Abstract
Binswanger’s disease (BD) is a progressive form of cerebral small vessel disease (SVD) affecting the white matter (WM) and other subcortical structures. Advances in imaging have increased interest in understanding has arisen in the definitions, clinical presentations, differential diagnoses, risk factors and complications of BD. Clinical and imaging features, neurophyschological profile and cerebrospinal fluid analysis aid in making the diagnosis. With recent developments in MRI methods and analysis of CSF and blood biomarkers, we have gained a greater understanding of the complex pathophysiology of the disease, which has helped with the diagnosis and prognosis of BD.
There is growing evidence that the WM injury in BD is related to endothelial dysfunction with secondary inflammatory response leading to breakdown of the neurovascular unit (NVU). This review summarizes current and future research directions, including pathophysiological mechanisms and potential therapeutic approaches.
Keywords: Binswanger’s disease, small vessel disease, vascular cognitive impairment, neuroinflammation, neurovascular unit, matrix metalloproteinases, subcortical ischemic vascular disease, leukoaraiosis, dynamic contrast enhanced MRI
INTRODUCTION
Vascular cognitive impairment (VCI), which is the second most common form of dementia after Alzheimer’s disease, is projected to increase, as the population grows older.[1] Different types of vascular injuries and vascular pathologies can cause or contribute to this heterogeneous disorder. Small vessel disease (SVD) is the major form of VCI and one most potentially amenable to treatment.[2] SVD also results from a variety of pathological processes, including lacunar strokes and progressive white matter (WM) injury. Binswanger’s disease (BD) is a form of VCI related to injury of the small vessels of the brain, characterized by extensive WM hyperintensities (WMHs) with gradual subcortical ischemia. These patients classically develop focal neurological findings, gait disturbances, and cognitive impairment.[3]
Currently BD is considered a subset to SVD patients and overlaps with other VCI and degenerative conditions (Figure 1). Elois Alzheimer first quoted the term in 1902 in reference to the case series described by Otto Binswanger eight years earlier. Binswanger wrote a long clinical-pathological description of a group of demented patients that had hypertension, gait disturbances with progressive decline.[4] Their brains showed “hardening of the arteries”, “diffuse pallor of the WM”, “multiple subcortical strokes” and “severe WM atrophy with relative sparing of the gray matter”.[4] Later, more clinical-pathological descriptions were added to the literature.[5] BD was primary a pathological diagnosis and rarely was diagnosed in living patients until the introduction of computer tomography (CT) and magnetic resonance imaging (MRI). Neuroimaging showed “WM pallor and rarefactions” and small subcortical strokes (lacunar strokes). Widespread use of imaging lead to an epidemic of radiologically-defined BD, especially in the elder population. However, some patients with WM changes on CT or brain MRI were asymptomatic or did not have the clinical features described by Binswanger. In the seventies and eighties, Alzheimer’s disease (AD) was recognized as the leading cause of cognitive impairment and dementia with less emphasis on importance of cerebrovascular impact. However, as more careful neuropathological studies were done, many patients with AD were found to have concomitant cerebrovascular changes, forcing a reassessment of the role of vascular disease in dementia. As the controversy raged over the definition of BD and the significance of the WMHs on MRI, the relevance of the initial description of the syndrome was overlooked.
Figure 1.
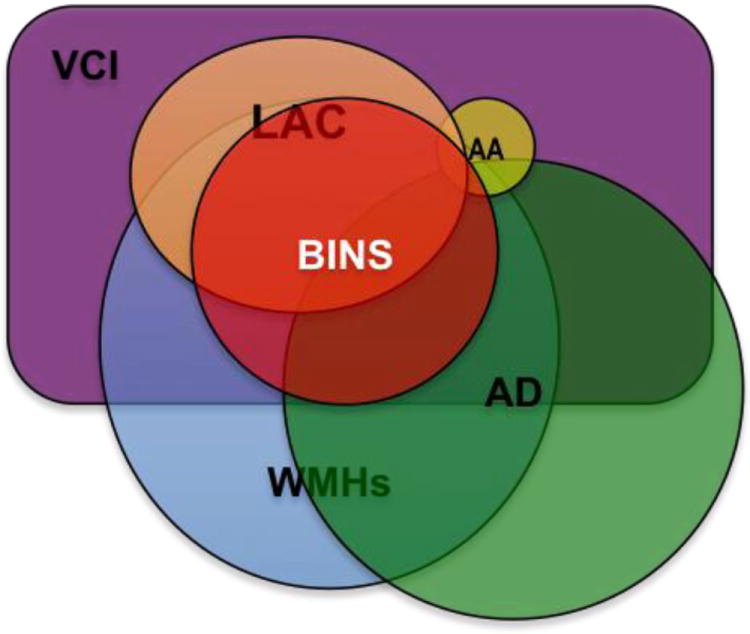
The most common cause of vascular cognitive impairment (VCI) is small vessels disease (SVD). The most common causes of SVD are depicted in this graph. These conditions commonly overlap, especially with aging.
LAC: (lacunar) Small subcortical ischemic strokes
AA: Amyloid angiopahty.
AD: Alzheimer’s disease.
BINS: Binswanger’s disease
WMHs: White matter hyperintensities or leukoaraiosis.
In this review, we argue that the term “Binswanger disease” is meaningful for the clinician. It defines a progressive medical condition. Other terms such as subcortical ischemic vascular disease (SIVD) or ischemic WM, subcortical microvascular ischemic changes, leukoaraiosis and WMHs are less helpful to the clinician. Indeed, most of these terms describe radiological concepts that are not bound to any clinical description. The lack of consensus on BD and multiple definitions used for various form of VCI has limited its clinical study. For example the epidemiology of BD is still not well studied.
In the following paragraphs we review current methods to reach a more certain diagnosis of the syndrome and postulate some treatment strategies based on the experience with other VCI conditions. We also provide an outlook on future developments in research and possible therapeutic options based on recent theories on neuroinflammation and neurovascular unit (NVU) dysfunction.
DIAGNOSES
Close to 20 years have passed since Bennett and Caplan reviewed and proposed a diagnostic criterion for BD.[6,7] Since then we have learned more about the pathophysiology, clinical features, imaging and comorbidities of this condition. Currently, BD can be diagnosed with greater certainty using clinical information, neuroimaging and ancillary tests.
Clinical features
Patients with BD often have different degrees of cognitive impairment. History reveals past episodes of “mini-strokes” or transient ischemic attacks that occurred. On physical examination there are usually upper motor signs, asymmetric hyperreflexia and mild parkinsonism. Symptoms are always steadily progressive, but often can show a waxing and waning pattern and at times a stepped course. Hypertension is almost always present and its absence should lead to questioning the diagnosis. Other vascular risk factors, including diabetes, pre-diabetes, smoking, hyperlipidemia, sleep apnea and atrial fibrillation while common are less helpful for defining for defining the disease (Table 1).
Table 1.
| CLINICAL FEATURES |
| Cognitive impairment |
| Pyramidal signs |
| Extrapyramidal signs |
| Hypertension |
| Ataxia/balance disturbances |
| Progressive symptoms |
| IMAGING FEATURES |
| White matter hyperinteinsities on T2 |
| Brain atrophy (mild to moderate) |
| Lacunar infarcts |
| Micro-bleeds |
| Enlarged perivascular spaces |
| Intracranial atherosclerosis |
| DIFFERENTIAL DIAGNOSIS |
| Amyloid angiopathy |
| WMHs of unknown cause |
| CNS vasculitis |
| CADASIL, CARASIL |
| Prior WM injuries. |
| Late onset multiple sclerosis |
| Adult onset leukodystropies |
| Chronic kidney disease. |
| Watershed ischemia (border-zone strokes) |
| Normal Pressure Hydrocephalus |
| Clinically irrelevant WMHs |
Executive function and processing speed are the most commonly affected cognitive functions.[8] Memory dysfunction is less common, but also can be seen in BD. Although these distinctive cognitive deficits are very well described and accepted by the medical community, they are not reliable for the diagnosis. Cognitive decline occurs but in a gradual slow progression. For office evaluation we usually prefer the Montreal Cognitive Assessment (MOCA) to the mini-mental status examination (MMSE). The MMSE which is used for AD where memory is impaired, is often in the normal range in BD, and the MOCA, which includes tests of executive function, is more often abnormal in BD patients, making it a better screening test.[9]
Imaging
We recommend advanced imaging with brain MRI for the diagnosis of BD. Protocol should include FLAIR, T2, echo gradient or susceptibility weighted imaging (SWI) for detection of blood products and MR angiogram. The imaging characteristics are comparable to the early pathological descriptions; the WMHs, WM atrophy and small subcortical strokes. Although there is no specific threshold for the size of WMHs in BD, they should always be present and they are not subtle. Classically the WMHs are divided into periventricular and deep by their location. Both types are commonly present, but the periventricular WM lesions, have stronger association with aging. [Reference at end of reference list] Small subcortical ischemic lesions or “lacunar strokes” are extremely frequent which argue for a common pathophysiological mechanism.[5] Enlarged perivascular spaces are frequently seen in T2 sequences.[10] Microbleeds are less common but if present are usually subcortical. The presence of numerous or cortical microbleeds should suggest amyloid angiopathy as the primary diagnosis.[11] Atherosclerosis with dolicholectasia of intracranial vasculature is also a common finding.[12] Brain atrophy is also a common finding with mild hippocampal atrophy that is less pronounced than in AD patients.[10] It is important to point out that although the imaging features are very suggestive of the disease process, the diagnosis cannot be made based only on imaging. The clinical features are crucial for the diagnosis of the disease and ancillary studies are sometimes needed.
Ancillary studies
Neuropsychological testing shows a broad spectrum of cognitive dysfunctions in these patients. Slowed processing speed and executive dysfunction are the most affected cognitive areas in SVD.[13] Not surprisingly memory and other cognitive domains are frequently also affected. A mixed type of dementia with both VCI and AD can be suspected especially if the memory domain appears to be the most affected. Depression might be discovered during the evaluation too. Cognitive impairment is less evident in highly intelligent patients, but discrepancies in the executive function and processing speed with the rest of cognitive domains should hint at the diagnosis.
Cerebrospinal fluid (CSF) is associated with an increase of albumin index without oligoclonal bands. Increased albumin reveals an opening of the BBB.[14] There is an increase of MMP-9 and normal or decreased levels of amyloid beta1-42 (Aβ42), although these latter tests are still mainly used in research.[15] Transcranial Doppler ultrasound shows high resistant flow pattern with high pulsatility signal but it is not specific for BD.[16] Perfusion imaging of the WM with a variety of brain imaging techniques have also shown decreased blood flow in the WM.[17,18] Spectroscopy of the WM shows a decrease of N-acetylaspartate (NAA) and creatine in these patients.[19]
Binswanger’s Disease Spectrum
Clinical diagnosis of BD can be initially challenging but as the clinical course is learned, the diagnosis can be made with greater certainty. For instance, WMHs are found incidentally after scanning the brains of healthy individuals. These subjects are functioning independently and deficits are not detectable on an office visit. However similar population studies with detailed neuropsychological evaluation have demonstrated a correlation of WMHs with mild cognitive impairment. WMHs also correlated with a higher risk of ICH and ischemic stroke at population level studies. Sub-clinical WMHs associated with hypertension might be a precursor of BD.
On the other hand, the higher prevalence of hypertension, WMHs, AD and vascular pathology in patients older than 70, makes the diagnosis difficult. Therefore in older patients we need to be careful to ascribe all these changes to BD pathology. Alternatively other diagnoses, such as AD, mixed disease or “normal aging” should be considered. Still, additional biomarkers that we discuss below could help to make this diagnosis more certain.
Comorbidity with Alzheimer’s Disease
A mixed type of dementia (AD+BD) is common. The comorbidity occurs because the prevalence of both conditions increases in older individuals. Diagnosing AD based on reduced Aβ42 in patients with SVD may be more difficult because fibrosed blood vessels may impede clearance of Aβ42 along with other metabolic waste products. Impairment of cerebral microcirculation of interstitial fluid is abnormal in SVD, which may result in increase waste products in the brains of AD patients, worsening and accelerating the progression of AD.[20]
TREATMENT
The American Heart Association (AHA) recently published treatment guidelines for patients with VCI.[1] Nevertheless, there are no specific clinical studies targeting therapies for BD, making it difficult to choose treatments for this group of patients. Although most studies that use subjects with VCI, vascular dementia and leukoaraiosis have included BD patients, they have not been specifically targeted for treatment trials. Therefore the real treatment effect of a specific therapy is still unknown. Despite the lack of rigorous studies, best treatments can still be inferred and generalized from multiple studies. Here we review treatments that aim to prevent progression, improve behavior and reduce complications.
Blood Pressure Reduction
Hypertension plays a central role in the pathogenesis of BD. Hypertension is a major risk factor for all types of symptomatic strokes, asymptomatic strokes and WMHs.[21] Blood pressure control reduces the incidence of stroke in both primary and secondary prevention.[22,23] There is compelling evidence that treatment of hypertension is beneficial in patients with SVD. Lacunar strokes are prevented with blood pressure control but the BP target is still debatable. Tight BP control (SPB<130) is emerging as more effective than less intense therapy (SBP 140-130) for stroke prevention. The recent secondary prevention of small subcortical stroke trial (SPS3) trial compared both BP regimens for secondary prevention in lacunar strokes but did not reach statistical significance.[24]
Hypertension has consistently been associated with cross-sectional measured WML burden and with longitudinally measured WML progression.[25,26]
In longitudinal studies, progression of WMHs is associated with uncontrolled hypertension and hypertension treatment reduces the growth of WMHs.[27,28] To date two randomized, placebo-controlled trials have been performed to investigate the effect of antihypertensive treatment on the progression of WML; the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) and the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial.[29] The PROGRESS achieved a small significant decrease of WMHs on brain MRI with large reduction in BP compare to placebo (mean SBP reduction: 9.5 mmHg). In the PRoFESS the reduction of BP was modest (mean SBP reduction: 3.3 mmHg) and no significant changes in WMH was observed.[30] Two recent observational studies found treatment of people with high systolic blood pressure to be related to less WML progression.[27,28]
Blood pressure control has also proved to reduce progression of cognitive impairment and onset of dementia associated with stroke and WMHs.[31] We recommend an aggressive management of hypertension especially in younger patients who can tolerate lower BP levels better than older ones. Older patients should be treated with hypertensive medications although targeting low BP levels is in this group is still controversial.
Antiplatelet therapy
The use of antiplatelet therapy is recommended based on the presence of lacunar strokes. Dual antiplatelet therapy is not recommended. In the SPS3 the use of dual antiplatelet therapy did not reduce recurrence of ischemic stroke outcomes and increased the rate of intracranial hemorrhages (ICH) in that patient population.[32] There is scarce evidence in the use of antiplatelets in patients with isolated WMHs or BD in the absence of clinical or radiological strokes. There is a 3 times higher risk of stroke in subjects with WMHs than in the general population.[33] BD carries a higher risk of ICH; therefore the benefit of ASA for stroke primary prevention versus the risk of increasing ICH in this group is unknown. Despite of the limitations, the use of antiplatelet therapy BD with radiological evidence of ischemic stroke seems reasonable. However, it is important to avoid treating patients without symptomatic stroke or TIA and amyloid angiopathy or other mimic conditions that could carry a higher risk of ICH.
Statins
Statins play a very important role in secondary stroke prevention.[34] Statins have many pleotropic properties such as reduction of atherosclerosis and inflammation.[35] Makers of endothelial dysfunction and vascular inflammation are common finding on patients with BD and SVD.[36] However the use of statins is not certain in BD patients with no history of clinical stroke or imaging confirmed stroke. The recent AHA guidelines recommends the use of statins in patients with high risk of stroke as primary prevention.[37] We recommend for the use of statins in BD patients with history of stroke or TIA, radiological stroke and an LDL >100mg/dl. Because statins might somewhat increase the risk of ICH in patients with SVD more information is required to understand how intensive the treatment should be.
Physical Activity
Physical activity prevents cognitive impairment in normal aging and reduces the speed of cognitive decline in patients with mild cognitive impairment (MCI).[38] Physical activity in participants with SVD is related to WMHs volume on conventional FLAIR and to WM microstructural integrity using WM MRI tractography analysis.[39] In large cohort of patients with leukoaraiosis from the LADIS group, physical activity reduced the risk for cognitive impairment and vascular dementia, independently of the severity of the WM lesions. [40]
The benefits of exercise and physical activity for stroke prevention and hypertension treatment of are also well stated. Theoretical benefits of physical activity on the pathophysiology of the disease are multiple: reduction of blood pressure, anti-inflammatory, increase of oxygen capacity, etc. The type (aerobic versus resistance) and amount of recommended exercise are still debated and an ongoing matter of study. Although resistance exercises (e.g. weight lifting) might have potential benefits, most of the human and animals studies have focused on the effects of aerobic exercise in cognition.[41] Therefore, there is more evidence to recommend aerobic exercise for prevention of BD.
Diet
High homocysteine levels are associated with risk of stroke and SVD. However there is no benefit of folic acid or vitamin supplements for the prevention of stroke, WHMs or improvement of cognitive function.[42-44] We recommend a low salt diet. A normal American diet has 3.5 to 4 gms of sodium per day.[45] A high salt diet raises blood pressure and increases the risk of stroke. Salt reduction plays an important role on reduction of blood pressure.[46] The American heart association recommends only 1.5 grams of sodium in patients with stroke. High sodium intake is also linked to increase in oxidative stress and worsen of vascular resistance.[47] However, the specifics of the role of salt and Binswanger’s pathology remain to be studied.
Physical Therapy and Rehabilitation
Patients with BD often have balance problems and parkinsonian features. The initiation of an exercise program should be preceded by a full evaluation of physical therapy and programing. Although the evidence of physical therapy as an effective treatment is unclear, it encourages patients to participate in physical activities, understanding their risks and limitations. Yoga and tai-chi most likely are beneficial, but studies are still needed.
Management of other medical conditions
Diabetes does not appear to be at the root of the BD pathology, but it is suspected to contribute to the worsening of the disease. Glucose control is recommended but tight glucose control should be avoided due to the detrimental effects of hypoglycemic events.
Restoring impaired vision and hearing helps to restore well-being in aging and demented patients in general.[48] Hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in older adults. Although still unclear that improvement of hearing with devices will affect cognitive decline.[49]
Acetylcholinesterase inhibitors and Memantine
Autopsy studies have demonstrated cholinergic deficits in patients with VCI.[50,51]
Several large clinical trials have examined the effect of cholinesterease inhibitors. Patients with VCI who received galantamine showed improved cognitive performance compared with placebo, though functional measures were unchanged.[52] In another double-blind placebo controlled trial of donepezil for VaD, statistically significant gains occurred for cognitive outcomes, dementia severely and global clinical-based improvement ratings.[53] However, favorable effects in functional status were again not obtained. In fact, only one study to date has reported benefits for activities of daily living.[54] Another placebo-controlled study compared donepezil to placebo in patients with CADASIL.[55] No significant effect was found on the primary outcome measure, though treatment improved some secondary endpoints. The lack of benefits for functional outcomes or dementia severity gain indicated uncertain clinical efficacy, a conclusion reinforced in a recent metanalysis.[56,57]
Relatively less is published about treatment of VCI patients with memantine, an N-methyl –D – aspartate antagonist approved for moderate to severe AD. Two large, placebo-controlled studies found statistically significant drug-placebo differences in cognitive outcome measures.[58] No effect on global or functional outcomes was demonstrated, however, undermining their clinical significance in a manner to cholinesterase inhibitors.
In conclusion human studies with cholinergic medications and memantine have not shown a well-defined clinical improvement and in our experience these medications might cause side effects and discomforts. These medications are not FDA approved in VCI or VaD patients and in general we do not recommend its widespread use in VCI or mixed dementia when the vascular component appears to be the primary cause.
Medications to avoid
There is some evidence that blood pressure variability and systolic spikes can decompensate patients and increase risk of vascular events.[59] ICH is also more common on patients with SVD and BD and can be potentially trigger by fluctuations of BP. In our experience we try to avoid medications that can cause rebound hypertension or hypertensive surges. We try to avoid medications that can cause confusion, drowsiness and psychosis especially in BD with severe cognitive impairment or dementia.
Because parkinsonism is a common BD finding, neuroleptics, prokinetic agents and medications that can worse parkinsonism should be used with caution.
Anticoagulation, especially with warfarin, should be considered only in atrial fibrillation, valve replacement or high-risk patients. There is a strong association with warfarin therapy and symptomatic ICH inpatients with severe WMHs. The Stroke Prevention in Reversible Ischemia Trail (SPIRIT) used an INR target of 3-4.5 and found that WMHs was a strong independent risk factor for ICH, with an odds ratio of 9.2.[60] The results were replicated in a hospital-based case-control, in which patients with a mean of INR of 3.2 had an odds ratio of 12.9 for symptomatic ICH.[61]
EXPERT COMMENTARY
BBB DISRUPTION IN BD
Evidence from imaging and pathological studies in BD suggests that disruption of the NVU is crucial in the progression of the WM injury. Human pathologic studies have shown evidence of endothelial dysfunction that is usually accompanied by NVU leakage of plasma components into the vessel wall and perivascular space. Autopsy studies of BD patients show arteriolosclerosis with fibrosis, fibrinoid necrosis and extravasation of serum-derived proteins in the white matter blood vessels.[62] Pathological studies reveal an increase in matrix metalloproteinase (MMP) containing inflammatory cells around blood vessels.[63,64] Macrophage/microglia activity around hypertensive, fibrosed vessels could lead to protease secretion and by-stander demyelination.[65] MMPs are detected in the cerebrospinal fluid in VCI. Thus, there is evidence that neuroinflammation is an important factor in white matter damage and oligodendrocyte death. MMPs are implicated in damage and repair processes: they disrupt the NVU, breakdown myelin, and modulate repair through facilitation of angiogenesis and neurogenesis.[66] While the constitutive MMP-2 is present in high concentration in astrocytic foot processes as reflected in the CSF, the inducible MMPs, MMP-3 and MMP-9, are released as part of the inflammatory response, opening the NVU and attacking myelin.[67] The breakdown of the NVU is more often seen in the patients with VCI than in the patients with AD with WM changes.
One of the first clinical methods to study the integrity of the NVU, consisted in measuring the cerebrospinal (CSF)/serum ratio for albumin; an increased CSF/serum albumin ratio indicates impaired NVU function.[68] Abnormal permeability results in an increase in CSF albumin ratio in patients with vascular dementia.[14,69] MMPs have also been measured in human CSF. Patients with BD have high CSF MMP-9 levels[15], elevated active form of MMP-3 and reduction in the MMP-2 index.[70] These CSF findings provide further evidence of an inflammatory NVU disruption.
In a hypertensive stroke-prone rat animal model of BD Jajal et al found an increase in MMP-3, MMP-9, and tumor necrosis factor-α; these inflammatory markers were associated with myelin breakdown and NVU disruption in the rat brain.[71] The endothelial dysfunction and increase leakage of the NVU in BD patients could be the direct cause of brain WM damage or a consequence of other reasons. Nevertheless, closure of the NVU could be surrogate biomarker of treatments for BD.
BBB imaging
Dynamic contrast-enhanced brain MRI (DCEMRI) can be used to visualize the leakage of Gd-DTPA across a disrupted NVU. Imaging studies using other qualitative contrast-enhanced MR methods have demonstrated NVU abnormalities in VCI patients when compared with normal controls.[72] Patients with BD and lacunar strokes have increase of NVU permeability in the WM compared with cortical stroke patients.[73] Permeability may increase with age, making age-matched controls important.
Using DCEMRI our group showed that patients with VCI had leakage of Gd-DTPA as measured at 1.5T that was elevated compared to a group of healthy controls. In a second study using a 3T MRI, we confirmed the increase in permeability in VCI patients compared to a group of early type-2 diabetes and to controls. Permeability maps in color facilitate the display of regions with NVU disruption, which add visual information to the quantitative assessments (FIGURE 3).
Figure 3.
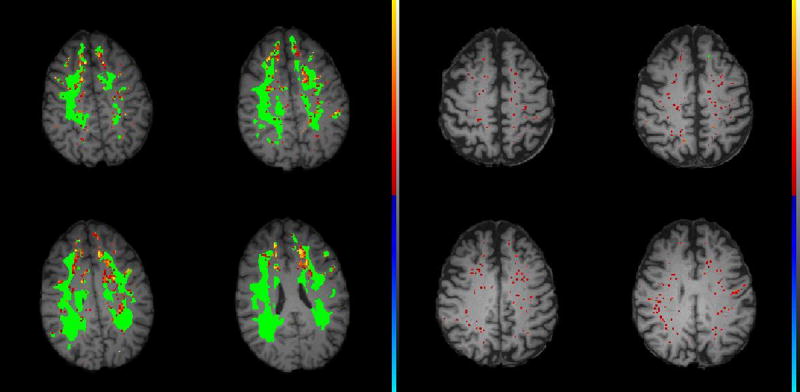
Dynamic contrast-enhanced MRI (DCEMRI) of a Binswanger patient (Left) and a control (Right). White matter hyperintensities on the FLAIR MRI are shown in green. NVU permeability scale is from red to yellow with yellow the highest permeability (see the color scale on the side of the images). The Binswanger patient has an increase in ki (yellow areas). The major changes in the permeability are located at the edge of the WMHs on FLAIR (green regions), suggesting that the areas of active inflammation are around rather than within the regions of gliosis. The control patient of a similar age does not have WMHs and the red dots, indicating increased leakage, are minimal and scattered.
NVU permeability maps in Binswanger’s disease
Patients with BD have increase of mean ki within the WM compared with patients with multi-infarct dementia and patients with WMHs of other etiologies.[74] In BD, patterns of NVU disruption suggest that the increased permeability tends to be around regions with WMHs. Using non-quantitative NVU imaging methods, Topakian et al showed an increase of NVU permeability in normal appeared WM in SVD subjects.[75] Such findings suggest a more generalized subcortical endothelial dysfunction/inflammatory process in patients with lacunar strokes. When these SVD patients are followed over long-term there is persistent NVU disruption in the WM and basal ganglia.[76]
We have performed repeat DCEMRI studies on a group of VCI patients that included those with BD. All of the patients showed persistence of the disruption of the NVU over the one to two year period between the studies. When we compared the regions of increased permeability in the two studies, we found a shift in regions with increased permeability; in fact, the areas of overlap were small. This suggests a continuous inflammatory response with new regions appearing and old ones disappearing. A preliminary analysis suggests that those with BD show growth in the regions of abnormal permeability, while the multiple stroke patients show some resolution. Further studies with larger numbers of patients will be needed to confirm this early finding. If these findings can be replicated, the DCEMRI method might be a biomarker of active disease that could be useful in both clinical trials and the understanding of disease progression.
White Matter Tractrography
Diffusion tensor Imaging (DTI) is an MR method used to assess the integrity of the WM tracts. Demyelination of the WM reduces the fractional anisotropy and increases the mean diffusivity.[77] This technique is more sensitive than MRI FLAIR for detecting the integrity of the WM.[78] For instance, in patients with lacunar strokes and leukoaraiosis, DTI is able to predict changes in normal appearing WM on FLAIR.[79,80] In a recent longitudinal MRI study, baseline DTI measures in visually normal appearing WM predicted the development of new WMLs at follow up.[81] DTI measures and FLAIR signal intensity were independently associated with WML development.[81] Cross sectional studies have shown correlation of DTI with cognitive impairment.[82] This correlation appears to be stronger than with WMHs volume on measured by FLAIR.[83] A longitudinal study has also demonstrated that the prediction of future cognitive decline was also better for DTI than volume WMHs of FLAIR.[84] Hence, DTI is a promising imaging biomarker to measure progression of disease.
Arterial stiffness
One central pathological feature of BD patients is arteriolosclerosis or lipohyalinosis of the small penetrating arterioles in the WM.[4,5,7] Besides arteriolosclerosis is the most important pathologic correlate of WMHs accumulation over time in aging.[85] However there is no imaging method able to offer direct visualization of the small WM penetrating arteries. These arterioles loss compliance becoming stiffer and have leakage of the arteriolar wall. These anatomic changes correlate with functional changes such as loss of arteriole elasticity. Indirect methods to measure these changes include transcranial Doppler and brain perfusion imaging. These perfusion studies might increase their sensitivity with the use of CO2 or vasodilators to challenge the arteriole vasoreactivity. Resting state MRI, which can detect cardiac pulsatility is a promising method for creating maps of the vascular resistance in the WM.[86]
We listed these new imaging methods with their advantages and disadvantages for the study and identification of BD patients (Table 2).
Table 2.
Possible Imaging biomarkers for BD.
| Anatomic-Physiologic measurements | Putative clinical use | Disadvantages | Reference | ||
|---|---|---|---|---|---|
|
| |||||
| Diffusion tensor Imaging (DTI) | MRI | WH tract integrity. | Monitoring Staging Predictive | Unknown reproducibility. | [78,80,81] |
|
| |||||
| Dynamic Contrast Enhancement MRI (DCEMRI) | MRI | Disruption of the BBB. | Diagnostic Monitoring? | Requires contrast. | [74,75,88] |
| Long acquisition time. | |||||
| Unknown reproducibility and variability | |||||
|
| |||||
| Perfusion Imaging | PWI, SPECT, PET, XeCT CTP. | White matter blood flow | Diagnostic Monitoring? | Variability and reproducibility issues. | |
| Some require contrast. | |||||
|
| |||||
| Cerebrovascular reserve imaging. | MRI, CTP, TCD. | Arteriole vasodilation response. | Diagnostic Monitoring? | Lack of validation | |
|
| |||||
| Resting State MRI | MRI | Pulsatility of the arteries. | Diagnostic Monitoring? | Lack of validation | [86] |
| Unknown reproducibility and variability | |||||
Clinical Scales
One of the major barriers in the study of BD is lack of consensus as to clinical definition. For difficult cases and standardization in clinical trials, a diagnostic multimodal approach with biomarkers might be helpful.[87] A new consensus on the clinical definition on this syndrome in the following years is expected. That will improve standardize patient identification for futures studies.
Another important barrier for future BD trials is the absence of outcome makers to measure treatment effectiveness. An ideal BD maker would be one that is clinically meaningful, representative of the disease’s underlying biological progression, efficient at detecting changes in response to treatment, reliable, reproducible and easy to set up across different trial sites. Once the major features important in the diagnosis of BD can be identified, it will be possible to use factor analysis to develop a scale that conforms with the diagnosis after long-term follow-up since autopsy verification is usually not available. This information will need to come from large cohorts of patients subjected to similar biomarker analysis.
Five-year view
The pathophysiology of BD is complex. New evolving treatments will target different mechanism of the disease. There is more evidence that inflammation and stiffness of the small vessels plays an important role in the pathophysiology of the disease. A logical next step will be the use of anti-inflammatory therapies to ameliorate the progression of disease. There are several anti-inflammatory compounds that have shown effectiveness in animal models. Inhibiting adhesion molecules can have therapeutic effects in chronic WM ischemia involving detrimental leukocyte trafficking. Minocycline is a drug with multiple neuroprotective actions, including broad anti-inflammatory properties, has shown promise in pre-clinical clinical trials in acute cerebral ischemia. The putative mechanisms of these agents make them ideal for models of persistent inflammation of chronic cerebral ischemia such as BD.
Reduction of stiffness of small arteries, improving its compliance and cerebral blood flow can in theory delay progression of the disease.
Once new clinical biomarkers have been chosen for the selection of BD patients, a next step will be to determine the speed of the progression of the disease. Not all BD patients worsen at the same pace. Identifying which patients are on higher risk for rapid disease progression will be essential to the design of larger trials. The challenge will be to combine a program of medical therapy with exercise and diet to reduce vascular risk factors and the accompanying inflammation to achieve optimal primary and secondary prevention.
Figure 2.
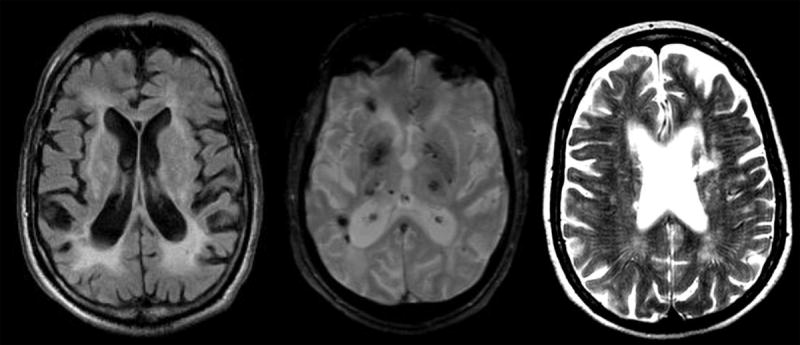
Imaging features of Binswanger’s disease.
A. Large white matter hyperintensities with white matter atrophy in FLAIR.
B. Subcortical microhemorrhages (black dots) on Echo gradient.
C. Dilated perivascular spaces on T2.
Key issues.
Binswanger’s disease is a form of cerebral small vessels disease characterized by cognitive impairment, gait disturbances with small subcortical ischemic strokes and ischemic WHMs. It is strongly associated with long standing hypertension and symptoms progress over time.
Advanced imaging, neuropsychological testing and CSF evaluation are aids to support the diagnosis of Binswanger’s disease.
Blood pressure control is the main therapy that most likely delays progression of the disease.
Antiplatelet therapy, statins diet and exercise are also play an important role in the medical management of this condition.
Avoid dual antiplatelet therapy or anticoagulation for secondary stroke prevention unless benefits are clearly more than risks (eg, afib, cardiac stent).
Converging evidence suggests that endothelial dysfunction and neuroinflamation leads to BBB disruption in BD patients. These changes can be measured by new MRI methods.
New clinical scales and outcome biomarkers will facilitate the selection and execution of clinical trials with BD patients.
As new pathophysiology is revealed, novel therapeutics will target other mechanism such as inflammation, arterial stiffness and clearance of cerebral waste material in the coming years.
Acknowledgments
Funding: 1) RO1# 5R01NS052305-07 to GAR 2) Bayer Pharmaceutical Corp.
Footnotes
Disclosures: Authors have nothing to disclose
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR. Binswanger’s disease--revisited. Neurology. 1995;45(4):626–633. doi: 10.1212/wnl.45.4.626. Review. [DOI] [PubMed] [Google Scholar]
- 4.Blass JP, Hoyer S, Nitsch R. A translation of Otto Binswanger’s article, ‘The delineation of the generalized progressive paralyses’. 1894. Archives of neurology. 1991;48(9):961–972. doi: 10.1001/archneur.1991.00530210089029. [DOI] [PubMed] [Google Scholar]
- 5.Babikian V, Ropper AH. Binswanger’s disease: a review. Stroke; a journal of cerebral circulation. 1987;18(1):2–12. doi: 10.1161/01.str.18.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Gilley DW, Fox JH. Clinical diagnosis of Binswanger’s disease. Journal of neurology, neurosurgery, and psychiatry. 1990;53(11):961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caplan LR. Binswanger’s disease--revisited. Neurology. 1995;45(4):626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. Journal of neurology, neurosurgery, and psychiatry. 2005;76(8):1140–1145. doi: 10.1136/jnnp.2004.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y, Sharma VK, Chan BP, et al. The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. Journal of the neurological sciences. 2010;299(1-2):15–18. doi: 10.1016/j.jns.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 10.Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. International journal of stroke : official journal of the International Stroke Society. 2013 doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet neurology. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Annals of neurology. 2005;57(4):472–479. doi: 10.1002/ana.20423. [DOI] [PubMed] [Google Scholar]
- 13.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain : a journal of neurology. 2005;128(Pt 9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson M, Zetterberg H, van Straaten E, et al. Cerebrospinal fluid biomarkers of white matter lesions - cross-sectional results from the LADIS study. Eur J Neurol. 2010;17(3):377–382. doi: 10.1111/j.1468-1331.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- 15.Adair JC, Charlie J, Dencoff JE, et al. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke; a journal of cerebral circulation. 2004;35(6):e159–162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 16.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2001;11(3):229–235. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 17.Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5(6):454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Yoshikawa T, Oku N, et al. Statistical parametric analysis of cerebral blood flow in vascular dementia with small-vessel disease using Tc-HMPAO SPECT. Cerebrovascular diseases. 2008;26(5):556–562. doi: 10.1159/000160213. [DOI] [PubMed] [Google Scholar]
- 19.Gasparovic C, Prestopnik J, Thompson J, et al. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. Journal of neurology, neurosurgery, and psychiatry. 2013;84(7):715–721. doi: 10.1136/jnnp-2012-303878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawes CM, Vander Hoorn S, Rodgers A International Society of H. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 22.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke; a journal of cerebral circulation. 2003;34(11):2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull F Blood Pressure Lowering Treatment Trialists C. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 24.Group SPSS. Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke; a journal of cerebral circulation. 2008;39(10):2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 26.Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68(3):214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- 27.Verhaaren BF, Vernooij MW, de Boer R, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61(6):1354–1359. doi: 10.1161/HYPERTENSIONAHA.111.00430. [DOI] [PubMed] [Google Scholar]
- 28.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)-Dijon Magnetic Resonance Imaging Study. Circulation. 2011;123(3):266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- 29.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112(11):1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 30.Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke; a journal of cerebral circulation. 2012;43(9):2336–2342. doi: 10.1161/STROKEAHA.111.648576. [DOI] [PubMed] [Google Scholar]
- 31.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet neurology. 2008;7(8):683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 32.Investigators SPS. Benavente OR, Hart RG, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. The New England journal of medicine. 2012;367(9):817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amarenco P, Bogousslavsky J, Callahan A, 3rd et al. High-dose atorvastatin after stroke or transient ischemic attack. The New England journal of medicine. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. Journal of atherosclerosis and thrombosis. 2011;18(5):351–358. doi: 10.5551/jat.7591. [DOI] [PubMed] [Google Scholar]
- 36.Hassan A, Hunt BJ, O’Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain : a journal of neurology. 2003;126(Pt 2):424–432. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 37.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 38.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. Journal of internal medicine. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 39.Gons RA, Tuladhar AM, de Laat KF, et al. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013;81(11):971–976. doi: 10.1212/WNL.0b013e3182a43e33. [DOI] [PubMed] [Google Scholar]
- 40.Verdelho A, Madureira S, Ferro JM, et al. Physical activity prevents progression for cognitive impairment and vascular dementia: results from the LADIS (Leukoaraiosis and Disability) study. Stroke; a journal of cerebral circulation. 2012;43(12):3331–3335. doi: 10.1161/STROKEAHA.112.661793. [DOI] [PubMed] [Google Scholar]
- 41.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic proceedings. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. The New England journal of medicine. 2006;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 43.Group VTS. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet neurology. 2010;9(9):855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 44.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 45.Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among US adults: NHANES 2003-2008. The American journal of clinical nutrition. 2012;96(3):647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. The New England journal of medicine. 2010;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-Sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. Journal of human hypertension. 2009;23(12):826–835. doi: 10.1038/jhh.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. American journal of ophthalmology. 2008;146(3):404–409. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA internal medicine. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomimoto H, Ohtani R, Shibata M, Nakamura N, Ihara M. Loss of cholinergic pathways in vascular dementia of the Binswanger type. Dementia and geriatric cognitive disorders. 2005;19(5-6):282–288. doi: 10.1159/000084553. [DOI] [PubMed] [Google Scholar]
- 51.Keverne JS, Low WC, Ziabreva I, Court JA, Oakley AE, Kalaria RN. Cholinergic neuronal deficits in CADASIL. Stroke; a journal of cerebral circulation. 2007;38(1):188–191. doi: 10.1161/01.STR.0000251787.90695.05. [DOI] [PubMed] [Google Scholar]
- 52.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359(9314):1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61(4):479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- 54.Black S, Roman GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke; a journal of cerebral circulation. 2003;34(10):2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 55.Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet neurology. 2008;7(4):310–318. doi: 10.1016/S1474-4422(08)70046-2. [DOI] [PubMed] [Google Scholar]
- 56.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet neurology. 2007;6(9):782–792. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 57.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) International clinical psychopharmacology. 2002;17(6):297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke; a journal of cerebral circulation. 2002;33(7):1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 59.Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 60.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53(6):1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 61.Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59(2):193–197. doi: 10.1212/wnl.59.2.193. [DOI] [PubMed] [Google Scholar]
- 62.Tomimoto H. Subcortical vascular dementia. Neurosci Res. 2011;71(3):193–199. doi: 10.1016/j.neures.2011.07.1820. [DOI] [PubMed] [Google Scholar]
- 63.Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathology & Applied Neurobiology. 1997;23(5):406–415. [PubMed] [Google Scholar]
- 64.Rosenberg GA, Sullivan N, Esiri MM. White matter damage is assoiciated with matrix metalloproteinases in vascular dementia. Stroke; a journal of cerebral circulation. 2001;32:1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 65.Cammer W, Bloom BR, Norton WT, Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci U S A. 1978;75:1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yong VW, Zabad RK, Agrawal S, Goncalves Dasilva A, Metz LM. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. Journal of the neurological sciences. 2007;259(1-2):79–84. doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liuzzi GM, Trojano M, Fanelli M, et al. Intrathecal synthesis of matrix metalloproteinase-9 in patients with multiple sclerosis: implication for pathogenesis. Mult Scler. 2002;8(3):222–228. doi: 10.1191/1352458502ms800oa. [DOI] [PubMed] [Google Scholar]
- 69.Wallin A, Blennow K, Fredman P, Gottfries CG, Karlsson I, Svennerholm L. Blood brain barrier function in vascular dementia. Acta neurologica Scandinavica. 1990;81(4):318–322. doi: 10.1111/j.1600-0404.1990.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 70.Candelario-Jalil E, Thompson J, Taheri S, et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2011;42(5):1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke; a journal of cerebral circulation. 2012;43(4):1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanyu H, Asano T, Tanaka Y, Iwamoto T, Takasaki M, Abe K. Increased blood-brain barrier permeability in white matter lesions of Binswanger’s disease evaluated by contrast-enhanced MRI. Dementia and geriatric cognitive disorders. 2002;14(1):1–6. doi: 10.1159/000058326. [DOI] [PubMed] [Google Scholar]
- 73.Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Annals of neurology. 2009;65(2):194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 74.Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. Journal of neurology, neurosurgery, and psychiatry. 2010;81(2):192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 76.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet neurology. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choudhri AF, Chin EM, Blitz AM, Gandhi D. Diffusion tensor imaging of cerebral white matter: technique, anatomy, and pathologic patterns. Radiologic clinics of North America. 2014;52(2):413–425. doi: 10.1016/j.rcl.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 78.O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57(12):2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- 79.Reijmer YD, Freeze WM, Leemans A, Biessels GJ Utrecht Vascular Cognitive Impairment Study G. The effect of lacunar infarcts on white matter tract integrity. Stroke; a journal of cerebral circulation. 2013;44(7):2019–2021. doi: 10.1161/STROKEAHA.113.001321. [DOI] [PubMed] [Google Scholar]
- 80.Maillard P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra. Stroke; a journal of cerebral circulation. 2011;42(7):1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maillard P, Carmichael O, Harvey D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR American journal of neuroradiology. 2013;34(1):54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt R, Ropele S, Ferro J, et al. Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study. Stroke; a journal of cerebral circulation. 2010;41(5):e402–408. doi: 10.1161/STROKEAHA.109.576629. [DOI] [PubMed] [Google Scholar]
- 83.Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke; a journal of cerebral circulation. 2008;39(7):1999–2005. doi: 10.1161/STROKEAHA.107.507475. [DOI] [PubMed] [Google Scholar]
- 84.Jokinen H, Schmidt R, Ropele S, et al. Diffusion changes predict cognitive and functional outcome: the LADIS study. Annals of neurology. 2013;73(5):576–583. doi: 10.1002/ana.23802. [DOI] [PubMed] [Google Scholar]
- 85.Erten-Lyons D, Woltjer R, Kaye J, et al. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81(11):977–983. doi: 10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makedonov I, Black SE, Macintosh BJ. BOLD fMRI in the white matter as a marker of aging and small vessel disease. PloS one. 2013;8(7):e67652. doi: 10.1371/journal.pone.0067652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke; a journal of cerebral circulation. 2014;45(5):1531–1538. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wardlaw JM, Doubal FN, Valdes-Hernandez M, et al. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2013;44(2):525–527. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]


