Abstract
Objective
The objective of this study is to examine how nursing homes changed their use of antipsychotic and other psychoactive medications in response to Nursing Home Compare’s initiation of publicly reporting antipsychotic use in July 2012.
Research Design and Subjects
The study includes all state recertification surveys (n = 40,415) for facilities six quarters prior and post the initiation of public reporting. Using a difference-in-difference framework, the change in use of antipsychotics and other psychoactive medications is compared for facilities subject to public reporting and facilities not subject to reporting.
Principal Findings
The percentage of residents using antipsychotics, hypnotics, or any psychoactive medication is found to decline after public reporting. Facilities subject to reporting experienced an additional decline in antipsychotic use (−1.94 vs. −1.40 percentage points) but did not decline as much for hypnotics (−0.60 vs. −1.21 percentage points). Any psychoactive use did not vary with reporting status, and the use of antidepressants and anxiolytics did not change.
Conclusion
Public reporting of an antipsychotic quality measure can be an effective policy tool for reducing the use of antipsychotic medications—though the effect many only exist in the short run.
Keywords: Nursing home compare, public reporting, quality, antipsychotics, psychoactive medications
Following the Food Drug Administration (FDA) approval of atypical antipsychotics in the late 1990s, the use of antipsychotic medications among nursing home residents increased rapidly over the next 15 years. Many of these residents were receiving antipsychotics to manage behavioral and psychiatric symptoms associated with dementia (Briesacher et al. 2005; Office of Inspector General [OIG] 2011). This led to widespread off-label use of antipsychotics, often viewed as a form of “chemical restraint” (Hughes, Lapane, and Mor 1999). In the mid-2000s, it became evident that these medications could result in significant negative health outcomes in persons with dementia.1 Subsequently, the FDA issued public health advisories cautioning that atypical antipsychotics (2005), and later all antipsychotics (2008), are associated with increased risk of mortality in persons with dementia.
Even with the public health advisories of the FDA, the quantity of antipsychotic medication use is still a concern as the national rates of antipsychotic use remain high (Kales et al. 2011). In March of 2012, the Centers for Medicare and Medicaid Services (CMS) launched a national initiative, the Partnership to Improve Dementia Care in Nursing Homes, in an attempt to reduce the unnecessary use of antipsychotic medications, especially for nursing home residents with dementia (CMS 2012, 2013). As part of this initiative, CMS distributed for public comment revised surveyor guidance related to antipsychotics, launched a website (https://www.nhqualitycampaign.org/), and began public reporting new quality measures on antipsychotic medication use on the Nursing Home Compare (NHC) website. The purpose of this initiative, including the public reporting of antipsychotic quality, was to improve behavioral health care practices and reduce inappropriate use of antipsychotics.
The focus of this paper is the public reporting of antipsychotic use on the NHC website. Since 2002, NHC has publically reported various measures of nursing home quality, with the intent of allowing consumers to compare the quality of nursing home care in their local area. By logging onto the NHC website, consumers and administrators are able to search nursing homes within a geographic area and compare their quality performance. Quality is currently reported using a 5-star system, but data on specific quality measures are also available, such as number of deficiencies, staffing levels, financial penalties, and a set of resident care quality measures.
NHC did not initially include antipsychotic use as a measure of quality. Starting in July of 2012, CMS began publically reporting an antipsychotic quality measure on NHC that attempts to approximate “inappropriate” use.2 This measure is also included in the calculations of the 5-star quality scores of facilities. The public reporting of an antipsychotic quality measure should reduce the use of antipsychotics in nursing homes subject to public reporting. But one of the unintended effects is that nursing homes may substitute other medications that have similar sedating properties for antipsychotics. This paper studies this issue by examining the changes in the use of antipsychotics and other psychoactive medications just prior to and after the period in which NHC began publicly reporting the long-stay antipsychotic quality measure.
Conceptual Model
The purpose of public reporting is to inform consumers about quality of care, allowing the consumers to better differentiate quality among providers, and thus incentivize providers to compete on quality. Empirical evidence suggests that the consumer response to public reporting of quality in nursing homes is small in magnitude (Stevenson 2006; Werner et al. 2012). Although consumers seem to be less responsive to reported quality, a majority of nursing home administrators regularly check quality scores on NHC and take actions to improve quality based on reported scores (Mukamel et al. 2007). The perception of providers that consumers may respond to this public information and the ability of providers to use this information to benchmark quality against their peers is an important driver to improve quality, with public reporting resulting in quality improvement in some reported measures (Mukamel et al. 2008; Werner et al. 2009; Grabowski and Town 2011).3 Administrators may alter behavior more than consumers because some measures of quality reported may be utilized in lawsuits or as marketing tools to support quality claims (i.e., deficiency-free).
While there is evidence that nursing homes modestly improve quality after public reporting in some reported dimensions, incentivizing one measure of quality can distort facility efforts to achieve other dimensions of quality, especially those dimensions of quality that receive less regulatory scrutiny (McKie 1970; Baker 2002; Bowblis and Lucas 2012; Bowblis et al. 2012; Lu 2012). In the case of NHC, Lu (2012) examined the shift in deficiency citations nursing homes received after the initial introduction of NHC. She found that the number of deficiencies reported in domains (Quality of Life and Administration) not related to publically reported quality measures in NHC significantly increased.
As this applies to the initiation of public reporting of the facility-level antipsychotic quality measure by NHC, it is expected that public reporting will have the intended effect of reducing the use of antipsychotic medications more in nursing homes subject to public reporting. Also because of other CMS efforts, it is expected that antipsychotic use would be lower in all facilities after the initiation of public reporting.4 However, because antipsychotic medications are often used to manage behavioral and psychiatric symptoms associated with dementia, and in some cases act as a “chemical restraint,” nursing homes may offset this reduction in antipsychotic use by switching to other medications that have similar sedative properties. These other medications, which include other psychoactive medications such as antidepressants, anxiolytics, and hypnotics (Hughes and Lapane 2005), are highly prevalent in nursing homes (Simoni-Wastila et al. 2014). This incentive to substitute may be weakened if there is sufficient regulatory and clinical burden to using these alternatives. However, if substitution does occur, this does not address the issue of managing dementia care, as many of these alternatives also have harmful side effects and are associated with the same quality of life issues as antipsychotics (American Geriatrics Society 2012). Any significant substitution can lead to future, unintended public policy concerns.
Methods
The primary source of data utilized in this study is the Certification and Survey Provider Enhanced Reports (CASPER). CASPER is CMS’s redesigned reporting system and is the successor to the Online Survey, Certification and Reporting (OSCAR) System. Both OSCAR and CASPER contain data validated onsite by state surveyors during the annual recertification process required for nursing homes to be eligible for Medicare and Medicaid reimbursement. These surveys occur no less often than every 15 months, with the average time between surveys of approximately 12 months, and data collected include characteristics on the facility and residents, aggregated to the facility level. State surveyors review the data and conduct checks by comparing the facility report against individual resident records, staffing records, and observations of residents during the onsite visit, making this data valid for research purposes (Feng et al. 2005).
Using the identification number of the facility and the survey date, CASPER data are converted into a panel dataset where the unit of observation is a nursing home survey. All state surveys from the 50 states and the District of Columbia that occurred between January 1, 2011 and December 31, 2013 are utilized.5 This period is chosen because it captures the state of the nursing home industry six quarters before and after CMS initiated public reporting of a long-stay antipsychotic quality measure in July of 2012. The final dataset contains 40,415 nursing home survey observations from 15,319 facilities.
Using information in CASPER, six measures of medication utilization are calculated. For each class of medication, the proportion of residents using a medication is calculated by dividing the number of residents using that medication class by the number of residents in the facility. The measure reflects use among all residents in the nursing home, both long- and short-stay residents. Medication utilization measures are calculated for antipsychotics, hypnotics, anxiolytics, antidepressants, and any psychoactive medication. Antibiotic use is also calculated to serve as a robustness check and is described below. The empirical strategy is to compare how the proportion of residents using psychoactive medications changes after public reporting was initiated.
Just comparing the pre-postdifferences in medication utilization rates after public reporting leaves open the possibility that the findings are an artifact of some other unmeasured factor, such as the other CMS efforts to reduce the use of antipsychotics or separate temporal trends. Therefore, we apply an approach commonly used in the literature (Glickman et al. 2007; Lindenauer et al. 2007; Werner et al. 2009; Konetzka et al. 2014) to identify a comparison or control group (i.e., nonreporting nursing homes) that is either not affected or impacted less by public reporting. This will allow for the identification of the effect of public reporting alone, but it should be noted that antipsychotic utilization rates have been rather constant since 2005. Therefore, any reduction in antipsychotic utilization rates around the time of public reporting for all facilities may be due to other CMS efforts described in the introduction.
The control group is identified using the antipsychotic quality scores for long-stay residents from NHC obtained from CMS for the third quarter of 2012. This quarter reflects the first quarter in which the measure was made publically available. Nursing homes that do not have at least 30 residents for calculating the quality measure are excluded from public reporting, and they make up the control group of facilities that are not impacted by public reporting for this study (1,866 facilities). The control group is found to be rather similar in other characteristics to facilities subject to reporting other than number of beds. In robustness checks, we find that control group facilities behave similarly as nursing homes subject to public reporting, making them an ideal control group.
By comparing facilities subject to public reporting to facilities not subject to public reporting, and examining the differences in how these facilities react to public reporting, a difference-in-difference equation is estimated as:
where the Mit is a measure of medication utilization, Reportit is an indicator variable if the facility is subject to public reporting of the antipsychotic quality measure, Postit is an indicator for a survey after public reporting is initiated, Xit are facility covariates, and δi is a facility fixed effect. The coefficient estimate of α3 identifies the effect of public reporting. This coefficient estimate reflects the average change in utilization for the six quarters after public reporting compared to the six quarters prior to public reporting among publically reporting facilities compared to those that do not publically report. It may also be the case that among facilities subject to public reporting that those with high utilization of antipsychotics may respond differently to public reporting than those with low utilization. Some regressions differentiate between these two groups where high utilization is defined as having over the median proportion of long-stay residents using antipsychotics in the third quarter of 2012 (21.5 percent).
A consideration that must be addressed is how fast nursing homes can react to public reporting as new medical plans need to be developed and medications withdrawn from residents. A pilot study found abrupt antipsychotic discontinuation to be feasible (Azermai et al. 2013), but gradual dose reduction is recommended. Guidelines on how to taper dosages are unclear, but the general goal is to wean individuals off antipsychotics in 4–6 weeks while alternatives to antipsychotics are tried (Gordon 2014). This implies that in the short run, the effect of public reporting may be smaller as nursing homes initially reduce dosage and find alternatives to antipsychotics. To allow for this potential effect, the above equation is also estimated to allow for a short run and longer run effect by breaking the post-implementation period into the three quarters immediately following the initiation of public reporting (short run) and the fourth to sixth quarters after public reporting (longer run).6
The advantage of the difference-in-difference approach is the effect of the policy can be identified and any unmeasured factors that affect all facilities are identified by the group of facilities not subject to public reporting. Furthermore, by including facility fixed effects in the regression, any unmeasured factors that are fixed over the study period are also accounted for in the regression. Any other factors that may affect the utilization rate of medication, such as facility structure, resident case mix, and staffing resources are captured in the facility control variable, Xit. The selection of these variables is guided by the previous literature on appropriate prescribing and responses to nursing home public reporting (Hughes, Lapane, and Mor 1999; Liperoti et al. 2003; Lau et al. 2004; Mukamel et al. 2008; Werner et al. 2009).
Although this approach has many advantages, a key assumption of the difference-in-difference analysis, the common trend assumption, must hold. As it applies in this case, absent public reporting, the trends in the proportion of residents using a medication at facilities subject to and not subject to public reporting should be the same. To test this assumption, regression models are estimated to compare the trends in the utilization of each medication in the six quarters prior to public reporting. These regressions did not find statistically significant evidence that these trends were different and support the validity of the difference-in-difference approach (results not shown).
Another validity check of this approach is to determine if a statistically significant effect is found for a medication that should not be affected by public reporting. There is no clinical reason why attempting to reduce the use of antipsychotics should affect the utilization of antibiotics. Therefore, as an additional robustness check, the proportion of residents using antibiotic medication is examined. The results are consistent with the difference-in-difference approach being valid. The results for these regressions are reported in the tables, but they are not discussed.
Results
Table1 reports the average proportion of residents using various medications for six quarters prior to and six quarters after the initiation of publically reporting the antipsychotic quality measure on NHC and the change in that proportion between the two periods. The proportions are reported for facilities that were not required to report quality and for those required to report, stratified by low- and high-use nursing homes. It should be noted that these proportions reflect the actual reported proportions and are not adjusted for differences in case mix or other characteristics.
Table 1.
Unadjusted Medication Use Rate by Public Reporting Status
| Average Rate: Six Quarters Prior to Public Reporting (%) | Average Rate: Six Quarters Post Public Reporting (%) | Difference Post-Prior (%) | |
|---|---|---|---|
| Antipsychotics | |||
| Nonreporting facilities | 24.828 | 23.954 | −0.874 |
| Low-use reporting facilities | 17.348 | 15.459 | −1.889 |
| High-use reporting facilities | 31.326 | 29.716 | −1.610 |
| Hypnotics | |||
| Nonreporting facilities | 9.284 | 8.124 | −1.160 |
| Low-use reporting facilities | 6.865 | 6.317 | −0.548 |
| High-use reporting facilities | 7.886 | 7.340 | −0.546 |
| Anxiolytics | |||
| Nonreporting facilities | 23.286 | 23.367 | 0.081 |
| Low-use reporting facilities | 20.272 | 20.538 | 0.266 |
| High-use reporting facilities | 24.740 | 25.037 | 0.297 |
| Antidepressants | |||
| Nonreporting facilities | 45.814 | 44.984 | −0.830 |
| Low-use reporting facilities | 46.998 | 45.735 | −1.263 |
| High-use reporting facilities | 51.632 | 50.778 | −0.854 |
| Psychoactives | |||
| Nonreporting facilities | 65.291 | 63.329 | −1.962 |
| Low-use reporting facilities | 61.546 | 60.026 | −1.520 |
| High-use reporting facilities | 69.245 | 67.615 | −1.630 |
| Antibiotics | |||
| Nonreporting facilities | 12.035 | 11.987 | −0.048 |
| Low-use reporting facilities | 9.193 | 9.289 | 0.096 |
| High-use reporting facilities | 8.440 | 8.755 | 0.315 |
Note. The period prior to and post public reporting refers to the six quarters before and after July 1, 2012. The number of nonreporting, low-use reporting, and high-use reporting facilities are 1,866, 6,707, and 6,746, respectively.
Facilities required to report the antipsychotic quality measure had higher antipsychotic use in the high-use facilities compared to low-use facilities in the period prior to public reporting (31.3 percent vs. 17.3 percent). Facilities not subject to reporting had 24.8 percent of residents using an antipsychotic. During the public reporting period, all three groups of facilities saw reductions in the proportion of residents using antipsychotic medications. For nonreporting facilities, the decline averaged 0.87 percentage points. Facilities required to publically report quality saw greater declines. These ranged from 1.89 percentage points for low-use and 1.61 percentage points for high-use facilities.
For the other medications, the unadjusted proportion of residents using hypnotics, antidepressants, and any psychoactive medication declined after public reporting for all three groups of facilities (−0.55 to −1.16, −0.83 to −1.26, −1.52 to −1.96 percentage points, respectively). Specifically for hypnotics, the size of the decline is about twice as large for nonreporting facilities compared to facilities required to report quality. All facility groups saw an increase in the proportion of residents using anxiolytics, with increases in the range of 0.08 to 0.30 percentage points.
Adjusting for differences in case mix and other characteristics, Table2 reports the difference-in-difference results, and full regression results are available in the online appendix. The first column of the table reports the change in the proportion using the specific class of medication for nonreporting facilities prior to and after public reporting. The next three columns report the additional change after public reporting for all, low-use, and high-use facilities subject to reporting. The final column reports the p-value of F-test to determine if the change for low compared to high use is statistically different from each other.
Table 2.
Change in Medication Use Prior and after Public Reporting of Antipsychotic Quality Measures
| Dependent Variables: Percentage of Residents Using | Nonreporting Facilities | Additional Change for Reporting Facilities† | |||
|---|---|---|---|---|---|
| All Reporting Facilities | Low-Use Reporting Facilities | High-Use Reporting Facilities | Test for Difference between Low and High Use (p-value) | ||
| Antipsychotics | −1.389*** (0.298) | −0.552* (0.308) | −0.614* (0.329) | −0.489 (0.317) | .5371 |
| Hypnotics | −1.213*** (0.268) | 0.613** (0.248) | 0.618** (0.259) | 0.608** (0.248) | .9247 |
| Anxiolytics | 0.059 (0.367) | 0.040 (0.412) | 0.095 (0.414) | −0.016 (0.437) | .6157 |
| Antidepressants | −0.618 (0.488) | −0.464 (0.472) | −0.574 (0.477) | −0.354 (0.511) | .3950 |
| Psychoactives | −2.041*** (0.492) | 0.204 (0.523) | 0.416 (0.545) | −0.009 (0.538) | .1614 |
| Antibiotics | −0.012 (0.387) | 0.174 (0.341) | 0.050 (0.357) | 0.298 (0.351) | .1304 |
Note. Change in pre-post is calculated using a difference-in-difference regression design. Regressions also control for ownership, chain membership, part of a CCRC, facility size, occupancy rate, payer mix, physical acuity, mental health acuity, presence of Alzheimer’s special care unit, nurse staffing levels, presence of mental health services, physician model, and facility fixed effects. All standard errors are adjusted for clustering within the state. The sample size is 40,415 observations.
Additional change reflects the additional change in post period for facilities publically reporting antipsychotic quality relative to facilities not reporting. The All column is the total effect for all facilities publically reporting quality, whereas Low- and High-use columns differentiate facilities based on the level of antipsychotic use. The Difference (p-value) column reports the p-value to determine if there is a difference in the change for low- compared to high-use reporting facilities.
p < .01
p < .05
p < .10.
The regressions in Table2, which compare the six quarters prior to and after the initiation of public reporting, confirm the summary statistic results reported in Table1. For antipsychotics, all facilities experienced a decline in the use of antipsychotics, with a larger decline for facilities subject to public reporting. For nonreporting facilities, the decline averaged 1.40 percentage points and for all facilities subject to reporting, the decline was 1.94 percentage points. The difference between the two groups is statistically significant. While the decline for high-use facilities is slightly smaller than low use (total decline of 1.88 vs. 2.00 percentage points), the difference is not statistically significant.
In the case of hypnotic medications, nonreporting facilities experienced a decline of 1.21 percentage points compared to a decline of 0.60 percentage points for facilities subject to reporting. The use of any psychoactive medication is found to decline after public reporting by 2.04 percentage points, but there is no statistical difference by reporting status. Though the direction of the coefficient estimates for anxiolytics and antidepressants is consistent with the results found in Table1, there are no statistically significant changes in the use of these medications.
One disadvantage of using the approach in Table2 is it compares the average effect for the six quarters prior to and after the initiation of public reporting. One might expect that the reactions of public reporting may take some time. In Table3, the effect of public reporting in the postperiod is differentiated into the short and longer run, for all facilities required to report regardless of use. For antipsychotics, the short run finds facilities subject to public reporting reduce the use of antipsychotics more than those that were not subject to public reporting by an additional 0.87 percentage points. However, in the longer run, the difference is only an additional 0.16 percentage points. Hypnotics are found to increase in facilities subject to reporting (0.52 percentage points) relative to those not reporting, but the effect is not statistically significant until the longer run (0.73 percentage points). Public reporting facilities have lower antidepressant use in the short run, but higher in the longer run. Public reporting is not found to not have a statistically significant effect in the short or longer run for all other medications.
Table 3.
Short versus Longer Run Effects of Public Reporting
| Dependent Variables: Percentage of Residents Using | Short Run (Quarters 1–3 after Public Reporting) | Longer Run (Quarters 4–6 after Public Reporting) | ||
|---|---|---|---|---|
| Nonreporting Facility Trend | Additional Change for Reporting Facilities | Nonreporting Facility Trend | Additional Change for Reporting Facilities | |
| Antipsychotics | −1.035*** (0.279) | −0.867*** (0.345) | −1.844*** (0.460) | −0.157 (0.434) |
| Hypnotics | −0.968*** (0.308) | 0.517 (0.312) | −1.541*** (0.361) | 0.727** (0.341) |
| Anxiolytics | −0.112 (0.529) | −0.035 (0.581) | 0.302 (0.436) | 0.145 (0.459) |
| Antidepressants | −1.182** (0.529) | −1.225* (0.712) | 0.239 (0.672) | 0.550 (0.538) |
| Psychoactives | −2.846*** (0.649) | −0.168 (0.694) | −0.893 (0.654) | 0.724 (0.668) |
| Antibiotics | −0.215 (0.377) | 0.314 (0.361) | 0.254 (0.489) | 0.001 (0.423) |
Note. Change in pre-post is calculated using a difference-in-difference regression design that allows for the post period to be in the short and longer run. The short run is the first three quarters after the initiation of public reporting and the longer run is four to six quarters after the initiation of public reporting. All regressions also control for ownership, chain membership, part of a continuing care retirement community (CCRC), facility size, occupancy rate, payer mix, physical acuity, mental health acuity, presence of Alzheimer’s special care unit, nurse staffing levels, presence of mental health services, physician model, and facility fixed effects. All standard errors are adjusted for clustering within the state. The sample size is 40,415 observations.
p < .01
p < .05
p < .10.
The results in Table3 are confirmed in Figures1 and 2, which graphically show the adjusted trends in medication use after the implementation of public reporting for the two classes of medications for which public reporting is found to have a statistically significant effect—antipsychotics and hypnotics. In both figures, the reporting and nonreporting facilities’ use of medications for the six quarters prior to public reporting (baseline) are indexed to 1. This allows for comparison of the rate of utilization after public reporting compared to the prereporting period, with values less than 1 implying that overall utilization rates have declined. For example, a value of 0.95 would imply utilization rates 95 percent of what they were in the baseline period.
Figure 1.
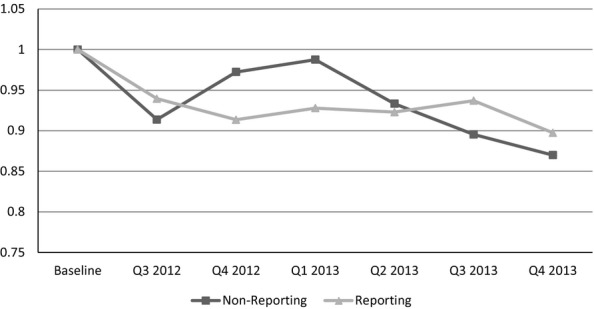
Trends after Reporting: AntipsychoticsNote. To construct this figure, the difference-in-difference regression is estimated allowing for the effect after public reporting to vary with the quarter after public reporting. The baseline period is the average proportion of residents using the medication in the six quarters prior to public reporting and is indexed to the value 1. Any number under 1 indicates a decline in the proportion of residents using that medication relative to the baseline period. Therefore, a value of 0.95 indicates for that group of facilities, the reported outcome is 95 percent of the level it was at baseline.
Figure 2.
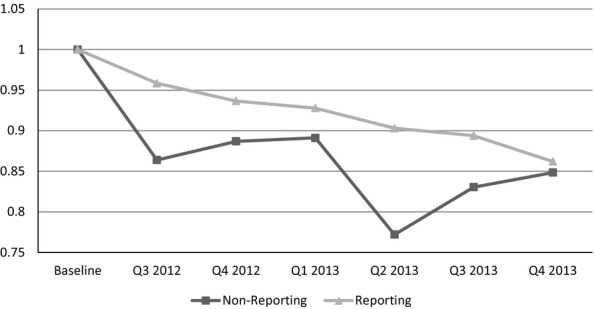
Trends after Reporting: HypnoticsNote. To construct this figure, the difference-in-difference regression is estimated allowing for the effect after public reporting to vary with each quarter after public reporting. The baseline period is the average proportion of residents using the medication in the six quarters prior to public reporting and is indexed to the value 1. Any number under 1 indicates a decline in the proportion of residents using that medication relative to the baseline period. For example, a value of 0.95 indicates for that group of facilities, the reported outcome is 95 percent of the level it was at baseline.
The use of antipsychotic medications for nonreporting and reporting facilities declined in the six quarters after public reporting was initiated. The use of antipsychotics declined faster for reporting facilities and remained high for nonreporting facilities until about the first quarter of 2013. Starting in the second quarter of 2013, the declines in antipsychotic use for both groups were about the same, but became larger for nonreporting in the last two quarters of 2013. In contrast, the decline from baseline in the use of hypnotics is larger for nonreporting facilities for all six quarters after the implementation of public reporting. Interestingly, the average decline becomes almost the same for both groups in the fourth quarter of 2013.
Discussion
The effort to improve quality through public reporting of an antipsychotic quality measure is found to have the intended effect of reducing the use of antipsychotic medications in the short run. While all facilities saw a reduction in the use of antipsychotics, facilities subject to public reporting saw declines in the proportion of residents using antipsychotics that were about 40 percent larger than facilities not subject to reporting. The size of the clinical effect is rather large and represents over 6,800 fewer residents receiving antipsychotics on a given day. Given the costs of these medications and that these drugs lead to 1 to 2 additional deaths per 100 persons (Schneider, Dagerman, and Insel 2005), the benefits of reducing antipsychotic use is rather large relative to the risk of continued use.
Though public reporting is having the intended effects, the difference-in-difference analysis does not tell the entire story. Early after NHC public reporting, antipsychotic use remained relatively level in nonreporting facilities and then started to decline steadily following the second quarter of 2013. By the last quarter of 2013, nonreporting facilities saw similar declines in the proportion of residents using antipsychotic medications to those facilities subject to public reporting. This pattern is consistent with public reporting incentivizing reporting facilities to initially act faster, but all facilities eventually experience similar decreases in the use of antipsychotics.
This is similar to the pattern found in physical restraints. In the late 1990s, there was a push to reduce the use of physical restraints and a physical restraint quality measure was publically reported starting in 2002. Initially, facilities that were subject to public reporting had a stronger incentive to reduce the use of physical restraints; however, the initiation of public reporting the use of physical restraints has declined in all nursing homes (Konetzka et al. 2014). Given our results only examine the six quarters after the initiation of public reporting, additional analysis with more recent data will be required to determine if antipsychotics follow the same long-term pattern found in the use of physical restraints.
These results suggest that public reporting may have the intended effect to incentivize facilities subject to reporting to fast-track specific quality improvement efforts. In the longer term, the combined efforts of the various CMS initiatives may have a similar impact on reducing the use of antipsychotics among all nursing homes regardless of reporting status. By stimulating nursing home staff and administrators to be aware of prescribing quality, public reporting of antipsychotic use may work by changing the culture of prescribing over time in all nursing homes (Hughes and Lapane 2005). Public reporting is an attempt to improve processes of care, which has already been found to be successful in reducing restraints.
All facilities are found to reduce the use of hypnotics. This is unexpected because hypnotics have similar sedative properties as antipsychotics, and if facilities are pressured to reduce the use of antipsychotics, they may switch to using hypnotics. Some substitution is found to be present as facilities subject to public reporting are found to decrease the use of hypnotics at a slower rate than facilities not subject to public reporting. In fact, the effect sizes for public reporting are similar, though in opposite directions for antipsychotics and hypnotics. A potential reason for this pattern is substitution to an alternative that is perceived as safe will occur, but substitution may occur to a lesser extent if the alternative faces clinical and/or regulatory scrutiny.7 Hypnotics faced this scrutiny as CMS changed regulatory guidelines related to antipsychotics and benzodiazepines starting in 2007 (CMS 2006) and hypnotics are known to cause serious adverse drug reactions (American Geriatrics Society 2012). The empirical result found in this paper may indicate CMS efforts have positive externalities to other medications, but additional investigation is warranted that uses resident-level data.
Our results should be interpreted with some caution. First, the results are based on facility-level and drug class data. By using individual resident-level clinical and drug data, the case mix and the specific agent(s) used by the resident could be identified. This would allow restricting the study population to residents with dementia and identify which psychoactive medications are being substituted for antipsychotics. Nonetheless, our data provide for the identification of general trends in the number of residents using antipsychotic and other psychoactive medications while controlling for facility-level case mix. Second, facilities not subject to reporting are smaller than the typical nursing home. As a robustness check, facilities of similar size were compared by restricting the sample to smaller facilities. These results were found to be robust, and in some cases, stronger than the ones reported. Finally, NHC reports separate antipsychotic quality measures for short-stay residents at the same time as long-stay residents. These two measures are found to be highly correlated, and many facilities subject to reporting for one measure are subject to reporting for the other. The effects of each of these measures need to be investigated using resident-level data that are restricted to the length of stay of the resident.
Conclusion
While most studies find little effect of public reporting on quality, and only in select measures (Mukamel et al. 2008; Werner et al. 2009; Grabowski and Town 2011), this study finds that public reporting of an antipsychotic quality measure can be an effective policy tool for reducing the use of antipsychotic medications in the short run. Though the absolute effect of public reporting is rather small, the clinical benefits are large given the high risk and limited benefit of antipsychotic use in nursing homes. It should be noted that this effect may only exist in the short run. In the long run, the effect of public reporting may not be sufficient alone but may need to be combined with other strategies, such as antipsychotic-specific deficiencies and financial penalties. These other strategies provide pressure to nursing homes to reduce the use of antipsychotic medications and provide CMS another mechanism to stimulate care process improvement. These other strategies may not be effective alone as public reporting would allow nursing homes to benchmark how their performance compares to peers. Therefore, there is still a role for public reporting even if the effects are small and become negligible in the longer run, as the public information provides nursing home administrators and policy makers benchmarks to identify the progress of all initiatives to reduce antipsychotic medications.
However, public reporting may have unintended consequences as nursing homes substitute care practices that face less regulatory scrutiny. While all facilities reduce the use of psychoactive medications in general, our finding that facilities subject to reporting reduced use of hypnotics more slowly may be consistent with some facilities substituting drugs that are not publically reported but with similar sedative properties as antipsychotics. We caution that while our study provides preliminary evidence that this form of substitution may already be occurring, it does not provide absolute proof and further research is needed to explore this issue.
In conclusion, public reporting of quality performance by itself seems to have a limited but positive role in improving quality (Werner et al. 2009). When combined with other strategies, it may be an effective mechanism enhancing other quality improvement efforts. However, policy makers need to be watchful for unintended consequences as nursing homes may switch to unreported care practices that may also cause harm. Either way, the effect of quality reporting on NHC and other CMS initiatives to reduce the use of antipsychotic medications overall seem to be having the intended impact.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We acknowledge the Farmer School of Business at Miami University for funding the cost of obtaining the data used in this study.
Disclosures: None.
Disclaimers: None.
Footnotes
These health outcomes include movement disorders, falls, hip fractures, infections, strokes, and increased risk of death (Schneider, Dagerman, and Insel 2005, 2006b; Schneider, Tariot, and Dagerman 2006a; Rochon et al. 2008; Ray et al. 2009; Huybrechts et al. 2012).
The measure used by NHC is the percent of nursing home residents who are receiving an antipsychotic medication, excluding those residents diagnosed with schizophrenia, Huntington’s disease, or Tourette’s syndrome (CMS, 2012). There is some debate as to whether this measures captures only “inappropriate” use (Lucas et al. 2014).
For example, Mukamel et al. (2008) examined five different publically reported measures but only found improvement in two—physical restraints and pain for short-stay residents. Werner et al. (2009) examined reporting of quality for postacute care and found improvement in two of three NHC measures—pain and improved walking. Finally, Grabowski and Town (2011) found very little evidence to suggest NHC resulted in better long-stay quality, but nursing homes in more competitive markets improved reported quality more than those in less competitive markets.
The use of antipsychotic medications has been relatively constant in nursing homes since 2005.
Some surveys from the fourth quarter of 2013 may be missing in the CASPER data utilized in the study. The CASPER data reflect all completed state surveys available as of December 2013, and because of slow upload times to the database, some surveys may not have been uploaded at the time the data were obtained.
The variable Postit in the equation above is replaced with two indicator variables that identify the short and longer run.
This is consistent with the findings of Konetzka et al. (2014), which show that the substitution of physical restraints for antipsychotics seems to end after the safety of atypical antipsychotic medications started to be questioned in 2005 (see Figure 3 in their paper).
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Summary Statistics and Full Regression Results.
References
- American Geriatrics Society. 2012. “ AGS Guide to the Management of Psychotic Disorders and Neuropsychiatric Symptoms of Dementia in Older Adults ” [accessed on February 15, 2014]. Available at http://dementia.americangeriatrics.org/GeriPsych_index.php.
- Azermai M, Petrovic M, Engelborghs S, Elseviers MM, Van der Mussele S, Debruyne H, Van Bortel L. Vander Stichele RH. The Effects of Abrupt Antipsychotic Discontinuation in Cognitively Impaired Older Persons: A Pilot Study. Aging & Mental Health. 2013;17(1):125–32. doi: 10.1080/13607863.2012.717255. [DOI] [PubMed] [Google Scholar]
- Baker G. Distortion and Risk in Optimal Incentive Contracts. Journal of Human Resources. 2002;37(4):728–51. [Google Scholar]
- Bowblis JR. Lucas JA. The Impact of State Regulations on Nursing Home Care Practices. Journal of Regulatory Economics. 2012;42:52–72. [Google Scholar]
- Bowblis JR, Crystal S, Intrator O. Lucas JA. Response to Regulatory Stringency: The Case of Antipsychotic Medication Use in Nursing Homes. Health Economics. 2012;21:977–93. doi: 10.1002/hec.1775. [DOI] [PubMed] [Google Scholar]
- Briesacher BA, Limcangco MR, Simoni-Wastila L, Doshi JA, Levens SR, Shea DG. Stuart B. The Quality of Antipsychotic Drug Prescribing in Nursing Homes. Archives of Internal Medicine. 2005;165:1280–5. doi: 10.1001/archinte.165.11.1280. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services [CMS] Interpretive Guidelines for Long-Term Facilities, Appendix PP-Guidance to Surveyors for Long-Term Care Facilities. Baltimore, MD: CMS; 2006. . In: State Operations Manual. [Google Scholar]
- Centers for Medicare and Medicaid Services [CMS] 2012. “ Description of Antipsychotic Medication Quality Measures on Nursing Home Compare ” [accessed on July 15, 2012]. Available at https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/AntipsychoticMedicationQM.pdf.
- Centers for Medicare & Medicaid Services [CMS] 2013. “ Advanced Copy: Dementia Care In Nursing Homes: Clarification to Appendix P State Operations Manual (SOM) and Appendix PP in SOM for F309 – Quality of Care and F329 – Unnecessary Drugs ” [accessed on February 15, 2014]. Available at http://www.Cms.gov/Medicare/Provider-Enrollment-and-Certification/Survey-CertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf.
- CMS Press Release. 2012. “ CMS Announces Partnership to Improve Dementia Care ” [accessed on May 30, 2012]. Available at http://www.CMS.gov.
- Feng Z, Katz PR, Intrator O, Karuza J. Mor V. Physician and Nurse Staffing in Nursing Homes: The Role and Limitations of the Online Survey Certification and Reporting (OSCAR) System. Journal of the American Medical Directors Association. 2005;6:27–33. doi: 10.1016/j.jamda.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Glickman SW, Ou F, DeLong ER, Roe MT, Lytle BL, Mulgund J, Rumsfeld JS, Gibler WB, Ohman EM, Schulman KA. Peterson ED. Pay for Performance, Quality of Care, and Outcomes in Acute Myocardial Infarction. Journal of the American Medical Association. 2007;297(21):2373–80. doi: 10.1001/jama.297.21.2373. [DOI] [PubMed] [Google Scholar]
- Gordon M. When Should Antipsychotics for the Management of Behavioral and Psychological Symptoms of Dementia Be Discontinued? Annals of Long Term Care: Clinical Care and Aging. 2014;22(4):24–9. “ ”. [Google Scholar]
- Grabowski DC. Town RJ. Does Information Matter? Competition, Quality and the Impact of Nursing Home Report Cards. Health Services Research. 2011;46(6 Pt 1):1698–719. doi: 10.1111/j.1475-6773.2011.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CM. Lapane KL. Administrative Initiatives for Reducing Inappropriate Prescribing of Psychotropic Drugs in Nursing Homes: How Successful Have They Been? Drugs and Aging. 2005;22(4):339–51. doi: 10.2165/00002512-200522040-00006. “ ”. [DOI] [PubMed] [Google Scholar]
- Hughes C, Lapane K. Mor V. Impact of Legislation on Nursing Home Care in the United States: Lessons for the United Kingdom. British Medical Journal. 1999;319(7216):1060–2. doi: 10.1136/bmj.319.7216.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts KF, Schneeweiss S, Gerhard T, Olfson M, Avorn J, Levin R, Lucas JA. Crystal S. Comparative Safety of Antipsychotic Medications in Nursing Home Residents. Journal of the American Geriatric Society. 2012;60(3):420–9. doi: 10.1111/j.1532-5415.2011.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Zivin K, Kim HM, Valenstein M, Chiang C, Ignacio RV, Ganoczy D, Cunningham F, Schneider LS. Blow FC. Trends in Antipsychotic Use in Dementia 1999-2007. Archives of General Psychiatry. 2011;68(2):190–8. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- Konetzka RT, Brauner DJ, Shega J. Werner RM. The Effects of Public Reporting on Physical Restraints and Antipsychotic Use in Nursing Home Residents with Severe Cognitive Impairment. Journal of the American Geriatrics Society. 2014;62(3):454–61. doi: 10.1111/jgs.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DT, Kasper JD, Potter DEB. Lyles A. Potentially Inappropriate Medication Prescriptions among Elderly Nursing Home Residents: Their Scope and Associated Resident and Family Characteristics. Health Services Research. 2004;39(5):1257–76. doi: 10.1111/j.1475-6773.2004.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenauer PK, Remus D, Roman S, Rothberg MB, Benjamin EM, Ma A. Bratzler DW. Public Reporting and Pay for Performance in Hospital Quality Improvement. New England Journal of Medicine. 2007;356(5):486–96. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- Liperoti R, Mor V, Lapane KL, Pedone C, Gambassi G. Benabei R. The Use of Atypical Antipsychotics in Nursing Homes. Journal of Clinical Psychiatry. 2003;64(9):1106–12. doi: 10.4088/jcp.v64n0918. [DOI] [PubMed] [Google Scholar]
- Lu SF. Multitasking, Information Disclosure, and Product Quality: Evidence from Nursing Homes. Journal of Economics and Management Strategy. 2012;21(3):673–705. [Google Scholar]
- Lucas JA, Chakravarty S, Bowblis JR, Gerhard T, Kalay E, Paek EK. Crystal S. Antipsychotic Medication Use in Nursing Homes: A Proposed Measure of Quality. International Journal of Geriatric Psychiatry. 2014;29(10):1049–61. doi: 10.1002/gps.4098. doi: 10.1002/gps.4098. [DOI] [PubMed] [Google Scholar]
- McKie JW. Regulation and the Free Market: The Problem of Boundaries. Bell Journal of Economics and Management Science. 1970;1(1):6–26. [Google Scholar]
- Mukamel DB, Spector WD, Zinn JS, Huang L, Weimer DL. Dozier A. Nursing Homes’ Response to the Nursing Home Compare Report Card. Journal of Gerontology: Social Sciences. 2007;62(4):S218–25. doi: 10.1093/geronb/62.4.s218. [DOI] [PubMed] [Google Scholar]
- Mukamel DB, Weimer DL, Spector WD, Ladd H. Zinn JS. Publication of Quality Report Cards and Trends in Reported Quality Measures in Nursing Homes. Health Services Research. 2008;43(4):1244–62. doi: 10.1111/j.1475-6773.2007.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Inspector General [OIG] 2011. “ Medicare Atypical Antipsychotic Drug Claims for Elderly Nursing Home Residents ” [accessed on March 1, 2014]. Available at https://oig.hhs.gov/oei/reports/oei-07-08-00150.pdf.
- Ray WA, Chung CP, Murray KT, et al. Atypical Antipsychotic Drugs and the Risk of Sudden Cardiac Death. New England Journal of Medicine. 2009;360:225–35. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon P, Normand S, Gomes T, et al. Antipsychotic Therapy and Short-Term Serious Events in Older Adults with Dementia. Archives of Internal Medicine. 2008;168:1090–6. doi: 10.1001/archinte.168.10.1090. [DOI] [PubMed] [Google Scholar]
- Schneider L, Dagerman K. Insel P. Risk of Death with Atypical Antipsychotic Drug Treatment for Dementia: Meta-Analysis of Randomized Placebo-Controlled Trials. Journal of the American Medical Association. 2005;294(15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- Schneider L, Dagerman K. Insel PS. Efficacy and Adverse Effects of Atypical Antipsychotics for Dementia: Meta-Analysis of Randomized, Placebo-Controlled Trials. American Journal of Geriatric Psychiatry. 2006b;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- Schneider L, Tariot P. Dagerman K. Effectiveness of Atypical Antipsychotic Drugs in Residents with Alzheimer’s Disease. New England Journal of Medicine. 2006a;355:1525–38. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Wei Y, Luong M, Rattinger C. Franey T. Huang, Zuckerman GB, Brandt IHN. Lucas JA. Quality of Psychopharmacological Medication Use in Nursing Home Residents. Research in Social and Administrative Pharmacy. 2014;10(3):494–507. doi: 10.1016/j.sapharm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Stevenson DG. Is a Public Reporting Approach Appropriate for Nursing Home Care? Journal of Health Politics, Policy and Law. 2006;31(4):773–810. doi: 10.1215/03616878-2006-003. “ ”. [DOI] [PubMed] [Google Scholar]
- Werner RM, Konetzka RT, Stuart EA, Norton EC, Polsky D. Park J. Impact of Public Reporting on Quality of Postacute Care. Health Services Research. 2009;44(4):1169–87. doi: 10.1111/j.1475-6773.2009.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner RM, Norton EC, Konetzka RT. Polsky D. Do Consumers Respond to Publically Reported Quality Information? Evidence from Nursing Homes. Journal of Health Economics. 2012;31:50–61. doi: 10.1016/j.jhealeco.2012.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Summary Statistics and Full Regression Results.


