Abstract
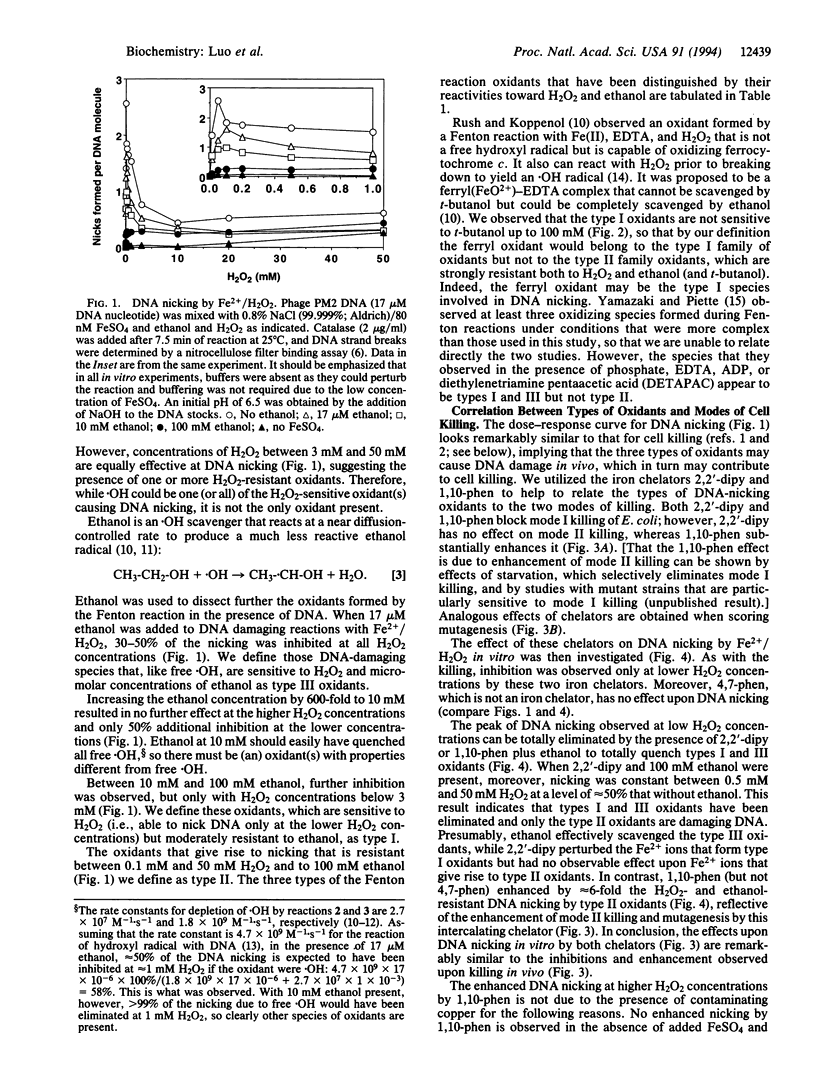
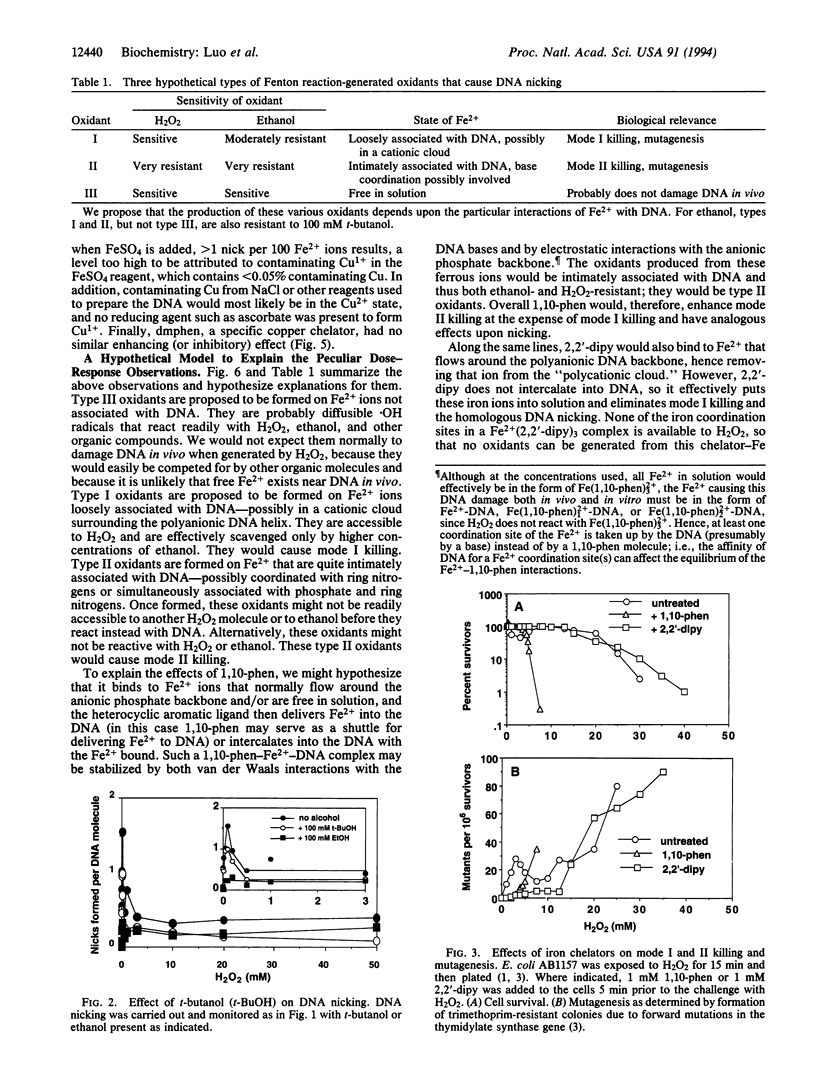
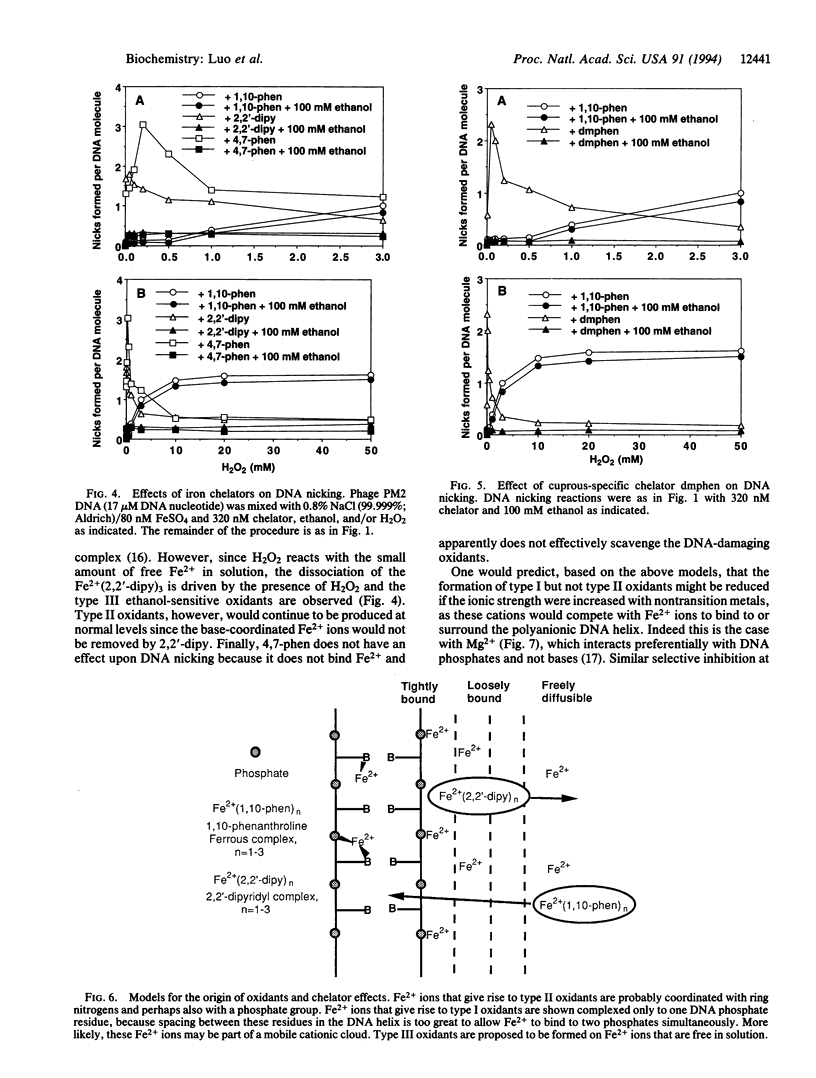
Exposure of Escherichia coli to H2O2 leads to two kinetically distinguishable modes of killing: mode I killing occurs maximally near 2 mM H2O2, whereas mode II killing is essentially independent of H2O2 concentrations up to 20 mM. A major portion of H2O2 toxicity is attributed to DNA damage caused by the iron-mediated Fenton reaction. By studying DNA damage during Fenton reactions in vitro, the same complex kinetics were observed and three types of oxidants were distinguished based upon their reactivities toward H2O2 and alcohols and upon iron-chelator effects. Type I oxidants are sensitive to H2O2 but moderately resistant to ethanol; type II oxidants are resistant to both H2O2 and ethanol; type III oxidants are sensitive to H2O2, ethanol, and t-butanol. To explain these results, we hypothesize that type I oxidants are generated upon Fe2+ associated with DNA only through electrostatic interactions and cause mode I killing of E. coli; type II oxidants arise upon Fe2+, which is at least partially base-associated, and cause mode II killing; type III oxidants arise on Fe2+ free in solution and probably do not cause killing. Therefore, particular interactions of DNA with transition metals should be considered to be an integral part of the chemistry and toxicity of H2O2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Green M. J., Hill H. A. Chemistry of dioxygen. Methods Enzymol. 1984;105:3–22. doi: 10.1016/s0076-6879(84)05004-7. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol. 1986 May;166(2):519–527. doi: 10.1128/jb.166.2.519-527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels H. B., Hunt J. W. Reactions of the hydroxyl radical with polynucleotides. Radiat Res. 1973 Oct;56(1):57–70. [PubMed] [Google Scholar]
- Nelson D. P., Kiesow L. A. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H 2 O 2 solutions in the UV). Anal Biochem. 1972 Oct;49(2):474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]



