Abstract
Objectives
Characterize longitudinal changes in the use of medical care in adult survivors of childhood cancer.
Data Sources
The Childhood Cancer Survivor Study, a retrospective cohort study of 5+ year survivors of childhood cancer.
Study Design
Medical care was assessed at entry into the cohort (baseline) and at most recent questionnaire completion. Care at each time point was classified as no care, general care, or survivor-focused care.
Data Collection
There were 6,176 eligible survivors. Multivariable models evaluated risk factors for reporting survivor-focused care or general medical care at baseline and no care at follow-up; and survivor-focused care at baseline and general care at follow-up.
Principal Findings
Males (RR, 2.3; 95 percent CI 1.8–2.9), earning <$20,000/year (RR, 1.6; 95 percent CI 1.2–2.3) or ≤high school education (RR, 2.5; 95 percent CI 1.6–3.8 and RR 2.0; 95 percent CI 1.5–2.7 for <high school and high school, respectively) were associated with no care at follow-up. Survivors with severe or life-threatening conditions at baseline (RR 0.5; 95 percent CI 0.3–0.6) were less likely to report no care at follow-up.
Conclusions
While the incidence of late effects increases over time for survivors, the likelihood of receiving survivor-focused care decreases for vulnerable populations.
Keywords: Childhood cancer survivors, health insurance, health care access, survivorship, delivery of health care
The improvement in long-term survival of children with cancer to over 83 percent is one of the major success stories in oncology (Howlader et al. 2013). Consequently, there are more than 360,000 childhood cancer survivors alive in the United States, two-thirds of whom will develop a chronic health problem (late effect) as a result of their cancer and/or its therapy (Hudson et al. 2003; Oeffinger et al. 2006; Geenen et al. 2007; Mariotto et al. 2009; Armstrong et al. 2014; Cox et al. 2014). These late effects include a variety of physical, psychological, and social conditions that result in excess early morbidity and a risk for premature mortality compared to age-matched norms in the general adult population (Zebrack et al. 2002; Wenzel et al. 2005; Gurney et al. 2009; Meadows et al. 2009; Friedman et al. 2010). The Institute of Medicine has advocated that cancer survivors receive lifelong medical care targeted at surveillance, prevention, and treatment of late effects (Hewitt, Weiner, and Simone 2003). We have previously defined the construct of regular medical care with targeted screening, prevention, and treatment of late effects as “survivor-focused care” (Nathan et al. 2008). Receipt of survivor-focused care provides opportunities for early detection and intervention to preserve health. As the cumulative risk for late effects increases as survivors age, an understanding of the factors that influence utilization of medical care over time is necessary to inform strategies that ensure lifelong receipt of survivor-focused care (Armstrong et al. 2009; Mulrooney et al. 2009; Nottage et al. 2011).
A previous study of 8,522 adult survivors of childhood cancer enrolled in the Childhood Cancer Survivor Study (CCSS) revealed that although most survivors (89 percent) reported at least one medical visit within the 2 years preceding the survey (completed in 2002–2003), less than one-third of survivors reported a survivor-focused visit (Nathan et al. 2008). Since that survey, several factors were expected to increase the proportion of survivors who receive survivor-focused care. First, because the risk for late effects increases over time, it is likely that survivors with emerging health problems would have increased medical care utilization (American Academy of Pediatrics Section on Hematology/Oncology, and Children’s Oncology Group’s 2009). Second, in 2003, the Children’s Oncology Group (COG) published guidelines that provided recommendations for the care of long-term survivors of childhood cancer (Landier et al. 2004). These guidelines recommend an annual survivor-focused follow-up visit. The COG guidelines were developed as a resource for clinicians who provide long-term care for childhood, adolescent, and young adult cancer survivors and include complementary patient education materials called “Health Links,” which were expected to impact health care utilization by increasing survivors’ knowledge and awareness of their risks. However, prior cross-sectional studies in the CCSS cohort suggest that survivor-focused medical visits decrease with increasing time from completion of cancer therapy (Oeffinger et al. 2004). In essence, as risk for late effects increases, survivor-focused care appears to decrease. These earlier cross-sectional studies did not allow for identification of factors that predict whether survivors will receive optimal or suboptimal medical care as they age. Knowledge of the factors that predict a decrease in the level of medical care over time is critical as survivors’ risk for morbidity and mortality increases as they age. Identification of these risk factors will inform the development and implementation of targeted interventions to address disparities in care.
The purpose of the present study was to characterize longitudinal changes in the use of survivor-focused care in a cohort of adult survivors of childhood cancer enrolled in the CCSS and to explore predictors of decreases in the level of medical care utilized by survivors over time.
Methods
Childhood Cancer Survivor Study
The methodology and characteristics of participants in the CCSS have been published previously (Robison et al. 2002, 2009; Leisenring et al. 2009). Briefly, the CCSS is a multi-institutional study of individuals who were diagnosed with cancer prior to 21 years, treated at one of 26 collaborating institutions in the United States and Canada between January 1, 1970, and December 31, 1986, and survived ≥5 years after diagnosis. The Institutional Review Board at all participating institutions approved the study. Informed consent was obtained from each participant.
Survivors were eligible if they had completed the “baseline” CCSS questionnaire administered between 10/1992–12/2002 and at least one follow-up questionnaire (the “2003” questionnaire which was administered between 11/2002–4/2005 and/or the “2007” questionnaire which was administered between 7/2007–11/2009). We excluded subjects who were <18 years at the time of the baseline questionnaire, had incomplete medical care or treatment records, were diagnosed with a second malignant neoplasm, or were deceased at last contact. We analyzed data regarding medical care utilization from the baseline questionnaire and the most recently completed subsequent questionnaire.
Primary Outcome Measures
Levels of Medical Care
The CCSS questionnaires include items that assess the medical care utilized by survivors within the 2 years preceding questionnaire administration. As previously reported, “participants were asked whether they had visited a health care provider (physician or nurse) within the preceding 2 years, whether the visit was related to their previous cancer, and whether their health care provider had given them advice on how to reduce their risks or discussed or ordered screening tests for cancer-related sequelae” (Nathan et al. 2008). Responses to these questions were used to categorize health care into one of three hierarchical and mutually exclusive groups: (1) no health care; (2) general medical care (defined as ≥1 medical visits to a health care provider [e.g., physician, nurse], none of which focused on their previous cancer or surveillance strategies or prevention of late effects); or (3) survivor-focused care (defined as a medical visit related to the prior cancer, or one at which the survivor was counseled about how to reduce risks for late effects or had surveillance tests ordered or discussed). The type of care received was classified independent of provider or the location of care. Study questionnaires are available at http://ccss.stjude.org.
Decrease in Levels of Medical Care
Participants’ level of medical care was categorized at two time points—baseline and at the last completed questionnaire. We defined two categories of decreased care: (1) a survivor who reported survivor-focused care or general medical care at baseline but no care at follow-up; or (2) a survivor who reported survivor-focused care at baseline but general care at follow-up.
Predictors of Decreases in the Level of Medical Care
Demographic and Treatment Variables
Sociodemographic data (gender, income, health insurance, education, and employment status) were obtained from the baseline and most recently completed questionnaire. Health insurance was classified as U.S. insured, Canadian resident, or no health insurance. Based on self-reported race/ethnicity, participants were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. Cancer diagnosis and treatment variables were abstracted from medical records.
Baseline Chronic Health Conditions and Health Status
We determined the prevalence and severity of chronic health conditions at baseline to assess the influence of chronic health conditions on health care utilization over time. As previously described, the severity of each chronic health condition was classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) as mild (grade 1), moderate (grade 2), severe (grade 3), or life-threatening or disabling (grade 4) (Oeffinger et al. 2006). Health status was assessed at baseline using a previously published set of domains (general health, emotional health; Derogatis 2000; Hudson et al. 2003), physical functioning (Ware and Sherbourne 1992), cancer-related pain, cancer-related anxiety, and fear (Hudson et al. 2003).
Statistical Analysis
We generated descriptive statistics for each of the sociodemographic, cancer diagnosis and treatment, chronic health conditions, health status, and levels of medical care variables at baseline and follow-up. Time-dependent sociodemographic factors were compared between baseline and the last follow-up. Statistical significance was evaluated using a bootstrap technique that takes into account the within-subject correlation between two time points (Efron and Ibshirani 1993). We examined decreased levels of survivor-focused medical care between baseline and the most recent follow-up in two ways. First, among survivors who reported survivor-focused or general care at baseline, we determined the proportion that reported no care at follow-up. Second, among those who reported survivor-focused care at baseline, we determined the proportion that reported general care at follow-up. A backward variable-selection method was employed to build separate summary models describing the independent and simultaneous associations of decreased care with baseline demographic and clinical factors. Multivariable logistic regression models were used to estimate the association between the variables in the final model with the decreased care over time (Savu, Liu, and Yasui 2010). Associations were quantified as odds ratios (OR) with corresponding large-sample 95 percent confidence intervals (CI). We conducted sensitivity analyses using the inverse probability weighting (IPW) technique to evaluate the potential for bias due to nonparticipation (Little and Rubin 2002). Although IPW results differed very little from unadjusted results, the IPW-adjusted results are presented. Statistical analyses were performed using SAS Version 9.2 (SAS Institute 1996). Two-sided statistical inferences were employed throughout the analyses.
Results
Of the 14,358 childhood cancer survivors who completed the baseline CCSS questionnaire, 7380 were eligible and 6,176 were included in the final analysis (see consort diagram in Figure1). We found statistically significant differences between those survivors who were eligible for inclusion in the analysis and the nonparticipants, which included the 2,342 who did not complete any follow-up questionnaire (either the 2003 or 2007 CCSS questionnaire) and the 727 survivors who had no medical records. Nonparticipants were more likely to be male, greater than 20 years from diagnosis, non-Hispanic black or Hispanic, less educated, lower income, and uninsured.
Figure 1.
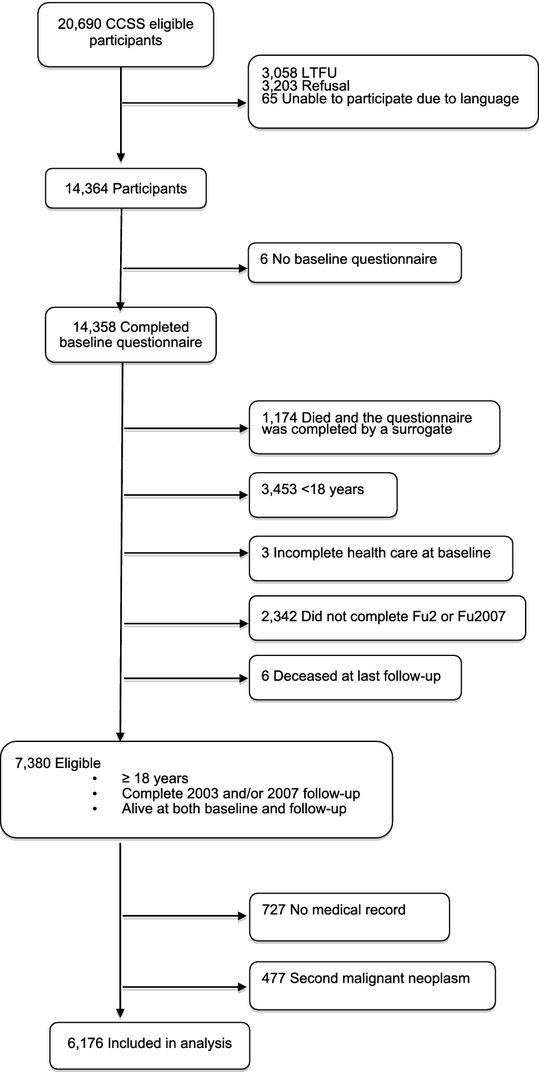
Consort Diagram of Eligible Participants
Table1 displays the diagnostic, treatment, and baseline sociodemographic characteristics of the participants. The most recently completed CCSS questionnaire was the 2007 questionnaire by 5,076 (82.2 percent) survivors and the 2003 questionnaire by 1,100 (17.8 percent) survivors. The mean time between the baseline and most recently completed questionnaire was 11.5 years (SD = 2.2) and the median time was 12.3 years (interquartile range = 10.3–13.0). A significant increase was found for the time-dependent characteristic of being insured in the United States (an increase from 80.2 to 83.7 percent, p < .001).
Table 1.
Cohort Characteristics at Baseline and Most Recent Follow-Up Questionnaire (2003 or 2007) (n = 6,176)
| Baseline Questionnaire | Most Recent Questionnaire | p-value* | |
|---|---|---|---|
| Age at questionnaire | |||
| Mean ± SD | 27.1 ± 6.0 | 38.6 ± 6.2 | |
| Median | 26.3 | 38.0 | |
| Range | 18.0–48.0 | 23.5–58.9 | |
| Age at diagnosis | |||
| Mean ± SD | 9.9 ± 5.6 | ||
| Gender, N (%) | |||
| Male | 3,227 (52.3) | ||
| Female | 2,949 (47.7) | ||
| Race/Ethnicity, N (%) | |||
| Non-Hispanic white | 5,592 (90.8) | ||
| Non-Hispanic black | 162 (2.6) | ||
| Hispanic | 246 (4.0) | ||
| Other | 156 (2.5) | ||
| Health insurance status, N (%) | |||
| Yes, United States | 4,875 (80.2) | 5,157 (83.7) | <.001 |
| No, United States | 859 (14.1) | 621 (10.1) | |
| Canadian resident | 348 (5.7) | 384 (6.2) | |
| Current annual household income, N (%) | |||
| <$20,000 | 1,159 (20.7) | 659 (11.4) | <.001 |
| $20,000–$39,999 | 1,668 (29.8) | 1,065 (18.4) | |
| $40,000–$59,999 | 1,254 (22.4) | 1,069 (18.4) | |
| $60,000+ | 1,519 (27.1) | 3,009 (51.9) | |
| Education (highest level of attainment), N (%) | |||
| <High school | 523 (8.9) | 198 (3.2) | <.001 |
| High school | 3,328 (56.9) | 2,731 (44.3) | |
| College graduate | 2,000 (34.2) | 3,234 (52.5) | |
| Current employment status, N (%) | |||
| Employed | 5,379 (88.2) | 4,721 (76.5) | <.001 |
| Unemployed | 723 (11.8) | 1,451 (23.5) | |
| Cancer diagnosis, N (%) | |||
| Leukemia | 1,915 (31.0) | ||
| CNS tumor | 794 (12.9) | ||
| Hodgkin lymphoma | 960 (15.5) | ||
| Non-Hodgkin lymphoma | 559 (9.1) | ||
| Wilms tumor | 431 (7.0) | ||
| Neuroblastoma | 248 (4.0) | ||
| Sarcoma | 604 (9.8) | ||
| Bone tumor | 665 (10.8.) | ||
| Radiation therapy (RT), N (%) | |||
| Both brain and chest | 113 (1.8) | ||
| Brain only | 1,889 (30.6) | ||
| Chest only | 1,188 (19.2) | ||
| RT, but not brain or chest | 1,019 (16.5) | ||
| RT, but brain/chest RT status unknown | 128 (2.1) | ||
| No RT | 1,839 (29.8) | ||
| Cardiotoxic therapies, N (%) | |||
| Anthracyclines, no chest RT | 1,793 (29.0) | ||
| Chest RT, no anthracyclines | 868 (14.1) | ||
| Anthracyclines + chest RT | 432 (7.0) | ||
| No anthracyclines, no chest RT | 2,950 (47.8) | ||
| Missing | 133 (2.2) | ||
| Alkylating agent dose, N (%) | |||
| None | 3,043 (49.3) | ||
| First tertile | 1,166 (18.9) | ||
| Second tertile | 852 (13.8) | ||
| Third tertile | 560 (9.1) | ||
| Missing | 555 (9.0) | ||
p-value for comparison of distribution between baseline and most recent questionnaire.
These data demonstrated a series of patterns in the receipt of longitudinal medical care. At baseline, of the 6,176 survivors included in the analysis, 696 (IPW proportion: 12.2 percent) reported no medical care, 2,965 (IPW proportion: 47.5 percent) reported general medical care, and 2,515 (IPW proportion: 40.3 percent) reported survivor-focused care. At their most recent follow-up, 473 (IPW proportion: 8.2 percent) reported no medical care, 3,840 (IPW proportion: 61.6 percent) reported general medical care, and 1,863 (IPW proportion: 30.2 percent) reported survivor-focused care. Figure2 displays the distribution of survivors who reported each level of care at their most recent follow-up, stratified by their level of care at baseline. Of the 5,480 participants who reported survivor-focused or general care at baseline, 348 (IPW proportion: 6.9 percent) reported no care at their most recent follow-up. Of the 2,515 survivors who reported survivor-focused care at baseline, 1,396 (IPW proportion: 55.6 percent) reported a lower level of care at follow-up, 1,270 (IPW proportion: 50.1 percent) reported general care, and 126 (IPW proportion: 5.5 percent) reported no care.
Figure 2.
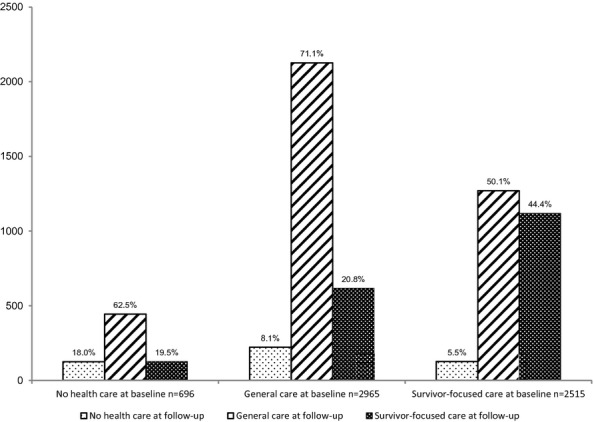
Number of Survivors Reporting Each Level of Care at Follow-Up Stratified by Level of Care at Baseline
Table2 presents the final multivariate model describing the association between baseline factors and the likelihood that survivors who reported some form of medical care at baseline (either survivor-focused or general) would report no medical care at follow-up. Results adjusted for potential bias due to differential nonparticipation using the IPW method are presented here and in Table3. Among survivors, male sex, an annual household income of <$20,000/year, and ≤high school education at baseline were independently associated with an increased risk of reporting no care at follow-up. In contrast, survivors who reported any chronic health condition at baseline were less likely to report no care at follow-up.
Table 2.
Multivariable Model of the Likelihood of Reporting a Decrease from Survivor-Focused or General Care Baseline to No Care at Most Recent Follow-Up*,†
| Baseline Predictor Variables | OR§ (95% CI) | p-value |
|---|---|---|
| Gender | ||
| Male | 2.3 (1.8–2.9) | <.001 |
| Female | Ref | |
| Race/ethnicity | ||
| Non-Hispanic white | Ref | |
| Non-Hispanic black | 1.6 (0.9–2.9) | .14 |
| Hispanic | 1.3 (0.7–2.2) | .40 |
| Other | 2.1 (1.2–3.7) | .01 |
| Current annual household income | ||
| <$20,000 | 1.6 (1.2–2.3) | .005 |
| $20,000–$39,999 | 1.4 (1.0–1.9) | .07 |
| $40,000–$59,999 | 1.0 (0.7–1.4) | .84 |
| $60,000+ | Ref | |
| Chronic disease status‡ | ||
| Grade 3–4 | 0.5 (0.3–0.6) | <.001 |
| Grade 1–2 | 0.7 (0.5–0.9) | .004 |
| Grade 0 | Ref | |
| Education | ||
| <High school | 2.5 (1.6–3.8) | <.001 |
| High school | 2.0 (1.5–2.7) | <.001 |
| College graduate | Ref | |
n = 2,965 general care at baseline, 2,515 survivor-focused care at baseline; among them 348 became no care.
Age at diagnosis, years since diagnosis, gender, race, insurance, income, education, ever had a job, general health, anxiety due to cancer, physical limitation, cancer pain, emotional health, chronic disease status, RT status, alkylating agent score, and anthracycline dose are all candidates of the model. Backward selection resulted in a model containing only the variables shown in the table.
At baseline, the severity of chronic health conditions reported by participants was scored according to NCI Common Terminology Criteria of Adverse Events version 3.
Results from analyses were adjusted using IPW to account for potential bias due to nonparticipation.
Table 3.
Multivariable Model of the Likelihood of Reporting a Decrease from Survivor-Focused Care at Baseline to General Care at Most Recent Follow-Up*,†
| Baseline Predictor Variables | OR¶ (95% CI) | p-value |
|---|---|---|
| Gender | ||
| Male | 1.3 (1.0–1.5) | .01 |
| Female | Ref | |
| Years since diagnosis | ||
| <10 years | 1.3 (0.9–1.8) | .21 |
| 10–<15 years | 1.4 (1.1–1.8) | .01 |
| 15–<20 years | 1.4 (1.1–1.8) | .009 |
| 20+ years | Ref | |
| Health insurance status | ||
| Yes, United States | Ref | |
| No, United States | 1.6 (1.2–2.1) | .003 |
| Canadian resident | 0.8 (0.6–1.0) | .07 |
| Physical limitation | ||
| Yes | 0.7 (0.5–0.9) | .002 |
| No | Ref | |
| Poor emotional health‡ | ||
| Yes | 0.6 (0.4–0.9) | .006 |
| No | Ref | |
| Pain | ||
| Adverse outcome | 0.8 (0.6–1.0) | .07 |
| No adverse outcome | Ref | |
| Chronic disease status§ | ||
| Grade 3–4 | 0.6 (0.5–0.7) | <.001 |
| Grade 1–2 | 0.6 (0.5–0.8) | <.001 |
| Grade 0 | Ref | |
| Radiation therapy | ||
| Both brain and chest | 0.3 (0.2–0.7) | .001 |
| Brain only | 0.6 (0.4–0.7) | <.001 |
| Chest only | 0.4 (0.3–0.6) | <.001 |
| RT, but not site brain or chest | 0.6 (0.5–0.8) | <.001 |
| Had RT but site for brain/chest unknown | 0.4 (0.2–0.8) | .004 |
| No RT | Ref | |
n = 2,515 survivor-focused care at baseline, among them, 1,270 became general care, 1,119 remained survivor-focused care, and 126 became no care.
Age at diagnosis, years since diagnosis, gender, race, insurance, income, education, ever had a job, general health, anxiety due to cancer, physical limitation, cancer pain, emotional health, chronic disease status, RT status, alkylating agent score, and anthracycline dose are all candidates of the model. Backward selection resulted in a model containing only the variables shown in the table.
GSI of ≥63 from the BSI, BSI = Brief Symptom Inventory-18; GSI: General Sensitivity Index (Derogatis 2000).
At baseline, the severity of chronic health conditions reported by participants were scored according to NCI Common Terminology Criteria of Adverse Events version 3.
Results from analyses were adjusted using IPW to account for potential bias due to nonparticipation.
Factors independently associated with the likelihood that patients who reported survivor-focused care at baseline were receiving general care at follow-up are provided in Table3. Uninsured survivors were more likely to report a reduction from survivor-focused to general care. Survivors who reported a physical limitation, decreased emotional health or pain at baseline, had a chronic health condition at baseline, or received radiation were less likely to report a decrease in care from survivor-focused to general care.
Discussion
In this study, we characterized the longitudinal patterns of medical care received by more than 6,000 adult survivors of childhood cancer over an average of 12 years. There were several notable findings that extend the observations of previously published cross-sectional studies on medical care utilization among childhood cancer survivors (Oeffinger et al. 2004; Skinner, Wallace, and Levitt 2006; Nathan et al. 2008). At entry into the CCSS cohort, almost 90 percent of survivors reported having received medical care within the preceding 2 years. By their most recent follow-up, 6.9 percent of these survivors reported that they were no longer receiving regular medical care of any kind. Survivors who were male, had a low household income, or limited education were at particular risk for receiving no medical care at follow-up, highlighting the impact that socioeconomic status has on access to and use of appropriate medical care. Survivors who reported no morbidity at baseline were also at risk for no longer receiving regular medical care, despite their well-established elevated risks for morbidity and mortality. This is unfortunate and indicates potentially missed opportunities for implementation of proactive risk-reducing interventions in asymptomatic survivors.
Uninsured survivors were at risk for a decrease in their level of care from survivor-focused care to general care over time. The importance of maintaining health insurance, particularly among those who are socially disadvantaged, is a timely finding given the passage of the Affordable Care Act in the United States in 2010. Research among adults with cancer, including data from the U.S. National Health Interview Survey (Weaver et al. 2010), demonstrates that cost is a significant barrier to receiving appropriate medical care, even among survivors with health insurance (American Cancer Society 2008; Finkelstein et al. 2009; Tangka et al. 2010). The Affordable Care Act includes key provisions that ensure access to care that is affordable, prohibits health insurers from charging different rates based on medical histories or gender, does not allow insurers to deny coverage for preexisting conditions, and allows young adults to remain on their parent’s health insurance plans until 26 years of age. These provisions are expected to benefit young adult survivors of childhood cancer directly and will hopefully ameliorate some of the observed disparities in care (Senate and House of Representatives of the United States of America 2010; Park et al. 2012).
The Institute of Medicine and international groups have strongly endorsed the need for the long-term care of survivors to include regular surveillance and prevention strategies focused on the specific risks arising from their prior cancer and its treatment (Hewitt, Weiner, and Simone 2003; Skinner, Wallace, and Levitt 2006; Blaauwbroek et al. 2008; Kremer et al. 2013). At baseline, only 40 percent of survivors in the cohort reported receiving such survivor-focused care. Despite our expectation that need for such care would increase as survivors aged and new late effects emerged, and the publication of guidelines for the care of survivors in North America and elsewhere, only 30 percent of the cohort continued to receive survivor-focused care at their most recent follow-up (Skinner, Wallace, and Levitt 2006; Kremer et al. 2013). Most of those who no longer reported survivor-focused care were still engaged with the medical system and reported receiving general medical care within the preceding 2 years. This finding suggests that rates of survivor-focused care might be improved through better partnerships with primary care physicians in survivors’ communities. In fact, two studies that surveyed a random sample of North American internists and family physicians assessed their comfort with knowledge regarding the care of childhood cancer survivors and revealed that although these physicians were willing to care for survivors, they were generally unfamiliar with the guidelines for survivor care. Among those that had one or more survivors in their practice, most had not received a treatment summary or care plan from the treating cancer center that outlined the necessary surveillance and health promotion counseling. (Nathan et al. 2013; Suh et al. 2014) Primary care providers have been shown to value the receipt of a treatment summary/survivorship care plan. One study demonstrated that summaries increase primary care providers’ knowledge about survivors’ cancer histories and recommended surveillance care and positively influence patient care (Shalom et al. 2011). Our findings build upon this research and suggest several opportunities for future research and program development. These include conducting randomized clinical trials assessing the impact that treatment summaries/survivorship care plans have on patient and provider reported outcomes for childhood cancer survivors, similar to those studies that have been conducted successfully conducted in adult cancer survivor populations (Grunfeld et al. 2011; van de Poll-Franse et al. 2011). Implementing core competency training for primary care providers that includes survivorship care might also increase comfort and knowledge, and would leverage new educational and other resources that have become available for general practitioners (National Cancer Survivorship Resource Center 2014; Nekhlyudov and Wenger 2014). The American Society of Clinical Oncology and other professional societies have published recommendations on how to expand and coordinate educational offerings for medical professionals in areas that are essential to survivorship care (McCabe et al. 2013). Lastly, development of interventions that focus on effective patient-centered communication and shared decision making between the provider and the cancer survivor have been shown to improve the quality of cancer care for survivors (Epstein and Street 2007; Nekhlyudov and Wenger 2014).
Those patients with poor emotional health, physical limitations, anxiety about their prior cancer, or the presence of a chronic medical condition at baseline were more likely continue receiving survivor-focused care, suggesting that existing physical or psychological sequelae of the childhood cancer are the primary drivers of the receipt of survivor-focused care. As the risk for late effects and premature mortality increases without apparent plateau as survivors age (Ganame et al. 2008; Nathan et al. 2008; Armstrong et al. 2009; Castellino et al. 2011; Nagarajan et al. 2011; Oeffinger et al. 2011), it is vital that strategies be developed to ensure that survivors who are well at the time they enter adulthood continue to engage in survivor-focused care. These asymptomatic survivors need to be equipped with tools that allow them to understand their prior cancer therapy and its long-term risks, and that empower them to seek appropriate care and engage in health promoting behaviors (Hewitt, Weiner, and Simone 2003; Hewitt, Sheldon, and Stovall 2005; Underwood et al. 2012).
Similar to the findings above targeting interventions to improve providers’ knowledge of survivorship care, patient-centered care research in young adult cancer survivors has demonstrated that the receipt of a survivorship care plan is associated with the survivors reporting increased confidence in seeking appropriate medical care (Casillas et al. 2011). Among survivors of childhood Hodgkin lymphoma, receipt of a one-page cancer survivorship care plan was shown to improve compliance with recommended screening (Oeffinger et al. 2011). Consequently, there are ongoing efforts in the United States and in Canada to ensure that all cancer survivors receive a survivorship care plan prior to transition out of the pediatric cancer environment, including a mandate from the Commission on Cancer by the American College of Surgeons (ACoS) that will make the provision of a survivorship care plan a mandatory requirement for accreditation as a cancer center in 2015 (Hewitt, Weiner, and Simone 2003; Hewitt, Sheldon, and Stovall 2005; Ganz, Casillas, and Hahn 2008; Stricker et al. 2011; Fashoyin-Aje, Martinez, and Dy 2012). It is recognized that the new survivorship care plan requirement from the Commission on Cancer is less applicable to free-standing children’s hospitals serving childhood cancer survivors. However, for those Cancer Centers that care for survivors of both pediatric and adult malignancies, this requirement will impact the delivery of patient-centered care. Currently, only a minority of young adult cancer survivors report receiving such plans even when cared for within cancer centers (Casillas et al. 2011; Nathan et al. 2013; Suh et al. 2014).
The findings of this study should be interpreted in the context of using self-reported data to classify medical care. Survivors who developed late effects of their therapy may be more likely to identify health care visits as being related to their prior cancer history. Survivors without any late effects may not have recognized that their medical care provider was adapting their visit to their prior cancer history resulting in an underestimation of the frequency of survivor-focused care by some participants. Future work exploring the correlation between the individual survivor’s knowledge regarding survivorship care to influence his or her willingness and actual receipt of recommended survivorship screening or health promotion is an important area for future investigation.
We identified differences between participants in the study and those who were nonparticipants. The cohort’s members represent a group that is more likely to have health insurance, to be educated, and to be employed when compared to nonparticipating survivors. Although we accounted for this by applying IPW in our analysis, our results may not be completely generalizable to the broader population of childhood cancer survivors. However, given this is one of the largest cohorts of long-term childhood cancer survivors and the first to identify predictors of a longitudinal decline in the level of medical care received by adult survivors of childhood cancer, these findings will inform the development of targeted interventions to improve the long-term rates of survivor-focused care and eliminate disparities in access to needed care.
Conclusions
While the incidence of chronic health conditions is increasing, less than a third of adult survivors of childhood cancer report regular survivor-focused care with rates of such care decreasing over time among specific vulnerable populations. Survivors who have significant chronic health conditions or psychological sequelae of their cancer are more likely to report receiving appropriate care. In contrast, survivors with low income and education levels may fail to receive any medical care at all. Uninsured survivors are at particular risk for having a decrease in their level of care from survivor-focused care to general care over time. Targeted interventions, such as transition planning visits and the provision of survivorship care plans created by the oncology team, will empower survivors to be knowledgeable health care consumers. Such interventions will be particularly important for at-risk populations so that preventive and risk-reducing opportunities are not lost. Future research exploring the impact of not having survivor-focused care on morbidity and mortality is an important area for future investigation.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Supported by Grant No. CA55727 (GT Armstrong, Principal Investigator) from the National Cancer Institute, by Cancer Center Support (CORE) Grant No. CA21765 (R. Gilbertson) to St. Jude Children’s Research Hospital, and by the American Lebanese Syrian Associated Charities.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- American Academy of Pediatrics Section on Hematology/Oncology, & Children’s Oncology Group. Long-Term Follow-Up Care for Pediatric Cancer Survivors. Pediatrics. 2009;123(3):906–15. doi: 10.1542/peds.2008-3688. doi: 10.1542/peds.2008-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. 2008. “ Insurance and Cost-Related Barriers to Cancer Care: Cancer Facts and Figures 2008 ” [accessed on May 29, 2010]. Available at http://www.cancer.org/downloads/accesstocare/CFF2008_Special_Section.pdf.
- Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL. Mertens AC. Late Mortality among 5-Year Survivors of Childhood Cancer: A Summary from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2328–38. doi: 10.1200/JCO.2008.21.1425. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM. Oeffinger KC. Aging and Risk of Severe, Disabling, Life-Threatening, and Fatal Events in the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2014;32(12):1218–27. doi: 10.1200/JCO.2013.51.1055. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauwbroek R, Tuinier W, Meyboom-de Jong B, Kamps WA. Postma A. Shared Care by Paediatric Oncologists and Family Doctors for Long-Term Follow-Up of Adult Childhood Cancer Survivors: A Pilot Study. The Lancet Oncology. 2008;9(3):232–8. doi: 10.1016/S1470-2045(08)70034-2. doi: 10.1016/S1470-2045(08)70034-2. [DOI] [PubMed] [Google Scholar]
- Casillas J, Syrjala KL, Ganz PA, Hammond E, Marcus AC, Moss KM. Friedman DL. How Confident are Young Adult Cancer Survivors in Managing Their Survivorship Care? A Report from the LIVESTRONG Survivorship Center of Excellence Network. Journal of Cancer Survivorship. 2011;5(4):371–81. doi: 10.1007/s11764-011-0199-1. doi: 10.1007/s11764-011-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P. Hudson MM. Morbidity and Mortality in Long-Term Survivors of Hodgkin Lymphoma: A Report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–16. doi: 10.1182/blood-2010-04-278796. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Nolan VG, Leisenring W, Yasui Y, Ogg SW, Mertens AC. Robison LL. Noncancer-Related Mortality Risks in Adult Survivors of Pediatric Malignancies: The Childhood Cancer Survivor Study. Journal of Cancer Survivorship. 2014;8(3):460–71. doi: 10.1007/s11764-014-0353-7. doi: 10.1007/s11764-014-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- Efron B. Ibshirani R. An Introduction to the Boostrap. Boca Raton, FL: Chapman and Hall/CRC; 1993. [Google Scholar]
- Epstein RM. Street RL., Jr . Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD: N. C. Institute; 2007. Vol. NIH Publication No. 07-6225. [Google Scholar]
- Fashoyin-Aje LA, Martinez KA. Dy SM. New Patient-Centered Care Standards from the Commission on Cancer: Opportunities and Challenges. Journal of Community and Supportive Oncology. 2012;10(3):107–11. doi: 10.1016/j.suponc.2011.12.002. doi: 10.1016/j.suponc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Tangka FK, Trogdon JG, Sabatino SA. Richardson LC. The Personal Financial Burden of Cancer for the Working-Aged Population. American Journal of Managed Care. 2009;15(11):801–6. [PubMed] [Google Scholar]
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M. Neglia JP. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganame J, Pignatelli RH, Eidem BW, Claus P, D’Hooge J, McMahon CJ. Mertens L. Myocardial Deformation Abnormalities in Pediatric Hypertrophic Cardiomyopathy: Are All Etiologies Identical? European Journal of Echocardiography. 2008;9(6):784–90. doi: 10.1093/ejechocard/jen150. doi: 10.1093/ejechocard/jen150. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Casillas J. Hahn EE. Ensuring Quality Care for Cancer Survivors: Implementing the Survivorship Care Plan. Seminars in Oncology Nursing. 2008;24(3):208–17. doi: 10.1016/j.soncn.2008.05.009. doi: 10.1016/j.soncn.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC. van Leeuwen FE. Medical Assessment of Adverse Health Outcomes in Long-Term Survivors of Childhood Cancer. Journal of the American Medical Association. 2007;297(24):2705–15. doi: 10.1001/jama.297.24.2705. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A. Levine MN. Evaluating Survivorship Care Plans: Results of a Randomized, Clinical Trial of Patients with Breast Cancer. Journal of Clinical Oncology. 2011;29(36):4755–62. doi: 10.1200/JCO.2011.36.8373. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B. Ness KK. Social Outcomes in the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009;27(14):2390–5. doi: 10.1200/JCO.2008.21.1458. doi: 10.1200/jco.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt M, Sheldon G. Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- Hewitt M, Weiner SL, editors; Simone JV, editor. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, D.C: The National Academies Press; 2003. [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse S, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ. Cronin KA. 2013. “ SEER Cancer Statistics Review, 1975-2010 ” [accessed on April 1, 2013]. Available at http://seer.cancer.gov/csr/1975_2010/
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG. Oeffinger KC. Health Status of Adult Long-Term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Journal of the American Medical Association. 2003;290(12):1583–92. doi: 10.1001/jama.290.12.1583. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G. Hudson MM. A Worldwide Collaboration to Harmonize Guidelines for the Long-Term Follow-Up of Childhood and Young Adult Cancer Survivors: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatric Blood & Cancer. 2013;60(4):543–9. doi: 10.1002/pbc.24445. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL. Hudson MM. Development of Risk-Based Guidelines for Pediatric Cancer Survivors: The Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. Journal of Clinical Oncology. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, Lanctot JQ. Yasui Y. Pediatric Cancer Survivorship Research: Experience of the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2319–27. doi: 10.1200/JCO.2008.21.1813. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJ. Rubin DB. Statistical Analysis with Missing Data. 2nd Edition. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L. Feuer EJ. Long-Term Survivors of Childhood Cancers in the United States. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(4):1033–40. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS. Hudson MM. American Society of Clinical Oncology Statement: Achieving High-Quality Cancer Survivorship Care. Journal of Clinical Oncology. 2013;31(5):631–40. doi: 10.1200/JCO.2012.46.6854. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M. Inskip PD. Second Neoplasms in Survivors of Childhood Cancer: Findings from the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009;27(14):2356–62. doi: 10.1200/JCO.2008.21.1920. doi: 10.1200/jco.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M. Leisenring WM. Cardiac Outcomes in a Cohort of Adult Survivors of Childhood and Adolescent Cancer: Retrospective Analysis of the Childhood Cancer Survivor Study Cohort. British Medical Journal. 2009;339:b4606. doi: 10.1136/bmj.b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, Mertens A. Marina N. Twenty Years of Follow-Up of Survivors of Childhood Osteosarcoma: A Report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625–34. doi: 10.1002/cncr.25446. doi: 10.1002/cncr.25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC. Oeffinger KC. Medical Care in Long-Term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2008;26(27):4401–9. doi: 10.1200/JCO.2008.16.9607. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ. Henderson TO. Family Physician Preferences and Knowledge Gaps Regarding the Care of Adolescent and Young Adult Survivors of Childhood Cancer. Journal of Cancer Survivorship. 2013;7(3):275–82. doi: 10.1007/s11764-013-0271-0. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- National Cancer Survivorship Resource Center. 2014. “ Cancer Survivorship E-Learning Series for Primary Care Physicians: A Program of the National Cancer Survivorship Resource Center ” [accessed on July 17, 2014]. Available at https://cancersurvivorshipcentereducation.org.
- Nekhlyudov L. Wenger N. Institute of Medicine Recommendations for Improving the Quality of Cancer Care: What Do They Mean for the General Internist? Journal of General Internal Medicine. 2014;29(10):1404–9. doi: 10.1007/s11606-014-2931-9. doi: 10.1007/s11606-014-2931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottage K, Lanctot J, Li Z, Neglia JP, Bhatia S, Hammond S. Armstrong GT. Long-Term Risk for Subsequent Leukemia after Treatment for Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Blood. 2011;117(23):6315–8. doi: 10.1182/blood-2011-02-335158. doi: 10.1182/blood-2011-02-335158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H. Robison LL. Health Care of Young Adult Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Annals of Family Medicine. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT. Robison LL. Chronic Health Conditions in Adult Survivors of Childhood Cancer. The New England Journal of Medicine. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Oeffinger KC, Hudson MM, Mertens AC, Smith SM, Mitby PA, Eshelman-Kent DA. Robison LL. Increasing Rates of Breast Cancer and Cardiac Surveillance among High-Risk Survivors of Childhood Hodgkin Lymphoma Following a Mailed, One-Page Survivorship Care Plan. Pediatric Blood & Cancer. 2011;56(5):818–24. doi: 10.1002/pbc.22696. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ER, Kirchhoff AC, Zallen JP, Weissman JS, Pajolek H, Mertens AC. Kuhlthau KA. Childhood Cancer Survivor Study Participants’ Perceptions and Knowledge of Health Insurance Coverage: Implications for the Affordable Care Act. Journal of Cancer Survivorship. 2012;6(3):251–9. doi: 10.1007/s11764-012-0225-y. doi: 10.1007/s11764-012-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Poll-Franse LV, Nicolaije KA, Vos MC, Pijnenborg JM, Boll D, Husson O. Kruitwagen RF. The Impact of a Cancer Survivorship Care Plan On Gynecological Cancer Patient and Health Care Provider Reported Outcomes (ROGY Care): Study Protocol for a Pragmatic Cluster Randomized Controlled Trial. Trials. 2011;12:256. doi: 10.1186/1745-6215-12-256. doi: 10.1186/1745-6215-12-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM. Zeltzer LK. Study Design and Cohort Characteristics of the Childhood Cancer Survivor Study: A Multi-Institutional Collaborative Project. Medical and Pediatric Oncology. 2002;38(4):229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS. Zeltzer LK. The Childhood Cancer Survivor Study: A National Cancer Institute-Supported Resource for Outcome and Intervention Research. Journal of Clinical Oncology. 2009;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS 9.2. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Savu A, Liu Q. Yasui Y. Estimation of Relative Risk and Prevalence Ratio. Statistics in Medicine. 2010;29(22):2269–81. doi: 10.1002/sim.3989. doi: 10.1002/sim.3989. [DOI] [PubMed] [Google Scholar]
- Senate and House of Representatives of the United States of America. Patient Protection and Affordable Care Act. United States Government; 2010. [accessed on January 1, 2015]. Available at http://burgess.house.gov/uploadedfiles/hr3590_health_care_law_2010.pdf. [Google Scholar]
- Shalom MM, Hahn EE, Casillas J. Ganz PA. Do Survivorship Care Plans Make a Difference? A Primary Care Provider Perspective. Journal of Oncology Practice. 2011;7(5):314–8. doi: 10.1200/JOP.2010.000208. doi: 10.1200/JOP.2010.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R, Wallace WH. Levitt GA. Long-Term Follow-Up of People Who Have Survived Cancer during Childhood. The Lancet Oncology. 2006;7(6):489–98. doi: 10.1016/S1470-2045(06)70724-0. doi: 10.1016/s1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- Stricker CT, Jacobs LA, Risendal B, Jones A, Panzer S, Ganz PA. Palmer SC. Survivorship Care Planning after the Institute of Medicine Recommendations: How Are We Faring? Journal of Cancer Survivorship. 2011;5(4):358–70. doi: 10.1007/s11764-011-0196-4. doi: 10.1007/s11764-011-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Daugherty CK, Wroblewski K, Lee H, Kigin ML, Rasinski KA. Henderson TO. General Internists’ Preferences and Knowledge about the Care of Adult Survivors of Childhood Cancer: A Cross-Sectional Survey. Annals of Internal Medicine. 2014;160(1):11–7. doi: 10.7326/M13-1941. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka FK, Trogdon JG, Richardson LC, Howard D, Sabatino SA. Finkelstein EA. Cancer Treatment Cost in the United States: Has the Burden Shifted over Time? Cancer. 2010;116(14):3477–84. doi: 10.1002/cncr.25150. doi: 10.1002/cncr.25150. [DOI] [PubMed] [Google Scholar]
- Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA. Fairley TL. Surveillance of Demographic Characteristics and Health Behaviors among Adult Cancer Survivors–Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveillance Summaries. 2012;61(1):1–23. [PubMed] [Google Scholar]
- Ware JE., Jr Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- Weaver KE, Rowland JH, Bellizzi KM. Aziz NM. Forgoing Medical Care Because of Cost: Assessing Disparities in Healthcare Access among Cancer Survivors Living in the United States. Cancer. 2010;116(14):3493–504. doi: 10.1002/cncr.25209. doi: 10.1002/cncr.25209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel L, DeAlba I, Habbal R, Kluhsman BC, Fairclough D, Krebs LU. Aziz N. Quality of Life in Long-Term Cervical Cancer Survivors. Gynecologic Oncology. 2005;97(2):310–7. doi: 10.1016/j.ygyno.2005.01.010. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Zeltzer LK, Whitton J, Mertens AC, Odom L, Berkow R. Robison LL. Psychological Outcomes in Long-Term Survivors of Childhood Leukemia, Hodgkin’s Disease, and Non-Hodgkin’s Lymphoma: A Report from the Childhood Cancer Survivor Study. Pediatrics. 2002;110(1):42–52. doi: 10.1542/peds.110.1.42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


