1. Introduction
Environmental factors are recognized as important determinants of life-long health trajectories (Cohen Hubal and others 2014; Grandjean and Landrigan 2006; Manciocco and others 2014; Sharma and others 2014). In studying the health effects arising from the interaction of environmental chemicals and human physiology, exposure assessment in most epidemiological studies is limited to a single toxicant or a small group of toxicants. However, humans are exposed to thousands of environmental chemicals which may exert effects jointly that are distinct to their individual effects (Kortenkamp and others 2007). The “Exposome” concept addresses this issue and encompasses the complete life long experience of environmental exposures from the prenatal period onwards (Rappaport 2011; Vrijheid 2014; Wild 2005; Wild 2012). Unlike the human genome, the exposome is dynamic and must be examined at key developmental stages to understand its role in human health. In this review, we propose the use of novel tooth matrix biomarkers to capture the composition and timing of the exposome retrospectively.
1.1 Importance of exposure timing
Alongside the growth of the exposome concept, is also the increasing body of evidence that internal and external exposure to chemicals (and their reaction products) exerts a variable influence on our physiology at different developmental stages (Selevan and others 2000). As a consequence, windows of susceptibility exist when vulnerability to environmental chemicals is heightened (Grandjean and Landrigan 2006). It, therefore, becomes important to look beyond how much exposure has been experienced (i.e. the dose) to also consider the timing of exposure. The prenatal period is particularly important when considering critical windows. During fetal life and early childhood, the tissues and organs of the body undergo periods of rapid growth, during which a toxic insult or nutrient deficiency can lead to long-term effects (Osmond and Barker 2000; Selevan and others 2000). Considering the brain as an example, the complexity of its developmental process underlies its unique sensitivity to the environment. As early as the second week of gestation, the neuro-ontogenic process in humans begins with the folding and fusion of ectoderm to form the neural tube (Tau and Peterson 2010). The development of the human central nervous system (CNS) involves the production of 100 billion nerve cells and 1 trillion glial cells. These neurons must undergo migration, synaptogenesis, selective cell loss, myelination, and selective synaptic pruning in stages that ebb and flow before development is complete (Faustman and others 2000). These processes commence early in the first month of gestation and continue well into the second trimester. For example, neuronal migration peaks between gestational weeks 12 and 20 and is largely complete by weeks 26–29 (Tau and Peterson 2010). Other critical processes in brain development continue postnatally (Andersen 2003).
Even weak inhibitory or excitatory signals imposed by environmental toxicants during specific CNS developmental stages can alter subsequent processes over-riding a normal growth trajectory towards a maladaptive phenotype. For example, neurotoxic chemicals can lead to permanent reductions in cell number (Bayer 1989) or altered synaptic architecture (Bressler and others 1999). In many cases, developmental processes occur sequentially, rather than concurrently; hence the observed specificity of exposure timing on health effects, as exposures might affect only a process that is operant at a specific life phase. Thus, when a person is exposed to a toxic chemical is as important as the dose. In this regard, our focus is on the prenatal period because the lack of exposure assessment tools that can directly measure fetal chemical exposure in large population based studies without imposing undue risk on the pregnancy remains an important barrier to environmental health research.
The semi-penetrable nature of the placenta coupled with the increased susceptibility to chemicals makes the prenatal period of critical interest to understanding the fetal environmental determinants of life long health trajectories. The placenta only partially (and in some cases negligibly) regulates a large number of potentially toxic chemicals. Polybrominated diphenyl ethers (PBDEs) can be transferred to the developing fetus across the placenta (and to children via breast milk) (Lorber 2008). A prior study has shown almost identical PBDE concentrations in maternal blood collected at delivery and in cord blood, suggesting that the placenta offers limited protection to the fetus (Mazdai and others 2003). Studies have also shown that bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT) and DDT metabolites levels are higher in fetal circulation than maternal levels (Waliszewski and others 2001). Similarly, manganese (Mn), an essential metal that can be neurotoxic at high levels, is actively transported across the placenta resulting in higher levels in fetal circulation (Takser and others 2004).
1.2 Challenges to studying fetal chemical programming
Epidemiologic studies that investigate fetal programming of long-term health trajectories face major challenges when trying to estimate the fetal exposome, and we focus on two barriers that are especially relevant to lower frequency health outcomes. Longitudinal birth cohort studies that collect biomarkers of environmental chemical exposure during pregnancy and then follow offspring into childhood provide the highest evidence study design to assess the impact of exposures during key developmental windows in humans. However, the expense and time required for such studies are major barriers to investigating lower frequency conditions with long latency periods. For example, to determine the fetal origins of a disorder that occurs at a frequency of 1:100 live births, a study would have to recruit 10,000 pregnant women and study the offspring prospectively until a stable clinical diagnosis can be made many years later. This is compounded by another, equally important barrier to uncovering fetal environmental exposures. Even in studies that commence with recruitment of pregnant women, measurements of maternal biomarkers do not necessarily provide accurate measures of fetal exposure for all chemicals. Reliance on maternal biomarkers of fetal exposure fails to account for variability in placental transport and metabolism, potentially overlooking the significant interplay at the maternal-fetal interface. Additionally, in population-based studies it is not feasible to obtain prospective fetal samples without imposing unacceptable health risks to both mother and child. Umbilical cord blood has been successfully collected at birth in epidemiologic studies and has provided valuable exposure information (Aylward and others 2014; Cooke 2014; Delvaux and others 2014; Lin and others 2013). However, for compounds with a short half-life in blood, cord blood levels can only provide information on the latter part of the third trimester. It is important to consider that even though case-control studies nested within large population cohorts may overcome the first barrier of requiring of long-term follow ups, the absence of a direct fetal measurement would remain a limitation.
To overcome these limitations, researchers have long sought a biomarker that is retrospective, objective, and capable of directly measuring fetal exposures to multiple chemicals. For health outcomes that occur at lower frequencies, this biomarker would be applied in population-based case-control designs. Unlike contemporary biomarkers that are cross-sectional, this novel biomarker would provide time-series exposure data similar to that obtained from a longitudinal study, whilst doing so retrospectively. In this perspective, we discuss a novel dental-matrix based biomarker that brings us closer to this ideal of retrospectively reconstructing the dynamic internal exposome. We provide a conceptual framework for this approach and provide data to support the utility of this biomarker in epidemiologic studies. We place emphasis on case-control studies of lower frequency and rare outcomes with long latency periods where prospective cohort studies would prove inefficient. Our discussion is limited to internal chemical exposures, although some of the concepts we introduce are also relevant to other domains of the exposome.
2. Teeth as a Novel Matrix for Reconstructing the Early Life Exposome
2.1 Aspects of Tooth Development Relevant to this Biomarker
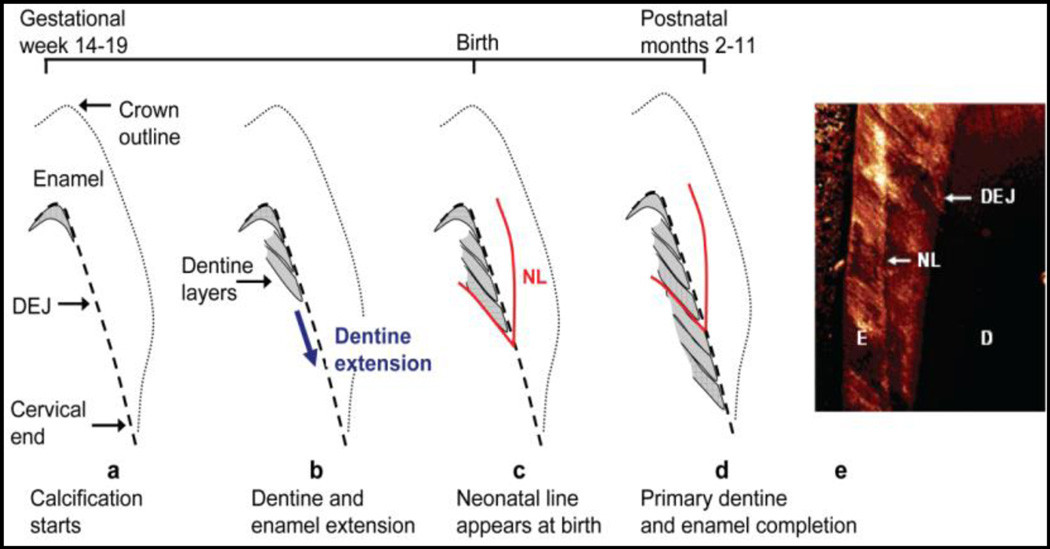
Between the 14th to 19th week of intrauterine development, the tooth germ enters the advanced bell stage characterized by the appearance of enamel and dentine at the future dentine-enamel junction (DEJ) on the cusp tip (Fig. 1a) (BKB Berkovitz 2009). Subsequently, enamel and dentine deposition occurs in a rhythmic manner, forming incremental lines akin to growth rings in both enamel and dentine (Fig. 1b). At birth, an accentuated incremental line, the neonatal line, is formed due to disturbances in the secretory cells during protein matrix deposition (Sabel and others 2008). A resultant change in crystal orientation and lower degree of mineralization has been detected at the neonatal line in enamel of human primary teeth (Figs. 1c,d,e). This line forms a clear histological landmark that demarcates pre- and postnatally formed parts of teeth (Sabel and others 2008). Beyond the neonatal line, teeth manifest daily growth lines, which allow chronological ages to be determined at various positions within tooth crowns and roots. We have previously validated this biomarker for certain metals (Mn, Pb, Ba, Sr) (Arora and others 2014; Arora and others 2012; Arora and others 2004; Arora and others 2011; Austin and others 2013) and validation for a range of organic targets is underway in our laboratory.
Fig. 1. Schematic of tooth development(Arora and others 2012).
(a) Earliest deposition of dentine (grey area) at DEJ at cusp tip (b) Continued extension of dentine (and enamel) towards the tooth cervix. (c) Neonatal line (NL), a histological feature, formed at the time of birth (d) Completion of enamel and primary dentine formation between 2 to 11 postnatal months depending on tooth type. Secondary dentine continues forming at pulpal margin (not shown). (e) Confocal laser scanning micrograph of NL in enamel. Reprinted with permission from Environ. Sci. Technol., 2012, 46 (9), pp 5118–5125. Copyright 2012 American Chemical Society."
2.2 Comparison of teeth with other biological matrices to uncover prenatal exposure timing retrospectively
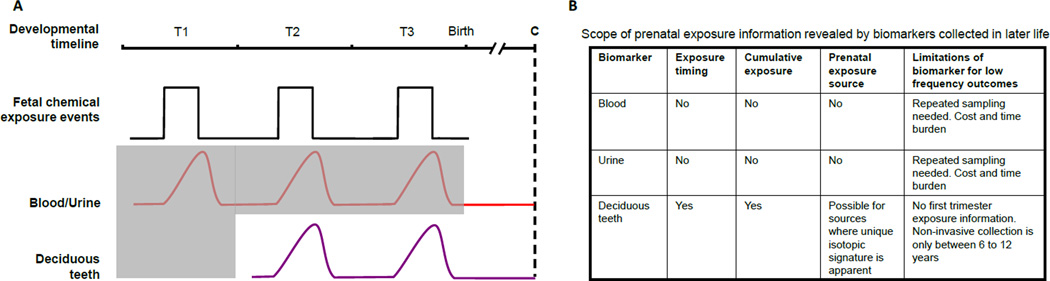
Biological matrices vary in their response to environmental chemical exposures. Here we contrast the prenatal exposure information that is gleaned from tooth matrix biomarkers with the most commonly used biological media (blood and urine; Fig. 2). We present a scenario where there are discrete exposure events to a chemical with a short half-life (< 1 month) during the prenatal period but the first sample is collected postnatally in childhood, adolescence or adulthood. In Figure 2a, it can be seen that maternal environmental exposure events may expose the fetus to chemicals during first (T1), second (T2) or third (T3) trimesters. For chemicals that cross the placental barrier, fetal blood levels rise in response to a limited exposure but return to pre-exposure levels due to the short half-life of the chemicals in blood unless exposure is sustained. In a case-control study, cconcentrations of chemicals in blood or urine at a collection time (C) in childhood or adulthood will not provide a direct measure of the timing or intensity of exposure during any trimester, or of cumulative exposure over the entire prenatal period. In the biologic matrices considered in this example, deciduous teeth alone would provide exposure timing and intensity over the 2nd and 3rd trimesters and during early childhood, and would also provide cumulative long-term exposure. In Figure 2, grey shaded areas represent the developmental times when exposure information is not available at postnatal sample collection. The table in Panel B summarizes the scope of the information from these biomarkers.
Fig. 2.
Conceptual framework of how blood, urine, and deciduous teeth collected postnatally compare in estimating prenatal exposure to chemicals that have a short half-life. Grey shaded area indicates time periods for which exposure information is missing when using a biomarker collected in childhood.
3. Key Aspects of Methodology
Five deciduous teeth were randomly selected for exploratory, non-targeted metabolite profiling. The main aims were to: (i) collect developmental stage specific dentine layers formed during (a) 2nd and 3rd trimester of two children (Child A and B), and (b) prenatal (pool of 2nd and 3rd trimester) and postnatal (up to 6 months from birth) phase of three children (Child C, D, and E), and (ii) perform an exploratory global screening of small molecules using a liquid chromatography coupled quadrupole time-of-flight mass spectrometry (QTOF-LC/MS) based metabolomics approach.
Method development for the trimester-specific tooth layer analysis has been described previously (Arora and others 2012; Austin and others 2013). Our analytical methodology consisted of isolating dentin layers from desired developmental time periods followed by extraction and concentration of the embedded analytes. Separation of analytes was undertaken using chromatographic separation and the final extract was introduced in to the tandem mass spectrometer as an aerosol using electrospray ionization (ESI). Metabolite profiling was undertaken using Agilent 6550 iFunnel QTOF with Jet Stream ESI and both positive and negative ions were detected sequentially using positive and negative operating modes (schematic presented in Fig. SI-1). We provide a detailed discussion of our approach including the analytical method and instrument operating conditions in Supplementary Information (Fig. SI-1, Table SI-1). Briefly, representative workflows applied in this study were: (i) hard tissue sample preparation for multi-omics studies (Blackwell 2013) (ii) non-targeted screening with an accurate-mass Q-TOF LC/MS (Zamboni 2012) and (iii) data mining. The computational tools used for targeted analysis in this study were (a) ‘Personal Compound Database (PCD)’ (Kuhlmann 2009a; Kuhlmann 2009b), which is a large database with accurate mass measurements for several thousand chemical compounds, and (b) ‘Find by Formula’ (Broecker 2010), which is a ‘feature extraction’ algorithm for ion extraction, formulae calculation, grouping based on user-specified adducts (e.g. sodium) and multimers (e.g. several halogens), reconstruction and verification of spectra, and matching with compounds present in the large chemical compounds databases compiled by Agilent Technologies. The tools used for untargeted screening in this study were (a) ‘Molecular Feature Extraction (MFE)’(Jenkins 2013), which is a unbiased algorithm that tests chromatographic covariance and chemical relationship, finds related ions including isotopes and ion adducts, and reconstruction of spectra and their verification against molecular formulae in the available large databases, and (b) ‘Batch Recursive Feature Extraction (BRFE)’ (MassHunterProfinder 2014), which improves quality of the identified target list and reduces the amount of manual interpretations. LC/MS spectra were curated for a threshold of 1500 counts for ion abundance and 70 percent for quality score, with a minimum of two ions requirement. Chemically qualified compounds were classified into three classes (i) known unknowns (with available chemical characterization in databases and reference standards), (ii) suspected unknowns (with a priori information from literature and available chemical characterization in databases, but no reference standards), (iii) unknown unknowns (with neither a priori information nor chemical characterization in databases or reference standards) (modified description of the terminology from Naegele (Naegele 2011) and Little et al (Little and others 2012)).
4. Multi-Chemical Exposure Profiles During the Prenatal and Early Childhood Periods
Preliminary QTOF-LC/MS data showed promising results to support the application of the dental matrix for reconstructing the prenatal exposome. Results of a typical non-targeted profile of organic compounds and metabolites accumulated in dentine formed during the prenatal period are demonstrated for Child C (Fig. SI-2). An unbiased MFE data mining algorithm on Child C generated 7,043 and 5,137 distinguished peaks in positive (Fig. SI-2a) and negative (Fig. SI-2b) mode, respectively.
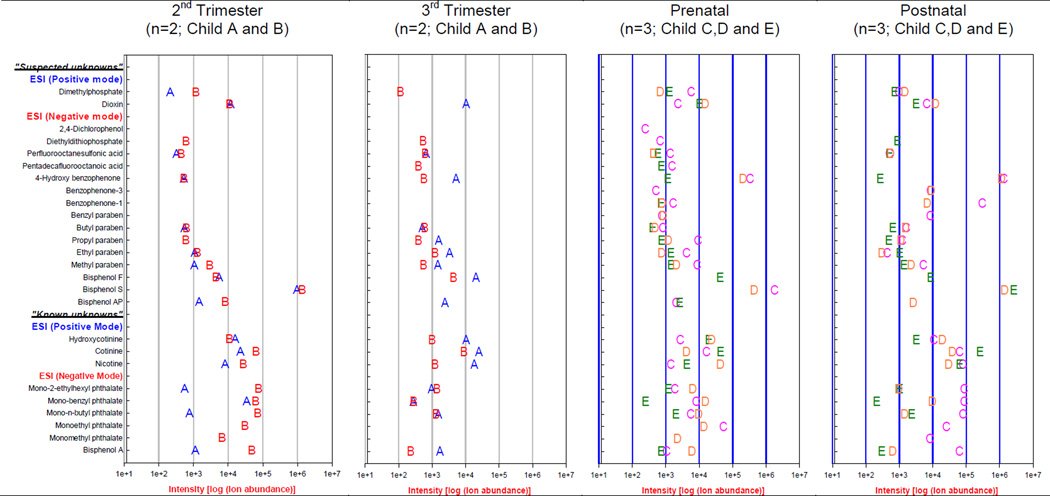
Results from the target analysis for known unknowns and screening for suspected unknowns in both negative and positive ESI mode are presented in Figure 3. Targeted analysis using reference standards identified bisphenol A, five phthalate metabolites (of 13 targeted) and three tobacco metabolites in the majority of time-specific dentine fractions from all five children (Fig. 3). This is amongst the first reports on the presence of bisphenol A in teeth. Moreover, for the first time in the tooth matrix we observed (i) mono-methyl phthalate, mono-ethyl phthalate, mono-butyl phthalate and mono-benzyl phthalate, in addition to mono-ethylhexyl phthalate, and (ii) hydroxycotinine in addition cotinine and nicotine reported in previous studies.(Garcia-Algar and others 2003; Marchei and others 2008; Pascual and others 2003) It is important to note, that neither the oxidative metabolites of di-ethylhexyl phthalate (e.g. mono-2-ethyl-5-hydroxyhexyl phthalate, mono-2-ethyl-5-oxohexyl phthalate) nor higher molecular weight phthalate metabolites (e.g. mono-octyl phthalate, mono-isononyl phthalate) were observed in the study samples. Bisphenol A and phthalates are widely dispersed contaminants in our environment and detection in our samples does not necessarily indicate that only the absorbed fractions have been measured. While we have undertaken precautions, including analyses of method blanks, we agree with concerns recently raised over the routine analyses of these compounds in non-traditional media, (Calafat and others 2013; Wolff and Swan 2010) and are undertaking (i) validation against other established biomarkers (Calafat and Needham 2009), (ii) monitoring oxidative-metabolites as reliable measures of phthalates exposure (Barr D.B and others 2003), (iii) simultaneous analysis of unconjugated BPA (free BPA) and BPA conjugates such as BPA-glucuronide and BPA-sulfate (mono and bi) to assess introduction of BPA from external sources during sample preparation (Vandenberg and others 2014; Volkel and others 2005), and (iv) application of NIST Standard Reference Material 3673 (Organic Contaminants in Non-Smokers’ Urine) as a quality control to assess recovery and contamination of phenol and phthalates metabolites.
Fig. 3.
Intensity and distribution of ‘known unknowns’ and ‘suspected unknown’ compounds in the pre- and postnatal tooth layers of the study children. QTOF-MS was operated in a dual electrospray ionization mode (positive and negative mode).
Furthermore, a suite of chemical contaminants and metabolites were compiled based on literature for prenatal and children’s exposures to environmental factors (e.g.(Bellinger 2013; Gonzalez-Alzaga and others 2014; Lyall and others 2014; Meeker 2012)). This list was used to screen the suspected unknowns using the PCD feature. We observed the following classes of chemicals with abundances varying between developmental time points (Fig. SI-1): structural analogs of bisphenol (Bisphenol S, Bisphenol F, Bisphenol AP), parabens (methyl, ethyl, propyl, butyl and benzyl), UV filters (benzophenone-1 and 3), polyflourinated compounds (pentadecafluorooctanoic acid, perfluorooctanesulfonic acid), and pesticides (diethyl dithiophosphate, dimethylphosphate, 2,4-dichlorophenol). Tandem mass spectrometry (MS/MS) experiments were not performed during this pilot phase and hence confirmation on suspected unknowns is currently unavailable.
Calibration curves and limits of detection were not calculated despite available reference standards for the known unknowns as our goal was not to quantify the targets. However, ion abundance was used as a benchmark to compare the occurrence and intensity of compounds across the tooth layer fractions from the study children. Our results demonstrate the vulnerability of conventional biomarkers, such as single measures of blood and urine, or the use of pulverized whole teeth, to exposure misclassification. For example, bisphenol A ion abundances (counts) in 2nd and 3rd trimester-specific dentine were similar in Child A (112,902 vs 169,687) but not in Child B (4,834,665 vs 23,108). Analysis of a single sample during the prenatal period would not capture the 200 fold higher intensity of bisphenol A in the 2nd compared to 3rd trimester of Child B. Similarly, nicotine ion abundances (counts) in pre- and post-natal specific dentine were 140,694 and 7,825,123 in Child C. Pulverized whole teeth would not capture the 55-fold change in nicotine levels from the pre- to postnatal period in Child C.
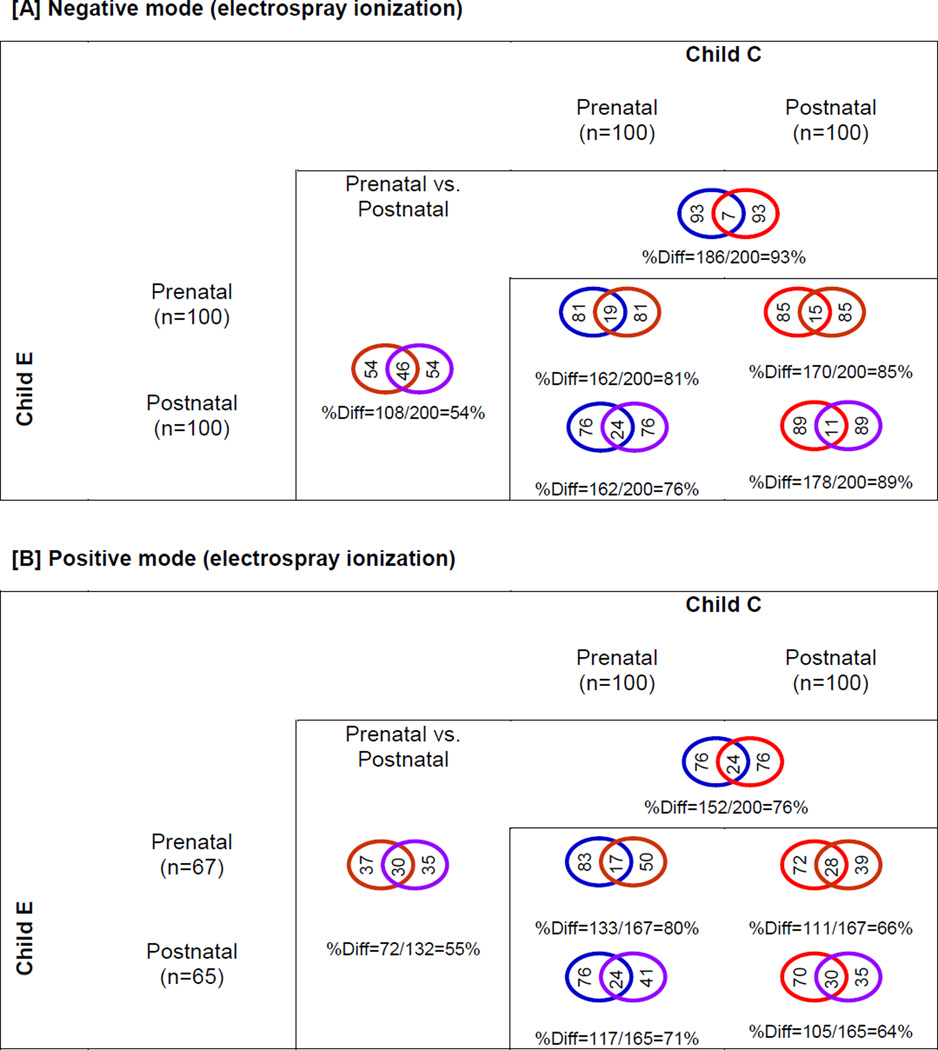
We used established computational methods to identify the compounds from the peaks we detected in our analyses. Molecular Formula Generation (MFG) algorithm available in the MFE was used to generate chemical formulas of the different peaks detected in our analyses based on overall scoring that takes into account the monoisotopic mass (ppm), isotope spacing (ppm) and distribution (%), and the matching of spacing, abundance and compound mass. MassHunter Profinder (MassHunterProfinder 2014) was used to perform BRFE with moderately strict filters, which condense the number of chemical signatures by minimizing false positives and negatives. The 100 most abundant compounds were selected based on ion intensity in each study sample, and comparisons were made for inter- and intra-child variability in nontargeted analysis profiles. Tables SI-2 and SI-3 give the highest prevalence compounds detected in each of the samples analyzed (pre- and post-natal of Child C and E) in negative and positive ESI mode, respectively. Characteristic alteration in metabolites pattern were observed between the pre- and post-natal samples for each child and between children, in both ionization modes (Fig. 4). Percent difference in peaks is graphically represented in Figure 5. For example, percent difference in detection frequency of the top prevalence compounds observed in negative ESI mode between (i) pre- and post-natal dentine was 93% for Child C (186 out of 200 peaks) and 54% for Child E (108/200 peaks), (ii) prenatal dentine of Child C and E was 81% (162/200 peaks), and (iii) postnatal dentine of both children was 89% (178/200 peaks) (Table SI-2, Fig. 5). Similarly, percent difference in detection frequency of the highest prevalence compounds observed in positive ESI mode between (i) pre- and post-natal dentine was 76% for Child C (152/200 peaks) and 55% for Child E (72/132 peaks), (ii) prenatal dentine of Child C and E was 80% (133/167 peaks), and (iii) postnatal dentine of Child C and E was 64% (105/165 peaks) (Table SI-3, Fig. 5). Of the 100 most prevalent compounds across the 4 samples analyzed, only 7% (22/309 peaks) were common in negative mode and only 6% (13/225 peaks) in positive mode in a pool of peaks from pre- and postnatal samples from two children (Table SI-2 and SI-3).
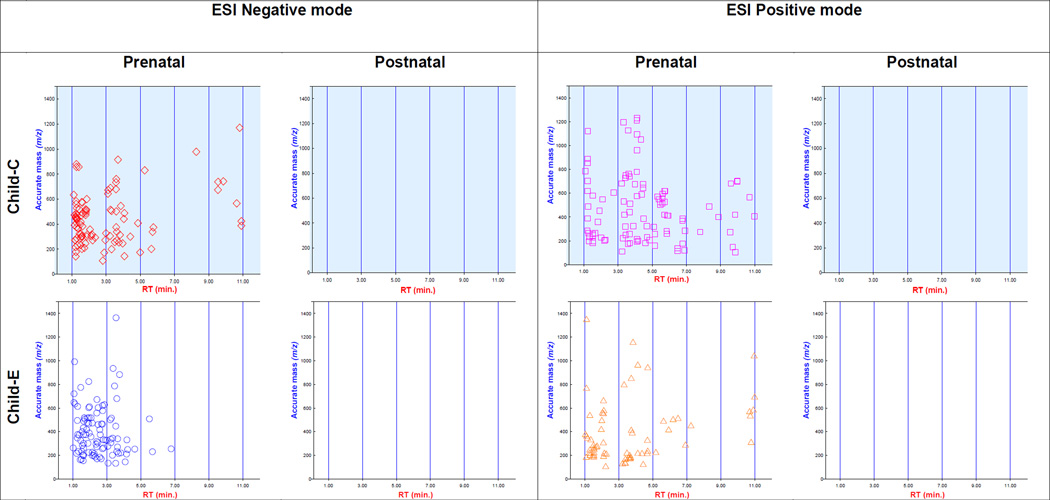
Fig. 4.
Scatter plots of retention time (min.) versus accurate mass (single isotopic m/z) of the top 100 ‘unknown unknowns’ obtained using the Batch Recursive Feature Extraction, and detected in both negative and positive electrospray ionization mode. Data from two children is depicted for graphical representation of chemical composition differences observed within (prenatal versus postnatal) and between (Child C versus E) children.
Fig. 5.
Venn representation of the common and dissimilar peaks among the pre- and post-natal dentine layers of two representative children from the study in both negative [A] and positive [B] electrospray ionization mode. The 100 most prevalent ‘unknown unknowns’ were obtained using the Batch Recursive Feature Extraction. We observed that only a small proportion of peaks were common between pre- and postnatal periods within the same child’s samples (for example, in negative mode, Child C had only 7% common peaks between the pre- and postnatal periods). Furthermore, when comparing the same time periods between children, we saw that only a small proportion of peaks were highly abundant for both children (for example, in negative mode, only 19% of peaks were common for both children during the prenatal period). These data rule out that the predominant compounds we have detected are generic housekeeping components of our matrix.
We grouped compounds with the same base molecular formula (for example C16H12N3O3 and C24H15N4O18 were added to the CxHyNzOa group) and compared the chemical composition of the predominant compounds between samples. Large differences in the base molecular formula detected between negative and positive ESI modes were also observed across the four samples (Table SI-4). For example, CxHyNzOaPj compounds were detected with a 14% frequency across the samples in positive ESI mode but were not detected at all in negative mode, and CxHyNzOaSb compounds were detected with a frequency of 21% in negative ESI mode but only 5% in positive mode. CxHyNzOa compounds occurred with the highest frequency in both ionization modes (i) 38% in prenatal and 51% in postnatal dental layers from Child C, and 50% and 37% from Child E, respectively, in negative mode, and (ii) 30% in prenatal and 40% in postnatal dental layers from Child C, and 36% and 37% from Child E, respectively, in positive mode. Together, these results highlight the differences in chemical signatures between individuals and across developmental times. These data also rule out the possibility that the predominant compounds we have detected are baseline housekeeping components of our matrix that would be present at a higher frequency across all teeth samples.
5. Conclusion and Perspectives
The archival nature of dentine, which captures and preserves important aspects of developmental history, provides an opportunity to retrospectively study health outcomes in response to early life environmental stressors including chemicals. We have shown here and in previous work that a surprisingly large number of chemical signatures can be recovered from teeth ranging from metals to organic compounds some of which have very short half-lives in blood and urine (Arora and others 2014; Arora and others 2012; Arora and others 2004; Arora and others 2011; Austin and others 2013). Dentine, which undergoes very limited remodeling, preserves both the timing and intensity of chemical signatures over the second and third trimesters (conceptual framework summarized in Fig. 2). Taking advantage of this property, we uncovered trimester-specific information for more than 12,000 unique signatures. Most importantly, this was done 7 to 10 years after the exposure event for the 5 teeth we have analyzed (Figs. 3, 4). Similar methods can be applied to permanent/adult teeth for the study of adult disease outcomes as we have recently proposed for adult neurodegeneration such as Parkinson’s disease (Hare and others, in press). An important consideration in case-control studies that collect biospecimens cross-sectionally at the time of clinical assessment is the degree to which the chemical measures in the biological media have been affected by the disease rather than being causally associated with disease onset.
While our discussion here has focused on teeth, there are other biological media, such as placenta and newborn hair, which could provide important information on prenatal exposures (Jin and others 2013; Sakamoto and others 2013; Varrica and others 2014; Wright and others 2006). Other biomonitoring approaches can also contribute to the prenatal exposome. Historical air pollution data as well as satellite-based estimation of particulate matter can be a rich source of information on the external exposome from many years ago (Hyder and others 2014; Kloog and others 2012). It is also important to note the limitations of using teeth for exposure assessment. At present, teeth cannot be used to uncover information on exposures during the first trimester, and because deciduous teeth shed between the ages of 6 to 12 years for most children, on-the-spot sampling is not possible. However, the non-invasive collection and stability at room temperature does offer an advantage when using naturally shed deciduous teeth. As with any biological media, the complex biology of teeth is another important consideration in understanding whether chemical signatures in teeth reflect exposure or some aspect of local tissue-specific metabolism. In this regard, we caution against methods that pulverize whole teeth or fragments of teeth, without regard for the developmental physiology of teeth, and in the process lose temporal exposure information.
Exposure to environmental chemicals during fetal and early childhood periods may alter life-long health trajectories, increasing the risk of conditions that are global priorities including neurodevelopmental disorders, cardiovascular disease and cancer. A major challenge to identifying critical developmental windows of heightened susceptibility is the absence of retrospective biomarkers that objectively measure the timing of environmental chemical exposures. This problem is further compounded when the susceptibility window occurs prenatally and the health outcome under study is uncommon. The development of tools for a comprehensive retrospective temporal exposome that encompasses the prenatal and early childhood periods would be an important advancement in better understanding the early life environmental determinants of long-term health trajectories.
Supplementary Material
Highlights.
Humans are exposed to thousands of chemicals, many of which cross the placenta
Susceptibility to toxicants and their metabolites is heightened during early life
Biomarkers to assess past exposure to environmental chemical mixtures are lacking
We propose to retrospectively reconstruct the exposome at different life stages using teeth
Tooth-matrix biomarkers will advance the study of environmental determinants of health
Acknowledgements
M.A. is supported by grants from the National Institute of Environmental Health Sciences: DP2ES025453 (New Innovator Award), R00ES019597. R.O.W is supported by P30ES023515; R01ES013744; R01ES021357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors declare no competing financial interests.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and biobehavioral reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arora M, Austin C, Sarrafpour B, Hernandez-Avila M, Hu H, Wright RO, Tellez-Rojo MM. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PloS one. 2014;9:e97805. doi: 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, Smith DR. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46:5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Chan SW, Kennedy BJ, Sharma A, Crisante D, Walker DM. Spatial distribution of lead in the roots of human primary teeth. J Trace Elem Med Biol. 2004;18:135–139. doi: 10.1016/j.jtemb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409:1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, Bishop D, Hare DJ, Doble P, Eskenazi B, Arora M. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498:216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward LL, Hays SM, Kirman CR, Marchitti SA, Kenneke JF, English C, Mattison DR, Becker RA. Relationships of chemical concentrations in maternal and cord blood: a review of available data. Journal of toxicology and environmental health Part B, Critical reviews. 2014;17:175–203. doi: 10.1080/10937404.2014.884956. [DOI] [PubMed] [Google Scholar]
- Barr DB DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environmental Health Perspectives. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA. Cellular aspects of brain development. Neurotoxicology. 1989;10:307–320. [PubMed] [Google Scholar]
- Bellinger DC. Prenatal Exposures to Environmental Chemicals and Children's Neurodevelopment: An Update. Safety and health at work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BKB Berkovitz GH, Moxham BJ. Oral Anatomy, Histology and Embryology. London: Elsevier; 2009. [Google Scholar]
- Blackwell A, Aja S, Zhou W, Graham D, Ronnett GV. Agilent Technologies. Inc Technical Overview 5991-3528EN; 2013. Multi-omics compatible protocols for preparation and extraction of biological samples for wide coverage in untargeted metabolomics experiments. [Google Scholar]
- Bressler J, Kim KA, Chakraborti T, Goldstein G. Molecular mechanisms of lead neurotoxicity. Neurochem Res. 1999;24:595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- Broecker S, Herre S, Pragst F, Kuhlmann F, Wust B, Zweigenbaum J, Kuhlmann F, Stone P, Imatani K. Agilent Technologies. Inc Application Note 5990-6419EN; 2010. Toxicological screening with the Agilent 6500 series accurate-mass Q-TOF LC/MS and the Personal Compound Database and Library using the Broecker, Herre and Pragst Accurate Mass Spectral Library. [Google Scholar]
- Calafat AM, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Longnecker MP, Rudel RA, Teitelbaum SL, Whyatt RM, Wolff MS. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast cancer research : BCR. 2013;15:403. doi: 10.1186/bcr3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Needham LL. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environmental Health Perspectives. 2009;117:1481–1485. doi: 10.1289/ehp.0901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Hubal EA, de Wet T, Du Toit L, Firestone MP, Ruchirawat M, van Engelen J, Vickers C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: results of a World Health Organization review. Regulatory toxicology and pharmacology : RTP. 2014;69:113–124. doi: 10.1016/j.yrtph.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke GM. Biomonitoring of human fetal exposure to environmental chemicals in early pregnancy. Journal of toxicology and environmental health Part B, Critical reviews. 2014;17:205–224. doi: 10.1080/10937404.2014.898167. [DOI] [PubMed] [Google Scholar]
- Delvaux I, Van Cauwenberghe J, Den Hond E, Schoeters G, Govarts E, Nelen V, Baeyens W, Van Larebeke N, Sioen I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res. 2014;132:24–32. doi: 10.1016/j.envres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying Children's susceptibility to environmental toxicants. Environ Health Perspect. 2000;108(Suppl 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Algar O, Vall O, Segura J, Pascual JA, Diaz D, Mutnoz L, Zuccaro P, Pacifici R, Pichini S. Nicotine concentrations in deciduous teeth and cumulative exposure to tobacco smoke during childhood. JAMA. 2003;290:196–197. doi: 10.1001/jama.290.2.196. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B, Lacasana M, Aguilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, Hernandez AF. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicology letters. 2014;230:104–121. doi: 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2.5 exposure and birth outcomes: use of satellite- and monitor-based data. Epidemiology. 2014;25:58–67. doi: 10.1097/EDE.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S, Fischer SM, Sana TR. Agilent Technologies. Inc Application Note 5991-2470EN; 2013. Compound identification, profiling and pathway analysis of the Yeast metabolome in Mass Profiler Professional. [Google Scholar]
- Jin L, Zhang L, Li Z, Liu JM, Ye R, Ren A. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reproductive toxicology. 2013;35:25–31. doi: 10.1016/j.reprotox.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Kloog I, Nordio F, Coull BA, Schwartz J. Incorporating local land use regression and satellite aerosol optical depth in a hybrid model of spatiotemporal PM2.5 exposures in the Mid-Atlantic states. Environ Sci Technol. 2012;46:11913–11921. doi: 10.1021/es302673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(Suppl 1):106–114. doi: 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann F, Stone P, Imatani K. Agilent Technologies. Inc Technical Overview 5990-4828EN; 2009a. Increased productivity for target compound screening using TOF-MS and accurate-mass databases with optional retention time. Part 1: Unique Capabilities of Agilent Software. [Google Scholar]
- Kuhlmann F, Stone P, Imatani K. Agilent Technologies. Inc Technical Overview 5990-4829EN; 2009b. Increased productivity for target compound screening using TOF-MS and accurate-mass databases with optional retention time. Part 2: Integrated, Automated Workflows. [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, Hsieh WS, Jeng SF, Su YN, Chen PC. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Little JL, Williams AJ, Pshenichnov A, Tkachenko V. Identification of "known unknowns" utilizing accurate mass data and ChemSpider. Journal of the American Society for Mass Spectrometry. 2012;23:179–185. doi: 10.1007/s13361-011-0265-y. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. Journal of exposure science & environmental epidemiology. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International journal of epidemiology. 2014;43:443–464. doi: 10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manciocco A, Calamandrei G, Alleva E. Global warming and environmental contaminants in aquatic organisms: the need of the etho-toxicology approach. Chemosphere. 2014;100:1–7. doi: 10.1016/j.chemosphere.2013.12.072. [DOI] [PubMed] [Google Scholar]
- Marchei E, Joya X, Garcia-Algar O, Vall O, Pacifici R, Pichini S. Ultrasensitive detection of nicotine and cotinine in teeth by high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2609–2612. doi: 10.1002/rcm.3636. [DOI] [PubMed] [Google Scholar]
- MassHunterProfinder. Agilent Technologies. Inc Technical Overview 5991-3947EN; 2014. MassHunter Profinder: Batch processing software for high quality feature extraction of mass spectrometry data. [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD. Exposure to environmental endocrine disruptors and child development. Archives of pediatrics & adolescent medicine. 2012;166:E1–E7. doi: 10.1001/archpediatrics.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele E. Agilent Technologies. Inc Application Note 5990-9112EN; 2011. Agilent 1290 Infinity LC: The ideal partner for MS – Part 5 Improved mass accuracy by enhanced separation of compounds of isobaric mass using Agilent 1290 Infinity LC technology. [Google Scholar]
- Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000;108(Suppl 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual JA, Diaz D, Segura J, Garcia-Algar O, Vall O, Zuccaro P, Pacifici R, Pichini S. A simple and reliable method for the determination of nicotine and cotinine in teeth by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:2853–2855. doi: 10.1002/rcm.1279. [DOI] [PubMed] [Google Scholar]
- Rappaport SM. Implications of the exposome for exposure science. Journal of exposure science & environmental epidemiology. 2011;21:5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- Sabel N, Johansson C, Kuhnisch J, Robertson A, Steiniger F, Noren JG, Klingberg G, Nietzsche S. Neonatal lines in the enamel of primary teeth--a morphological and scanning electron microscopic investigation. Arch Oral Biol. 2008;53:954–963. doi: 10.1016/j.archoralbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Yasutake A, Domingo JL, Chan HM, Kubota M, Murata K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: potential use as indicators for prenatal exposure. Environment international. 2013;60:106–111. doi: 10.1016/j.envint.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma BM, Bharat GK, Tayal S, Nizzetto L, Cupr P, Larssen T. Environment and human exposure to persistent organic pollutants (POPs) in India: a systematic review of recent and historical data. Environment international. 2014;66:48–64. doi: 10.1016/j.envint.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res. 2004;95:119–125. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Gerona RR, Kannan K, Taylor JA, van Breemen RB, Dickenson CA, Liao C, Yuan Y, Newbold RR, Padmanabhan V, Vom Saal FS, Woodruff TJ. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environmental health : a global access science source. 2014;13:25. doi: 10.1186/1476-069X-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrica D, Tamburo E, Milia N, Vallascas E, Cortimiglia V, De Giudici G, Dongarra G, Sanna E, Monna F, Losno R. Metals and metalloids in hair samples of children living near the abandoned mine sites of Sulcis-Inglesiente (Sardinia, Italy) Environ Res. 2014;134:366–374. doi: 10.1016/j.envres.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Volkel W, Bittner N, Dekant W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug metabolism and disposition: the biological fate of chemicals. 2005;33:1748–1757. doi: 10.1124/dmd.105.005454. [DOI] [PubMed] [Google Scholar]
- Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69:876–878. doi: 10.1136/thoraxjnl-2013-204949. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzon RM, Silva CS, Siliceo J. Organochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum, and milk from inhabitants of Veracruz, Mexico. Arch Environ Contam Toxicol. 2001;40:432–438. doi: 10.1007/s002440010194. [DOI] [PubMed] [Google Scholar]
- Wild CP. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Wild CP. The exposome: from concept to utility. International journal of epidemiology. 2012;41:24–32. doi: 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Swan SH. Phthalate biomarkers in pediatric research. Pediatrics. 2010 [Google Scholar]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Zamboni N, Fischer SM. Agilent Technologies. Inc Application Note 5990-9762EN; 2012. High-throughput, high-efficiency metabolome profiling using the Agilent 6550 iFunnel Q-TOF LC/MS system. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.