Abstract
Acinar cells in pancreatitis die through apoptosis and necrosis, the roles of which are different. The severity of experimental pancreatitis correlates directly with the extent of necrosis and inversely, with apoptosis. Apoptosis is mediated by the release of cytochrome c into the cytosol followed by caspase activation, whereas necrosis is associated with the mitochondrial membrane potential (ΔΨm) loss leading to ATP depletion. Here, we investigate the role of Bcl-2 proteins in apoptosis and necrosis in pancreatitis. We found up-regulation of prosurvival Bcl-2 proteins in pancreas in various experimental models of acute pancreatitis, most pronounced for Bcl-xL. This up-regulation translated into increased levels of Bcl-xL and Bcl-2 in pancreatic mitochondria. Bcl-xL/Bcl-2 inhibitors induced ΔΨm loss and cytochrome c release in isolated mitochondria. Corroborating the results on mitochondria, Bcl-xL/Bcl-2 inhibitors induced ΔΨm loss, ATP depletion and necrosis in pancreatic acinar cells, both untreated and hyperstimulated with CCK-8 (in vitro pancreatitis model). Together Bcl-xL/Bcl-2 inhibitors and CCK induced more necrosis than either treatment alone. Bcl-xL/Bcl-2 inhibitors also stimulated cytochrome c release in acinar cells leading to caspase-3 activation and apoptosis. However, different from their effect on pronecrotic signals, the stimulation by Bcl-xL/Bcl-2 inhibitors of apoptotic responses was less in CCK-treated than control cells. Therefore, Bcl-xL/Bcl-2 inhibitors potentiated CCK-induced necrosis but not apoptosis. Correspondingly, transfection with Bcl-xL siRNA stimulated necrosis but not apoptosis in the in vitro pancreatitis model. Further, in animal models of pancreatitis Bcl-xL up-regulation inversely correlated with necrosis, but not apoptosis. Results indicate that Bcl-xL and Bcl-2 protect acinar cells from necrosis in pancreatitis by stabilizing mitochondria against death signals. We conclude that Bcl-xL/Bcl-2 inhibition would aggravate acute pancreatitis, whereas Bcl-xL/Bcl-2 up-regulation presents a strategy to prevent or attenuate necrosis in pancreatitis.
Keywords: Pancreatic acinar cell, Bcl-xL, CCK, Mitochondrial membrane potential, Cytochrome c release, Caspase-3
Introduction
Acinar cell death is a major pathological response of acute pancreatitis; in particular, parenchymal necrosis is a major cause of severe complications and mortality in human pancreatitis [1,2]. In models of acute pancreatitis acinar cells die through both necrosis and apoptosis. The severity of experimental pancreatitis correlates directly with the extent of necrosis and inversely, with apoptosis [2–8]. Thus, elucidating the mechanisms that mediate acinar cell death in pancreatitis is important for understanding the mechanism of this disease and is of clinical relevance.
Mechanisms underlying these major forms of cell death are different [9–12], although they both involve mitochondria. Apoptosis is mediated by the release of cytochrome c from mitochondria into the cytosol. Once in cytosol, cytochrome c causes activation of specific cysteine proteases, the caspases (e.g., the major effector caspase-3), which execute apoptotic cell death [9–13]. On the other hand, necrosis is mediated by the loss of mitochondrial membrane potential (ΔΨm). Which ultimately leads to depletion of cellular ATP and necrosis [9,11,12,14]. Depolarization is mediated by opening of the mitochondrial permeability transition pore (PTP), a multi-subunit complex formed by proteins residing in both inner and outer mitochondrial membrane. PTP opening is associated with swelling of mitochondrial matrix and consequent rupture of the outer mitochondrial membrane [11,15,16], which allows the release of cytochrome c. Recent data on mice lacking cyclophilin D [15,17] show, however, that cytochrome c can be released independent of PTP, through the channels in the outer mitochondrial membrane [9,11,15–17]. We have recently showed [18–20] that in isolated pancreatic mitochondria PTP mediates loss of ΔΨm but not cytochrome c release.
Bcl-2 family proteins are important regulators of cell death, particularly apoptosis [9,11,21]. They act through regulating of mitochondrial outer membrane permeabilization, which mediates cytochrome c release into cytosol [9,11,21]. Much less is known on the role of Bcl-2 proteins in the regulation of mitochondrial depolarization leading to necrosis [22,23].
Bcl-2 proteins are subdivided into 3 groups on the basis of their Bcl-2 homology (BH) domains. The “prosurvival” members, such as Bcl-2 itself and Bcl-xL, contain four BH domains (BH1–BH4). The “pro-apoptotic” members, such as Bax and Bak, contain three BH domains; and the “BH3-only” proapoptotic proteins, such as Bad, Puma and Noxa, only contain the BH3 domain.
Each of the 3 groups of the Bcl-2 family proteins has specific functional roles in the regulation of apoptosis [9,11,13,24]. In particular, the pro-apoptotic Bax and Bak form channels in the outer mitochondrial membrane through which cytochrome c is released into the cytosol (this permeability system remains poorly characterized) [13,25]. The BH3-only proteins facilitate Bax/Bak channel formation, and thus cytochrome c release and apoptosis [9,11]. On the other hand, the prosurvival Bcl-xL and Bcl-2 inhibit apoptosis by sequestering BH3-only proteins (as well as Bax and Bak) [9,11]. Bcl-2 can also block PTP opening, thus preventing loss of ΔΨm and subsequent necrosis [22,23]. Small-molecule pharmacological inhibitors of the prosurvival Bcl-xL and Bcl-2 have recently been developed and became a valuable tool to study the roles of these proteins [26–28].
We and others showed that cytochrome c release and mitochondrial depolarization occur and mediate acinar cell death in pancreatitis [5,29,30]. However, there is little known on the roles of Bcl-2 proteins in apoptotic and necrotic cell death in pancreatitis [2,31].
Here, we measured changes in the levels of various Bcl-2 proteins in models of acute pancreatitis and found marked up-regulation of the prosurvival protein Bcl-xL (as well as Bcl-2) in both total pancreatic tissue and pancreatic mitochondria. Using pharmacological Bcl-xL/Bcl-2 inhibitors and Bcl-xL knockdown with Bcl-xL siRNA transfection, we assessed the role of Bcl-xL and Bcl-2 in the regulation of ΔΨm, cytochrome c release and subsequent necrosis and apoptosis in isolated pancreatic mitochondria, intact pancreatic acinar cells and in acinar cells hyperstimulated with CCK-8, the experimental system considered in vitro model of acute pancreatitis [5,7,29,32–36].
The results indicate that by preventing mitochondrial depolarization and subsequent ATP depletion, Bcl-xL and Bcl-2 protect acinar cells in pancreatitis against necrosis (rather than apoptosis). They suggest that Bcl-xL/Bcl-2 inhibition, which is applied in clinical trials to stimulate apoptotic death of cancer cells, would likely increase necrosis and thus the severity of acute pancreatitis. By contrast, Bcl-xL/Bcl-2 up-regulation or stabilization may represent a promising strategy to prevent or attenuate necrosis in pancreatitis.
Materials and methods
Materials
Antibodies against Bcl-xL, Bcl-2, and p44/42 MAP kinase (ERK1/2) were from Cell Signaling (Beverly, MA); Bax and Bak, Bid, Bim from Santa Cruz Biotechnology (Santa Cruz, CA); COX IV, from Molecular Probes (Eugene, OR). Cerulein was from Peninsula Laboratories (Belmont, CA); CCK-8, from American Peptide (Sunnyvale, CA). The Bcl-xL/Bcl-2 inhibitor 3-iodo-5-chloro-N-[2-chloro-5-(4 chlorophenyl)- sulphonyl)phenyl]-2-hydroxybenzamide (BH3I-2′) was from Calbiochem (La Jolla, CA); ethyl 2-amino-6-bromo-4-(1-cyano-2-ehtoxy-2-oxoethyl)-4H-chromene-3-car-boxylate (HA 14-1), from ALEXIS Biochemicals (San Diego, CA). Other reagents were from Sigma Chemical (St. Louis, MO).
Animal models of acute pancreatitis
Cerulein pancreatitis was induced in male (200–250 g) Spra-gue–Dawley rats and male (25–30 g) Swiss Webster CD-1 mice as described previously [5,35,36] by up to 7 hourly intraperitoneal (i.p.) injections of 50 µg /kg cerulein. Control animals received injections of physiological saline. In the cerulein models, animals were sacrificed at 0.5, 4 or 7 h after the 1st cerulein (or saline) injection. L-arginine pancreatitis was induced in Sprague-Dawley rats as described previously [5], by 2 hourly i.p. injections of 2.5 g/kg L-arginine; controls received similar injections of saline. Rats were sacrificed 24 h after the 1st injection. Choline-deficient, ethionine supplemented (CDE) diet pancreatitis was induced as described previously [37] in 5-wk old CD-1 mice weighing 14.5 ± 0.2 g. Both the CDE and control diet were obtained from Harlan Teklad (Madison, WI) and were provided fresh to the animals every 12 h in 3-g aliquots. At each feeding, the CDE diet was supplemented with 0.5% ethionine. Mice were sacrificed 72 h after the initiation of the diet.
The development of pancreatitis was confirmed by measurements of serum amylase and lipase levels (using the Hitachi 707 analyzer at Antech Diagnostics, Irvine, CA), and of histological changes as analyzed on H&E stained pancreatic tissue sections.
Care and handling of the animals were approved by the Animal Research Committee of the VA Greater Los Angeles Healthcare System, in accordance with the National Institutes of Health guidelines.
Isolation of pancreatic acinar cells
Isolation of pancreatic acinar cells from rats was performed using a collagenase digestion procedure as described previously [5,29,35,36]. For treatments, the isolated acinar cells were incubated at 37 °C in 199 medium with or without 100 nM CCK-8 (CCK) and other agents as described in corresponding figures.
Prolonged culture of mouse acinar cells and transfection
Isolated pancreatic acinar cells are short-lived. To measure the effect of Bcl-xL knockdown with siRNA, we established a prolonged (up to 36 h) culture of mouse pancreatic acinar cells. Mouse pancreatic acinar cells were cultured according to [38] on collagen IV in DMEM medium containing 15% FBS, 5 ng/ml EGF, 0.25 µg/ml amphotericin B, 0.5 mM IBMX, 0.2 mg/ml soybean trypsin inhibitor, 100 U/ml penicillin, 100 µg/ml streptomycin. Acinar cells cultured in these conditions maintain phenotype and do not de-differentiate into ductal cells [38]. Cultured acinar cells were transfected with Bcl-xL siRNA using SMARTpool™ from Dharmacon (Lafayette, CO). For negative control, we used ON-TARGET siCONTROL Non-Targeting pool; for positive control, the siGLOcyclophillin B siRNA labeled with fluorescent CX-rhodamine (all from Dharmacon). Transfections (100 pmoles of each siRNA) were performed using the Amaxa electroporation system (Amaxa Transfection, Gaithersburg, MD). Transfected cells were then transferred to 199 medium containing no growth factors and incubated for 3 h with and without 100 nM CCK-8.
Isolation of pancreatic mitochondria and measurements of respiration and mitochondrial membrane potential (ΔΨm)
Mitochondria were isolated from rat or mouse pancreas using previously described procedures [18]. Briefly, pancreas was dissected, minced, and homogenized in a medium containing 250 mM sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 0.5% BSA, and 0.25 mg/ml soybean trypsin inhibitor. The homogenate was centrifuged at 800×g for 10 min to sediment cell debris, nuclei, and zymogen granules. The resulting supernatant was centrifuged at 6000×g for 15 min, and the pellet washed by centrifugation and re-suspended in 200 ml of a medium containing 250 mM sucrose and 10 mM Tris-HCl (pH 7.4). Mitochondria suspensions contained 20–30 mg protein/ml, as determined by the Bradford assay (Bio-Rad Laboratories, Hercules, CA).
The medium used in mitochondria functional assays contained 250 mM sucrose, 22 mM KCl, 22 mM triethanolamine (pH 7.4), 3 mM MgCl2, 5 mM KH2PO4, 0.5% BSA, and 1 mM EGTA. In all experiments on isolated mitochondria, 10 mM succinate was used as the respiratory substrate. The measurements were performed at room temperature.
Respiration rate and ΔΨm were simultaneously recorded in the mitochondria suspension (1 mg protein/ml) in a 1-ml custom-made chamber. Oxygen consumption was measured using a Clark-type electrode (Instech Lab., Plymouth Meeting, PA) connected to an oxygen meter (Yellow Springs Instruments, Yellow Springs, OH). Quality of mitochondria preparations was assessed by measuring the ratio of oxygen uptake in the presence of ADP to that in the absence of ADP (respiratory control ratio). The value of respiratory control ratio in the presence of succinate was >3 in all mitochondria preparations, indicating mitochondria functional integrity. The membrane potential was monitored as in [18] in the presence of 2 µM tetraphenyl phosphonium (TPP+) using a TPP+-sensitive electrode [39] connected to an amplifier (Vernier Software, Beaverton, OR). TPP+ is redistributed to mitochondria according to membrane potential. An increase in ΔΨm results in TPP+ uptake by mitochondria and, correspondingly, in a decrease in external TPP+ concentration measured by the electrode.
Measurements of ΔΨm in pancreatic acinar cells
Measurements of ΔΨm in pancreatic acinar cells were performed by use of the Mitochondrial Membrane Potential Detection Kit (Cell Technology Inc., Mountain View, CA) according to manufacturer’s instructions. Briefly, cells were re-suspended in the assay buffer, incubated with the ΔΨm-sensitive fluorescent dye JC-1 for 20 min at 37 °C, washed twice in PBS, and then the “red” (excitation 550 nm, emission 600 nm) and “green” (excitation 485 nm, emission 535 nm) fluorescence were measured in a Shimadzu RF-1501 spectrofluorometer. Mitochondrial depolarization (i.e., loss of ΔΨm) manifests itself by a decrease in the red/ green fluorescence ratio.
Western blot analysis
Western blot analysis was performed on homogenates of pancreatic tissue or isolated mitochondria, or on membrane and cytosolic fractions, as previously described [5,18,29,35,36]. Briefly, snap-frozen pancreatic tissue was homogenized on ice in RIPA buffer supplemented with 1 mM PMSF and a protease inhibitor cocktail containing pepstatin, leupeptin, chymostatin, antipain and aprotinin (5 µg/ml each), rotated for 20 min at 4 °C, and centrifuged at 16,000×g for 15 min at 4 °C. The supernatant was collected and stored at −80 °C. Protein concentration was determined by the Bradford assay. Proteins were separated by SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes. Nonspecific binding was blocked by 1-h incubation of the membranes in 5% (w/v) nonfat dry milk inTris-buffered saline (pH 7.5). Blots were then incubated for 2 h at room temperature (or overnight at 4 °C) with primary antibodies in the antibody buffer containing 1% (w/v) nonfat dry milk in TTBS (0.05% (v/v) Tween-20 in Tris-buffered saline), washed 3 times with TTBS, and finally incubated for 1 h with a peroxidase-labeled secondary antibody in the antibody buffer. Blots were developed for visualization using enhanced chemiluminescence (ECL) detection kit (Pierce, Rockford, IL). Band intensities on the immunoblots were quantified by densitometry using the Scion imaging software (Scion Corporation, Frederick, MD).
Measurements of Bcl-xL mRNA expression by reverse transcription and polymerase chain reaction (RT-PCR)
The procedures for RNA isolation and conventional RT-PCR were as we described previously [35–37]. Briefly, total RNA was obtained from pancreatic tissue using TRI reagent (Molecular Research Center, Cincinnati, OH) and its quality assessed in Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). RNA was reverse-transcribed with the SuperScript II preamplification kit (GIBCO-BRL, Rockville, MD) and subjected to either real-time or conventional semiquantitative RT-PCR using gene specific, intron-spanning primers. Negative controls were performed by omitting the RT step or cDNA template from the PCR amplification.
Real-time RT-PCR was carried out in iQ5 Real Time PCR Detection System (Bio-Rad Laboratories) using primers designed with Beacon Designer software (Table 1). In these experiments, cDNA derived from 50 ng total RNA was used in each sample. mRNA expression was quantified by the double delta Ct method relative to that for the acidic ribosomal phosphoprotein P0 (ARP) used as a reference (housekeeping) control. We have previously shown [36] that pancreatic ARP mRNA expression is not affected by experimental pancreatitis. In semiquantitative RT-PCR, the target ARP and Bcl-xL sequences were amplified at the annealing temperature 58.5 °C during 20 or 27 cycles, respectively, to yield visible products within linear amplification range. In these experiments, cDNA derived from 400 ng total RNA was used in each sample. Resulting RT-PCR products were run on agarose gel and visualized by staining with ethidium bromide. Band intensities of the RT-PCR products were quantified using the Scion imaging software.
Table 1.
RT-PCR primers used for analysis of Bcl-xL gene expression in pancreas.
| mRNA | Forward primer | Reverse primer | Product size (bp) | GenBank accession no. |
|---|---|---|---|---|
| Mouse ARP | 5′-cgtcctggcattgtctgtgg | 5′-catctgattcctccgactcttcc | 185 | NM_007475 |
| Mouse Bcl-xL | 5′-tgaatgaccacctagagccttg | 5′-cagaaccacaccagccacag | 155 | NM_009743 |
| Rat ARP | 5 ′-actgactacaccttcccactg | 5′-tcctccgactcttcctttgc | 155 | NM_022402 |
| Rat Bcl-xL (transcript variant 3) | 5 ′-tgaccacctagagccttg | 5′-gaactacaccagccacag | 149 | NM_001033670 |
| Rat Bcl-xL (transcript variant 1) | 5 ′-cacctcctcccgacctatgat | 5′-cccggttgctctgagacattt | 208 | NM_001033672 |
Note. ARP, acidic ribosomal protein P0.
Cytochrome c release
To measure cytochrome c release from isolated mitochondria, we used aliquots of the same mitochondria suspensions in which measurements of ΔΨm were performed. After incubating in various conditions described in the corresponding figures, mitochondria were centrifuged at 16,000×g for 10 min at 4 °C, and cytochrome c levels in the mitochondria pellet and the incubation medium (supernatant) were measured by Western blot analysis as previously described [18]. Aliquots for measurements of cytochrome c release were taken after 10 min of mitochondria incubation with and without inhibitors.
To measure cytochrome c release in pancreatic acinar cells [5,18,29], the cells were homogenized in a glass Dounce homogenizer (loose fit, 80 strokes) in a buffer containing 250 mM sucrose, 20 mM HEPES-KOH (pH 7.0), 10 mM KCl, 1 mM EGTA, 2 mM MgCl2,1 mM EDTA, 1 mM dithiothreitol (DTT),1 mM PMSF, and the above-specified protease inhibitors’ cocktail. Nuclei were removed by centrifugation at 1,000×g for 10 min at 4 °C. Postnuclear supernatant was centrifuged at 100,000×g for 1 h, and both the pellet (mitochondria-enriched membrane fraction) and supernatant (the cytosolic fraction) were collected separately and used for Western blotting.
ATP determination
Acinar cells were resuspended in a lysis buffer (100 mM Tris, 4 mM EDTA, pH 7.8), boiled for 2 min, centrifuged (1,000×g for 1 min), and ATP level was measured in the supernatant using luciferin/ luciferase-based ATP determination kit (Molecular Probes), according to manufacturer’s instructions. Luminescence was measured in a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA). ATP levels were normalized to protein content in the samples.
Caspase-3 activity
Caspase–3 activity was measured using a fluorogenic assay as described previously [5,18,29]. Acinar cells were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.5% Igepal CA-630 and 0.5 mM EDTA, centrifuged at 16,000×g for 15 min, and the supernatant collected. Proteolytic reactions were carried out at 37 °C in a buffer containing 25 mM HEPES (pH 7.5), 10% sucrose, 0.1% CHAPS and 10 mM DTT, using the substrate Ac-DEVD-AMC specific for caspase-3. Cleavage of this substrate relieves AMC (7-amino-4-methylcoumarin), which emits fluorescent signal with excitation at 380 nm and emission at 440 nm. Fluorescence was measured in a Shimadzu RF-1501 spectrofluorometer and calibrated using a standard curve for AMC. The data are expressed as mol AMC/mg protein/min.
Quantification of necrosis
Necrosis in rat pancreatic acinar cells was determined by the release of LDH into the incubation medium, as previously described [5,29,35]. LDH activity was measured using Cytotoxicity Detection Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. Necrosis in prolonged culture of trans-fected mouse acinar cells was determined as a percentage of cells stained positively with trypan blue.
Quantification of necrosis in pancreatic tissue was performed on sections stained with H&E, as previously described [5]. Cells with swollen cytoplasm, loss of plasma membrane integrity, and leakage of organelles into interstitium were considered necrotic.
Quantification of apoptosis
In pancreatic tissue, apoptosis was quantified on sections by use of TUNEL assay to measure DNA breaks, as described previously [5]. Briefly, tissue was fixed in 4% buffered formaldehyde, embedded in paraffin, and 6-µm-thick sections were adhered to glass slides. Sections were stained using terminal deoxynucleotidyl transferase and FITC-labeled dUTP according to the manufacturer’s protocol (Promega, Madison, WI).
Apoptosis in rat pancreatic acinar cells, and in prolonged culture of transfected mouse acinar cells was quantified by use of Hoechst 33258 or propidium iodine staining to visualize nuclear chromatin morphology, as described previously [5,18,29,35]. Briefly, cells were plated on polylysine-coated glass coverslips, fixed with methanol at −20°C for 10 min, and stained with 8 µg/ml Hoechst 33258 or 1 µg/ml propidium iodine. The slides were examined by fluorescence microscopy. Cells with nuclei containing condensed and/or fragmented chromatin were considered apop-totic. For quantification of apoptosis, a total of at least 3,000 acinar cells were counted on pancreatic tissue sections or cell smears for each condition.
Statistical analysis of data
This was done by using two-tailed Student’s t-test. p value <0.05 was considered statistically significant.
Results
Changes in pancreatic levels of Bcl-2 proteins in models of acute pancreatitis
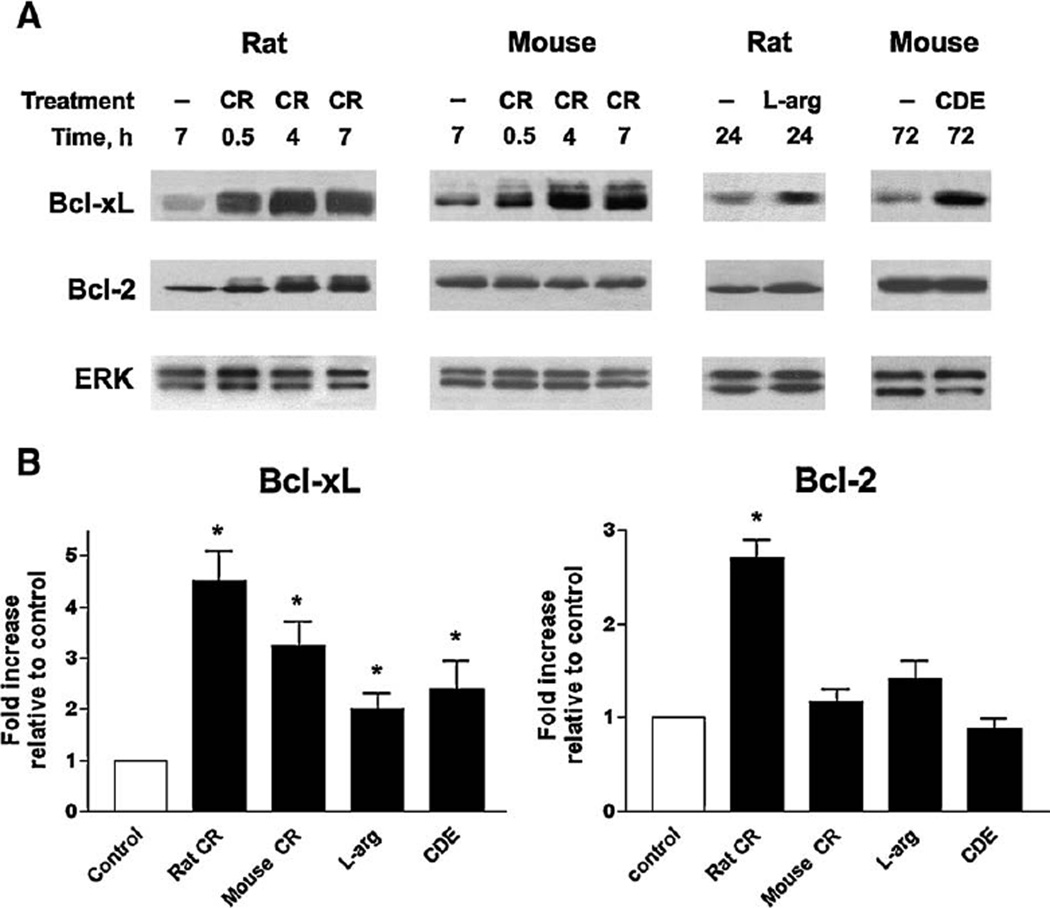
Western blot analysis showed that the prosurvival proteins Bcl-xL and Bcl-2 were present in normal rat and mouse pancreas, and were up-regulated in rodent models of acute pancreatitis (Fig. 1). Up-regulation of pancreatic Bcl-xL protein was detected in all models examined, namely pancreatitis induced by cerulein in rats and mice, by L-arginine in rats, and by choline-deficient ethionine supplemented (CDE) diet in mice. The extent of Bcl-xL up-regulation in fully developed pancreatitis was maximal in the rat cerulein model (~4.5-fold), and minimal (~2-fold) in the rat L-arginine model (Fig. 1B). Differently, pancreatic Bcl-2 level increased markedly in rat cerulein pancreatitis but not other models (Fig. 1). The upregulation of Bcl-xL and Bcl-2 occurred early in the development of cerulein pancreatitis, being already evident 30 min after the induction of pancreatitis (Fig. 1A).
Fig. 1.
Pancreatic levels of prosurvival proteins Bcl-xL and Bcl-2 are up-regulatedin rodent modelsof acute pancreatitis. Pancreatitis was induced in rats and mice as described in Materials and methods by administration of cerulein (CR), L-arginine (L-arg) or choline-deficient ethionine supplemented (CDE) diet. Control animals received saline injections (in the CR and L-arg models) or control diet (in the CDE model). (A) Bcl-xL and Bcl-2 protein levels were measured by Western blot analysis in pancreatic tissue of control and pancreatitic animals at indicated times after the induction of pancreatitis. Blots were re-probed for ERK1/2 to confirm equal protein loading. In this and other figures, Western blot data represent experiments that were repeated with similar results on at least three animals in each group. (B) Western blot was performed as in (A) on pancreatic tissue from rats and mice with fully developed pancreatitis: at 7hintheCR models,24 h in the L-arg model, and 72 h in the CDE model. The intensities of Bcl-xL and Bcl-2 bandson the immunoblots were quantifiedby densitometry and normalizedtothatofERK1/2 (loading control) inthe same sample. The mean ratio of Bcl-xL/ ERK1/2 (or Bcl-2/ERK1/2) intensities in animals with pancreatitis was further normalized to that in control group at the same time point. Values are means±SE from at least 3 animals per group. *p<0.05 compared with control.
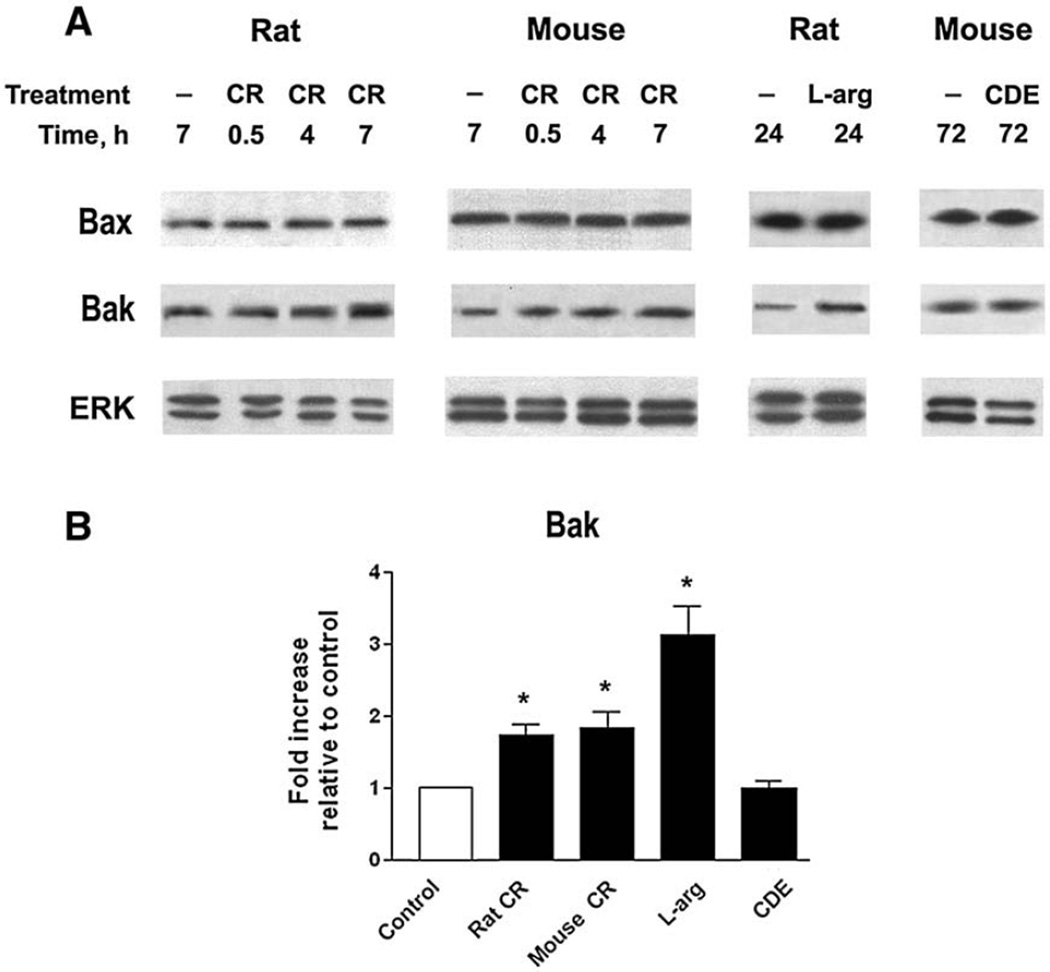
Pancreatic levels of the key pro-apoptotic protein Bax did not change in the models of pancreatitis tested (Fig. 2A). Another key pro-apoptotic Bcl-2 protein, Bak, was markedly (~3-fold) upregulated in the rat L-arginine model, and to a smaller extent, in mouse and rat cerulein pancreatitis (Figs. 2A, B).
Fig. 2.
Changes in pancreatic levels of pro-apoptotic Bax and Bak proteins in models of acute pancreatitis. Pancreatitis was induced in rats and mice as described in the legend to Fig. 1. (A) Bax and Bak protein levels were measured by Western blot analysis in pancreatic tissue of control and pancreatitic animals at indicated times after the induction of pancreatitis. Blots were re-probed for ERK1/2 to confirm equal protein loading. (B) Western blot was performed as in (A) on pancreatic tissue from rats and mice with fully developed pancreatitis: at 7 h in the CR models, 24 h in L-arg model, and 72 h in the CDE model. The intensities of Bak band on the immunoblots were quantified by densitometry and normalized to that of ERK1/2 (loading control) in the same sample. The mean ratio of Bak/ERK1/2 intensities in animals with pancreatitis was further normalized to that in control group at the same time point. Values are means±SE from at least 3 animals per group. *p<0.05 compared with control.
We also measured the levels of pro-apoptotic BH3 only proteins, Bim and Bid, in models of pancreatitis induced by cerulein in rat and mice. Rat cerulein pancreatitis is characterized by greater apoptosis and low necrosis, whereas mouse cerulein model has low apoptosis and high necrosis [2,4,5]. Western blot analysis showed no increase in Bim levels in these models of pancreatitis (not illustrated), indicating against its major role in the regulation of cell death in pancreatitis. The levels of Bid were too low to detect both in normal pancreas and in models of pancreatitis.
Bcl-xL and Bcl-2 levels in pancreatic mitochondria increase during cerulein pancreatitis
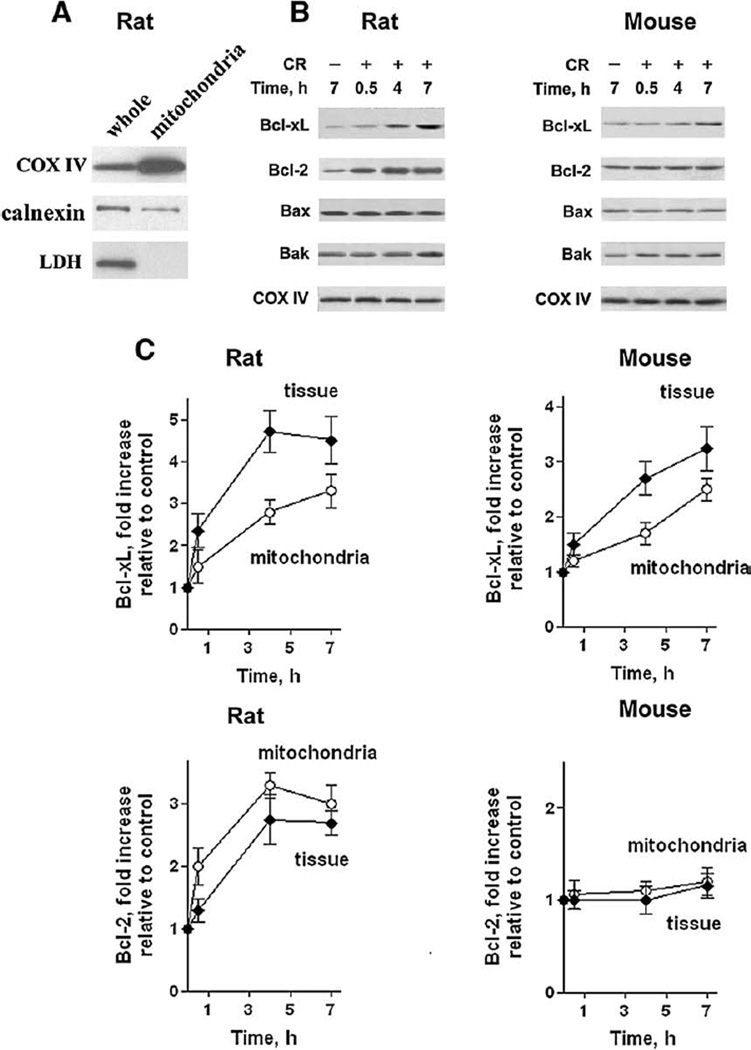
Death responses are regulated by Bcl-2 proteins localized in the mitochondria [9,11,13]. Therefore, an important question is whether the increases in pancreatic levels of Bcl-xL and Bcl-2 that we observed in models of pancreatitis (Fig. 1) translated into corresponding increases in mitochondrial levels of these proteins. For these measurements we used pancreatic mitochondria isolated from rats and mice as we have recently described in detail [18]. We also showed that as compared to whole tissue homogenates, mitochondrial preparations were enriched in mitochondrial marker cytochrome c oxidase IV, contained less ER marker calnexin, and no cytosolic marker LDH (Fig. 3A).
Fig. 3.
Mitochondrial levels of Bcl-xL and Bcl-2 increase during cerulein pancreatitis in parallel with their total levels in pancreas. (A) Characterization of the preparation of isolated rat pancreatic mitochondria was performed by measuring mitochondrial marker COX IV, endoplasmic reticulum marker calnexin, and cytosolic marker lactate dehydrogenase (LDH) in whole tissue homogenate (whole) and isolated mitochondria (mitochondria) by Western blot analysis. (B, C) Cerulein (CR) pancreatitis was induced in rats and mice as described in Materials and methods. Animals were sacrificed at indicated times. The levels of the various Bcl-2 proteins were measured by Western blot analysis in mitochondria isolated from pancreas of control and pancreatitic animals at indicated times after the induction of pancreatitis. Blots were re-probed for the mitochondrial marker COX IV to confirm equal protein loading. (C) The intensities of Bcl-xL and Bcl-2 bands on mitochondria immunoblots were quantified by densitometry and normalized to that of COX IV (loading control) in the same sample. The mean ratio of Bcl-xL/COX IV (or Bcl-2/COX IV) intensities in mitochondria from animals with pancreatitis was further normalized to that in control group at the same time point. The quantification of Bcl-xL and Bcl-2 protein levels in the whole pancreatic tissue was done as described in Fig. 1B. Values are means ±SE from at least 3 animals per group.
We found that in the course of cerulein pancreatitis, the mitochondrial levels of Bcl-2 proteins changed in parallel with those in total pancreas (Fig. 3). Same as their total levels in pancreas, the mitochondrial levels of Bcl-xL increased in both rat and mouse cerulein pancreatitis, whereas mitochondrial Bcl-2 increased only in the rat but not mouse cerulein model (Fig. 3). Furthermore, the kinetics of these proteins’ up-regulation in pancreatic mitochondria paralleled that in total pancreas (Fig. 3B). These data indicate that the increases in mitochondrial levels of Bcl-xL and Bcl-2 are due to the up-regulation of total levels of these proteins in pancreas.
The mitochondrial levels of pro-apoptotic Bax and Bak did not significantly change during cerulein pancreatitis in rats or mice (Fig. 3A). Therefore, our subsequent experiments focused on the roles of Bcl-xL and Bcl-2 in death responses of pancreatitis.
Pancreatic mRNA expression of Bcl-xL is up-regulated in cerulein pancreatitis
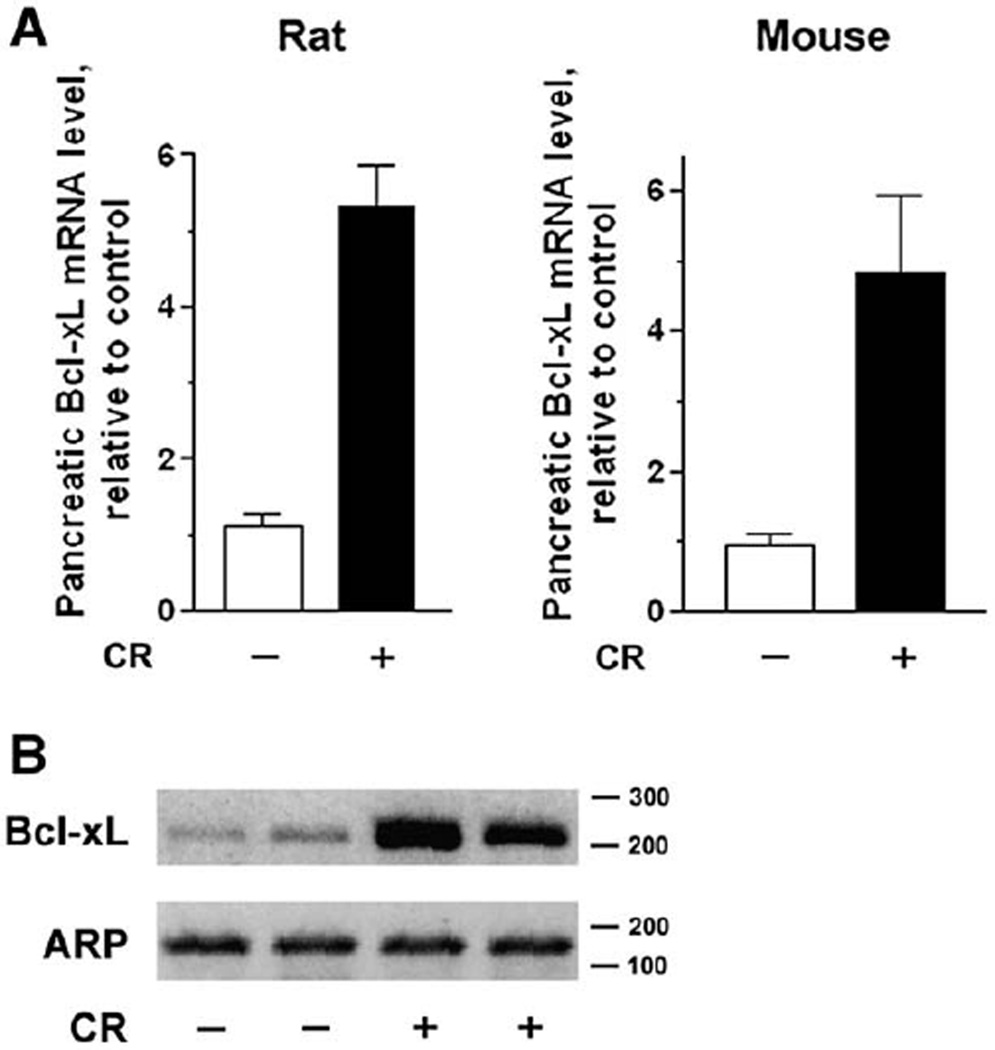
Because pancreatic Bcl-xL protein levels greatly increased during rat and mouse cerulein pancreatitis (Fig. 1), we asked whether such up-regulation was at the mRNA level. The bcl-X gene contains multiple promoters, and its transcription may generate several splice variants [40–42]. The main Bcl-xL transcript is termed in the rat “transcript variant 3” and codes for protein isoform 2 with molecular mass of approximately 26 kDa. Quantitative analysis, using real time RT-PCR, showed that the levels of this transcript increased several-fold during cerulein pancreatitis in both rat and mouse (Fig. 4A). Although characterization of alternative Bcl-xL splicing was not the purpose of our study, we tested whether pancreatitis also induced mRNA expression of a different transcript from the bcl-X gene (termed in the rat “transcript variant 1”, coding for protein isoform 1). Semiquantitative RT-PCR using primers specific for this transcript (Table 1), showed a 3–4 fold increase in the pancreatic level of this mRNA in rat cerulein pancreatitis (Fig. 4B).
Fig. 4.
Pancreatic levels of Bcl-xL mRNA increase in cerulein pancreatitis. Total RNA was extracted from pancreatic tissue of rats and mice with fully developed cerulein (CR) pancreatitis (at 7 h), as well as from control animals. RNA was subjected to RT-PCR as described in Materials and methods, using primers presented in Table 1. The gene for acidic ribosomal protein P0 was used as a reference (“housekeeping”) gene. (A) Expression of the main Bcl-xL transcript (transcript variant 3 in the rat) was quantified by real time RT-PCR and normalized to that in control group. Values are means ± SE from 3 animals per group. (B) Expression of rat Bcl-xL transcript variant 1 was measured by semiquantitative RT-PCR Each lane represents an individual animal; shown are the results for 2 rats per group. Numbers to the right of the gel are DNA size markers in base-pairs.
The results in Fig. 4 indicate that Bcl-xL up-regulation in cerulein pancreatitis is mediated at least in part through transcriptional activation.
Pharmacological Bcl-xL/Bcl-2 inhibitors induce both loss of ΔΨm and cytochrome c release in isolated pancreatic mitochondria
To assess the functional role of Bcl-xL and Bcl-2 in mitochondria-mediated necrosis and apoptosis of pancreatitis, we applied 2 structurally different pharmacological inhibitors of Bcl-xL and Bcl-2, HA14-1 and BH3I-2′ [26–28]. Both inhibitors specifically bind to the hydrophobic pocket of Bcl-xL and Bcl-2, thus preventing interaction of these proteins with pro-apoptotic members of the Bcl-2 family, such as Bax or “BH3-only” proteins [26–28]. For example, our [43] and literature [44] data showed that HA14-1 and BH3I-2′ displace recombinant Bax from complexes with recombinant Bcl-xL and Bcl-2. Because the active domains of Bcl-xL and Bcl-2 have similar structures [45], HA14-1 and BH3I-2′ inactivate both of these proteins.
The effects of HA14-1 and BH3I-2′ on ΔΨm of isolated pancreatic mitochondria were measured with membrane potential-sensitive TPP+ electrode. The quality of mitochondrial preparations was assessed by measuring respiratory control ratio, as described in the Methods section. We recently published that Ca 2+ at micromolar concentrations rapidly depolarizes pancreatic mitochondria, and that pancreatic mitochondria maintain ΔΨm and functional activity only if isolated in the presence of EGTA [18]. Therefore the experiments with isolated mitochondria (Figs. 5, 6) were performed in Ca2+-free medium (see Materials and methods and Ref. [18]).
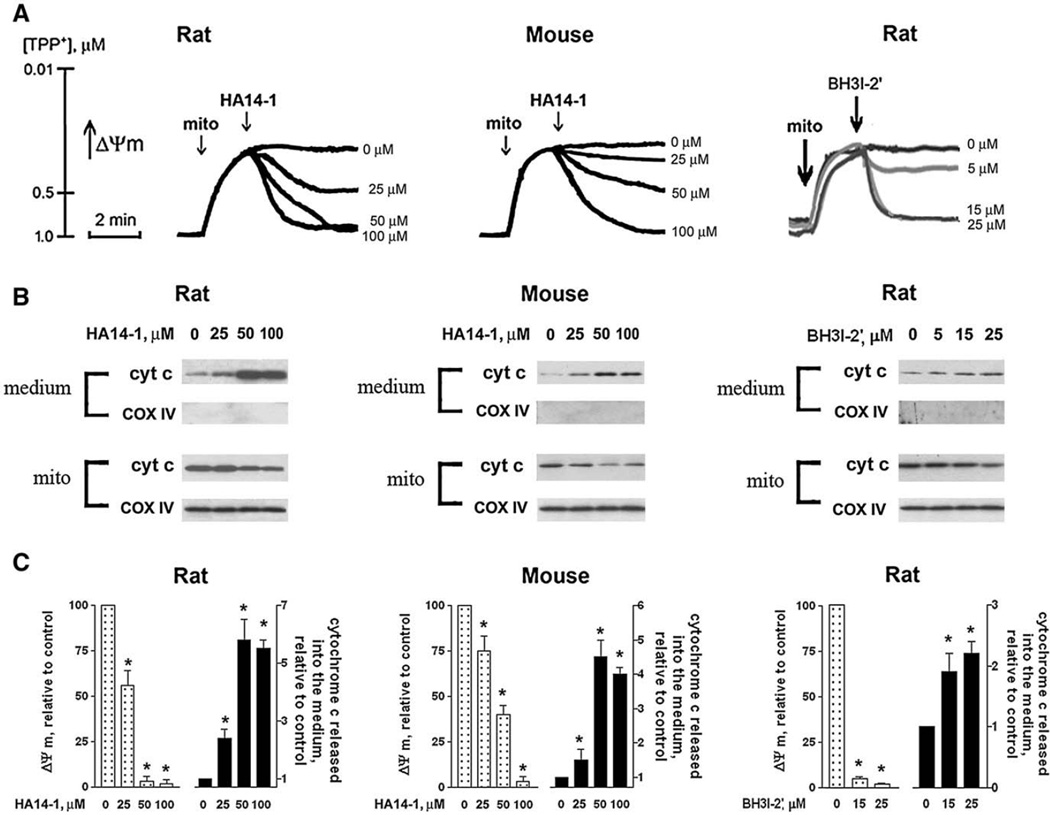
Fig. 5.
Bcl-xL/Bcl-2 inhibitors induce loss of ΔΨm and cytochrome c release in isolated pancreatic mitochondria. Mitochondria were isolated from rat and mouse pancreas as described in Materials and methods and incubated in the presence of 10 mM succinate for indicated times (A) or for 10 min (B, C), with and without the Bcl-xL/Bcl-2 inhibitors HA14-1 or BH3I-2′. (A) Changes in the mitochondrial membrane potential (ΔΨm) were measured in mitochondria suspension (mito) using TPP+-selective electrode. At the end of each experiment, 15 µM CCCP (a mitochondrial uncoupler) was added to the mitochondria suspension to completely dissipate ΔΨm and thus calibrate changes in ΔΨm (not shown). (B) Cytochrome c (cyt c) levels were measured by Western blot analysis both in the incubation medium and the mitochondria pellet (mito). Blots were re-probed for the mitochondrial marker COX IV to assess the quality of mitochondria separation and to confirm equal protein loading. ΔΨm and cytochrome c were measured in the same mitochondria preparations. (C) The band intensity of cytochrome c released from mitochondria into the incubation medium was densitometrically quantified. Values for ΔΨm and cytochrome c are means ± SE from at least 3 different preparations, normalized to those in mitochondria incubated in the absence of the Bcl-xL/Bcl-2 inhibitors (control). *p< 0.05 compared with control (i.e., mitochondria incubated without the inhibitors).
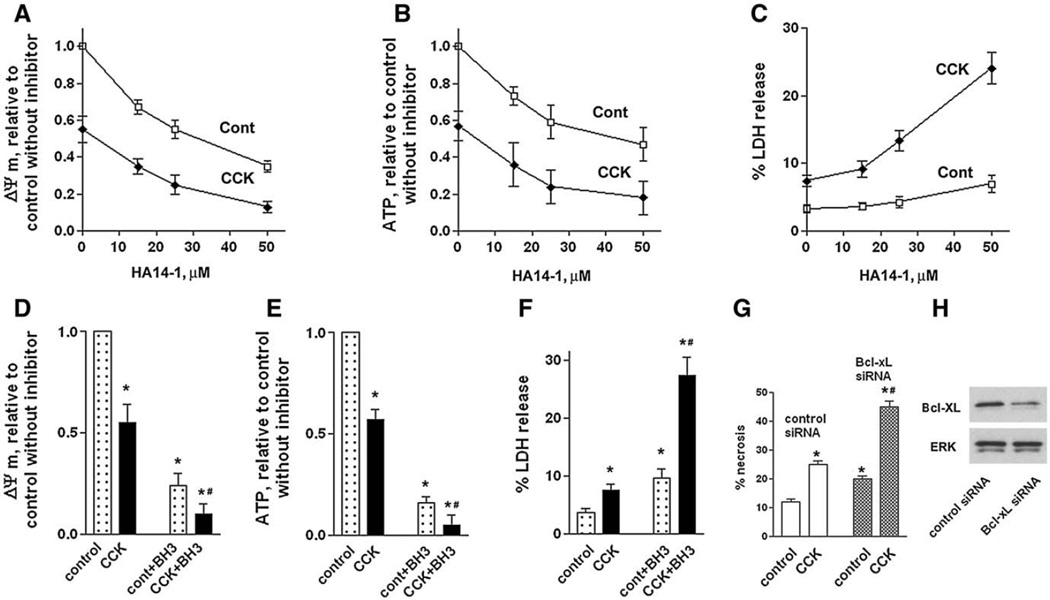
Fig. 6.
Bcl-xL/Bcl-2 inhibitors induce mitochondrial depolarization, ATP depletion, and necrosis in pancreatic acinar cells. These effects are greater in CCK-hyperstimulated acinar cells than in unstimulated cells. (A–F) Rat pancreatic acinar cells were incubated for 3 h with and without 100 nM CCK-8 and indicated concentrations of HA14-1 (HA) or 15 µM BH3I-2′ (BH3). (G, H) Mouse pancreatic acinar cells were cultured for 24 h in DMEM medium, as described in Materials and methods, transfected with control or Bcl-xL siRNA, and then incubated for 3 h in 199 medium with and without 100 nM CCK-8. ΔΨm was measured in acinar cells loaded with the ΔΨm-sensitive dye JC-1 (A D); cellular ATP, by use of luciferin/luciferase-based ATP determination kit (B, E). Necrosis was measured (C,F) by the percentage of total cellular LDH released into the extracellular medium by LDH release or (G) as percentage of cells stained positively with trypan blue. (H). Bcl-xL levels in cells transfected with control and Bcl-xL siRNA were measured with Western blot. Blots were re-probed for ERK1/2 to confirm equal protein loading. ΔΨm and ATP values were normalized to those in cells incubated without CCK in the absence of the inhibitors (control). Values are means ± SE (n = 3). *p < 0.05 compared with cells incubated without CCK in the absence of the inhibitor, (control), or cells transfected with control siRNA. #p< 0.05 compared to cells incubated with CCK in the absence of the inhibitor.
Both HA14-1 and BH3I-2′ dose-dependently decreased TPP+ uptake by mitochondria, indicating loss of ΔΨm (Fig. 5A). Previous publications [46,47] showed that the Bcl-xL/Bcl-2 inhibitors depolarized mitochondria isolated from liver and potentiated Ca2+-induced depolarization in mitochondria isolated from HeLa cells.
We next measured the effects of the inhibitors on cytochrome c release from isolated mitochondria (Fig. 5B). The levels of cytochrome c both in the medium and in mitochondrial pellets were measured with Western blot. The results show that both HA14-1 and BH3I-2′ induced cytochrome c release in mitochondria isolated from rat and mouse pancreas (Fig. 5B). Thus, HA14-1 and BH3I-2′ dose-dependently induced both depolarization and cytochrome c release in mitochondria isolated from rat and mouse pancreas (Figs. 5A, B), suggesting that Bcl-xL and/or Bcl-2 are required to protect pancreatic mitochondria against the signals, namely ΔΨm loss and cytochrome c release, that lead to apoptosis and necrosis, respectively. Of note, at the maximal doses applied (50–100 µM for HA14-1 and 15–25 µM for BH3I-2′) the inhibitors caused complete dissipation of ΔΨm, as the addition of the mitochondrial uncoupler CCCP did not further decrease ΔΨm (not shown).
The dose-dependencies of the effects of the Bcl-xL/Bcl-2 inhibitors on ΔΨm and cytochrome c release were in the same range, but not identical (Fig. 5C). For example, 50 µM HA14-1 induced maximal cytochrome c release in mouse mitochondria but only ~60% depolarization. Also, the mouse and rat mitochondria displayed somewhat different sensitivity to the same inhibitor; for example, depolarization induced by 50 µM HA14-1 in mouse mitochondria was much less than in the rat (Figs. 5A, C).
Bcl-xL/Bcl-2 inhibitors potentiate acinar cell necrosis in the in vitro model of pancreatitis
To corroborate the findings on isolated pancreatic mitochondria, we performed experiments on intact acinar cells, both unstimu-lated and hyperstimulated with supramaximal (100 nM) CCK. Supramaximal CCK induces pancreatitis-like changes in acinar cells, such as activation of trypsinogen and the pro-inflammatory transcription factor NF-κB, sustained increase in free cytosolic Ca2 +, necrosis, and apoptosis [5,7,29,32–36]. Therefore, this system is considered in vitro model of acute pancreatitis.
Similar to what we found in isolated pancreatic mitochondria (Fig. 5), both HA14-1 and BH3I-2′ caused mitochondrial depolarization in untreated and CCK-hyperstimulated acinar cells (Figs. 6A, D). Of note, the incubation of acinar cells with supramaximal CCK by itself decreased ΔΨm by ~50% (Fig. 6A), in accord with previous results from our group and others [5,29,30].
Mitochondrial depolarization induced in acinar cells by CCK hyperstimulation or Bcl-xL/Bcl-2 inactivation was associated with a dramatic decrease in cellular ATP and increased necrosis (measured by LDH release) (Figs. 6B, C, E, F). Importantly, combination of Bcl-xL/Bcl-2 inhibitors and CCK produced a greater depolarization, decrease in cellular ATP and necrosis than either treatment alone.
To confirm the effects of pharmacologic inhibitors we measured the effect of Bcl-xL knockdown with siRNA transfection on acinar cell necrosis (Figs. 6G, H). For this purpose, we established a prolonged (up to 36 h) culture of mouse pancreatic acinar cells [38]. Transfection with Bcl-xL siRNA increased necrosis in the prolonged culture of mouse acinar cells treated with and without CCK (Fig. 6G). Consistent with the effect of pharmacologic Bcl-xL/Bcl-2 inhibitors, the extent of necrosis was the greatest in cells transfected with Bcl-xL siRNA and treated with CCK.
The results in Fig. 6 indicate that Bcl-xL and Bcl-2 protect acinar cells, both untreated and hyperstimulated with CCK, against loss of ΔΨm, ATP depletion, and necrosis.
Bcl-xL/Bcl-2 inhibitors induce less apoptosis in CCK-hyperstimulated pancreatic acinar cells than in unstimulated cells
As we showed before [5,18,29], supramaximal CCK stimulates cyto-chrome c release in rat pancreatic acinar cells resulting in caspase activation and apoptosis. Cytochrome c release also mediates the basal apoptosis in untreated acinar cells [29].
HA14-1 and BH3I-2′ both stimulated cytochrome c release, the activity of key effector caspase-3, and apoptosis in untreated acinar cells (Figs. 7A – F). These findings suggest that Bcl-xL and/or Bcl-2, at the basal level of their expression, protect acinar cells against apoptosis.
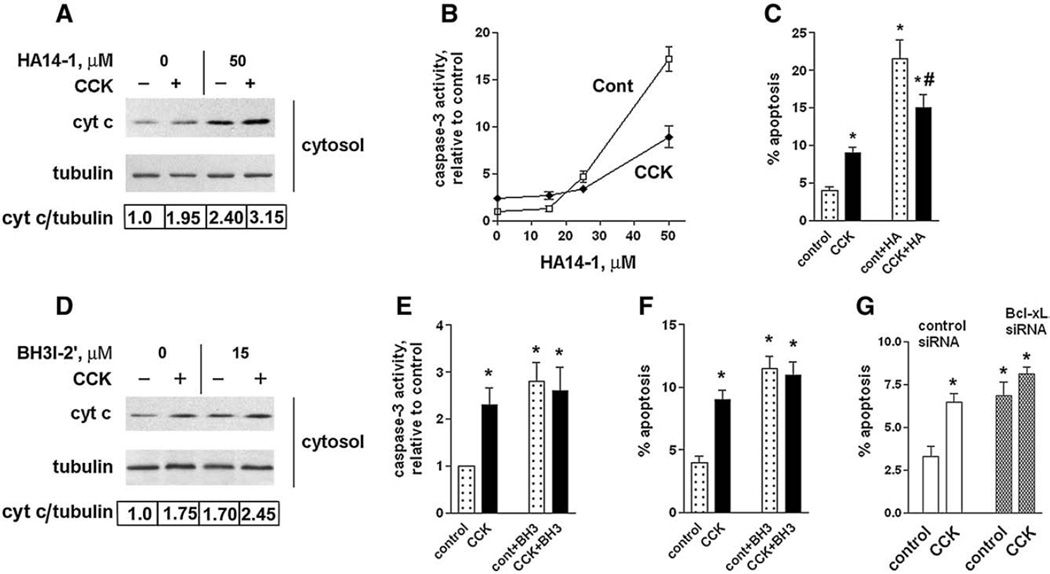
Fig. 7.
Bcl-xL/Bcl-2 inhibitors induce cytochrome c release, caspase-3 activation, and apoptosis in pancreatic acinar cells. These effects are less in CCK-hyperstimulated acinar cells than in unstimulated cells. (A–F) Rat pancreatic acinar cells were incubated for 3 h with and without 100 nM CCK-8 and indicated concentrations of HA14-1 (HA) or 15 µM BH3I-2′ (BH3). (G) Mouse pancreatic acinar cells were cultured 24 h in DMEM medium, as described in Materials and methods, transfected with control or Bcl-xL siRNA, and then incubated for 3 h in 199 medium with and without 100 nM CCK-8. (A D). Cytochrome c levels were measured in cytosolic fractions by immunobloting. Blots were re-probed for α-tubulin to confirm equal protein loading. The intensities of cytosolic cytochrome c and α-tubulin bands in the same sample were densitometrically quantified, and their ratio normalized to that in control (i.e., cells without CCK and the inhibitors) and presented below the blots. (B, E). Caspase-3 activity was measured by a fluorogenic assay using DEVD-AMC as a substrate. (C, F, G). Apoptosis was measured by the percentage of cells with apoptotic nuclear morphology using Hoechst 33258 staining. For each condition, at least 1000 cells were counted in 3 different acinar cell preparations. The inhibitor concentrations used were 50 µM HA14-1 (HA) or 15 µM BH3I-2′ (BH3). Values are means ± SE (n = 3). *p< 0.05 compared to cells incubated without CCK in the absence of the inhibitor. #p< 0.05 compared to cells incubated with CCK in the absence of the inhibitor.
Bcl-2/Bcl-xL inhibitors stimulated apoptosis in both control cells and cells treated with CCK. However, in contrast with what we observed for necrosis, the stimulatory effects of the Bcl-xL/Bcl-2 inhibitors on apoptotic signals were much less pronounced in CCK-treated than in untreated cells. For example, the induction of caspase-3 activity by 50 µM HA14-1 in CCK-hyperstimulated and unstimulated acinar cells (Fig. 7B) was, respectively, 3.7-fold versus 17.2-fold. That is, the effect of the Bcl-xL/Bcl-2 inhibitor in CCK-treated cells was ~5 times less than in cells non-treated with CCK. Therefore, as a quite surprising result, the combination of supramaximal CCK and Bcl-xL/Bcl-2 inhibitors decreased apoptosis over that seen with the Bcl-xL/Bcl-2 inhibitors alone. In other words, in the presence of the Bcl-xL/Bcl-2 inhibitors supramaximal CCK did not induce more apoptosis; on the contrary, there was less apoptosis in CCK-hyperstimulated than in unstimulated acinar cells (Fig. 7C). BH3I-2′ was much less potent than HA14-1 in causing caspase-3 activation and apoptosis – opposite to its effect on necrosis and pronecrotic signals (Fig. 6).
Transfection with Bcl-xL siRNA increased apoptosis in prolonged culture of mouse acinar cells (Fig. 7G). Consisitent with the effect of Bcl-xL/Bcl-2 inhibitors on apoptosis (Figs. 7C, F), CCK did not significantly stimulated apoptosis in cells transfected with BcL-xL siRNA (Fig. 7G).
In sum, the results of Figs. 6 and 7 show that the inactivation or knockdown of Bcl-xL and Bcl-2 increased both necrosis and apoptosis in acinar cells treated with and without CCK. The stimulatory effects of Bcl-xL/Bcl-2 inhibitors on necrosis were similar in untreated and CCK-treated cells (the in vitro model of pancreatitis). In contrast to their effect on necrosis, Bcl-xL/Bcl-2 inhibitors induced less apoptosis in CCK-hyperstimulated than in control cells. Thus, inactivation or knockdown of Bcl-xL/Bcl-2 in CCK-treated cells potentiated mitochondrial depolarization, ATP depletion and necrosis, but diminished the cytochrome c release, caspase-3 activation and apoptosis.
Pancreatic Bcl-xL up-regulation in models of acute pancreatitis inversely correlates with necrosis but not apoptosis
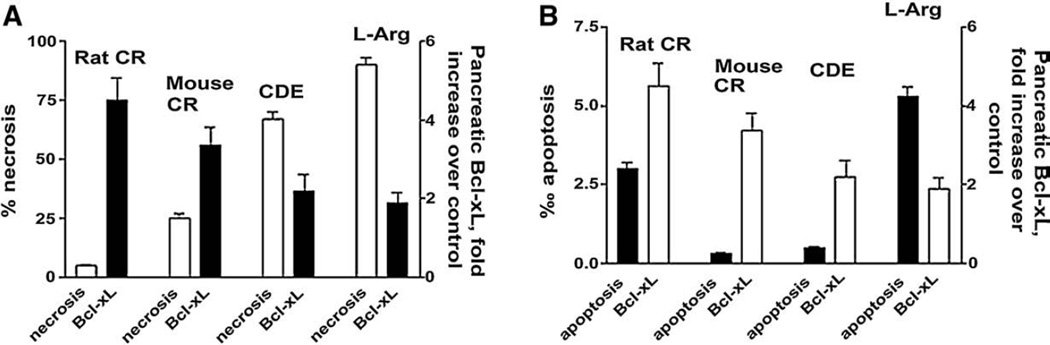
As we discussed in the Introduction, the severity of pancreatitis correlates with the extent of pancreatic necrosis. Correspondingly, experimental models of mild pancreatitis have low necrosis rate, whereas models of severe pancreatitis are associated with high necrosis. [2–7,31] . The results presented in the Fig. 8 show that the extent of Bcl-xL and Bcl-2 upregulation inversely correlates with necrosis and severity of the disease. In particular, in rat cerulein pancreatitis, which is a mild disease with low necrosis, Bcl-xL and Bcl-2 were upregulated 4.5 and 2.5-fold, correspondingly. By contrast, in the models of severe necrotizing pancreatitis (CDE diet model or L-arginine model), there was no upregulation of Bcl-2, and Bcl-xL was only increased by 2-fold. Thus, the levels of both Bcl-xL and Bcl-2 were 2–3 fold greater in mild (low necrosis) versus severe (high necrosis) models of pancreatitis. These data are consistent with our findings that inactivation of Bcl-xL and Bcl-2 increases acinar cell necrosis (Fig. 6). They suggest that several-fold increase in intrapancreatic Bcl-2 and Bcl-xL could be critical to decrease necrosis in pancreatitis.
Fig. 8.
The extent of Bcl-xL up-regulation inversely correlates with pancreatic necrosis, but not apoptosis, in models of acute pancreatitis. Pancreatitis was induced in rats and mice as described in Fig. 1 byadministration of cerulein (CR), L-arginine (L-arg) or choline-deficient ethionine supplemented diet (CDE). Control animals received saline injections (in the CR and L-arg models) or control diet (in the CDE model). Pancreatic levels of Bcl-xL were measured by Western blot analysis as shown in Fig. 1. Necrosis (A) and apoptosis (B) were measured on pancreatic tissue sections by use of, respectively, H&E staining (necrotic cells per 100 acinar cells) and TUNEL assay (apoptotic cells per 1000 acinar cells). Measurements were performed on tissue from animals with fully developed pancreatitis: at 7 h after the induction of CR pancreatitis, 24 h in the L-arg model, and 72 h in the CDE model. Values are means±SE from at least 3 animals per group.
Consistent with the results on acinar cells (Fig. 7), we found that the extent of Bcl-xL up-regulation did not correlate with apoptosis rate in rodent models of acute pancreatitis (Fig. 8). For example, the extent of Bcl-xL up-regulation was about the same in CDE model, which has a very low rate of apoptosis, and the L-arginine model, with the highest apoptosis rate (Fig. 8).
Discussion
We have recently shown [5,18,29] that mitochondrial permeabi-lization, manifested by loss of ΔΨm and cytochrome c release, occurs and mediates acinar cell death in experimental pancreatitis. In the present study we investigate the roles of the prosurvival Bcl-2 proteins in the regulation of cytochrome c release and mitochondria depolarization mediating apoptosis and necrosis in pancreatitis, respectively.
We show that pancreatic levels of various Bcl-2 proteins change in experimental models of acute pancreatitis. In particular, the key prosurvival protein Bcl-xL was up-regulated in all 4 models of pancreatitis examined, indicating that its up-regulation is a common event in experimental acute pancreatitis. Differently, another prosurvival protein, Bcl-2, increased only in rat cerulein but not the other models of pancreatitis. Up-regulation of the proapoptotic Bak was mostly in L-arginine pancreatitis; and there were no changes in the pancreatic level of Bax, another key proapopotic member of the Bcl-2 family (during the acute phase of pancreatitis that we studied).
Importantly, we found that the increases in total pancreatic levels of Bcl-xL and Bcl-2 during cerulein pancreatitis were associated with corresponding increases in their levels in pancreatic mitochondria. Mitochondria are the principal site of the effects of Bcl-2 family proteins on death responses [9,11,13]. The observed changes in mitochondrial levels of Bcl-2 proteins closely paralleled those in total pancreas, with regard to both the kinetics and model specificity. For example, mitochondrial Bcl-xL levels increased in both rat and mouse cerulein pancreatitis, whereas mitochondrial Bcl-2 only increased in the rat but not mouse cerulein model.
The observed increase in Bcl-xL protein was associated with increased mRNA expression in both rat and mouse cerulein pancreatitis; thus, a likely mechanism of Bcl-xL increase in pancreatitis is its transcriptional up-regulation. Interestingly, we found an increase in the pancreatic level of not only the main transcript but also an alternative splice variant from the bcl-X gene. Transcriptional regulation of this gene [40–42] has not been studied in pancreatitis. One regulator of Bcl-xL gene expression in a number of cell types is the transcription factor NF-κB [41]. Of note, pancreatic NF-κB activation is an early and prominent event in various experimental models of acute pancreatitis [36]. Using mice deficient in NF-κB proteins we found [48] that pancreatic Bcl-xL expression is, indeed, under control of NF-κB. In addition to transcriptional up-regulation, other mechanisms, e.g., increased protein stability, may also be involved because the increases in Bcl-xL (as well as Bcl-2) protein were already pronounced within 30 min after induction of cerulein pancreatitis.
In the present study we focus on the roles of the prosurvival Bcl-xL and Bcl-2 in the regulation of mitochondrial polarity and cytochrome c release and their corresponding death responses, necrosis and apoptosis in pancreatitis.
To investigate the functional role of Bcl-xL and Bcl-2 in pancreatitis we applied the recently introduced small-molecule Bcl-xL/Bcl-2 inhibitors, HA14-1 and BH3I-2′, which became a major tool in studying the roles of these proteins in death responses [26–28]. Bcl-xL and Bcl-2 have the same structure of the “catalytic groove” through which they interact with pro-apoptotic proteins [45]; therefore, HA14-1 and BH3I-2′ inactivate both Bcl-xL and Bcl-2. Of note, HA14-1 and BH3I-2′ are structurally different [26,27]. We also measured the effects of Bcl-xL knockdown with siRNA on death responses in the in vitro model of pancreatitis.
A critical finding of the study is that inactivation of pro-survival Bcl-xL and Bcl-2 proteins with pharmacologic inhibitors or Bcl-xL siRNA increases necrosis but not apoptosis in in vitro model of pancreatitis (CCK-hyperstimulated acinar cells). In agreement with these data we found that in animal models of pancreatitis the extent of Bcl-xL/Bcl-2 upregulation inversely correlates with necrosis. Bcl-xL and Bcl-2 upregulation was several-fold greater in models of mild pancreatitis than in severe necrotizing experimental pancreatitis. Differently, there was no correlation between Bcl-xL/Bcl-2 levels and apoptosis in pancreatitis. These results are important because as we discussed above, necrosis is a major factor mediating severity of pancreatitis, whereas apoptosis is associated with mild forms of the disease [2,4,5].
To obtain insights into the mechanisms underlying such effects of Bcl-xL/Bcl-2 in pancreatitis we first measured the effects of the inhibitors on isolated pancreatic mitochondria. We found that the Bcl-xL/Bcl-2 inhibitors induced both depolarization and cytochrome c release in rat and mouse pancreatic mitochondria. These data indicate that Bcl-xL/Bcl-2 proteins protect pancreatic mitochondria against both depolarization (leading to necrosis) and cytochrome c release (mediating apoptosis).
To corroborate the findings on isolated mitochondria, we assessed the effects of Bcl-xL/Bcl-2 inactivation on necrosis, apoptosis and the underlying signaling in pancreatic acinar cells, both untreated and hyperstimulated with CCK. The results on intact acinar cells, in accord with those on isolated pancreatic mitochondria, provide evidence that Bcl-xL and Bcl-2 protect acinar cells against loss of ΔΨm and its consequences, namely the cellular ATP depletion and necrosis. Bcl-xL/Bcl-2 inhibitors acted in concert with CCK to stimulate loss of ΔΨm, and ATP depletion in acinar cells. That is, both ΔΨm and ATP were lower in cells treated with the combination of Bcl-xL/Bcl-2 inhibitors and CCK, than in cells treated with the inhibitors alone or CCK alone.
Differently, although the Bcl-xL/Bcl-2 inhibitors induced cytochrome c release, caspase-3 activation and apoptosis in unstimulated cells, the effects of CCK on apoptotic signals (especially caspase-3 activation) were much less pronounced in the presence of Bcl-xL/Bcl-2 inhibitors. Therefore, counterintuitively, supramaximal CCK did not induce more apoptosis in the presence of Bcl-xL/Bcl-2 inhibitors; on the contrary, there was less apoptosis in CCK-hyperstimulated than in unstimulated acinar cells.
Thus, Bcl-xL/Bcl-2 inactivation in pancreatic acinar cells had drastically different effects on ΔΨm and subsequent necrosis versus cytochrome c release and subsequent apoptosis. Both pharmacologic analysis and transfection with Bcl-xL siRNA indicate that Bcl-xL/Bcl-2 inactivation potentiated CCK-induced necrosis while essentially blocking the CCK-induced apoptosis, and therefore shifted the pattern of death response in the in vitro model of pancreatitis towards necrosis.
As discussed above, these results can be explained by the interplay of oppositely directed mechanisms triggered by Bcl-xL/ Bcl-2 inactivation in acinar cells. Although Bcl-xL/Bcl-2 inactivation per se stimulates cytochrome c release, it also greatly facilitates ΔΨm loss and ATP depletion. Loss of ΔΨm and ATP depletion not only stimulates necrosis, but also inhibits apoptosis. Loss of ΔΨm, as we have shown [18], negatively regulates cytochrome c release from pancreatic mitochondria. Depletion of cellular ATP blocks caspase activation downstream of cytochrome c [49]. Because the levels of ΔΨm and ATP are much lower in cells hyperstimulated with CCK than in control cells, the overall effect of Bcl-2/Bcl-xL inhibitors in CCK-treated cells is inhibition of apoptosis.
Our data further suggest that the negative effects of ΔΨm loss and ATP depletion on caspase activation and apoptosis in acinar cells may be of “threshold” nature. Indeed, the conditions in which acinar cells retained a significant part of ΔΨm and ATP (e.g., in the presence of 50 µM HA14-1 alone) allowed caspase-3 activation and apoptosis to proceed; whereas a profound loss of ΔΨm and ATP (e.g., under combined effect of 50 µM HA14-1 and CCK hyper-stimulation) curtailed caspase activation and apoptosis.
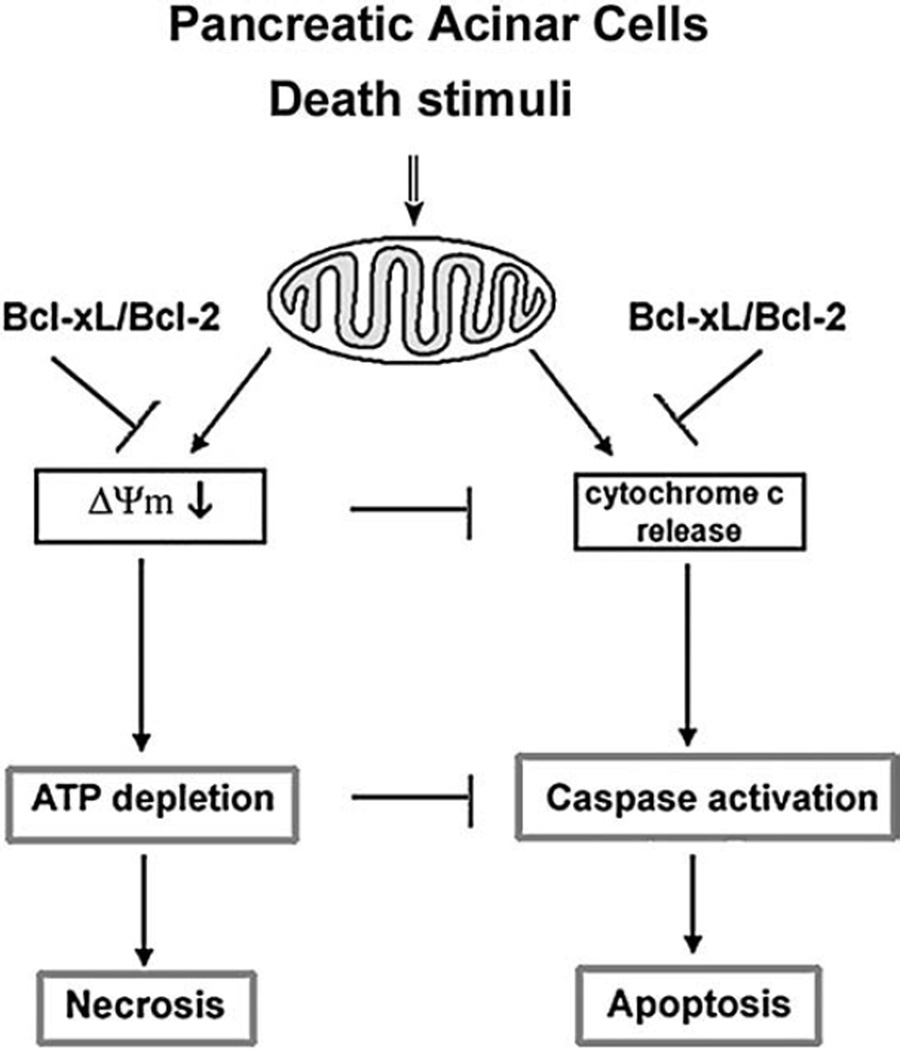
The above-discussed mechanisms of regulation of acinar cell death responses by Bcl-xL and Bcl-2, based on the results of our study, are depicted in Fig. 9. Combination of Bcl-xL/Bcl-2 inactivation and pancreatitis causes pronounced mitochondrial depolarization, which leads to ATP depletion and necrosis. Depolarization and ATP depletion limits cytochrome c release [18] and caspase activation [9,49] leading to inhibition of apoptosis.
Fig. 9.
Schematic illustrating the regulation of necrosis and apoptosis in pancreatic acinar cells by Bcl-xL and Bcl-2.
Interestingly, in cancer cells the effects of Bcl-xL/Bcl-2 inactivation on death responses differ from what we found in pancreatic acinar cells. In various cancer cells, including pancreatic cancer, Bcl-xL/Bcl-2 inhibitors greatly stimulate apoptosis and thus are considered a promising tool for cancer treatment [50–52]. The different effects of Bcl-xL/Bcl-2 inactivation in cancer versus pancreatitis are due likely to the different roles of mitochondria in cancer and normal cells. In cancer cells, ATP production is mostly through glycolysis and, therefore, loss of ΔΨm does not result in severe ATP depletion [53]. Further, as we showed for pancreatic cancer cells [54], mitochondrial depolarization does not limit cytochrome c release in cancer cells. Thus, the major effect of Bcl-x/Bcl-2 inhibitors in cancer cells is increased apoptosis resulting from stimulation of cytochrome c release. Differently, our results demonstrate that the predominant effect of the small-molecule Bcl-xL/Bcl-2 inhibitors on pancreatitis is ATP depletion and necrosis.
In summary, our results suggest that up-regulation of the prosurvival proteins Bcl-xL and Bcl-2 is a key protective mechanism against necrosis in pancreatitis. We found that Bcl-xL and Bcl-2 levels increase in models of pancreatitis, both in the whole pancreas and pancreatic mitochondria. The findings on isolated mitochondria and acinar cells indicate that these proteins protect pancreatic acinar cells against necrosis by preventing mitochon-drial depolarization and subsequent ATP depletion. Our results suggest that low levels of Bcl-xL and Bcl-2 in pancreatitis would facilitate necrosis and limit apoptosis, thus making the disease more severe. The results further suggest that Bcl-xL/Bcl-2 inhibition, which is considered a promising strategy to stimulate apoptotic death of cancer cells, would likely increase necrosis and thus the severity of acute pancreatitis. By contrast, approaches aimed at Bcl-xL/Bcl-2 up-regulation (or stabilization) may present a novel strategy to prevent or attenuate necrosis in pancreatitis.
Acknowledgment
This study was supported by NIH Grant DK59936 (to A.S.G.), by the American Gastroenterology Association Foundation Designated Research Scholar Award in Pancreatitis (to O.A.M.) and by the Department of Veteran Affairs (to S.J. P.). We thank Dr. A. Lugea for help with animal models of pancreatitis, and Drs. G. Eibl and E. Angst for help with using iQ5 real-time PCR detection system.
Abbreviations
- ΔΨm
mitochondrial membrane potential
- ARP
acidic ribosomal phosphoprotein P0
- CCK
cholecystokinin-8
- CDE
choline-deficient, ethionine supplemented (diet)
- DTT
dithiothreitol
- PTP
mitochondrial permeability transition pore
- TPP+
tetraphenyl phosphonium ion
REFERENCES
- 1.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 3.Gukovskaya AS, Perkins P, Zaninovic V, Sandoval D, Rutherford R, Fitzsimmons T, Pandol SJ, Poucell-Hatton S. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996;110:875–884. doi: 10.1053/gast.1996.v110.pm8608898. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am. J. Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 5.Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J. Biol. Chem. 2006;281:3370–3381. doi: 10.1074/jbc.M511276200. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M, Wallig MA, Hofbauer B, Lee HS, Frossard JL, Steer ML, Saluja AK. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem. Biophys. Res. Commun. 1998;246:476–483. doi: 10.1006/bbrc.1998.8519. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am. J. Physiol.: Gasterointest. Liver Physiol. 2004;286:G189–G196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 8.Saluja A, Hofbauer B, Yamaguchi Y, Yamanaka K, Steer M. Induction of apoptosis reduces the severity of caerulein-induced pancreatitis in mice. Biochem. Biophys. Res. Commun. 1996;220:875–878. doi: 10.1006/bbrc.1996.0498. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J. Biomed. Sci. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. BioEssays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 12.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 13.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopou-lou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp. Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 15.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim. Biophys. Acta. 2006;1757:1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 18.Odinokova I, Sung KF, Mareninova OA, Hermann K, Evtodienko Yu, Andreyev A, Gukovsky I, Gukovskaya AS. Mechanisms regulating cytochrome c release pancreatic mitochondria. GUT. 2009;58(3) doi: 10.1136/gut.2007.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odinokova IV, Sung KF, Mareninova OA, Hermann K, Gukovsky I, Gukovskaya AS. Mitochondrial mechanisms of death responses in pancreatitis. J. Gastroenterol. Hepatol. 2008;23(Suppl 1):S25–S30. doi: 10.1111/j.1440-1746.2007.05271.x. [DOI] [PubMed] [Google Scholar]
- 20.Odinokova IV, Sung KF, Hermann K, Mareninova OA, Gukovsky I, Gukovskaya AS. Bcl-xL and Bcl-2 prosurvival proteins protect against necrosis in pancreatitis. Pancreas. 2007;35:420. [Google Scholar]
- 21.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 25.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat. Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 27.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin. Oncol. 2003;30:133–142. doi: 10.1053/j.seminoncol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J. Biol. Chem. 2002;277:22595–22604. doi: 10.1074/jbc.M202929200. [DOI] [PubMed] [Google Scholar]
- 30.Voronina SG, Barrow SL, Gerasimenko OV, Petersen OH, Tepikin AV. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J. Biol. Chem. 2004;279:27327–27338. doi: 10.1074/jbc.M311698200. [DOI] [PubMed] [Google Scholar]
- 31.Frossard JL, Rubbia-Brandt L, Wallig MA, Benathan M, Ott T, Morel P, Hadengue A, Suter S, Willecke K, Chanson M. Severe acute pancreatitis and reduced acinar cell apoptosis in the exocrine pancreas of mice deficient for the Cx32 gene. Gastroenterology. 2003;124:481–493. doi: 10.1053/gast.2003.50052. [DOI] [PubMed] [Google Scholar]
- 32.Saluja AK, Bhagat L, Lee HS, Bhatia M, Frossard JL, Steer ML. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am. J. Physiol. 1999;276:G835–G842. doi: 10.1152/ajpgi.1999.276.4.G835. [DOI] [PubMed] [Google Scholar]
- 33.Steer ML. Early events in acute pancreatitis, Bailliere’s Best Pract. Res. Clin. Gastroenterol. 1999;13:213–225. doi: 10.1053/bega.1999.0020. [DOI] [PubMed] [Google Scholar]
- 34.Sutton R, Criddle D, Raraty MG, Tepikin A, Neoptolemos JP, Petersen OH. Signal transduction, calcium and acute pancreatitis. Pancreatology. 2003;3:497–505. doi: 10.1159/000075581. [DOI] [PubMed] [Google Scholar]
- 35.Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J. Clin. Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am. J. Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 37.Lu SC, Gukovsky I, Lugea A, Reyes CN, Huang ZZ, Chen L, Mato JM, Bottiglieri T, Pandol SJ. Role of S-adenosylmethionine in two experimental models of pancreatitis. FASEB J. 2003;17:56–58. doi: 10.1096/fj.01-0752fje. [DOI] [PubMed] [Google Scholar]
- 38.Sphyris N, Logsdon CD, Harrison DJ. Improved retention of zymogen granules in cultured murine pancreatic acinar cells and induction of acinar-ductal transdifferentiation in vitro . Pancreas. 2005;30:148–157. doi: 10.1097/01.mpa.0000147086.15867.ab. [DOI] [PubMed] [Google Scholar]
- 39.Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- 40.Habens F, Lapham AS, Dallman CL, Pickering BM, Michels J, Marcusson EG, Johnson PW, Packham G. Distinct promoters mediate constitutive and inducible Bcl-XL expression in malignant lymphocytes. Oncogene. 2007;26:1910–1919. doi: 10.1038/sj.onc.1209979. [DOI] [PubMed] [Google Scholar]
- 41.Pecci A, Viegas LR, Baranao JL, Beato M. Promoter choice influences alternative splicing and determines the balance of isoforms expressed from the mouse bcl-X gene. J. Biol. Chem. 2001;276:21062–21069. doi: 10.1074/jbc.M008665200. [DOI] [PubMed] [Google Scholar]
- 42.Sevilla L, Zaldumbide A, Pognonec P, Boulukos KE. Transcrip-tional regulation of the bcl-x gene encoding the anti-apoptotic Bcl-xL protein by Ets, Rel/NFkappaB, STAT and AP1 transcription factor families. Histol. Histopathol. 2001;16:595–601. doi: 10.14670/HH-16.595. [DOI] [PubMed] [Google Scholar]
- 43.Ohno I, Gukovskaya AS, Odinokova I, Pandol SJ. Bcl-2/Bcl-xL mediate the proapoptotic effects of ROS in pancreatic cancer. Gastroenterology. 2007;132:A437. [Google Scholar]
- 44.Qian J, Voorbach MJ, Huth JR, Coen ML, Zhang H, Ng SC, Comess KM, Petros AM, Rosenberg SH, Warrior U, Burns DJ. Discovery of novel inhibitors of Bcl-xL using multiple high-throughput screening platforms. Anal. Biochem. 2004;328:131–138. doi: 10.1016/j.ab.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 45.Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milanesi E, Costantini P, Gambalunga A, Colonna R, Petronilli V, Cabrelle A, Semenzato G, Cesura AM, Pinard E, Bernardi P. The mitochondrial effects of small organic ligands of BCL-2: sensiti-zation of BCL-2-overexpressing cells to apoptosis bya pyrimidine-2,4,6-trione derivative. J. Biol. Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 47.An J, Chen Y, Huang Z. Critical upstream signals of cytochrome c release induced by a novel Bcl-2 inhibitor. J. Biol. Chem. 2004;279:19133–19140. doi: 10.1074/jbc.M400295200. [DOI] [PubMed] [Google Scholar]
- 48.Zhao G, Reyes CN, Hermann K, Sung KF, Gukovskaya AS, Hoffman A, Pandol SJ, Gukovsky I. Genetic deletions of NF-kB proteins amerliorate cerulein-induced pancreatitis. Pancreas. 2007;35:420. [Google Scholar]
- 49.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill J, Manion M, Schwartz P, Hockenbery DM. Promises and challenges of targeting Bcl-2 anti-apoptotic proteins for cancer therapy. Biochim. Biophys. Acta. 2004;1705:43–51. doi: 10.1016/j.bbcan.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Manion MK, Fry J, Schwartz PS, Hockenbery DM. Small-molecule inhibitors of Bcl-2. Curr. Opin. Investig. Drugs. 2006;7:1077–1084. [PubMed] [Google Scholar]
- 52.Mohammad RM, Wang S, Banerjee S, Wu X, Chen J, Sarkar FH. Nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL, (−)-Gossypol, enhances biological effect of genistein against BxPC-3 human pancreatic cancer cell line. Pancreas. 2005;31:317–324. doi: 10.1097/01.mpa.0000179731.46210.01. [DOI] [PubMed] [Google Scholar]
- 53.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 54.Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]