. 2003;201:181–4. doi: 10.1016/s0304-3835(03)00468-3. [DOI] [PubMed] [Google Scholar]
- 35.Le Marchand L, Haiman CA, van den BD, Wilkens LR, Kolonel LN, Henderson BE. T29C polymorphism in the transforming growth factor β1 gene and postmenopausal breast cancer risk: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2004;13:412–5. [PubMed] [Google Scholar]
- 36.Jin Q, Hemminki K, Grzybowska E, et al. Polymorphisms and haplotype structures in genes for transforming growth factor β1 and its receptors in familial and unselected breast cancers. Int J Cancer. 2004;112:94–9. doi: 10.1002/ijc.20370. [DOI] [PubMed] [Google Scholar]
- 37.Ewart-Toland A, Chan JM, Yuan J, Balmain A, Ma J. A gain of function TGFB1 polymorphism may be associated with late stage prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:759–64. [PubMed] [Google Scholar]
- 38.Kang D, Lee KM, Park SK, et al. Lack of association of transforming growth factor-β1 polymorphisms and haplotypes with prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Trial. Cancer Epidemiol Biomarkers Prev. 2007;16:1303–5. doi: 10.1158/1055-9965.EPI-06-0895. [DOI] [PubMed] [Google Scholar]
- 39.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiss M, Barcellos-Hoff MH. Transforming growth factor-β in breast cancer: a working hypothesis. Breast Cancer Res Treat. 1997;45:81–95. doi: 10.1023/a:1005865812918. [DOI] [PubMed] [Google Scholar]
- 41.Roberts AB, Wakefield LM. The two faces of transforming growth factor β in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kretzschmar M. Transforming growth factor-β and breast cancer: transforming growth factor-β/SMAD signaling defects and cancer. Breast Cancer Res. 2000;2:107–15. doi: 10.1186/bcr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.