Abstract
Objective
To compare rates of contraception between reproductive-aged cancer survivors and women in the general U.S. population. Among survivors, the study examined factors associated with use of contraception and emergency contraception.
Methods
This study analyzed enrollment data from an ongoing national prospective cohort study on reproductive health after cancer entitled the Fertility Information Research Study. We compared current contraceptive use in survivors with that of the general population ascertained by the 2006–2010 National Survey for Family Growth. Log-binomial regression models estimated relative risks for characteristics associated with use of contraception, World Health Organization tiers I–II (sterilization and hormonal) contraceptive methods, and emergency contraception in survivors.
Results
Data from 295 survivors (mean age 31.6 ± 5.7 years, range 20–44 years) enrolled in this prospective study (85% response rate) were examined. Age-adjusted rates of using tiers I–II contraceptive methods were lower in survivors than the general population (34% [28.8–40.0] compared with 53% [51.5–54.5], P<.01). Only 56% of survivors reported receiving family planning services (counseling, prescription or procedure related to birth control) since cancer diagnosis. In adjusted analysis, receipt of family planning services was associated with both increased use of tiers I–II contraceptive methods (relative risk 1.3, 95% confidence interval [CI] 1.1–1.5) and accessing emergency contraception (relative risk 5.0, 95% CI 1.6–16.3) in survivors.
Conclusion
Lower rates of using Tiers I–II contraceptive methods were found in reproductive-aged cancer survivors compared to the general population of U.S. women. Exposure to family planning services across the cancer care continuum may improve contraception utilization among these women.
Clinical Trial Registration
ClinicalTrials.gov, www.clinicaltrials.gov, NCT01843140.
INTRODUCTION
In the United States, there are more than 400,000 reproductive-aged women with a history of cancer.1 These cancer survivors have unique family planning needs. While many cancer treatments increase risks of infertility and premature ovarian aging, most survivors retain ovarian function and potential fertility after cancer treatment.2-5 Many survivors also seek to attain social and cancer-related milestones prior to pregnancy.6 Therefore, effective contraception is needed until pregnancy is desired.
While qualitative studies indicate that safe and reliable contraception is a key concern for survivors, awareness of contraceptive options is limited, and contraception rates are low.7-10 Survivors can underestimate their risk for pregnancy, one potential outcome of which is unintended pregnancy.9,10 Supporting this finding are several studies that suggest increased rates of therapeutic abortion in this population.11,12 However, it is unclear if these concerning contraceptive practices deviate from those of the general U.S. population and what exposures drive these behaviors. Additionally, unintended pregnancy and emergency contraception are not described in survivors.13
The primary objective was to compare contraception rates between reproductive-aged cancer survivors and women in the general U.S. population. We hypothesized that survivors would have lower rates of contraception compared to the general population, as reported by the National Survey of Family Growth, the population-based U.S. fertility survey.14,15 Second, the study examined factors associated with survivors’ use of any contraception, more effective contraceptive methods, and emergency contraception.
MATERIALS AND METHODS
This is a analysis of enrollment data from an ongoing national prospective cohort study entitled the Fertility Information Research Study (NCT01843140). Participants were recruited through referrals from diverse sources: social media outreach by cancer advocacy groups (60%), six university-based fertility preservation programs (26%), FERTLINE—the Oncofertility Consortium’s telephone hotline (6%), community outreach, or word of mouth (8%).16 Between May 2011 and February 2013, 352 female survivors ages 18–44 years were approached; 295 survivors were enrolled from 44 states (85% response rate). The institutional review board at University of California, San Diego approved this study.
Eligible survivors provided informed consent to participate and completed annual questionnaires by telephone interview or the Internet. These analyses used data from the enrollment questionnaire of 289 participants (98% of enrolled) who were ages 20-44 to be comparable in age to the general population data reported by the National Survey for Family Growth.14,15 The enrollment questionnaire ascertained demographic, cancer, hysterectomy, oophorectomy, pregnancy intention, fertility, and pregnancy information.17-19 Current contraceptive use, emergency contraception use, and receipt of family planning services were assessed using questions derived from the 2006-2010 National Survey for Family Growth Cycle.15 (See the Appendix online at http://links.lww.com/xxx.)
Contraceptive practices in survivors were compared to those reported by the National Survey for Family Growth, a population-based study conducted by the CDC to provide reliable national data on marriage, divorce, contraception, infertility, and the health of American women.15 In the 2006-2010 cycle, a nationally representative sample of 12,279 women ages 15-44 was interviewed using standardized questionnaires (78% response rate).20 Weights were used to adjust for different sampling, response and coverage rates to generate accurate national estimates.20
Descriptive statistics were calculated as frequencies and percentages, or median and interquartile ranges (IQR). The primary comparison was current contraceptive use between survivors and the general population. Secondarily, current use of World Health Organization (WHO) Tiers I and II contraceptive methods and emergency contraception use after cancer diagnosis were examined within survivors.
For current contraceptive use, National Survey for Family Growth methods were used to categorize survivors.14 Survivors were categorized by the most effective method for preventing pregnancy they reported using.14 Survivors who reported no contraception were assigned standardized reasons in the following order: surgically sterile (eg, hysterectomy, bilateral oophorectomy), seeking pregnancy, pregnant or postpartum, sexually active and not sexually active.14 We further categorized contracepting participants by WHO Tiers, which rate methods by effectiveness in preventing pregnancy.21 Tiers I–II included female and male sterilization, intrauterine device, pill and other hormonal methods. Tiers III–IV included condom, periodic abstinence, withdrawal, and other methods such as cervical cap.21
To compare age-adjusted rates of contraception between survivors and the general population, sampling weights were used and the survivor population was age-standardized using the general population as the standard.14 For the general population, contraception rates are calculated from the entire population, including women who are surgically sterile, pregnant or postpartum, seeking pregnancy, and not sexually active.14 Accordingly, survivor contraception rates were also estimated including the entire population of survivors. SAS PROC SURVEYFREQ was used to estimate proportions and 95% confidence intervals and p-values for comparisons between populations.
Secondarily, within the survivor population, log-binomial regression models estimated relative risks (RR) for characteristics associated with current contraception use and secondary outcomes.22,23 For current contraceptive use and use of Tiers I–II methods, the analyses excluded survivors who were not at risk of unintended pregnancy, i.e. survivors who are seeking pregnancy, pregnant or postpartum, surgically sterile or not seeking pregnancy. For emergency contraception use after cancer diagnosis, only surgically sterile survivors were excluded, because sexual activity, attempting pregnancy, and pregnancy or postpartum states can change over time since cancer diagnosis. Variables associated with outcomes at p<0.05 in bivariable analyses were included in final adjusted models. Statistical significance was set at p<0.05. All analyses were conducted using SAS software v9.3 (Cary, NC).
RESULTS
Two hundred eight-nine survivors (98%) were included in this analysis after excluding for age younger than 20 (n=3) and missing contraception data (n=3) (Table 1). Mean age (standard deviation) at enrollment was 31.6 (5.7) years. Median time since cancer diagnosis (interquartile range) was 2.4 (1.1-5.1) years. Most survivors were white (79%), college graduates (85%), and in partnered relationships (58%). The most common cancer types were breast cancer (32%), lymphoma (25%), and gynecologic (cervix, uterus, ovary) (10%). Eighty percent underwent chemotherapy, while 63% underwent surgery and 48% underwent radiation. In this cohort, 56% reported receiving family planning services since cancer diagnosis, and 50% reported receiving them in the past 12 months. Twenty-nine participants (10%) reported emergency contraception use. Among 31 participants with a pregnancy after cancer diagnosis, 5 reported an unintended pregnancy, with 2 resulting from contraceptive failure. Three participants with unintended pregnancies underwent pregnancy termination.
Table 1.
Descriptive characteristics of reproductive-aged female cancer survivors and women in the general U.S. population
| Participant Characteristics | Cancer Survivors n=289 n (%) | General Population n=51,277* n (%) |
|---|---|---|
| Demographics | ||
| Current Age, years | ||
| Mean | 31.6±5.7 | 32.1±11.3 |
| 20 to 24 | 38 (13.2) | 10,365 (20.2) |
| 25 to 29 | 81 (28.0) | 10,535 (20.6) |
| 30 to 34 | 83 (28.7) | 9,188 (18.0) |
| 35 to 39 | 69 (23.9) | 10,538 (20.6) |
| 40 to 44 | 18 (6.2) | 10,539 (20.6) |
| Race | ||
| White | 227 (78.8) | 37,872 (73.9) |
| Black | 9 (3.1) | 7,767 (15.1) |
| Other | 52 (18.1) | 5,638 (11.0) |
| Ethnicity | ||
| Hispanic | 27 (9.4) | 8,570 (16.7) |
| Non-Hispanic | 261 (90.6) | 42,707 (83.3) |
| Body Mass Index, kg/m2 | ||
| < 25 | 162 (56.1) | 22,188 (43.6) |
| 25 to 29.9 | 64 (22.1) | 12,497 (24.5) |
| ≥ 30 | 63 (21.8) | 16,245 (31.9) |
| Education: College Graduate | 239 (84.5) | 15,118 (29.5) |
| Income | ||
| ≤ $50,000 | 95 (32.9) | 29,878 (58.3) |
| > $50,000 | 138 (47.7) | 21,399 (41.7) |
| Declined to Answer | 56 (19.4) | -- |
| Current Health Insurance | 274 (95.1) | -- |
| Reproductive Characteristics | ||
| Partnered Relationship Status | 167 (57.8) | 31,900 (62.2) |
| Live Birth | 65 (22.5) | 33,654 (65.6) |
| Ever Been Pregnant | 103 (35.6) | 37,184 (72.5) |
| Desire to Have a Baby in the Future | 235 (81.3) | 27,481 (53.6) |
| Received Family Planning Services | ||
| In the Past 12 Months | 145 (50.4) | 20,710 (40.4) |
| Since Cancer Diagnosis | 162 (56.3) | -- |
| Ever Use of Emergency Contraception since Cancer Diagnosis | 29 (10.0) | -- |
| Unintended Pregnancy after Cancer Diagnosis | 5 (1.7) | -- |
| Cancer Characteristics | ||
| Cancer Diagnosis | ||
| Breast | 91 (31.5) | -- |
| Lymphoma | 71 (24.5) | -- |
| Gynecologic (cervix/uterus/ovary) | 28 (9.7) | -- |
| Blood/leukemia | 22 (7.6) | -- |
| Thyroid | 15 (5.2) | -- |
| Other | 62 (21.5) | -- |
| Cancer Stage | ||
| I | 59 (20.4) | -- |
| II | 86 (29.8) | -- |
| III | 56 (19.4) | -- |
| IV | 23 (7.9) | -- |
| Unknown | 65 (22.5) | -- |
| Cancer Treatment | ||
| Surgery | 183 (63.3) | -- |
| Chemotherapy | 230 (79.6) | -- |
| Radiation Therapy | 140 (48.4) | -- |
| Bone Marrow or Stem Cell Transplant | 16 (5.5) | -- |
| Time since Cancer Diagnosis, years | ||
| Median (IQR) | 2.4 (1.1 - 5.1) | -- |
| < 2 | 122 (42.4) | -- |
| ≥ 2 | 166 (57.6) | -- |
| Comorbid Medical Conditions† | ||
| 0 | 96 (33.2) | -- |
| 1 or more | 193 (66.8) | -- |
Abbreviation: IQR, interquartile range
General population as reported by the National Survey for Family Growth 2006-2010 cycle.15 Numbers are expressed in thousands and are based on applying sampling weights to 9995 respondents aged 20-44 years. Cancer-related variables were not collected by the survey.
Note: Due to missing data, some variables do not add up to 289 for cancer survivors or 51,276,864 for women in the general population.
Comorbid medical conditions included lung disease, hypertension, diabetes, overweight/obese, thyroid disorders, mood disorders, eating disorders, rheumatologic diseases, inflammatory bowel disease, neurologic disorders.
Figure 1 and Table 2 depict current contraceptive use in survivors and compare rates between survivors and the general population. Survivors were less likely to use a contraceptive method than women in the general population (57% vs. 69%, p<0.01). Moreover, survivors were less likely to use Tiers I–II methods compared to the general population (34% vs. 53%, p<0.01). Among those not using contraception, survivors were more likely to be surgically sterile for non-contraceptive indications (8% survivors vs. 0.4% general population) and less likely to be pregnant or postpartum (0.4% survivors vs. 5% general population). The proportion of women who were sexually active but did not report other reasons for non-contraception (surgical sterility, pregnant or postpartum, seeking pregnancy) was not different between survivors (10%) and the general population (8%).
Fig 1.
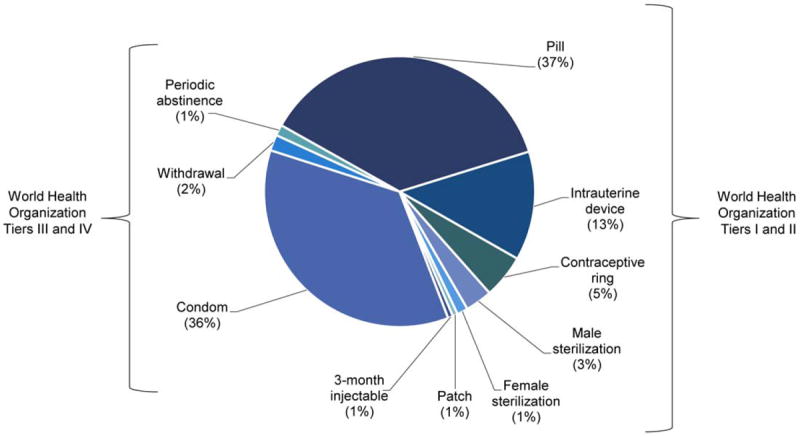
Contraceptive methods used by reproductive-aged cancer survivors (n=154), categorized by the most effective method.
Table 2.
Comparison of age-adjusted rates of contraceptive practices between the cancer survivor and general populations
| Contraceptive Status and Method | Cancer Survivors n=289 | General Population n=51,277* | P value |
|---|---|---|---|
| Percent (95% CI) | |||
| Using contraception | 57.4 (51.5 - 63.2) | 68.6 (67.3 - 70.0) | <0.01 |
| Contraception methods | <0.01 | ||
| WHO Tiers I/II† | 34.2 (28.8 - 40.0) | 53.0 (51.5 - 54.5) | |
| Female Sterilization† | 0.3 (0.0 - 1.9) | 19.9 (18.7 - 21.1) | |
| Male Sterilization | 4.2 (2.2 - 7.1) | 7.5 (6.7 - 8.4) | |
| Intrauterine Device | 5.9 (3.5 - 9.3) | 4.0 (3.5 - 4.6) | |
| Implant, Lunelle or Patch | 0.3 (0.0 - 1.9) | 0.9 (0.6 - 1.1) | |
| Pill | 20.8 (16.2 - 25.9) | 17.2 (16.1 - 18.4) | |
| 3-month Injectable (Depo-Provera) | 0.3 (0.0 - 1.9) | 2.0 (1.7 - 2.3) | |
| Contraceptive Ring | 2.4 (1.0 - 4.9) | 1.5 (1.1 - 1.8) | |
| WHO Tiers III/IV† | 23.2 (18.4 - 28.5) | 15.6 (14.5 - 16.6) | |
| Condom† | 20.4 (15.9 - 25.5) | 11.0 (10.1 - 11.9) | |
| Periodic Abstinence (family planning/calendar rhythm) | 1.7 (0.6 - 4.0) | 0.8 (0.6 - 1.1) | |
| Withdrawal† | 0.7 (0.1 - 2.5) | 3.4 (2.9 - 4.0) | |
| Other Methods | 0.0 (0.0 - 1.3) | 0.3 (0.1 - 0.5) | |
| Not using contraception | <0.01 | ||
| Surgically Sterile (female) † | 8.3 (5.4 - 12.1) | 0.4 (0.2 - 0.7) | |
| Nonsurgically Sterile‡(female or male) | -- | 1.9 (1.5 - 2.3) | |
| Pregnant or Postpartum† | 0.4 (0.0 - 1.9) | 4.7 (4.1 - 5.2) | |
| Seeking Pregnancy | 9.0 (6.0 - 12.9) | 5.4 (4.7 - 6.1) | |
| Not Sexually Active | 14.9 (11.0 - 19.5) | 11.0 (10.1 - 11.9) | |
| Sexually Active | 10.0 (6.8 - 14.1) | 7.9 (7.2 - 8.7) | |
Abbreviations: WHO, World Health Organization
General population as reported by the National Survey for Family Growth 2006-2010 cycle.15 Numbers are expressed in thousands and are based on applying sampling weights to 9995 respondents aged 20-44 years.
denotes non-overlapping 95% CIs of the proportions in the survivor and general populations.
For survivors, nonsurgically sterile is unknown and not included in the above table.
Notes: Overall comparisons performed using SAS PROC SURVEYFREQ with age-group weights to standardize for age. P-values from chi-square tests of homogeneity.
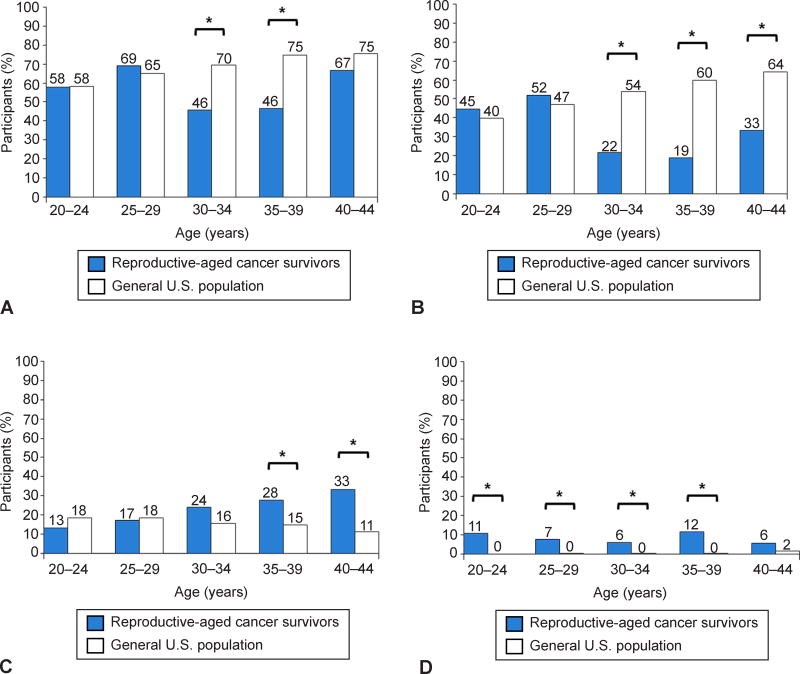
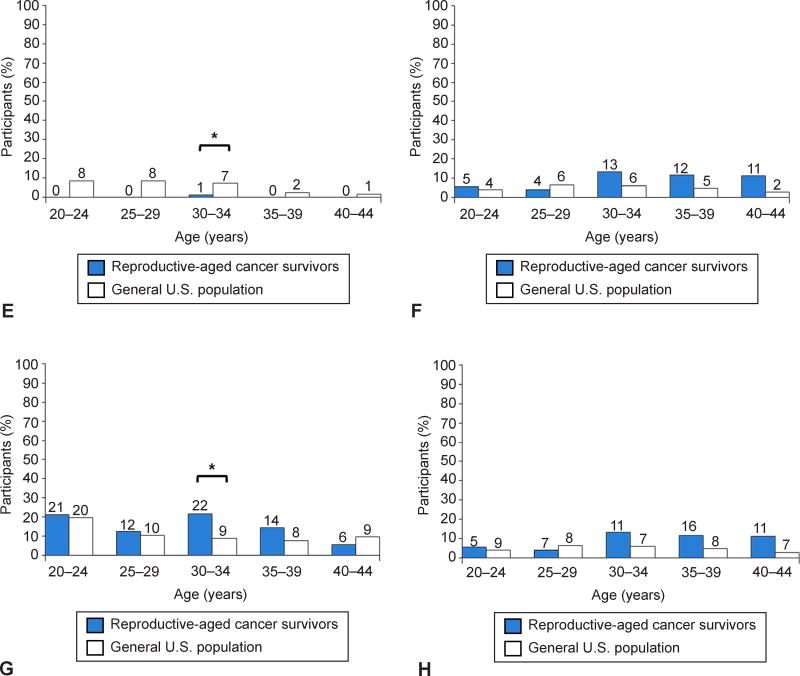
Several age-specific differences were observed between survivors and the general population (Figure 2). Lower contraception rates were seen in 30-34 and 35-39 years old survivors compared to the general population. Rates of using Tiers I–II contraceptive methods were also significantly lower in survivors ages 30 and older.
Fig 2.
Comparison of contraceptive practices between reproductive-aged cancer survivors (shown in blue) and women in the general U.S. population, as assessed by the National Survey of Family Growth (shown in white): use of contraception (A), use of World Health Organization (WHO) tiers I-II methods (B), use of WHO tiers III-IV methods (C), surgically sterile (D), pregnant or postpartum (E), seeking pregnancy (F), not sexually active (G), sexually active (H). The total number of survivors by age group is as follows: aged 20–24 years (n=38), aged 25– 29 years (n=81), aged 30– 34 years (n=83), aged 35– 39 years (n=69), and aged 40–44 years (n=18). *Nonoverlapping 95% confidence intervals of the proportions in the survivor and general populations.
Sensitivity analyses were performed in comparisons of the survivor and general populations. Since non-contraceptive surgical sterility was substantially higher in survivors, we excluded these women from both populations without substantively different results from the main analysis (Table 3). Restricting analyses to those at risk of unintended pregnancy (excluding sterility for non-contraceptive indications, pregnant or postpartum, seeking pregnancy, not sexually active), survivors still had lower contraception rates than the general population (84% vs. 90%, p=0.009), as well as lower rates of using Tiers I–II methods compared to the general population (51% vs. 69%, p<0.001).
Table 3.
Comparison of age-adjusted rates of contraceptive practices between the cancer survivor and general populations, excluding participants who are surgically sterile for non-contraceptive indications
| Contraceptive Status and Method | Cancer Survivors =265 | General Population n=51,277* | P value |
|---|---|---|---|
| Percent (95% CI) | |||
| Using contraception | 60.8 (54.9 - 66.7) | 69.0 (67.6 - 70.3) | <0.01 |
| Contraception methods | <0.01 | ||
| WHO Tiers I/II† | 36.7 (30.8 - 42.6) | 53.2 (51.8 - 54.7) | |
| Female Sterilization† | 0.7 (0.0 - 1.6) | 19.9 (18.7 - 21.1) | |
| Male Sterilization† | 1.9 (0.2 – 3.6) | 7.5 (6.7 - 8.4) | |
| Intrauterine Device | 7.9 (4.6 - 11.1) | 4.0 (3.5 - 4.6) | |
| Implant, Lunelle or Patch | 0.4 (0.0 - 1.2) | 0.9 (0.6 - 1.1) | |
| Pill | 22.0 (17.0 - 27.1) | 17.4 (16.2 - 18.5) | |
| 3-month Injectable (Depo-Provera) | 0.4 (0.0 - 1.2) | 2.0 (1.7 - 2.3) | |
| Contraceptive Ring | 3.5 (1.3 - 5.8) | 1.5 (1.1 - 1.8) | |
| WHO Tiers III/IV† | 24.1 (18.9 - 29.3) | 15.7 (14.6 - 16.7) | |
| Condom† | 21.8 (16.8 - 26.9) | 11.1 (10.2 - 12.0) | |
| Periodic Abstinence (family planning/calendar rhythm) | 1.2 (0.0 - 2.5) | 0.8 (0.6 - 1.1) | |
| Withdrawal† | 1.2 (0.0 - 2.5) | 3.5 (2.9 - 4.0) | |
| Other Methods | 0.0 (0.0 – 0.0) | 0.3 (0.1 - 0.5) | |
| Not using contraception | <0.01 | ||
| Nonsurgically Sterile‡(female or male) | -- | 1.9 (1.5 - 2.3) | |
| Pregnant or Postpartum† | 0.3 (0.0 - 1.0) | 4.7 (4.1 - 5.3) | |
| Seeking Pregnancy | 9.7 (6.1 - 13.3) | 5.4 (4.8 - 6.1) | |
| Not Sexually Active | 17.6 (13.0 - 22.2) | 11.1 (10.2 – 12.0) | |
| Sexually Active | 11.4 (7.5 – 15.2) | 7.9 (7.2 - 8.7) | |
Abbreviations: WHO, World Health Organization
Notes: Overall comparisons performed using SAS PROC SURVEYFREQ with age-group weights to standardize for age. P-values from chi-square tests of homogeneity.
General population as reported by the National Survey for Family Growth 2006-2010 cycle15. Numbers are expressed in thousands and are based on applying sampling weights to 9995 respondents aged 20-44 years.
denotes non-overlapping 95% CIs of the proportions in the survivor and general populations.
For survivors, nonsurgically sterile is unknown and not included in the above table.
One hundred eighty-four survivors (64%) were at risk of unintended pregnancy after excluding those not sexually active, seeking pregnancy, pregnant or postpartum, or surgically sterile from non-contraceptive indications. Among those at risk of unintended pregnancy, 154 (84%) reported use of contraception; 94 (51%) used Tiers I–II methods. Compared to users of Tiers III–IV methods, survivors who used Tiers I–II methods were more likely to have received family planning services (Table 4). Survivors who used Tiers I–II methods were also younger, further out from their cancer diagnosis, more likely to be obese, and less likely to be partnered or have breast cancer. In adjusted analyses, only receipt of family planning services in the past 12 months remained significantly associated with use of Tiers I–II methods (RR 1.3, 95%CI 1.1-1.5).
Table 4.
Characteristics associated with use of WHO Tiers I/II vs. WHO Tiers III/IV contraceptive methods among reproductive-aged cancer survivors who reported using a method of contraception (n=154)
| Characteristic | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P value | RR | (95% CI) | P value | |
| Receipt of family planning services in the past 12 months (n=100) (vs. no receipt) | 3.26 | (2.01 - 5.28) | <0.01 | 1.28 | (1.07 - 1.53) | <0.01 |
| Age ≥ 31 years (n=72) (vs. <31) | 0.62 | (0.47 - 0.82) | <0.01 | 0.98 | (0.81 - 1.18) | 0.81 |
| Body mass index, kg/m2 | ||||||
| 25 to 29.9 (n=34) (vs. < 25) | 1.15 | (0.83 - 1.59) | 0.41 | 1.06 | (0.86 - 1.31) | 0.58 |
| ≥ 30 (n=29) (vs. <25) | 1.54 | (1.19 - 1.98) | <0.01 | 1.12 | (0.86 - 1.45) | 0.40 |
| Partnered (n=92) (vs. not partnered) | 0.77 | (0.60 - 0.98) | 0.03 | 0.96 | (0.81 - 1.14) | 0.64 |
| Breast cancer diagnosis (n=51) (vs. non-breast) | 0.45 | (0.30 - 0.67) | <0.01 | 0.93 | (0.76 - 1.13) | 0.45 |
| < 2 years since cancer diagnosis (n=87) (vs. ≥ 2 years) | 0.66 | (0.49 - 0.88) | <0.01 | 0.91 | (0.77 - 1.08) | 0.28 |
Abbreviation: WHO, World Health Organization
Notes: Risk ratio estimates from log-binomial regression models, adjusted model included all variables shown in the table. P-values from Wald tests of regression coefficients. The analyses excluded survivors who were not at risk of unintended pregnancy (i.e., not sexually active, seeking pregnancy, pregnant/postpartum, or surgically sterile).
Thirty (16%) survivors who were at risk of unintended pregnancy reported no current contraceptive use. In unadjusted analyses, no contraceptive use was associated with older age (≥31 vs. <31, RR 2.3, 95%CI 1.1-4.7) and partnered status (RR 3.6, 95%CI 1.3-10.0), and inversely associated with receipt of family planning services (RR 0.4, 95%CI 0.2-0.8). In adjusted analyses, only partnered status was associated with a higher rate of non-contraception (RR 3.0, 95%CI 1.1-8.3), while age (RR 1.4, 95%CI 0.7-3.1) and family planning services (RR 0.6, 95%CI 0.3-1.1) were attenuated.
Emergency contraception use after cancer diagnosis was examined in 263 survivors, excluding 26 who were surgically sterile from non-contraceptive indications. Twenty-nine participants (11%) reported using emergency contraception after cancer diagnosis. In unadjusted analyses, receipt of family planning services was associated with higher emergency contraception use (RR 6.5, 95%CI 2.0-21.0). Additionally, emergency contraception use was associated with younger age (<31 vs. ≥31, RR 3.6, 95%CI 1.6-8.1), non-breast cancer diagnosis (RR 3.0, 95%CI 1.1-8.5), and longer time since cancer diagnosis (≥2 years vs. <2 years, RR 3.5, 95%CI 1.4-9.0). In adjusted models (Table 5), only younger age and receipt of family planning services was significantly associated with higher emergency contraception use.
Table 5.
Risk of emergency contraception use since cancer diagnosis by receipt of family planning services and cancer type in reproductive-aged cancer survivors (n=263)
| Characteristic | Age-adjusted* | Fully adjusted† | ||||
|---|---|---|---|---|---|---|
| RR | (95% CI) | P value | RR | (95% CI) | P value | |
| Receipt of family planning services since cancer diagnosis (n=149) (vs. no receipt) | 5.31 | (1.64 - 7.21) | <0.01 | 5.02 | (1.55 - 16.26) | <0.01 |
| Non-breast cancer diagnosis (n=176) (vs. breast cancer) | 2.07 | (0.72 - 5.94) | 0.17 | 1.74 | (0.63 - 4.81) | 0.29 |
Age-adjusted risk ratio estimates from log-binomial regression models including age as above/below mean age of 31 years.
Fully adjusted risk ratio estimates from log-binomial regression models including variables shown in the table and age as above/below mean age of 31 years.
Notes: P-values from Wald tests of regression coefficients. The analyses excluded survivors who were surgically sterile.
DISCUSSION
This study showed lower rates of contraception in reproductive-aged cancer survivors than in women in the general U.S. population. Survivors also used less reliable methods, incurring risks of unintended pregnancy. Exposure to family planning services in survivors may improve contraception utilization, as receipt of family planning services in cancer survivorship was significantly related to utilization of more effective forms of contraception and accessing emergency contraception.
Among survivors, 57% reported current contraception, consistent with recent reports.7,8 Low contraception rates among survivors motivated this study to compare contraceptive practices with the general population and determine factors related to the behavior. The lower contraception rate in survivors compared to the general population may be partially explained by more non-contraceptive sterility among survivors. However, sensitivity analyses excluding these women, women not at risk of unintended pregnancy, or breast cancer survivors (data not shown) all demonstrated significantly lower contraception rates in survivors.
Moreover, lower utilization of Tiers I–II methods was observed in survivors compared to the general population, which is concerning as rates of unintended pregnancy are significantly higher with Tiers III–IV methods such as condoms compared with Tiers I–II methods.24 One explanation may be concern about hormonal exposure in women with estrogen-sensitive tumors or hypercoagulable states.13 Accordingly, we found that those using Tiers III–IV methods were more likely to have breast cancer and be within 2 years of diagnosis compared to Tiers I–II users. Lower utilization of Tiers I–II methods was also attributable to low uptake of long-acting reversible contraception (LARC), despite that copper IUDs are recommended for women with estrogen-sensitive tumors.13 To improve LARC uptake, increased access to information and healthcare providers knowledgeable on contraceptive effectiveness and safety are needed.
Among survivors, receipt of family planning services increased use of Tiers I–II contraceptive methods by twofold and emergency contraception by fivefold, suggesting receipt of family planning services may improve contraception care. This finding is consistent with Maslow et al, who found a six-fold increased odds of using Tiers I–II methods in survivors who received family planning services.7 In contrast, fertility preservation counseling did not affect contraception choices, raising the question of how fertility preservation interactions can be optimized for family planning.8 Unfortunately, only half of survivors reported receiving contraception-related counseling, testing, prescriptions, or procedures since cancer diagnosis, despite likely more access to healthcare than women without chronic medical conditions.
Strengths of this study include diversity of cancers represented and data on reproductive health outcomes after cancer, allowing us to consider important covariates. By race and ethnicity, the study’s survivor population was similar to the general U.S. population of reproductive-aged cancer survivors. For example, reproductive-aged U.S. female cancer survivors are 76% non-Hispanic white, 9% black, 4% Asian, 14% Hispanic.25 Several strategies were undertaken to compare survivors and the general population, including age adjustment and use of National Survey for Family Growth methods. Inclusion of all survivors, regardless of cancer treatment or menstrual pattern, makes our study more generalizable than prior studies. While limited, this study described prevalence and factors related to emergency contraception use in survivors.
Sample size was a limitation. Post-hoc sample size calculations showed 80% power to detect a difference in rates of 8% between the survivor and general populations. Our study of 295 women is among the largest to evaluate contraception after cancer; however, the cohort size and limited variability of some characteristics restricted our ability to adjust for potential confounding factors other than age, such as race, ethnicity and education. Given the sociodemographic characteristics of survivors, they likely represent the upper bound of contraception use that might be observed in a more diverse survivor population. The study was not designed to compare rates of unintended pregnancy, an important clinical outcome. The low unintended pregnancy rate may reflect short time since cancer treatment for ovarian recovery of our survivors or different risks of unintended pregnancy after cancer, to be answered in future studies. The small numbers of survivors who used emergency contraception or were not contracepting while at risk of an unintended pregnancy impacted the number of covariates log-binomial models could accommodate, although estimates did not appreciably change with logistic models.23 Other study limitations include: self-reported study variables, inability to determine causation with the cross-sectional design, and generalizability of survivors who enrolled in a study on reproductive health. We lack information on religion, insurance coverage, potential ambivalence about unintended pregnancy, and other possible reasons for less effective contraceptive choices.
The study provides novel evidence of lower rates of using effective contraceptive methods in reproductive-aged cancer survivors compared to the general U.S. population. Increased access to family planning services may improve survivorship care and prevent unintended pregnancy in this vulnerable population.
Supplementary Material
Acknowledgments
Supported by NIH UL1 RR024926 pilot and HD-058799-01, and by the American Cancer Society MRSG-08-110-01-CCE and 120500-PFT-11-008-01-CPPB. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
The authors thank the Samantha Roberts for her contribution to this study.
Footnotes
Presented at the American Society of Reproductive Medicine Annual Meeting; Honolulu, Hawaii, Oct 19-22 2014.
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Sally A. Dominick, Department of Reproductive Medicine, Moores Cancer Center, University of California, San Diego.
Mamie R. McLean, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, University of Alabama at Birmingham.
Brian W. Whitcomb, Department of Biostatistics & Epidemiology, School of Public Health & Health Sciences, University of Massachusetts, Amherst.
Jessica R. Gorman, Department of Family and Preventive Medicine, Moores Cancer Center, University of California, San Diego.
Jennifer E. Mersereau, Department of Obstetrics and Gynecology, University of North Carolina, Chapel Hill.
Janet M. Bouknight, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, University of Alabama at Birmingham.
H. Irene Su, Department of Reproductive Medicine, Moores Cancer Center, University of California, San Diego.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Retrieved April 16, 2014. [Google Scholar]
- 2.Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. The Lancet Oncology. 2013;14:873–81. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunsford AJ, Whelan K, McCormick K, McLaren JF. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertility and Sterility. 2014;101:227–31. doi: 10.1016/j.fertnstert.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 4.Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–9. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DM, Sklar CA, Boice JD, Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27:2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominick SA, Whitcomb BW, Gorman JR, Mersereau JE, Chung K, Su HI. Factors associated with pregnancy attempts among female young adult cancer survivors. Journal of Cancer Survivorship. 2014;8:571–9. doi: 10.1007/s11764-014-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maslow BS, Morse CB, Schanne A, Loren A, Domchek SM, Gracia CR. Contraceptive use and the role of contraceptive counseling in reproductive-aged women with cancer. Contraception. 2014;90:79–85. doi: 10.1016/j.contraception.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Quinn MM, Letourneau JM, Rosen MP. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception. 2014;89:466–71. doi: 10.1016/j.contraception.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young Australian women with breast cancer. Health Care for Women International. 2006;27:94–110. doi: 10.1080/07399330500377580. [DOI] [PubMed] [Google Scholar]
- 10.Karaoz B, Aksu H, Kucuk M. A qualitative study of the information needs of premenopausal women with breast cancer in terms of contraception, sexuality, early menopause, and fertility. International Journal of Gynaecology and Obstetrics. 2010;109:118–20. doi: 10.1016/j.ijgo.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Green DM, Whitton JA, Stovall M, Mertens AC, Donaldson SS, Ruymann FB, et al. Pregnancy outcome of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. American Journal of Obstetrics and Gynecology. 2002;187:1070–80. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 12.Winther JF, Boice JD, Jr, Svendsen AL, Frederiksen K, Olsen JH. Induced abortions in Danish cancer survivors: a population-based cohort study. Journal of the National Cancer Institute. 2009;101:687–9. doi: 10.1093/jnci/djp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel A, Schwarz EB. Cancer and contraception. Release date May 2012 SFP Guideline #20121. Contraception. 2012;86:191–8. doi: 10.1016/j.contraception.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. National Health Statistics Reports. 2012:1–25. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Survey of Family Growth. 2006-2010. NSFG: Public Use Data Files, Codebooks, and Documentation. Available at: http://www.cdc.gov/nchs/nsfg/nsfg_2006_2010_puf.htm. Retrieved March 3, 2015.
- 16.Gorman JR, Roberts SC, Dominick SA, Malcarne VL, Dietz AC, Su HI. A diversified recruitment approach incorporating social media leads to research participation among young adult-aged female cancer survivors. Journal of Adolescent and Young Adult Oncology. 2014;3:59–65. doi: 10.1089/jayao.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network Guidelines for Treatment of Cancer by Site. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Retrieved March 3, 2015.
- 18.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available at: http://seer.cancer.gov/. Retrieved March 3, 2015.
- 19.Pierce JP, Faerber S, Wright FA, Rock CL, Newman VA, Flatt SW, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Controlled Clinical Trials. 2002;23:728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 20.Lepkowski JM, Mosher WD, Davis KE, Groves RM, Van Hoewyk J. The 2006-2010 National Survey of Family Growth: sample design and analysis of a continuous survey. Vital and Health Statistics Series 2, Data Evaluation and Methods Research. 2010:1–36. [PubMed] [Google Scholar]
- 21.World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP) Knowledge for Health Project. Family Planning: A Global Handbook for Providers (2011 update) Baltimore and Geneva: CCP and WHO; 2011. [Google Scholar]
- 22.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. American Journal of Epidemiology. 1986;123:174–84. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 23.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157:940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 24.WHO Guidelines Approved by the Guidelines Review Committee. Medical Eligibility Criteria for Contraceptive Use: A WHO Family Planning Cornerstone. 4. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 25.National Cancer Institute. Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program. Available at http://seer.cancer.gov/faststats/. Retrieved April 20, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.