Abstract
Objective
To evaluate prognostic risk factors for survival in women with low grade serous epithelial ovarian cancer (LGSC).
Methods
A multicenter retrospective analysis of patients with LGSC was conducted. Potential epidemiologic risk factors evaluated included obesity, age, parity, race, smoking, oral contraceptive pill and/or hormonal replacement therapy use, and previous hysterectomy or surgery on fallopian tubes and/or ovaries. Additional factors included stage, extent of debulking, residual disease, and disease status.
Results
Eighty-one patients were identified, and pathological diagnosis was independently confirmed. Median age of diagnosis was 56 years (range: 21 to 86). Thirty-four percent were obese, and 80% had optimally debulked disease. Forty-six percent were alive, 14% with disease; while 25% were dead of disease; 2% died of intercurrent disease; and 27% had an unknown status. In a univariate analysis, optimal surgical debulking was associated with improved PFS (p=0.01), DSS (p=0.03), and OS (p<0.001 and BMI with worse OS (p=0.05). On multivariate analysis, obesity (HR=2.8; 95% CI=1.05-7.3; p=0.04) and optimal tumor debulking (HR=0.05; 95% CI=0.008-0.29; p=0.001) were a significant predictor of OS.
Conclusions
In a multivariate analysis, obesity and optimal tumor cytoreduction were significant predictors of OS. However, obesity was not associated with worse DSS, suggesting that mortality of obese patients with LGSC may result from other co-morbidities. Interventions addressing obesity may improve survival for women diagnosed with LGSC and further study is warranted to address the role of obesity in LGSC.
Keywords: low grade serous ovarian cancer, obesity
Introduction
Ovarian cancer comprises a heterogeneous group of tumors with a wide variation in clinical behaviors, histologies, and molecular features. Low grade serous ovarian carcinomas (LGSC) represent about 10% of all ovarian cancers (1). In 2004, Malpica et al. proposed a two-tier grading system for ovarian cancer that classified Grade 2 and 3 tumors as high grade and Grade 1 tumors as low grade (2). LGSC comprises one subtype of type 1 epithelial ovarian cancers (EOCs) as proposed by Kurman and Shih (3). Patients diagnosed with LGSC are younger, (4, 5) live longer, (5, 6) and their disease is more likely to be confined to the ovary (5). Although LGSC may arise de novo, LGSC may arise from benign serous adenofibromas and serous tumors of low malignant potential (7). Due to the indolent nature of LGSC, optimal surgical debulking remains the frontline treatment because recurrent or persistent disease traditionally responds poorly to chemotherapeutics, (8) and patients may ultimately die from the burden of their recurrent disease (9).
Due to the prolonged disease course, potentially modifiable risk factors could alter the course of disease. To our knowledge, only one other study has investigated modifiable factors that may contribute to outcomes in women with LGSC. Schlumbrecht et al. found that smoking had a negative association with overall survival (OS) and progression free survival (PFS). Although not significant, patients who received hormonal consolidation (tamoxifen, letrozole, or leuprolide) after primary chemotherapy therapy had longer OS and PFS (10). When viewed as a single entity, non-modifiable risk factors for EOC include a family history of ovarian cancer, increasing age, early age of menarche and late age of menopause. Protective factors for development of EOC include increasing parity, a history of oral contraceptive use, oophorectomy, bilateral tubal ligation, and previous hysterectomy. Modifiable risk factors suspected to increase development of ovarian cancer include hormone-replacement therapy, high fat diet, obesity, smoking history, alcohol use, and inactivity (11). The relationship between body mass index (BMI), obesity, and ovarian cancer is uncertain, and there is minimal data about obesity and outcomes in women with LGSC.
Due to the rather indolent course of LGSC and lack of information regarding prognostic factors and conflicting results regarding obesity, we sought to evaluate prognostic modifiable and non-modifiable risk factors for women with this disease.
Materials and Methods
Each institution obtained Institutional Review Board approval. A database was created to identify all patients diagnosed with LGSC between January 1996 and December 2010. When available, archival pathology slides were reviewed by gynecologic pathologists (SB, SW, and MD) to verify that the neoplasms were LGSC. Pathologic inclusion criteria were based on the two-tier grading system for serous ovarian carcinoma originally described by Malpica et al (2). Briefly, serous ovarian tumors with:
relatively uniform round to oval nuclei with mild to moderate atypia and evenly distributed chromatin,
≤ 12 mitotic figures/10 high power fields, and 3. definitive stromal invasion >5mm, were considered LGSC. When pathology slides were not available, the original pathology report was reviewed and grade 1 disease was used as a surrogate for LGSC.
From medical charts, demographic data were abstracted. Patients who were lost to follow-up were excluded from analysis. Epidemiologic risk factors for EOC that were evaluated included obesity (BMI ≥ 30), BMI, age, parity, race, smoking, history of oral contraceptive pill/and or hormone replacement therapy use, previous hysterectomy, and previous surgery on fallopian tubes and/or ovaries. Other variables evaluated included stage of disease, residual disease after debulking, extent of debulking, and disease status. Information regarding residual disease and extent of debulking were obtained from the operative report. Optimal debulking was defined as residual disease less than 1 cm. BMI, defined as kilograms/meter squared (kg/m2), was used to classify patients as underweight (<18.5 kg/m2), normal weight (≥18.5 to <25.0 kg/m2), overweight (≥25.0 to < 30.0 kg/m2), and obese (≥30.0 kg/m2). Cox proportional hazards modeling adjusted for age at diagnosis, status of tumor debulking, BMI, and history of use of hormone replacement therapy because these factors were thought to be relevant clinically.
Statistical analysis
Both univariate and multivariate Cox proportional hazards regression models were used to predict OS, disease specific survival (DSS) and PFS. Multivariate Cox proportional hazards modeling was conducted using the backward selection technique with an α = 0.50 (12) using the following candidate predictors: age at diagnosis, tumor debulking, obesity, BMI and a history of hormone replacement therapy use. OS was calculated from time of diagnosis to death due to any cause. For those still alive, it was censored at the last follow-up visit. DSS was calculated from time of diagnosis to death due to disease and was censored for deaths due to other causes or at last follow-up visit for those still alive. PFS was calculated from diagnosis until first recurrence or death, whichever occurred first, and was censored for those still alive without recurrence at last follow-up visit. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Eighty-one eligible patients were identified. Median age of diagnosis was 56 years (range: 21 to 86). Thirty-four percent were obese (Table 1), and 90% had optimally debulked disease. At the study conclusion, 46% were alive, 14% with disease; while 25% were dead of disease; 2% died of intercurrent disease; and 27% had an unknown status (Table 1). The diagnosis of LGSC was confirmed by review of glass slides for 58 (71.6%) and by review of original pathology reports for the remaining patients (23; 28.3%).
Table 1.
Clinicodemographic variables for patients with LGSC.
| Prognostic factor | Subgroups | N | Percentage |
|---|---|---|---|
| BMI | 17 to < 25 | 28 | 35 |
| 25 to < 30 | 18 | 22 | |
| 30 to <35 | 19 | 23 | |
| 35 to < 40 | 6 | 7 | |
| 40 or more | 3 | 4 | |
| Not reported | 7 | 9 | |
| History of HRT use | Yes | 10 | 12 |
| No | 59 | 73 | |
| Not reported | 12 | 15 | |
| Previous surgery on tubes/ovaries | Yes | 12 | 85 |
| No | 69 | 15 | |
| Previous hysterectomy | Yes | 20 | 25 |
| No | 61 | 75 | |
| History of OCP use | Yes | 7 | 9 |
| No | 65 | 80 | |
| Not reported | 9 | 11 | |
| Smoking history | Previous history or current | 15 | 19 |
| Non-smoker | 66 | 81 | |
| Debulking | Optimal | 65 | 80 |
| Sub-optimal | 7 | 9 | |
| Not reported | 9 | 11 | |
| Stage | I | 20 | 25 |
| II | 6 | 7 | |
| III | 48 | 59 | |
| IV | 5 | 6 | |
| Not reported | 2 | 2 | |
| Race | White | 59 | 73 |
| Black | 15 | 19 | |
| Asian | 1 | 1 | |
| Hispanic | 2 | 2 | |
| Unknown | 4 | 5 | |
| Parity | 0 | 19 | 23 |
| 1 | 11 | 14 | |
| 2 | 24 | 30 | |
| 3 | 11 | 14 | |
| 4 | 5 | 6 | |
| >5 | 3 | 3 | |
| Not reported | 8 | 10 | |
| Medical history | CHF | 2 | 2 |
| Previous MI | 3 | 4 | |
| CAD | 1 | 1 | |
| Previous stroke | 2 | 2 | |
| Hypertension | 23 | 28 | |
| Diabetes mellitus | 5 | 6 | |
| Deep vein thrombosis | 5 | 6 | |
| Family history of ovarian cancer | Yes | 28 | 35 |
| No | 53 | 65 | |
| Disease status | No evidence of disease | 26 | 32 |
| Alive with disease | 11 | 14 | |
| Dead of disease | 20 | 25 | |
| Dead of intercurrent causes | 2 | 2 | |
| Unknown | 10 | 12 | |
| Lost to follow-up | 12 | 15 |
In a univariate analysis, BMI was associated with OS. Increasing BMI was associated with a 1.4-fold increased risk of death (p=0.05) (Table 2). More specifically, a 5-unit increase in BMI was associated with a 1.4 fold increase risk in death, while a 10-unit increase was associated with a 2.1 fold increase risk of death. For instance a person with BMI of 35 or 40 is 2.1 times more at risk of death than a person with a BMI of 25 or 30, respectively. Obesity, defined as a BMI ≥30.0 kg/m2, demonstrated a marginal association with worse OS (hazard ratio (HR)=2.08; confidence interval (CI) 0.93-4.66; p=0.07) in the univariate analysis. Using a multivariate Cox proportional hazards modeling to predict overall survival using age of diagnosis, status of tumor debulking, BMI, and history of use of hormone replacement therapy, obesity was associated with OS. The obese condition was associated with a 2.8 fold increase risk of death (p=0.04) (Table 3).
Table 2.
Univariate Cox proportional hazards modeling of potential prognostic variables to predict PFS, DSS, and OS.
| PFS | DSS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Obesity | 1.47 | 0.08-2.71 | 0.2 | 1.66 | 0.57-4.88 | 0.5 | 2.08 | 0.93-4.66 | 0.07 |
| BMI | 1.09 | 0.85-1.40 | 0.5 | 1.29 | 0.79-2.13 | 0.3 | 1.43 | 1.00-2.07 | 0.05 |
| History of HRT use | 0.77 | 0.32-1.82 | 0.5 | 0.49 | 0.11-2.18 | 0.3 | 0.49 | 0.14-1.66 | 0.3 |
| Previous surgery on tubes/ovaries | 0.83 | 0.39-1.77 | 0.6 | 1.61 | 0.51-5.07 | 0.4 | 1.47 | 0.59-3.68 | 0.4 |
| Previous hysterectomy | 0.74 | 0.36-1.53 | 0.4 | 0.75 | 0.24-2.32 | 0.6 | 0.55 | 0.21-1.45 | 0.2 |
| History of OCP use | 1.77 | 0.69-4.54 | 0.2 | -- | -- | -- | -- | -- | -- |
| Smoking | 0.75 | 0.34-1.68 | 0.5 | 0.24 | 0.03-1.83 | 0.2 | 0.50 | 0.15-1.67 | 0.3 |
| Residual disease | 1.73 | 1.09-2.76 | 0.02 | 1.04 | 0.51-2.12 | 0.9 | 0.92 | 0.51-1.65 | 0.8 |
| Optimal tumor debulking | 0.29 | 0.11-0.77 | 0.01 | 0.08 | 0.01-0.74 | 0.03 | 0.05 | 0.01-0.23 | <0.001 |
| Caucasian race | 1.16 | 0.56-2.41 | 0.7 | 0.62 | 0.20-1.94 | 0.4 | 0.71 | 0.29-1.77 | 0.5 |
| Parity | 1.09 | 0.93-1.29 | 0.3 | 1.26 | 0.91-1.75 | 0.2 | 1.21 | 0.92-1.58 | 0.2 |
| Family history of ovarian cancer | 1.01 | 0.56-1.81 | 1.0 | 1.59 | 0.59-4.31 | 0.4 | -- | -- | -- |
| Age at diagnosis | 1.02 | 0.94-1.11 | 0.7 | 1.02 | 0.90-1.17 | 0.7 | 1.60 | 0.74-3.48 | 0.2 |
Table 3.
Multivariate Cox proportional hazards modeling to predict overall survival for obesity and optimal tumor debulking.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Obesity | 2.8 | 1.05-7.3 | 0.04 |
| Optimal tumor debulking | 0.05 | 0.008-0.29 | 0.001 |
Surgical debulking and extent of residual disease were also associated with clinical outcome (Table 2 and 3). In a univariate Cox proportional hazards model, lower extent of residual disease (p=0.02) was associated with improved PFS, while optimal surgical debulking was associated with improved PFS (p=0.01), DFS (p=0.03), and OS (p<0.001) (Table 2). The majority of patients (94.3%) received adjuvant chemotherapy after initial cytoreduction. Primary regimens included carboplatin/paclitaxel (80%), carboplatin (1.43%), carboplatin/topotecan (1.43%), carboplatin/paclitaxel/doxorubicin (1.43%), carboplatin/docetaxel (2.86%), cisplatin/paclitaxel (2.86%), platinum agent/cyclophosphamide (2.86%), and xyotax (1.43%). Since a high proportion of patients received chemotherapy, this variable was not tested in the predictive survival models. Hormonal therapy was also unable to be included in the predictive survival models because only four patients received tamoxifen after initial cytoreduction. Hormonal therapy was also unable to be included in the predictive survival models because only four patients received tamoxifen after initial cytoreduction. Debulking status was included in the multivariate model, however, due to the limited number of patients who were suboptimally cytoreduced (n=7), we could not reliably explore associations between tumor reduction and survival (Table 3).
Prediction of DSS and PFS using the backward selection technique did not identify two or more variable models; therefore, multivariate models could not be constructed for DSS or PFS. There was a trend toward worse median survival for obese women with LGSC compared to non-obese women (6.5 versus 9.5 years; p= 0.07) (Figure 1). There were no significant differences in DSS (8.4 versus 12.2 years; p=0.5) and PFS (1.5 versus 2.6 years; p=0.2) in obese and non-obese women with LGSC (Figure 2). There was no significant difference in optimal cytoreduction rates in obese and non-obese patients (88% in obese versus 93% in non-obese, p=0.7).
Figure 1.
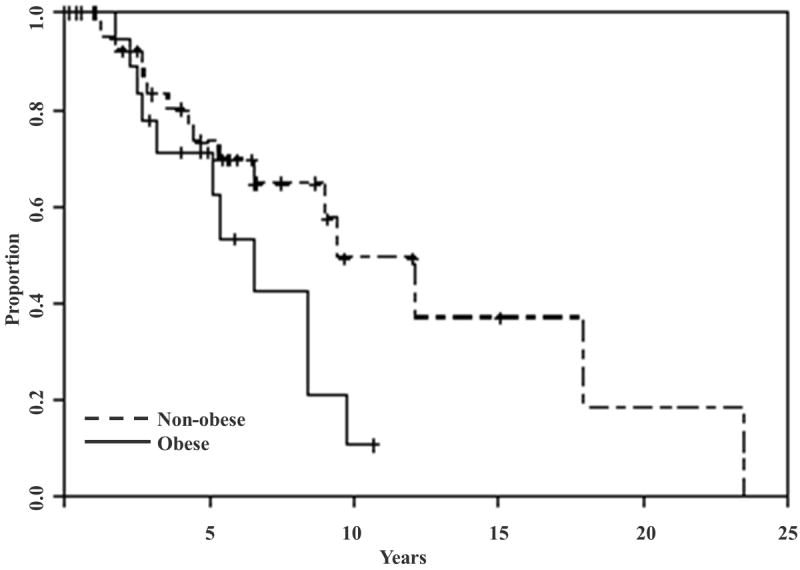
Overall survival stratified by obesity status. There was a trend toward worse median survival for obese women with LGSC compared to non-obese women (6.5 versus 9.5 years; p= 0.07).
Figure 2.
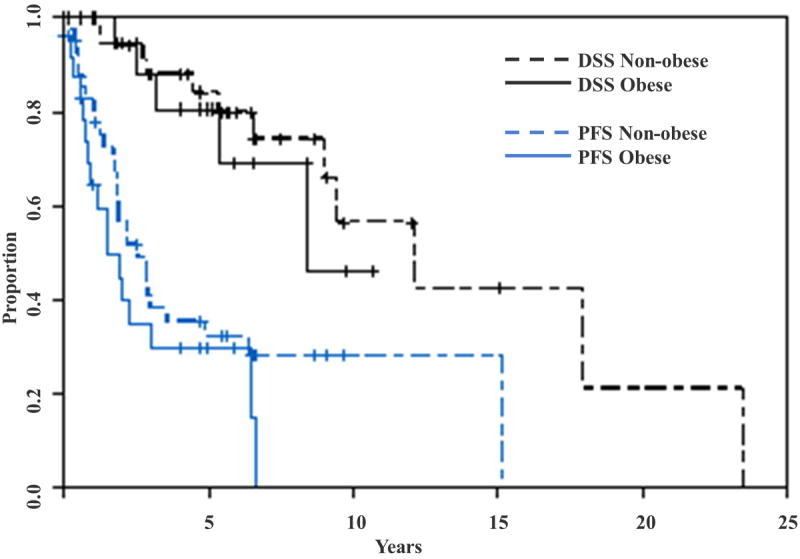
Disease-specific and progression-free survival stratified by obesity status. There were no significant differences in DSS (8.4 versus 12.2 years; p=0.5) and PFS (1.5 versus 2.6 years; p=0.2) in obese and non-obese women with LGSC.
Discussion
BMI and obesity were predictors of survival for women with LGSC. We evaluated body habitus based on BMI and obesity in order to capture the effect of different weight classification on survival. In our univariate models, BMI and obesity were associated with a significant 1.4-fold and a marginal 2.1-fold increased risk of death, respectively, but neither was associated with worse DSS. In our multivariate analysis, obesity was significantly associated with a 2.8-fold increased risk of death. In the multivariate model, the association between BMI and OS was lost. This is due to the high correlation between BMI and obesity. At the time of the analysis, 25% of the study population died. Longer follow-up is needed to determine the magnitude of obesity’s effect on survival in the patient population with a less aggressive tumor biology. Previously, Schlumbrecht et al. reported that patients with a BMI ≥ 35 kg/m2 were identified as having a greater likelihood of dying (HR, 2.53; 95% CI, 1.19-5.38; p = 0.02) (10). While one of the cutoffs for the study by Schlumbrecht et al. was BMI ≥ 35 and ours used BMI ≥ 30, the hazards ratios were comparable (2.53 versus 2.8). Furthermore, BMI assessed as a continuous variable was also associated with worse survival outcomes (HR=1.02, 95% CI, 1.00-1.1, p=0.05).
In contrast to our study, Schlumbrecht found that, residual disease after surgery was not statistically significant (p=0.29) for overall survival in a Cox univariate analysis. Tumor debulking has long been associated with improved outcomes in women with EOC (13). We were unable to explore associations between debulking status and survival due to the limited number of patients who were suboptimally cytoreduced. But, it is also important to note that optimal debulking rates did not differ in obese and non-obese women with LGSC. The majority of our patients received chemotherapy, so we were unable to test chemotherapy as a variable in our survival models due to the small number of patients who did not receive it. The effect of chemotherapy on LGSC warrants further study. Gershenson et al. reported relative insensitivity of LGSC to chemotherapy due to an observed low rate of patients who were clinically disease-free after adjuvant platinum-based chemotherapy (52%) (4). In a follow-up study, overall response rate to chemotherapy in 108 patients was only 3.8% in patients with recurrent LGSC (8). While chemotherapy does seem to have a less efficacious role in LGSC than in high grade disease, the majority of patients continue to receive frontline therapy, and chemotherapy does play an important role in the treatment of women with LGSC (14).
The relationship between BMI, obesity, and ovarian cancer is uncertain. Multiple studies have suggested that obesity does not negatively impact surgical outcomes, clinicopathological factors, (15) prognosis, (16, 17) or survival (15, 18) in all patients with EOC, when controlling for optimal debulking status (18) and whether the patient received optimal doses of chemotherapy or hormonal therapy (16). Other studies suggest that after adjustment for confounders including stage, grade, age, histology, residual disease, there is a trend toward shorter PFS in patients with a normal BMI, but OS is not significantly related to BMI (19). Conversely, results from other retrospective studies suggest that obesity was independently associated with both shorter time to recurrence and OS (20). BMI prior to and after diagnosis, as well as weight gain during adulthood, has also been associated with an increase in ovarian cancer mortality (21). Meta-analyses have attempted to better elucidate these conflicting results and suggest possible relationships between obesity in early adulthood and higher mortality among patients with EOC, (22) and slightly worse survival than non-obese women (23). Due to the variation between all of the studies, no definitive conclusions may be drawn, however. These studies take into account all patients with EOC, and so far no attempts have been made to understand the impact of obesity of LGSC.
Given the obesity epidemic, it would be helpful to improve our understanding of its effect on ovarian cancer biology. The role of obesity in LGSC may be multi-factorial. Given the lack of association between obesity and DSS and PFS, mortality of obese patients with LGSC may result from other co-morbidities secondary to obesity. Patients diagnosed with LGSC represent a unique population as compared to those diagnosed with other types of EOC. LGSC patients tend to live longer and have more indolent disease. The natural history of this disease may render lifestyle modifications worthwhile, particularly if the tumor microenvironment is adversely affected by excess adipose tissue. The GOG is currently evaluating the impact of diet and exercise in survival and recurrence rates in women with newly diagnosed stage II-IV ovarian cancer after successful first-line therapy (GOG225). Data from this study may help determine if lifestyle modifications targeting obesity will improve survival in women with LGSC.
According to the World Health Organization (WHO), overweight and obesity are the fifth leading risk for global deaths, and at least 2.8 million adults die each year as a result. Over 1.4 billion adults (age 20 and older) were overweight according to 2008 WHO estimates (24). Obesity comprises a major risk factor for complications including Type 2 diabetes, hypertension, hyperlipidemia, coronary artery disease and stroke. In our study, 28% of patients were diagnosed with hypertension which may contribute to higher incidence of coronary artery disease and/or stroke. However, only 6% of our study participants had diabetes. Our study did not take into account those patients with impaired fasting glucose or undiagnosed diabetes, and it is possible that we underestimated the percentage of patients with diabetes and LGSC. It is very likely that the association between obesity and worse survival we observed is secondary to obesity-related co-morbid conditions. In a prospective trial of more than 900,000 adults, BMI was associated with significantly higher rates of death in men and women due to a variety of cancers including esophagus, colon and rectum, liver, gallbladder, pancreas, and kidney. Furthermore, women with a higher BMI had a trend of increased risk of death due to cancers of the breast, uterus, cervix, and ovary (25).
In addition to obesity related illnesses, the increase risk of death in obese patients with LGSC may also be due to direct effects of obesity on carcinogenesis. Tumors in an obese environment may behave differently. Increased adipose tissue influences the bioavailability of sex steroids through multiple pathways. Obesity increases insulin resistance, bioavailability of sex hormones, and chronic inflammation that may promote the growth, rate or metastases of certain tumors (26). Leptin, found at higher concentrations in obese patients, induces expression of vascular endothelial growth factor, and may promote angiogenesis (27). The role of obesity on ovarian cancer in the literature provides conflicting evidence. It is possible that certain histopathologic types of ovarian cancer are more influenced by the obese environment. Furthermore, the association between obesity and risk of developing EOC may vary depending on tumor grade and the specific type of ovarian cancer. Increased height and obesity may be associated with an increased risk of endometroid types of EOC (28, 29). Elevated BMI has been associated with an increased risk for borderline serous (OR 1.24), invasive endometroid cancers (OR 1.17), and invasive mucinous cancers (OR 1.19). There was no association with BMI and invasive serous cancers (OR 0.98) except for in premenopausal women (OR 1.11), but there was an increased risk for LGSC (OR 1.13) associated with higher BMI (30).
This study is limited by a small sample size. Also, we did not analyze outcomes related to treatment with chemotherapy or hormonal therapy due to the small number of patients who received and did not receive this type of therapy, respectively. We also did not evaluate outcomes after additional surgery if recurrence did occur. We used BMI calculated at the time of diagnosis and did not take into account BMI during adolescence, if BMI changed post-operatively, or BMI at the conclusion of treatment. Recent studies suggest that adolescent exposure to obesity is associated with worse OS in women who develop EOC (22).
Pathology was reviewed for over 70% of patients included in this study to incorporate newer definitions of low grade disease. For the remaining patients, pathology was not reviewed often because the original slides were returned to the referring laboratories, but the diagnosis of Grade 1 disease was used as a surrogate for low grade disease. Additionally, 27% of our study population was unknown or lost to follow-up. Although the mean ages and BMIs between the lost to follow-up group was similar to the group with outcome data, this could potentially affect our results. Future prospective and/or studies that address BMI over the course of a patient’s lifetime could validate the trends observed in this series.
Despite these limitations, our findings indicate that obesity and tumor cytoreduction are associated with overall survival outcomes in women with LGSC. Obesity is a modifiable risk factor that was associated with worse survival in women with LGSC. In addition, obesity may be related to DSS and PFS, but we were unable to detect an association due to our limited sample size. Further study is warranted to address the role of obesity and weight loss interventions in women with LGSC.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Disclosures: No funding was received for this work.
References
- 1.Seidman JD, Horkayne-Szakaly I, Cosin JA, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103(2):703–8. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih IM. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am J Surg Pathol. 2010;34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108(2):361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 5.Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008;198(4):459 e1–8. doi: 10.1016/j.ajog.2008.01.035. discussion e8-9. [DOI] [PubMed] [Google Scholar]
- 6.Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Onco. 2008;108(3):510–4. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Crispens MA, Bodurka D, Deavers M, Lu K, Silva EG, Gershenson DM. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstet Gynecol. 2002;99(1):3–10. doi: 10.1016/s0029-7844(01)01649-0. [DOI] [PubMed] [Google Scholar]
- 8.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Schmeler KM, Gershenson DM. Low-grade serous ovarian cancer: a unique disease. Curr Oncol Rep. 2008;10(6):519–23. doi: 10.1007/s11912-008-0078-8. [DOI] [PubMed] [Google Scholar]
- 10.Schlumbrecht MP, Sun CC, Wong KN, Broaddus RR, Gershenson DM, Bodurka DC. Clinicodemographic factors influencing outcomes in patients with low-grade serous ovarian carcinoma. Cancer. 2011;117(16):3741–9. doi: 10.1002/cncr.25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–37. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 12.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 13.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 14.Romero I, Sun CC, Wong KK, Bast RC, Jr, Gershenson DM. Low-grade serous carcinoma: new concepts and emerging therapies. Gynecol Oncol. 2013;130(3):660–6. doi: 10.1016/j.ygyno.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Suh DH, Kim HS, Chung HH, et al. Body mass index and survival in patients with epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(1):70–6. doi: 10.1111/j.1447-0756.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 16.Barrett SV, Paul J, Hay A, Vasey PA, Kaye SB, Glasspool RM. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. 2008;19(5):898–902. doi: 10.1093/annonc/mdm606. [DOI] [PubMed] [Google Scholar]
- 17.Kotsopoulos J, Moody JR, Fan I, et al. Height, weight, BMI and ovarian cancer survival. Gynecol Oncol. 2012;127(1):83–7. doi: 10.1016/j.ygyno.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Matthews KS, Straughn JM, Jr, Kemper MK, et al. The effect of obesity on survival in patients with ovarian cancer. Gynecol Oncol. 2009;112(2):389–93. doi: 10.1016/j.ygyno.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Backes FJ, Nagel CI, Bussewitz E, Donner J, Hade E, Salani R. The impact of body weight on ovarian cancer outcomes. Int J Gynecol Cancer. 2011;21(9):1601–5. doi: 10.1097/IGC.0b013e31822d2aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavelka JC, Brown RS, Karlan BY, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107(7):1520–4. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Irwin ML, Risch HA. Pre- and post-diagnosis body mass index, weight change, and ovarian cancer mortality. Gynecol Oncol. 2011;120(2):209–13. doi: 10.1016/j.ygyno.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang HS, Yoon C, Myung SK, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2011;21(9):1525–32. doi: 10.1097/IGC.0b013e31822eb5f8. [DOI] [PubMed] [Google Scholar]
- 23.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res. 2012;5(7):901–10. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 24.Organization WH. [January 17, 2013];Obesity and overweight Fact sheet N311. 2012 Mar; http://www.who.int/mediacentre/factsheets/fs311/en/
- 25.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 27.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5(3):153–65. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95(16):1244–8. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 29.Farrow DC, Weiss NS, Lyon JL, Daling JR. Association of obesity and ovarian cancer in a case-control study. Am J Epidemiol. 1989;129(6):1300–4. doi: 10.1093/oxfordjournals.aje.a115249. [DOI] [PubMed] [Google Scholar]
- 30.Olsen CM, Nagle CM, Whiteman DC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20(2):251–62. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]


