Abstract
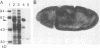
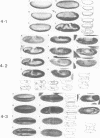
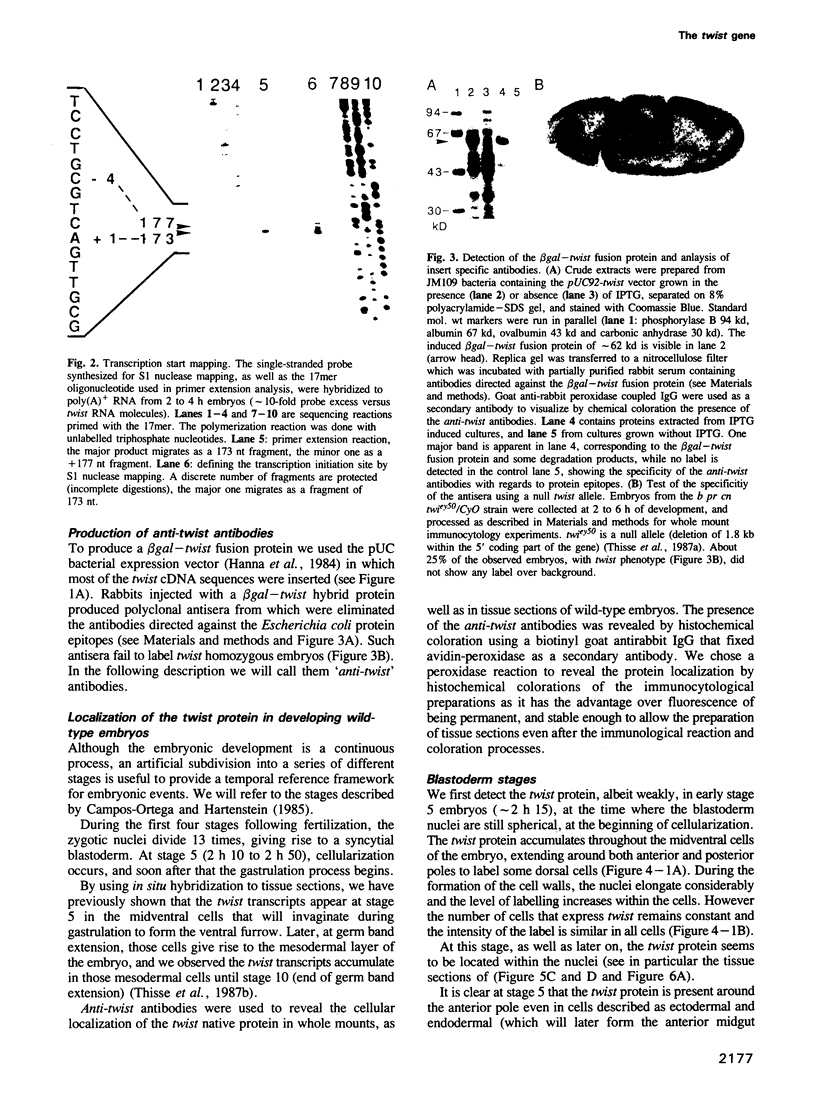
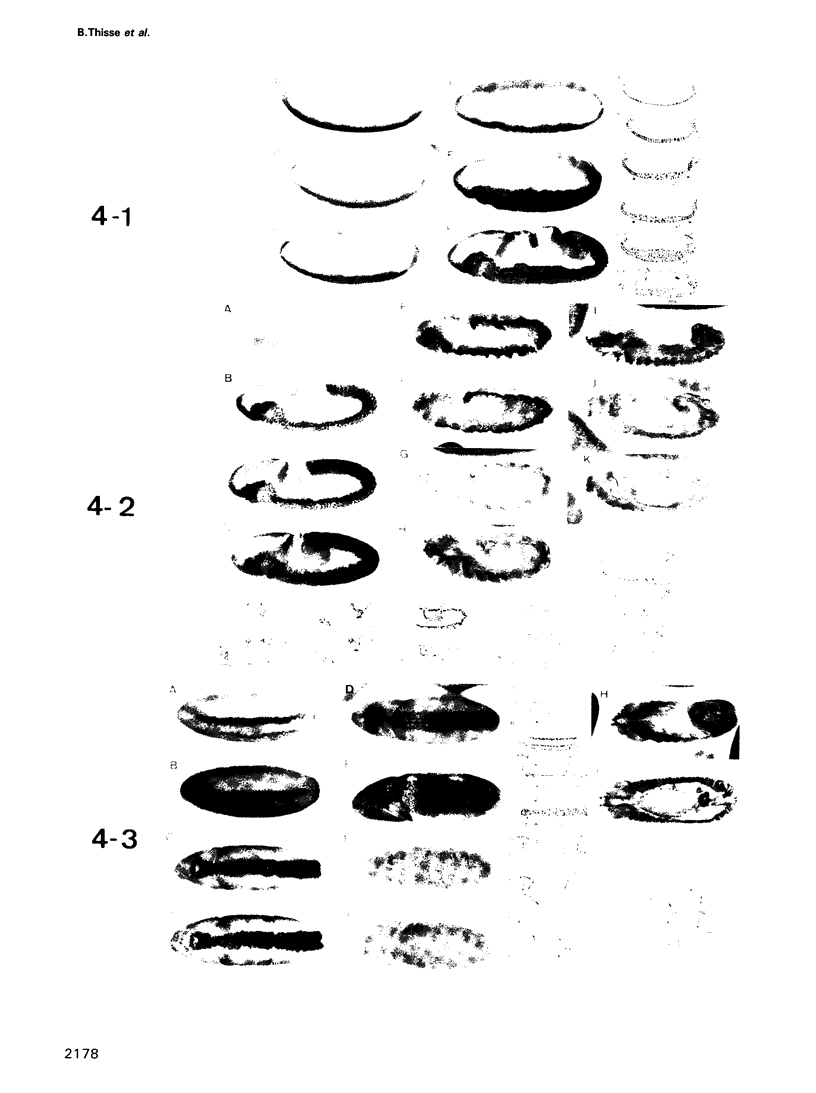
The twist gene is involved in the establishment of germ layers in Drosophila embryos: twist homozygous mutant embryos fail to form the ventral furrow at gastrulation and lack mesoderm and all internal organs. We have determined the sequence of the twist gene, that contains 'CAX' repeats in its 5' moiety, and codes for a protein of 490 amino acids. We have raised anti-twist antibodies that were used to study the distribution of the twist protein in whole mounts and tissue sections of wild-type embryos. Twist protein appears to be a nuclear protein at all developmental stages. It is present over both poles and in the midventral region (endoderm and mesoderm anlagen) at cellular blastoderm stage; later in development, it is detected within the mesodermal layer until its differentiation into somatopleura and splanchnopleura in which some cells are still labelled by anti-twist antibodies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogaert T., Brown N., Wilcox M. The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987 Dec 24;51(6):929–940. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carrel S., Gerber H., Barandun S. Preparation of polyacrylamide gels which contain protein and their use as high capacity immunosorbents. Nature. 1969 Jan 25;221(5178):385–386. doi: 10.1038/221385a0. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle H. J., Harding K., Hoey T., Levine M. Transcripts encoded by a homoeo box gene are restricted to dorsal tissues of Drosophila embryos. Nature. 1986 Sep 4;323(6083):76–79. doi: 10.1038/323076a0. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Zerges W., Schedl P., Jarry B. P., Vergis W. Molecular organization of the rudimentary gene of Drosophila melanogaster. J Mol Biol. 1986 May 5;189(1):25–36. doi: 10.1016/0022-2836(86)90378-5. [DOI] [PubMed] [Google Scholar]
- Gaul U., Seifert E., Schuh R., Jäckle H. Analysis of Krüppel protein distribution during early Drosophila development reveals posttranscriptional regulation. Cell. 1987 Aug 14;50(4):639–647. doi: 10.1016/0092-8674(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M., Goodman W. Identification in transgenic animals of the Drosophila decapentaplegic sequences required for embryonic dorsal pattern formation. Genes Dev. 1987 Aug;1(6):615–625. doi: 10.1101/gad.1.6.615. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Irish V. F., Gelbart W. M. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1987 Oct;1(8):868–879. doi: 10.1101/gad.1.8.868. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., Poole S. J., Wright D. K., O'Farrell P. H. Sequence conservation in the protein coding and intron regions of the engrailed transcription unit. EMBO J. 1986 Dec 20;5(13):3583–3589. doi: 10.1002/j.1460-2075.1986.tb04686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Dietrich U., Tepass U., Bremer K. A., Weigel D., Vässin H., Campos-Ortega J. A. EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J. 1987 Mar;6(3):761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Merrill D. A., Naughton M. A., Geczy C. Letter: Acrylamide affinity chromatography for immunohistochemistry. Purification of specific antibodies. J Histochem Cytochem. 1975 Feb;23(2):146–148. doi: 10.1177/23.2.46878. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Sedat J. Localization of antigenic determinants in whole Drosophila embryos. Dev Biol. 1983 Sep;99(1):261–264. doi: 10.1016/0012-1606(83)90275-0. [DOI] [PubMed] [Google Scholar]
- Naidet C., Sémériva M., Yamada K. M., Thiery J. P. Peptides containing the cell-attachment recognition signal Arg-Gly-Asp prevent gastrulation in Drosophila embryos. Nature. 1987 Jan 22;325(6102):348–350. doi: 10.1038/325348a0. [DOI] [PubMed] [Google Scholar]
- Regulski M., Harding K., Kostriken R., Karch F., Levine M., McGinnis W. Homeo box genes of the Antennapedia and bithorax complexes of Drosophila. Cell. 1985 Nov;43(1):71–80. doi: 10.1016/0092-8674(85)90013-3. [DOI] [PubMed] [Google Scholar]
- Roussel G., Neskovic N. M., Trifilieff E., Artault J. C., Nussbaum J. L. Arrest of proteolipid transport through the Golgi apparatus in Jimpy brain. J Neurocytol. 1987 Apr;16(2):195–204. doi: 10.1007/BF01795303. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Doyle H., Hoey T., Levine M. Molecular characterization of the zerknüllt region of the Antennapedia gene complex in Drosophila. Genes Dev. 1987 Dec;1(10):1268–1279. doi: 10.1101/gad.1.10.1268. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Frasch M., Doyle H., Levine M. Maternal regulation of zerknüllt: a homoeobox gene controlling differentiation of dorsal tissues in Drosophila. Nature. 1987 Dec 10;330(6148):583–586. doi: 10.1038/330583a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Maternal-Zygotic Gene Interactions during Formation of the Dorsoventral Pattern in Drosophila Embryos. Genetics. 1983 Nov;105(3):615–632. doi: 10.1093/genetics/105.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston R. D., Gelbart W. M. Decapentaplegic transcripts are localized along the dorsal-ventral axis of the Drosophila embryo. EMBO J. 1987 Sep;6(9):2785–2791. doi: 10.1002/j.1460-2075.1987.tb02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wirz J., Fessler L. I., Gehring W. J. Localization of the Antennapedia protein in Drosophila embryos and imaginal discs. EMBO J. 1986 Dec 1;5(12):3327–3334. doi: 10.1002/j.1460-2075.1986.tb04647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zusman S. B., Wieschaus E. F. Requirements for zygotic gene activity during gastrulation in Drosophila melanogaster. Dev Biol. 1985 Oct;111(2):359–371. doi: 10.1016/0012-1606(85)90490-7. [DOI] [PubMed] [Google Scholar]