Summary
Prior studies have demonstrated an association between prophylactic cranial irradiation (PCI) and subsequent decline in the Hopkins Verbal Learning Test (HVLT). In this analysis, prophylactic cranial irradiation is also associated with a higher rate of decline in self-reported cognitive functioning (SRCF). This study provides novel observations regarding the absence of a close correlation between decline in HVLT and decline in SRCF, suggesting that they may represent distinct elements of the cognitive spectrum.
Purpose
Prophylactic cranial irradiation (PCI) has been associated with decline in tested cognitive functioning, using the Hopkins Verbal Learning Test (HVLT). The purpose of this study was to assess the impact of PCI on self-reported cognitive functioning (SRCF), a functional scale on EORTC QLQ-C30.
Methods and Materials
RTOG 0214 randomized patients with locally advanced non-small cell lung cancer to PCI or observation. RTOG 0212 randomized patients with limited-disease small cell lung cancer to high- or standard-dose PCI. In both trials, HVLT-recall (R) and -delayed recall (DR) and SRCF were assessed at baseline (following locoregional therapy but before PCI or observation) and at 6 and 12 months (mos). Patients developing brain relapse prior to follow-up evaluation were excluded. Decline was defined using the reliable change index method and correlated with receipt of PCI versus observation using logistic regression modeling. Fisher's exact test correlated decline in SRCF with HVLT decline.
Results
Of the eligible patients pooled from RTOG 0212 and RTOG 0214, 410 (93%) receiving PCI and 173 (96%) undergoing observation completed baseline HVLT or EORTC QLQ-C30 testing and were included in this analysis. PCI was associated with a higher risk of decline in SRCF at 6 mos (Odds Ratio (OR), 3.60, 95% confidence interval (95%CI), 2.34-6.37, p<0.0001) and 12 mos (OR 3.44, 95%CI 1.84-6.44, p<0.0001). HVLT-R decline at 6 and 12 mos was also associated with PCI (p=0.002 and p=0.002, respectively), but was not closely correlated with decline in SRCF at the same time points (p=0.05 and p=0.86, respectively).
Conclusions
In lung cancer patients who do not develop brain relapse, PCI is associated with decline in HVLT-tested and self-reported cognitive functioning. Decline in HVLT and decline in SRCF are not closely correlated, suggesting that they may represent distinct elements of the cognitive spectrum.
Keywords: Lung cancer, cognitive functioning, RTOG 0212, RTOG 0214, prophylactic cranial irradiation
Introduction
Recent clinical trials have demonstrated cognitive impairment as an adverse effect of cranial irradiation, based on recall and delayed recall testing using the Hopkins Verbal Learning Test (HVLT). For instance, RTOG 0214 was a phase III trial of prophylactic cranial irradiation (PCI) versus observation in patients with locally-advanced NSCLC [1]. RTOG 0214 tested HVLT as a secondary endpoint and observed greater decline in HVLT in the PCI cohort as compared to the observation cohort at 1-year follow-up [2]. Similar findings have been demonstrated in the setting of brain metastases [3].
However, whether receipt of cranial irradiation is associated with subsequent decline in self-reported cognitive functioning (SRCF), as assessed using quality of life (QOL) questionnaires, remains ill defined. Slotman et al. attempted to address this question in a phase III trial of PCI versus observation for extensive-disease small cell lung cancer and observed a two-fold increase in the proportion of patients experiencing SRCF decline with PCI, although this result did not reach statistical significance [4]. Similarly, RTOG 0214 demonstrated a trend for greater decline in SRCF with PCI, but this finding lost statistical significance on multivariate analysis [2]. One potential reason for the absence of statistical significance in these findings may have been limited sample size, as RTOG 0214 was not able to reach target accrual and both trials reported significant non-compliance with QOL follow-up.
To overcome this limitation, we pooled neurocognitive and quality of life data from RTOG 0214 with data from RTOG 0212, a phase II trial of high-dose versus standard-dose PCI for limited-stage small cell lung cancer. In addition to using HVLT for cognitive function testing, both RTOG trials utilized the same QOL instrument, EORTC Core Quality of Life Questionnaire (QLQ-C30), in which self-reported cognitive functioning is specifically assessed as a two-item functional scale.
Methods and Materials
The details regarding patient eligibility and treatment on RTOG 0212 and RTOG 0214 have been previously described [1,5]. Briefly, eligibility for RTOG 0212 was limited to patients with limited-disease SCLC with complete response to chemotherapy and consolidative chest radiotherapy; Zubrod performance status of ≤1; and, RTOG neurologic function class of 1 or 2. Eligibility on RTOG 0214 was limited to patients with stage IIIA/B NSCLC with stable disease or complete/partial response after potentially curative therapy; no evidence of brain or extracranial metastases; and, resolution to grade ≤2 of any acute or subacute grade ≥3 toxicities from prior therapy. All patients signed an institutional review board-approved, study-specific consent form.
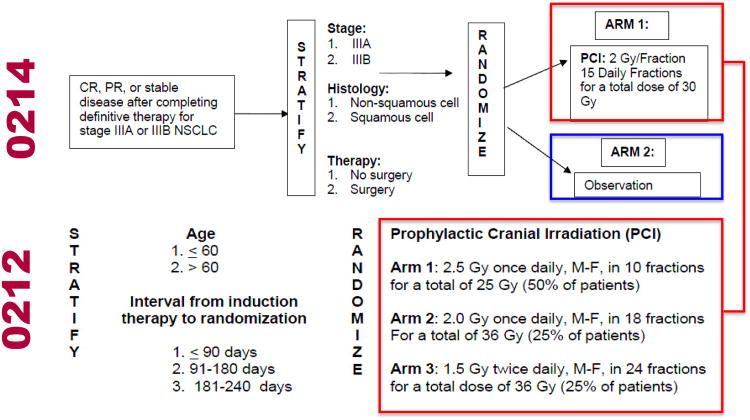
RTOG 0212 randomized patients to standard-dose PCI (25 Gy in 10 daily fractions) or high-dose PCI (36 Gy). Those randomized to the high-dose PCI underwent a second randomization to receive PCI in 18 daily fractions of 2.0 Gy per fraction or 24 twice-daily fractions of 1.5 Gy per fraction [5]. RTOG 0214 randomized patients to PCI (30 Gy in 15 daily fractions) or observation [1] (Figure 1). Both trials are registered with ClinicalTrials.gov, numbers NCT00057746 (RTOG 0212) and NCT00048997 (RTOG 0214).
Figure 1. Study schema.
QOL and HVLT Assessments
In both studies, self-reported outcomes were captured prospectively using the EORTC QLQ-C30. EORTC QLQ-C30 is a 30-item self-report questionnaire containing multiple QOL domains (scales) [6]. For this analysis, global quality of life as well as each symptom and functional scale, including SRCF, were analyzed separately. Specifically, SRCF is a two-item functioning scale captured with questions pertaining to concentration and memory. As with other functional scales, higher scores indicate better functioning. EORTC QLQ-C30 scores were converted to lie in a range between 0-100, according to the guidelines of EORTC [7]. EORTC QLQ-C30 has been previously shown to be a reliable and valid instrument in patients with lung and other cancer diagnoses [6,8].
Both trials tested cognitive function prospectively using HVLT, a well-validated and reliable assessment of list-learning memory, including encoding, retrieval and retention of new information over time [9]. HVLT incorporates 6 different forms, helping to mitigate practice effects of repeated administrations. Each form includes 12 nouns (targets) with 4 words drawn from 3 semantic categories, which differ across the 6 forms. The test involves memorizing a list of 12 targets for 3 consecutive trials (Recall) and recalling the 12 targets after a 20-minute delay (Delayed Recall). Raw scores can range from 0-36 for HVLT-Recall (HVLT-R) and 0-12 for delayed recall (HVLT-DR).
In both studies, baseline HVLT and EORTC QLQ-C30 were completed following definitive loco-regional therapy but before the initiation of PCI or observation. Serial follow-up HVLT and EORTC QLQ-C30 testing was performed at 6 and 12 months after study entry. Since patient follow-up did not always occur at exactly 6 and 12 months follow-up, data obtained within 4 weeks before or after these time points were included. Data collected at additional time points of 24, 36 and 48 months after study entry were not included in this analysis due to substantial long-term non-compliance. Each patient served as his/her own control, as the difference in scores obtained at baseline and at 6 and 12 months after randomization were calculated.
Statistical Methods
Data from RTOG 0212 and RTOG 0214 were pooled for this exploratory analysis, evaluating whether receipt of PCI predicted for decline in HVLT and/or SRCF. To minimize the confounding effects of intracranial relapse, all patients who developed an intracranial relapse prior to follow-up evaluation were excluded. Patients with missing follow-up assessments were excluded from analysis of the applicable time point. Comparisons of categorical patient characteristics were done using chi-square test statistics, and comparisons of continuous patient characteristics were done using the F-statistic from analysis of variance.
Baseline comparisons of continuous HVLT scores were done using the Kruskal-Wallis two-sided rank test to compare means. Follow-up scores were analyzed using the reliable change index (RCI) method [10], which allows for changes from baseline to be classified as either a decline, stability, or improvement in function. Logistic regression analysis [11] was used to evaluate whether use of PCI was predictive of decline in HVLT or EORTC QLQ-C30 functional or symptom scale. These models were adjusted for factors that were predictive for decline, such as baseline score, age, gender, education level, marital status, PCI dose or baseline Zubrod performance status. Baseline score was evaluated continuously and as a categorical variable (impaired vs. unimpaired). Baseline HVLT and EORTC QLQ-C30 scores were considered impaired if the score was ≥ 1.5 standard deviations (SDs) worse than the mean of the normative age-adjusted distribution [12,13]. For PCI dose, patients were categorized as standard dose PCI (2.5 Gy × 10 on RTOG 0212 or 2.0 Gy × 15 on RTOG 0214), high dose PCI (2.0 Gy × 18 or 1.5 Gy × 25 on RTOG 0212) or no PCI. Associations between decline in SRCF, HVLT, or other EORTC QLQ-C30 functional or symptom scale was tested using the Fisher's exact test, with agreement evaluated using the Kappa correlation statistic [14,15].
To prevent inflation of type I error, the decision was made a priori to assign statistical significance for analyses of any EORTC QLQ-C30 functional or symptom scale, including SRCF, to p-values <0.0001. Otherwise, statistical significance was assigned to p-values <0.05.
Statistical Analysis Software® (SAS Institute, Cary, NC) version 9.2 was used for all statistical analyses.
Results
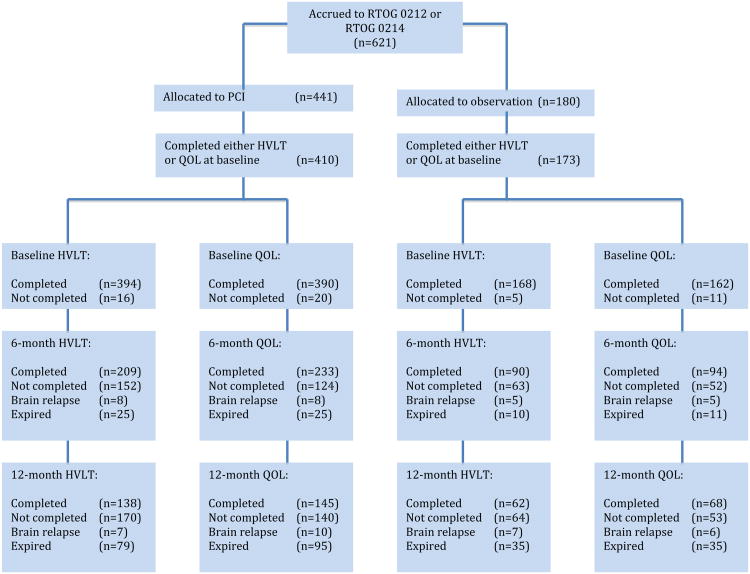
A total of 621 patients were accrued to RTOG 0212 (n=265) and RTOG 0214 (n=356). Of these, 252 patients (95%) on RTOG 0212 and 331 patients (93%) on RTOG 0214 completed either baseline HVLT or EORTC QLQ-C30 testing and were included in this analysis (Figure 2). Of the 410 patients treated with PCI, 158 came from the NSCLC study RTOG 0214, and 252 came from the SCLC study RTOG 0212. All 173 patients who did not receive PCI came from RTOG 0214. Comparison of PCI to observation cohorts demonstrated that patients treated with PCI were more likely to attain an educational level of high school equivalence or higher (p=0.02), compared to patients treated without PCI (Table 1). Otherwise, PCI and observation cohorts were similar with respect to age, gender, partner status, and baseline Zubrod performance status. Compliance with HVLT and EORTC QLQ-C30 assessments at 6 and 12 months follow-up did not differ between PCI and observation cohorts (Figure 2).
Figure 2. CONSORT Flowchart.
PCI: prophylactic cranial irradiation; HVLT: Hopkins Verbal Learning Test; QOL: quality of life.
Table 1. Patient Characteristics.
| PCI (n=410) | No PCI (n=173) | p value | |
|---|---|---|---|
|
| |||
| Age | 0.87 | ||
| Median | 62 | 62 | |
| Range | 39-86 | 39-83 | |
|
| |||
| Gender | 0.44 | ||
| Male | 242 (59%) | 108 (62%) | |
| Female | 168 (41%) | 65 (38%) | |
|
| |||
| Education Level | 0.02 | ||
| < High School | 96 (25%) | 57 (35%) | |
| High School | 146 (39%) | 44 (27%) | |
| > High School | 137 (36%) | 61 (38%) | |
| Unknown | 31 | 11 | |
|
| |||
| Partner Status | 0.07 | ||
| Partnered | 258 (65%) | 125 (73%) | |
| Not Partnered | 140 (35%) | 47 (27%) | |
| Unknown | 12 | 126 | |
|
| |||
| Zubrod Performance Status* | 0.09 | ||
| 0 | 133 (50%) | 102 (59%) | |
| 1 | 123 (46%) | 67 (39%) | |
| 2−3 | 9 (3%) | 4 (2%) | |
Baseline Zubrod performance was collected on 105 (43.2%) of the patients enrolled on RTOG 0212 and all patients enrolled on RTOG 0214.
Abbreviations: PCI, prophylactic cranial irradiation.
Higher baseline HVLT-R and HVLT-DR scores were observed among patients receiving PCI (p=0.02 and p=0.02, respectively) and high-dose PCI (p=0.004 and p=0.01, respectively) (Table e1; www.redjournal.org). Patient factors associated with higher baseline HVLT-R scores included female gender (p<0.0001), more advanced education level (p<0.0001), partnered status (p=0.04), and age ≤60 (p<0.0001). Patient factors associated with higher baseline HVLT-DR scores were female gender (p<0.0001), more advanced education level (p<0.0001), and age ≤60 (p=0.03). Comparisons of baseline EORTC QLQ-C30 scores demonstrated no significant associations of SRCF with any patient factors.
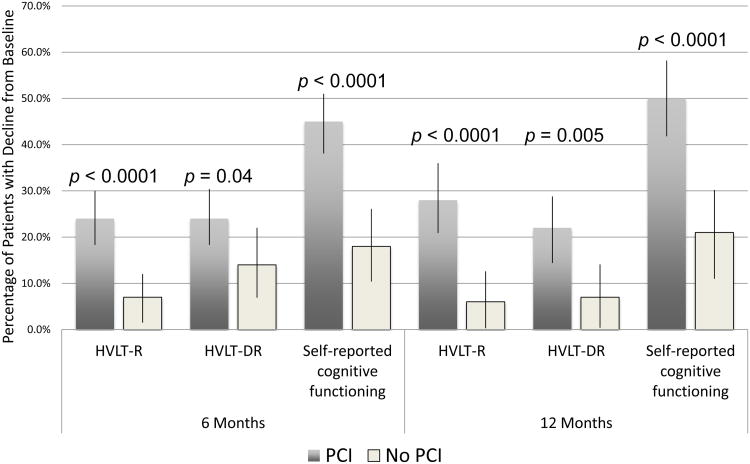
At 6 and 12 months follow-up, PCI was associated with higher rates of decline in HVLT-R, HVLT-DR, and SRCF (Figure 3). No other associations were observed between PCI and decline in global health status/quality of life or other EORTC QLQ-C30 functional or symptom scales (data not shown). For HVLT-R and –DR, baseline impairment was associated with lower rates of decline at 6 months (p=0.0003 and p=0.001, respectively) and 12 months (p=0.002 and p=0.03). Similar results were observed when baseline score was assessed continuously (data not shown). For SRCF, baseline score assessed continuously or categorically (impaired vs. unimpaired) was not associated with subsequent decline at 6 or 12 months. Age>60 was associated with higher rates of HVLT-DR decline at 12 months (p=0.02).
Figure 3. Prophylactic cranial irradiation (PCI) is associated with decline in both tested and self-reported cognitive functioning.
HVLT-R: Hopkins Verbal Learning Test-Recall; HVLT-DR: Hopkins Verbal Learning Test-Delayed Recall. Black bars represent 95% confidence intervals. For HVLT-Recall and –Delayed Recall comparisons, statistical significance was assigned to p<0.05. For SRCF comparisons, statistical significance was assigned to p<0.0001 to prevent inflation of type I error from multiple testing of EORTC QLQ-C30.
In a multivariate logistic regression model of HVLT-R decline at 6 and 12 months, both receipt of PCI (p=0.002 and p=0.002, respectively) and baseline HVLT-R impairment (p=0.0002 and p=0.003, respectively) remained independently predictive (Table 2). Association between PCI and HVLT-DR trended to statistical significance (6 months, p=0.08; 12 months, p=0.06) after adjusting for baseline impairment. Similar results were observed when baseline score was assessed continuously (data not shown).
Table 2.
Logistic regression models of association of PCI with decline in HVLT-Recall, HVLT-Delayed Recall, and self-reported cognitive function.
| Factor | Decline in … | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| HVLT-Recall | HVLT-Delayed Recall | Self-Reported Cognitive Function | ||||
|
| ||||||
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |
|
|
|
|
|
|
|
|
| No PCI (RL) vs. PCI | ||||||
| Odds Ratio | 3.91 | 4.96 | 1.89 | 2.49 | 3.60 | 3.44 |
| 95% CI | 1.68-9.08 | 1.84-13.38 | 0.94-3.81 | 0.96-6.48 | 2.34-6.37 | 1.84-6.44 |
| p | 0.002 | 0.002 | 0.08 | 0.06 | <0.0001 | <0.0001 |
| Baseline Impairment (RL) vs. No Impairment | ||||||
| Odds Ratio | 4.62 | 4.13 | 4.51 | 3.33 | NA* | NA* |
| 95% CI | 2.09-10.22 | 1.63-10.43 | 1.85-10.97 | 1.10-10.09 | ||
| p | 0.0002 | 0.003 | 0.0009 | 0.03 | ||
| Age ≤60 (RL) vs. >60 | ||||||
| Odds Ratio | NA* | NA* | NA* | 2.52 | NA* | NA* |
| 95% CI | 1.06-5.99 | |||||
| p | 0.04 | |||||
Abbreviations: HVLT, Hopkins Verbal Learning Test; PCI, prophylactic cranial irradiation; RL, referent level; 95% CI, 95% confidence interval.
N/A: Factor not significant on univariate logistic regression analysis.
For HVLT-Recall and –Delayed Recall comparisons, statistical significance was assigned to p<0.05. For SRCF comparisons, statistical significance was assigned to p<0.0001 to prevent inflation of type I error from multiple testing of EORTC QLQ-C30.
Analyses for correlation of SRCF decline with decline in HVLT-R or HVLT-DR demonstrated no significant associations at 6 months (HVLT-R, Kappa 0.113, p=0.05; HVLT-DR, Kappa 0.155, p=0.01) or 12 months follow-up (HVLT-R, Kappa -0.023, p=0.74; HVLT-DR, Kappa 0.046, p=0.50) (Table 3). At 6 and 12 months follow-up, decline in HVLT-R or HVLT-DR was also not correlated with decline in any other EORTC QLQ-C30 symptom or functional scale (data not shown). Correlative analyses of SRCF decline with other EORTC QLQ-C30 symptom or functional measures demonstrated fair agreement with decline in self-reported physical functioning at 6 months (Kappa=0.241, p<0.0001) and 12 months (Kappa=0.299, p<0.0001) and increasing fatigue (Kappa=0.260, p<0.0001) and appetite loss (Kappa=0.263, p<0.0001) at 6 months (Table 3).
Table 3.
Correlations between decline in self-reported cognitive functioning and decline in HVLT or other EORTC QLQ-C30 functional or symptom scales at 6 and 12 months follow-up.
| Self-Reported Cognitive Functioning | ||||
|---|---|---|---|---|
|
|
||||
| Decline | No decline | p value* | ||
| HVLT-Recall | 6 Months | 0.05 | ||
| Decline | 24 (9%) | 28 (10%) | ||
| No decline | 68 (25%) | 147 (55%) | ||
| Kappa (p**) | 0.113 (0.05) | |||
| 12 Months | 0.86 | |||
| Decline | 16 (9%) | 25 (14%) | ||
| No decline | 60 (33%) | 83 (45%) | ||
| Kappa (p**) | -0.023 (0.74) | |||
|
| ||||
| HVLT-Delayed Recall | 6 Months | 0.02 | ||
| Decline | 27 (11%) | 27 (11%) | ||
| No decline | 60 (24%) | 133 (54%) | ||
| Kappa (p**) | 0.155 (0.01) | |||
| 12 Months | 0.54 | |||
| Decline | 14 (8%) | 17 (10%) | ||
| No decline | 52 (31%) | 83 (50%) | ||
| Kappa (p**) | 0.046 (0.50) | |||
|
| ||||
| Global Health Status/QOL | 6 Months | 0.02 | ||
| Decline | 45 (14%) | 52 (16%) | ||
| No decline | 74 (23%) | 150 (47%) | ||
| Kappa (p**) | 0.126 (0.02) | |||
| 12 Months | 0.44 | |||
| Decline | 27 (13%) | 33 (16%) | ||
| No decline | 59 (28%) | 93 (44%) | ||
| Kappa (p**) | 0.055 (0.41) | |||
|
| ||||
| Physical Functioning | 6 Months | <0.0001 | ||
| Decline | 46 (14%) | 33 (10%) | ||
| No decline | 73 (23%) | 170 (53%) | ||
| Kappa (p**) | 0.241 (<0.0001) | |||
| 12 Months | <0.0001 | |||
| Decline | 38 (18%) | 20 (9%) | ||
| No decline | 48 (23%) | 106 (50%) | ||
| Kappa (p**) | 0.299 (<0.0001) | |||
|
| ||||
| Role Functioning | 6 Months | 0.0002 | ||
| Decline | 50 (16%) | 45 (14%) | ||
| No decline | 69 (21%) | 158 (49%) | ||
| Kappa (p**) | 0.207 (0.0002) | |||
| 12 Months | 0.007 | |||
| Decline | 31 (15%) | 24 (11%) | ||
| No decline | 55 (26%) | 102 (48%) | ||
| Kappa (p**) | 0.180 (0.006) | |||
|
| ||||
| Emotional Functioning | 6 Months | 0.05 | ||
| Decline | 27 (8%) | 28 (9%) | ||
| No decline | 92 (29%) | 175 (54%) | ||
| Kappa (p**) | 0.100 (0.04) | |||
| 12 Months | 0.005 | |||
| Decline | 29 (14%) | 21 (10%) | ||
| No decline | 57 (27%) | 105 (50%) | ||
| Kappa (p**) | 0.183 (0.004) | |||
|
| ||||
| Social Functioning | 6 Months | 0.0003 | ||
| Decline | 40 (12%) | 31 (10%) | ||
| No decline | 79 (25%) | 172 (53%) | ||
| Kappa (p**) | 0.200 (0.0001) | |||
| 12 Months | 0.03 | |||
| Decline | 27 (13%) | 23 (11%) | ||
| No decline | 59 (28%) | 103 (49%) | ||
| Kappa (p**) | 0.141 (0.03) | |||
|
| ||||
| Fatigue | 6 Months | <0.0001 | ||
| Decline | 64 (20%) | 57 (18%) | ||
| No decline | 54 (17%) | 146 (45%) | ||
| Kappa (p**) | 0.260 (<0.0001) | |||
| 12 Months | 0.002 | |||
| Decline | 42 (20%) | 35 (17%) | ||
| No decline | 44 (21%) | 91 (43%) | ||
| Kappa (p**) | 0.214 (0.002) | |||
|
| ||||
| Nausea and Vomiting | 6 Months | 0.04 | ||
| Decline | 27 (8%) | 27 (8%) | ||
| No decline | 92 (29%) | 176 (55%) | ||
| Kappa (p**) | 0.106 (0.03) | |||
| 12 Months | 0.55 | |||
| Decline | 14 (7%) | 16 (8%) | ||
| No decline | 72 (24%) | 110 (52%) | ||
| Kappa (p**) | 0.040 (0.46) | |||
|
| ||||
| Pain | 6 Months | 0.0006 | ||
| Decline | 49 (15%) | 46 (14%) | ||
| No decline | 70 (22%) | 156 (49%) | ||
| Kappa (p**) | 0.192 (0.0005) | |||
| 12 Months | 0.03 | |||
| Decline | 33 (16%) | 30 (14%) | ||
| No decline | 53 (25%) | 96 (45%) | ||
| Kappa (p**) | 0.152 (0.02) | |||
|
| ||||
| Dyspnea | 6 Months | 0.47 | ||
| Decline | 27 (9%) | 38 (12%) | ||
| No decline | 92 (29%) | 164 (51%) | ||
| Kappa (p**) | 0.043 (0.40) | |||
| 12 Months | 0.87 | |||
| Decline | 18 (8%) | 28 (13%) | ||
| No decline | 68 (32%) | 98 (46%) | ||
| Kappa (p**) | -0.014 (0.82) | |||
|
| ||||
| Insomnia | 6 Months | 0.05 | ||
| Decline | 34 (11%) | 38 (12%) | ||
| No decline | 85 (27%) | 163 (51%) | ||
| Kappa (p**) | 0.105 (0.05) | |||
| 12 Months | 0.06 | |||
| Decline | 30 (14%) | 29 (14%) | ||
| No decline | 55 (26%) | 96 (46%) | ||
| Kappa (p**) | 0.127 (0.06) | |||
|
| ||||
| Appetite Loss | 6 Months | <0.0001 | ||
| Decline | 56 (17%) | 44 (14%) | ||
| No decline | 63 (20%) | 159 (49%) | ||
| Kappa (p**) | 0.263 (<0.0001) | |||
| 12 Months | 0.002 | |||
| Decline | 27 (13%) | 16 (8%) | ||
| No decline | 57 (27%) | 108(52%) | ||
| Kappa (p**) | 0.209 (0.0008) | |||
|
| ||||
| Constipation | 6 Months | 0.10 | ||
| Decline | 34 (11%) | 41 (13%) | ||
| No decline | 85 (26%) | 162 (50%) | ||
| Kappa (p**) | 0.091 (0.09) | |||
| 12 Months | 0.009 | |||
| Decline | 29 (14%) | 22 (10%) | ||
| No decline | 56 (27%) | 103 (49%) | ||
| Kappa (p**) | 0.177 (0.006) | |||
|
| ||||
| Diarrhea | 6 Months | 0.58 | ||
| Decline | 15 (5%) | 21 (7%) | ||
| No decline | 104 (32%) | 181 (56%) | ||
| Kappa (p**) | 0.026 (0.54) | |||
| 12 Months | 0.06 | |||
| Decline | 13 (6%) | 8 (4%) | ||
| No decline | 73 (35%) | 117 (55%) | ||
| Kappa (p**) | 0.099 (0.04) | |||
|
| ||||
| Financial Difficulties | 6 Months | 0.01 | ||
| Decline | 25 (8%) | 21 (7%) | ||
| No decline | 93 (29%) | 181 (57%) | ||
| Kappa (p**) | 0.124 (0.008) | |||
| 12 Months | 0.11 | |||
| Decline | 17 (8%) | 14 (7%) | ||
| No decline | 69 (33%) | 110 (52%) | ||
| Kappa (p**) | 0.094 (0.09) | |||
Abbreviations: HVLT, Hopkins Verbal Learning Test.
P-value from Fisher's exact test. To prevent inflation from type 1 error due to multiple testing, statistical significance was assigned to p-values <0.0001.
P-value from Z-test. To prevent inflation from type 1 error due to multiple testing, statistical significance was assigned to p-values <0.0001.
Discussion
In this analysis of pooled HVLT and quality of life data from RTOG 0212 and RTOG 0214, we observed at least a three-fold elevated risk of decline in SRCF 6 and 12 months following PCI as compared to observation in lung cancer patients who did not develop brain relapse. This adverse quality of life effect of PCI was selective for SRCF and was not similarly appreciated with global health status/quality of life or any other EORTC QLQ-C30 symptom or functional scale. Similar results, presented in abstract form [16], have been observed in the EORTC trial of adjuvant whole-brain radiotherapy versus observation following surgical resection or radiosurgery for 1 to 3 brain metastases. Using the EORTC QLQ-C30 questionnaire to examine health-related quality of life, patients who underwent whole-brain radiotherapy were noted to have decline in SRCF at 12 months follow-up. The summation of these and our findings provides the first evidence of a cognitive functioning-specific quality of life detriment to cranial irradiation and emphasizes the importance of counseling patients on cranial irradiation-induced cognitive decline not just captured on intensive memory testing but also self-reported.
Another salient finding of this study is the poor agreement between HVLT and SRCF in detecting decline in cognitive functioning, raising important questions about our current understanding of cognitive decline following cranial irradiation. Firstly, prior studies have shown a decline in list-learning recall and delayed recall, as captured by HVLT, between 3 and 12 months after cranial irradiation [2,3,17]. However, the findings from our study question whether this HVLT decline is pertinent to self-reported quality of life and/or perceptible to patients. Secondly, prior studies have demonstrated a potentially selective effect of cranial irradiation on HVLT and not on other cognitive domains, assessed using conventional cognitive tests [17]. However, our study demonstrates an effect of PCI on SRCF in the absence of a close correlation between SRCF and HVLT. This finding suggests that cranial irradiation may induce decline in another as yet unidentified cognitive domain that may be more closely correlated with SRCF. To address both of these questions and further enhance our understanding of the cognitive effects of cranial irradiation, future prospective trials of cranial irradiation should expand beyond HVLT and the two-item cognitive function scale of EORTC QLQ-C30 to more comprehensively assess the multiple dimensions of human cognition.
Independent of the effects of PCI or age, patients with baseline impairment in HVLT-R or –DR were significantly less likely to develop subsequent HVLT decline. This may be due in part to the limited score range of HVLT. The reliable change index defines decline as an absolute score reduction outside a test's standard error. However, to achieve this absolute score reduction, patients who start at a substantially lower baseline score would require a larger percentage decrease in their follow-up score. This becomes especially problematic in HVLT-DR, for instance, where the score range is 0-12 and where baseline impairment for ages 70-86 is defined as a score less than 4.50 [12]. These findings highlight the importance of investigating cognitive tests with larger score ranges, as is currently being done on RTOG 0925 and RTOG 0933.
At 6 and 12 months, statistically significant correlations were observed between SRCF decline and decline in self-reported physical functioning. In addition, SRCF decline was correlated with increasing fatigue and appetite loss at 6 months, but these associations were lost at 12 months. These findings suggest that SRCF decline may be due, in part, to declining performance status, iatrogenic causes (e.g., pain medications), or extra-cranial disease progression. However, the absence of any effects of PCI on self-reported physician functioning, fatigue and appetite loss, and the relatively low kappa statistics suggesting fair strength of agreement [18], indicate that PCI-associated decline in SRCF is likely not explained by these effects alone.
The results of this study should be placed within the context of the established intracranial control and survival benefits of PCI in certain clinical settings of small cell lung cancer. Prior studies of whole brain radiotherapy in patients with brain metastases have observed an association between intracranial tumor progression and neurocognitive function[17,19]. Similar studies have not been conducted in the PCI setting. In this study, an attempt to evaluate HVLT and EORTC QLQ-C30 decline in patients who developed brain relapse was limited due to substantial non-compliance following brain relapse. However, given the aforementioned findings in the brain metastasis setting, it is reasonable to assume that the emergence of intracranial metastases may also have potentially adverse neurocognitive effects.
The findings of this study do contrast with those reported by Li et al.[20], who observed a significant correlation between neurocognitive function and quality of life at baseline and 4 months following whole-brain radiotherapy in patients with brain metastases. However, both our study and Li et al. were similar in observing no such correlation at 6 months follow-up. In addition, our study has a number of methodological differences from the Li et al. study. Firstly, the first follow-up evaluation in our study was 6 months, preventing an evaluation of potential correlations at earlier time points. Secondly, our study correlated decline of HVLT with decline in quality of life, whereas Li et al. correlated raw HVLT and quality of life scores. Lastly, Li et al. examined all patients with brain metastases following whole-brain radiotherapy, irrespective of intracranial progression. Our study specifically excluded patients with brain relapse to avoid the confounding effects of intracranial disease.
Pooling data from RTOG 0212 and RTOG 0214 was feasible due to the uniformity of quality of life and HVLT instruments and the serial assessment time points (6 and 12 months follow-up). However, pooling these data raises concerns over the interpretation of data from two distinct disease processes. For instance, in this study, the observation cohort consisted entirely of patients with NSCLC, while the PCI cohort was a mixed population of SCLC and NSCLC patients. To address this concern, we excluded any patients who developed brain relapse prior to follow-up evaluation. In addition, while RTOG 0214 randomized patients to PCI or observation, RTOG 0212 randomized patients to high- or standard-dose PCI. Prior analysis of RTOG 0212 has demonstrated an association between high-dose PCI and increased chronic neurologic toxicity. However, in that analysis, high-dose PCI was not specifically associated with greater HVLT decline. Similarly, to elucidate the HVLT and quality of life impact of radiation dose in this pooled analysis, we separated patients receiving low-dose PCI (25 Gy) from those receiving high-dose PCI (36 Gy) on RTOG 0212 and pooled them with all patients receiving PCI (30 Gy in 15 fractions) on RTOG 0214. Comparisons demonstrated no difference between high- and low-dose PCI in terms of HVLT or SRCF decline, which permitted inclusion of these patients into a singular PCI cohort.
In conclusion, for lung cancer patients who do not develop brain relapse, PCI is associated with decline in not just HVLT-tested but also SRCF at 6 and 12 months follow-up. However, HVLT decline and SRCF decline are not closely correlated, suggesting that they may represent distinct elements of the cognitive spectrum.
Supplementary Material
Acknowledgments
This study was presented in abstract form at the 2011 Annual Meeting for the American Society of Radiation Oncology (ASTRO). This project was supported by RTOG grant U10 CA21661, CCOP grant U10 CA37422, and from the National Cancer Institute (NCI) and 2009 PA Department of Health Formula Grant 4100050889. This manuscript's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI or the PA Department of Health.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gore EM, Bae K, Wong SJ, et al. Phase iii comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: Primary analysis of radiation therapy oncology group study rtog 0214. J Clin Oncol. 2011;29:272–278. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun A, Bae K, Gore EM, et al. Phase iii trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 4.Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: Short-term health-related quality of life and patient reported symptoms: Results of an international phase iii randomized controlled trial by the eortc radiation oncology and lung cancer groups. J Clin Oncol. 2009;27:78–84. doi: 10.1200/JCO.2008.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase ii randomized trial radiation therapy oncology group (rtog) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. International journal of radiation oncology, biology, physics. 2011;81:77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer qlq-c30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 7.Fayers P, Aaronson NK, Bjordal K, et al. Eortc qlq-c30 scoring manual. 3. Brussels, Belgium: EORTC Publications; 2001. [Google Scholar]
- 8.Osoba D, Zee B, Pater J, et al. Psychometric properties and responsiveness of the eortc quality of life questionnaire (qlq-c30) in patients with breast, ovarian and lung cancer. Qual Life Res. 1994;3:353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Benedict RH, Schretlen D, et al. Construct and concurrent validity of the hopkins verbal learning test-revised. Clin Neuropsychol. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of consulting and clinical psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Agresti A. Categorical data analysis. New York: Wiley; 1990. [Google Scholar]
- 12.Benedict RH, Schretlen D, Groniger L, et al. Hopkins verbal learning test-revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 13.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–202. [Google Scholar]
- 14.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 15.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. Hoboken: John Wiley & Sons; 2003. [Google Scholar]
- 16.Soffietti R, Mueller RP, Abacioglu MU, et al. Quality of life results of an eortc phase iii randomized trial of adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases of solid tumors. J Clin Oncol. 2010;28:15s. suppl; abstr 9036. [Google Scholar]
- 17.Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;(33):159–174. [PubMed] [Google Scholar]
- 19.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. International journal of radiation oncology, biology, physics. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Bentzen SM, Renschler M, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.