Abstract
Soccer is played by more than 250 million people worldwide. Repeatedly heading the ball may place soccer players at high risk for repetitive subconcussive head impacts (RSHI). This study evaluates the long-term effects of RSHI on neurochemistry in athletes without a history of clinically diagnosed concussion, but with a high exposure to RSHI. Eleven former professional soccer players (mean age 52.0±6.8 years) and a comparison cohort of 14 age- and gender-matched, former non-contact sport athletes (mean age 46.9±7.9 years) underwent 3T magnetic resonance spectroscopy (MRS) and neurocognitive evaluation. In the soccer players a significant increase was observed in both choline (Cho), a membrane marker, and myo-inositol (ml), a marker of glial activation, compared with control athletes. Additionally, ml and glutathione (GSH) were significantly correlated with lifetime estimate of RSHI within the soccer group. There was no significant difference in neurocognitive tests between groups. Results of this study suggest an association between RSHI in soccer players and MRS markers of neuroinflammation, suggesting that even subconcussive head impacts affect the neurochemistry of the brain and may precede neurocognitive changes. Future studies will need to determine the role of neuroinflammation in RSHI and the effect on neurocognitive function.
Key words: : brain metabolism, MR spectroscopy, repetitive subconcussive brain trauma, soccer
Introduction
Soccer is played by more than 250 million people worldwide. It is thus the most popular sport in the world, although hits to the head and concussions are quite common due to head-to-head or other body part, head-to-ground, and head-to-goal collisions.1–3 However, less is known about the short- and long-term consequences of repeatedly heading the ball in soccer. In most instances, heading does not result in symptoms of concussion, despite the fact that studies have demonstrated an average g-force of 16–28g4 and peak forces of up to 60g when heading the ball.5 The term “subconcussive” was introduced to describe impact to the head that produces neuronal changes similar to those in concussion, but without the symptoms of concussion.6 Nonetheless, repeatedly heading the ball places soccer players at high risk for repetitive subconcussive head impacts (RSHI). As soccer players perform, on average, 6–12 headings per game, a player's career likely involves the accumulation of thousands of headings.7–11
Of note, previous findings demonstrate alterations in the brain's white matter microstructure in professional soccer players even in the absence of concussive brain trauma. 12Another study reports an association between alterations in cerebral white matter microstructure and frequency of heading the ball in amateur players.13 Further, soccer players have been shown to have a higher risk for impaired neurocognitive function later in life.9,14,15 Alterations in gray matter, although likely to play a role in this impairment, have yet to be systematically investigated in athletes with a high exposure to RSHI.
A recent study using magnetic resonance spectroscopy (MRS) reveals long-term alterations of brain chemistry in individuals with a history of concussion sustained while participating in contact sports during college years, more than 3 decades prior to the study.16 Specifically, this study reported increased myo-inositol (mI), a chemical found primarily in glial cells, and an imbalance of choline (Cho), which becomes MR-visible in greater concentration during membrane turnover. These findings suggest increased glial cell activation and damage to cell membranes.16 The latter finding is not surprising given that MRS is sensitive to the underlying pathophysiological changes that occur in sports-related head injury.17 Studies in the acute stages of injury have shown reductions in N-acetyl aspartate (NAA),18 as well as changes in glutamate (Glu) and mI.16 Another recent study in collegiate hockey players19 also showed reduced NAA in female but not male hockey players when comparing pre- versus post-season MRS. The study's authors indicated the need to further examine the effects of subconcussive brain trauma, particularly in the light of observable diffusion tensor imaging changes throughout the season.
The aim of this study was to evaluate neurochemistry by using MRS in former professional soccer players without a known history of concussion, but with a history of extensive heading and associated RSHI, compared with former professional, age-matched, non-contact sport athletes. In addition, the association between brain chemical concentrations, neurocognitive performance, and estimated number of headers was assessed. To the best of our knowledge, this is the first study to use MRS to examine possible effects of subconcussive impacts to the head in soccer players.
Methods
Participants
Inclusion criteria for the soccer players were: participation in at least one season in one of the three highest national levels of German soccer leagues (first, second, or third Bundesliga), being male, age between 40 and 70 years, and right-handed. Exclusion criteria were: history of suspected or diagnosed concussion or other traumatic brain injury, neurological disorder, psychiatric illness, or being left-handed. Thirteen of 16 interested players met study criteria and were included in the study cohort. Three interested players were excluded from participation due to a history of moderate to severe traumatic brain injury sustained outside playing soccer. Of those included in the study, the imaging data of two subjects did not pass quality control due to motion artifacts.
The final study group consisted of 11 former professional soccer players (mean age 52.0±6.8 years) who had played at least one season of professional soccer and were still active at a recreational level. A comparison cohort of 14 athletes (mean age 46.9±7.9 years), participating in non-contact sports, was recruited from competitive athletic clubs and group matched on age, handedness, and gender. These subjects were former professional athletes and were still actively participating in the same non-contact sports at the time of the study. Non-contact sports included table tennis (n=4), running (n=7), or ballroom dancing (n=3), all sports with equal physical activity level of intensity to soccer, as measured by maximum oxygen consumption,20–23 but with low exposure to repetitive brain trauma. The comparison cohort exclusion criteria were: history of suspected or diagnosed concussion or other traumatic brain injury, organized training in a contact sport, neurological disorder, psychiatric illness, or left-handedness.
The local ethics committee approved the study and written informed consent was obtained from each participant.
Clinical information, neurocognitive evaluation, and balance test
A semi-structured interview was performed to acquire detailed information about training habits and lifestyle, including number of headings performed per week during the last year prior to the study, and position in the field. Players were asked how many headers they performed per week during the past 12 months prior to the study. The rationale behind asking for this time period is that self-report is known to be less reliable when asking about a time period that is further in the past. To obtain a lifetime estimate of headers, the number of headers per week during the last year was multiplied by the total years of organized training in soccer. A lifetime estimate of headings was calculated by multiplying the estimated number of headers performed during the last year by the total years played.
All study participants underwent a brief neurocognitive and balance examination by an examiner who was blinded to the athlete's sport. The following tests were selected for their utility in showing impairments in patients with mild traumatic brain injury: Trailmaking Test (TMT) parts A and B, Rey-Osterrieth Complex Figure (ROCF) test, and Balance Error Scoring System (BESS). TMT A measures visual search and psychomotor speed and TMT B measures these same functions as well as cognitive flexibility.24 The ROCF test is a measure of visuoconstruction, planning and organization, and visual memory.25 For this test, participants were asked to reproduce a complex line-drawn figure (copy condition), followed by a 5-min delay (short delay recall condition) and a 30-min delay (delayed recall condition). BESS is a measure of balance consisting of three different positions (double leg, single leg, tandem) on two different surfaces (foam and firm) over 20 sec, respectively. The athlete's performance was rated by the examiner according to a defined protocol.26
MR imaging data acquisition
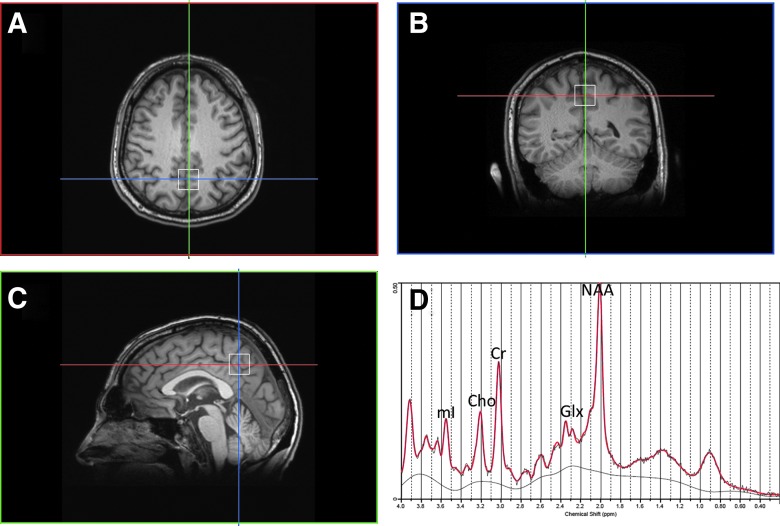
Data acquisition was performed in a supine position on a 3T MR scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil array. The scanning protocol included the following structural sequences: three-dimensional (3D) magnetization prepared rapid-acquisition gradient echo (MP-RAGE) sequence acquired in a sagittal orientation and a motion insensitive 3D T2-BLADE sequence acquired in a transversal orientation. Imaging parameters were as follows: MP-RAGE: repetition time (TR)=1800 msec, echo time (TE)=3.06 msec, field of view (FOV)=256 mm, voxel size=1×1×1 mm3, integrated parallel acquisition techniques (iPAT), acceleration factor 2; 3D T2-BLADE: TR=3000 msec, TE=400 msec, FOV=250 mm, voxel size=1×1×1 mm3, slices=160, iPAT, acceleration factor 2. MRS was acquired with the following parameters: single voxel point-resolved spectroscopy (PRESS) using a short TE=30 msec, TR=2000 msec, voxel size=20×20×20 mm3, 128 averages. The voxel was localized in the posterior cingulate gyrus using anatomical landmarks in all three planes from the 3D MP-RAGE sequence as shown in Figure 1A–C. Special care was taken to maximize the amount of gray matter in the voxel and to avoid the corpus callosum and the scalp tissue. Each voxel underwent automated optimization including 3D shimming, transmit gain, frequency adjustment, and water suppression. Magnetic field homogeneity was optimized for the selected spectroscopy volume of interest by manual shimming to a line width of <14 Hz full-width half maximum (FWHM) of the water signal. An unsuppressed water spectrum was then acquired using the same parameters except no water suppression and 16 averages. Total time of data acquisition was 5 min. After acquisition, screen shots of the voxel location and spectra were obtained for quality control. The posterior cingulate was selected for the MRS acquisition for several reasons. First, it is the same region measured in a cohort of American football players with chronic repetitive brain injury, which also provides the closest comparison to this cohort of subjects.27 Second, it has been utilized across a number of other brain injury studies, primarily acute and severe head trauma,28 thus providing a secondary comparison across the spectrum of brain injury. Third, positron emission tomography (PET)29 and functional magnetic resonance imaging (fMRI)30 studies have also found changes in the posterior cingulate after head injury. Moreover, it is one of the most homogeneous parts of the brain and thus enables excellent technical quality as well as high reproducibility.31 In addition, placement of the area of interest in this region is also a good indicator of neurochemical changes in other parts of the brain.31,32 As a result, neurochemical changes in the posterior cingulate are likely to be conservative such that if we have significant findings in this location, future studies in other brain regions will evince similar if not stronger effects.
FIG. 1.
(A) Location of the MRS voxel in the posterior cingulate gyrus, axial plane. (B) coronal plane. (C) Sagittal plane. (D) Representative spectra of a senior soccer player using LCModel for analysis and reconstruction. Cho, choline; CR, creatinine; Glx, glutamate/glutamine; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate. Color image is available online at www.liebertpub.com/neu
MR spectroscopy analysis
Spectra were exported as DICOM files and raw data files were extracted for post-processing. The data file and unsuppressed water file for each subject were then quantified using a user-independent time-domain fitting routine that uses a basis set of concentration-calibrated model spectra of individual chemicals to estimate the concentrations of similar brain chemistry from in vivo spectral data (LCModel, Provencher). This method exploits the full spectroscopic information of each chemical and not just isolated resonances. A representative spectrum from a soccer player is shown in Figure 1D. All spectra were quality controlled by examining the line width or FWHM of the unsuppressed water spectrum and signal-to-noise ratio (SNR). Cramer-Rao lower bounds (CRLB) were calculated for each neurochemical estimation. Only those measures with a CRLB <20% were used for data analysis. The following neurochemicals were quantified: NAA, creatine (Cr), Cho, Glu, GSH, and mI. In addition, lipid and macromolecule resonances were also characterized at 0.9, 1.3, and 2.0 ppm. To account for subject-to-subject variability in coil loading, the unsuppressed water signal for each subject was measured and accounted for and ratios to Cr were calculated in the LCModel output.
Statistical analysis
An independent sample t test was performed to evaluate differences between the age-matched groups where significance (p), degrees of freedom (df), and t value (t) are reported. MRS neurochemical ratios were correlated with cognitive measures and balance as well as with number of headings performed per week during the last year and lifetime estimate of headings using Spearman rank correlation. A p value <0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Results
Heading history, neurocognitive and balance evaluation
The mean age when organized soccer training started was 11.5±4.3 years. The number of headers performed per week over the past year ranged between 1 and 70 (mean 32.7). The athletes played in the following positions: defense (n=3), midfield offensive (n=4), midfield defensive (n=2), and goalkeeper (n=2).
All soccer players and controls tested within normal range for age for TMT parts A and B, ROCF test, and BESS. There were no significant group differences found in the neuropsychological measures or for the balance test. Results are listed in Table 1.
Table 1.
Summary of the Results of the Neuropsychological and Neurological Evaluation
| Soccer players (n=11) | Athletic controls (n=14) | P value | ||
|---|---|---|---|---|
| ROCF | Immediate recall T-score | 48.7 (11.0) | 54 (15.8) | 0.18 |
| Delayed recall T-score | 47.8 (14.8) | 55 (11.5) | 0.20 | |
| TMT A | Score | 102.6 (4.1) | 100.6 (5.3) | 0.32 |
| TMT B | Score | 108.4 (4.2) | 107.4 (5.3) | 0.63 |
| BESS | Total score | 17.6 (6.7) | 18.6 (8.8) | 0.77 |
There was no significant group difference for the following tests: ROCF (Rey-Osterrieth Complex Figure test; T-score, where a score between 40 and 60 is considered normal), TMT A and B (Trailmaking Test A and B; standard score, the higher the score the better; a score between 90 and 110 is considered normal), and BESS (Balance Error Scoring System; raw score, the higher the worse). All results are given as mean (standard deviation). P values obtained using two-sided Student's t test.
MR spectroscopy
Significant biochemical differences were found between the two groups (p<0.05), as shown in Figure 2. Higher ratios of Cho/Cr were measured in the soccer players (0.21+0.03) than in their athlete controls (0.19+0.02; p=0.04, df=17, t=2.17). mI/Cr levels were also found to be increased in the soccer players (0.89+0.04) compared with athlete controls (0.83+0.09; p=0.04, df=20, t=2.24). There were no differences in NAA/Cr concentrations between the two groups (soccer players: 1.16+0.08, controls: 1.17+0.11; p=0.9), although there were some senior soccer players that appeared to have higher NAA than some controls. Similarly, their macromolecule/lipid region from 0.9 to 1.5 ppm also appears elevated in senior soccer players. However, LCModel did not adequately fit those resonances. Total Cr (phosphocreatine+creatine) was compared between the players and controls and was not significantly different (soccer players: 5.7+0.6, controls: 5.8+0.5, p=0.11) and therefore it was used as a ratio to normalize data between each subject and the two groups. There were also no significant differences in Glu or GSH measures between the two groups.
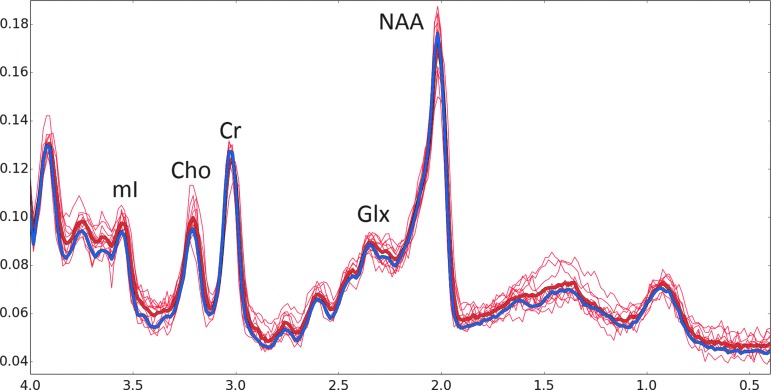
FIG. 2.
Stackplot of magnetic resonance spectroscopy (MRS) spectra. All control spectra were averaged together, Fourier transformed, and displayed in a thick blue line. Each individual senior soccer player spectra is shown in red. The average of all of the soccer players is shown in a thick red line. Major peaks of N-acetylaspartate (NAA), glutamate/glutamine (Glx), creatine (Cr), choline (Cho), and myo-inositol (mI) are labeled. Note the increase in Cho and mI when spectra are scaled to Cr.
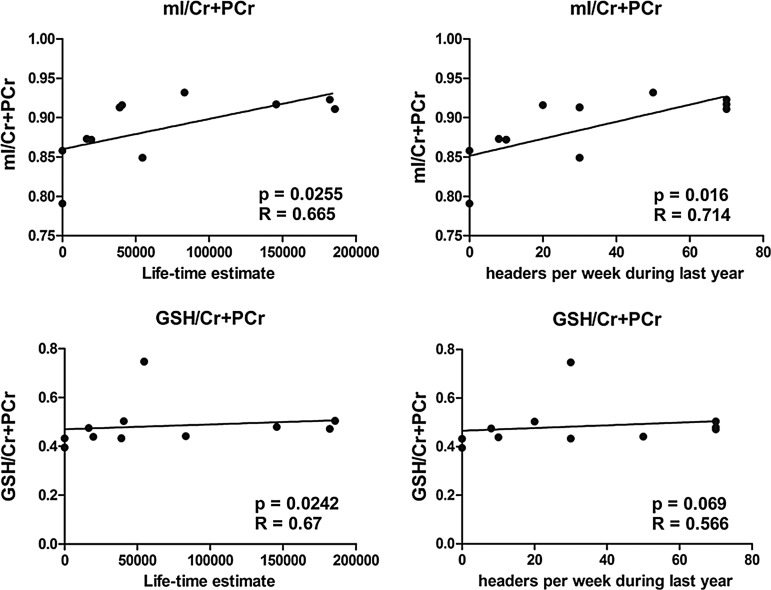
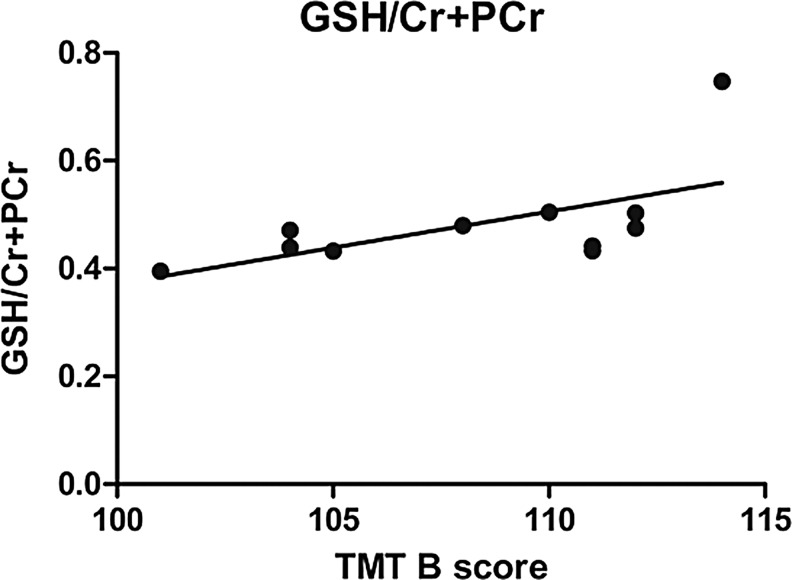
Neurochemical levels of all soccer players were correlated with the number of headings performed per week during the last year, as well as a lifetime estimate of headings. A significant positive correlation was found between mI/Cr and number of headings performed per week during the last year, as well as lifetime estimate of headings. GSH/Cr levels were significantly correlated with lifetime estimate of headings but not with number of headings performed per week during the last year (Fig. 3). In addition, test results of TMT B correlated with GSH/Cr+PCr (Fig. 4). No significant correlation was found for the remaining cognitive measures (ROCF, TMT A), BESS, or for age at start of soccer training.
FIG. 3.
Correlation between myo-inositol (mI) and glutathione (GSH) and estimated exposure to subconcussive head impact. Cr, creatine; PCr, total creatine (phosphocreatine+creatine).
FIG. 4.
Correlation between glutathione (GSH) and TrailmakingTest (TMT) B. Cr, creatine; PCr, total creatine (phosphocreatine+creatine).
Discussion
This study analyzed MRS brain biochemistry and neurocognitive performance in former professional soccer players without a history of suspected or diagnosed concussion, but with an extensive history of heading the ball and associated RSHI, compared with age- and gender-matched non-contact sport athletes. Significant increases were seen in both Cho, a membrane marker, and mI, a marker of glial activation, in the soccer players compared with the control athletes. Additionally, mI and GSH were significantly correlated with lifetime estimate of headings and performance on the TMT B.
Increased Cho results from membrane disruption that may be secondary to demyelination or to diffuse axonal injury, although some studies have suggested its role in astrocytosis33 and neuroinflammation34 in studies of acute head injury. Further, mI, found primarily in astrocytes and microglia, functions as an osmolyte and is thought to increase as a result of glial proliferation due to reactive astrocytosis and microglial activation.35,36 Cho/Cr and mI/Cr levels in the two goalkeepers were lower than the mean of the soccer players and therefore closer to the Cho/Cr and mI/Cr levels in the control group, as would be anticipated.
It is especially noteworthy that GSH/Cr levels were significantly correlated with exposure to RSHI. GSH is an anti-oxidant that removes or reduces damaging reactive oxygen species such as free radicals and peroxides and is therefore directly involved in oxidative stress and neuroinflammation. Increased GSH may be a mechanism for early compensatory or neuroprotective response to oxidative stress brought on by neuroinflammation.37 The combination of changes in Cho and mI, taken together with the correlation of GSH, implicate a neuroinflammatory process in these soccer players despite their lack of history of concussion. GSH also correlated with test results from the TMT B, which measures visual search, psychomotor speed, and cognitive flexibility. This correlation suggests a possible association between GSH, a measure of neuroinflammation, and subconcussive brain trauma, despite the fact that controls and players did not differ on TMT B. A prior PET study using 11C-PBR28, also a putative biomarker for inflammation, has shown that increased binding inversely correlated with performance on TMT B tests, as well as with reduced gray matter volume in the posterior cingulate.38 This study and our results support an effect of neuroinflammation on neurocognitive performance. It should be noted that the TMT B is a multidimensional task relying on several neurocognitive domains for its successful (i.e., quick) completion, including psychomotor speed, visual attention and scanning, working memory, and the ability to maintain and shift cognitive response sets.39 As such, it has been found to be sensitive to many types of neurological disorders and many areas of specific brain injury. The correlation between TMT B and putative inflammatory biomarkers may be a reflection of the test's sensitivity to diverse neural insult.
To date, it is not known how increased MRS markers for neuroinflammation translate to short- long-term functional deficits in athletes. It is, however, known that soccer players are prone to develop neurological and neurocognitive impairment later in life,9,14,15 although in the current study soccer players' neurocognitive functioning and balance were within the normal range. Of further note, in our study the lifetime estimate of exposure to subconcussive head impacts from heading the ball was correlated with increased markers for neuroinflammation in the soccer players, suggesting an association between subconcussive brain trauma and neuroinflammatory processes in former professional soccer players. We posit that findings of chronic inflammation may be importantly implicated in more long-term neurodegenerative processes and may be consistent with reports that soccer players are at increased risk for amyotrophic lateral sclerosis.40 Future studies need to focus on the effects of neuroinflammation, which may be an early indicator of later neurodegenerative processes, and which may be extant long before the clinical and cognitive impairments are observed in these players. Further, these findings suggest that MRS measures may be more sensitive to detecting subtle neurochemical abnormalities than are neuropsychological measures, at least early in the course of head impacts and their effect on brain chemistry.
It is important to note that we did not find any spectroscopic evidence of neurodegeneration as NAA, a putative neuronal marker, was found to be the same in both groups. It is of interest to note, however, that several subjects appeared to have increased NAA, which is contrary to the general observation of decreased NAA in mild traumatic brain injury studies.17 Johnson and colleagues had also observed an increase in those individuals who suffered multiple concussions as opposed to single concussions, which they posit may arise from adaptation of neurons to injury or oxidative stress.41 The evidence of increased GSH with a greater number of headers would strongly support the latter hypothesis. Further, what sets this study apart from previous studies is that our subjects did not have a history of clinically symptomatic concussion. The maintenance of NAA may be explained by the fact that players have not yet reached the threshold of neuronal degeneration that would be observed by decreases in NAA.
The relationship between increased MRS markers for neuroinflammation and concussive head injury has been previously reported in older ice hockey and American football players.16 However, our study focused on former professional soccer players without a history of a diagnosed concussion, thus demonstrating that such changes are extant in athletes subjected to repetitive subconcussive impacts. A recent study by Poole and colleagues examined high school American football athletes before and after a season in which they demonstrated reductions in NAA, Cr, Cho, glutamate/glutamine (Glx), and mI despite the lack of concussions, which they attribute to subconcussive blows.42 Our study contrasts with this study in several ways as we examine athletes at a professional level and at a much older age. Further, our subjects did not have a history of clinically symptomatic concussion. However, in the recent study by Poole and colleagues, the football players may not have sustained a concussion during the examined season but could have had a history of concussion prior to that season. Our study removes the potential effects of previous concussions on brain chemistry.
Study limitations
There are several limitations to this study that need to be considered. First, the study is preliminary and has a small sample size and thus needs to be confirmed in a larger sample, although many of the findings were quite robust. A second limitation is that the information pertaining to history of subconcussion and concussion was based on athlete's self-report. Thus, the calculation of lifetime estimate of headers provides only a rough estimate of the true exposure to RSHI. We note, however, that the rough estimate of headers showed some promising findings that need to be confirmed in future studies. A third limitation of the study is that information about number of headers does not take into account the forces that apply while heading the ball or the frequency of heading the ball during professional play compared with post-professional recreational play. It also does not take into account other mechanisms of head impact such as head-body or head-ground impacts. Another limitation of the study was the use of a single voxel location. While we think that the posterior cingulate is highly sensitive to the effects of concussions, other brain regions such as the frontal and temporal lobes are likely more vulnerable to the effects of subconcussive injury, although they suffer from technical issues such as susceptibility artifacts. We note, however, that our findings using posterior cingulate are likely conservative in that if we have significant findings in this location, future studies in other brain regions will evince similar if not stronger effects. Thus future studies need to include a larger sample, more detailed information on education and socioeconomic background, as well as more objective measures of frequency and forces that occur during exposure to RSHI and perhaps exploration of other brain locations for probing brain chemistry changes using MRS.
Conclusion
Results of this study suggest a possible association between RSHI from heading the ball in soccer, and neuroinflammation, in former professional soccer players, compared with athletic controls. Future studies, including longitudinal analyses, are needed to clarify the time course underlying changes in neurochemistry, as well as in the association of these changes with neurocognition, motor functioning, and quality of life.
Acknowledgments
This study was supported by the Else Kröner-Fresenius-Stiftung, Germany (I.K.). This work was also partially funded by grants from the Department of Defense (W81XWH-10-1-0835: A.P.L.; W81XWH-07-CC-CSDoD: R.Z., M.E.S.), the National Institutes of Health (R01-NS078337: A.P.L., M.E.S., R.A.S.) and a VA Merit Award (M.E.S.). Michael Mayinger was supported by the Petraeic Legate Foundation, Germany.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Maher M.E., Hutchison M., Cusimano M., Comper P., and Schweizer T.A. (2014). Concussions and heading in soccer: a review of the evidence of incidence, mechanisms, biomarkers and neurocognitive outcomes. Brain Inj. 28, 271–285 [DOI] [PubMed] [Google Scholar]
- 2.Bjorneboe J., Bahr R., and Einar Andersen T. (2014). Video analysis of situations with a high-risk for injury in Norwegian male professional football; a comparison between 2000 and 2010. Br. J. Sports Med. 48, 774–778 [DOI] [PubMed] [Google Scholar]
- 3.O'Kane J.W., Spieker A., Levy M.R., Neradilek M., Polissar N.L., and Schiff M.A. (2014). Concussion among female middle-school soccer players. JAMA. Pediatr. 168, 258–264 [DOI] [PubMed] [Google Scholar]
- 4.Naunheim R.S., Bayly P.V., Standeven J., Neubauer J.S., Lewis L.M., and Genin G.M. (2003). Linear and angular head accelerations during heading of a soccer ball. Med. Sci. Sports Exerc. 35, 1406–1412 [DOI] [PubMed] [Google Scholar]
- 5.Hanlon E., and Bir C. (2012). Real-time head acceleration measurement in girls' youth soccer. Med. Sci. Sports Exerc. 44, 1102–1108 [DOI] [PubMed] [Google Scholar]
- 6.Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 7.Tysvaer A., and Storli O. (1981). Association football injuries to the brain. A preliminary report. Br. J. Sports Med. 15, 163–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekblom B. (1986). Applied physiology of soccer. Sports Med. 3, 50–60 [DOI] [PubMed] [Google Scholar]
- 9.Matser E.J., Kessels A.G., Lezak M.D., Jordan B.D., and Troost J. (1999). Neuropsychological impairment in amateur soccer players. JAMA. 282, 971–973 [DOI] [PubMed] [Google Scholar]
- 10.Straume-Naesheim T.M., Andersen T.E., Dvorak J., and Bahr R. (2005). Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br. J. Sports Med. 39 Suppl 1, i70–i77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford A., Stephens R., Fernie G., and Potter D. (2009). Do UK university football club players suffer neuropsychological impairment as a consequence of their football (soccer) play? J. Clin. Exp. Neuropsychol 31, 664–681 [DOI] [PubMed] [Google Scholar]
- 12.Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., and Shenton M.E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 308, 1859–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton M.L., Kim N., Zimmerman M.E., Kim M., Stewart W.F., Branch C.A., and Lipton R.B. (2013). Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 2013. 268, 850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tysvaer A.T., and Lochen E.A. (1991). Soccer injuries to the brain. A neuropsychologic study of former soccer players. Am. J. Sports Med. 19, 56–60 [DOI] [PubMed] [Google Scholar]
- 15.Tysvaer A.T., Storli O.V., and Bachen N.I. (1989). Soccer injuries to the brain. A neurologic and electroencephalographic study of former players. Acta. Neurol. Scand. 80, 151–156 [DOI] [PubMed] [Google Scholar]
- 16.Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Theoret H., and Lassonde M. (2013). Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex 23, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 17.Lin A.P., Liao H.J., Merugumala S.K., Prabhu S.P., Meehan W.P., 3rd, and Ross B.D. (2012). Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012. 6, 208–223 [DOI] [PubMed] [Google Scholar]
- 18.Vagnozzi R., Signoretti S., Cristofori L., Alessandrini F., Floris R., Isgro E., Ria A., Marziale S., Zoccatelli G., Tavazzi B., Del Bolgia F., Sorge R., Broglio S.P., McIntosh T.K., and Lazzarino G. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain 133, 3232–3242 [DOI] [PubMed] [Google Scholar]
- 19.Chamard E., Theoret H., Skopelja E.N., Forwell L.A., Johnson A.M., and Echlin P.S. (2012). A prospective study of physician-observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. Neurosurg. Focus 33, E4: 1–7 [DOI] [PubMed] [Google Scholar]
- 20.Suchomel A. (2010). A comparison of exercise intensity on different player levels in table tennis. International Journal of Table Tennis Sciences 6, 79–82 [Google Scholar]
- 21.Daniels J., and Daniels N. (1992). Running economy of elite male and elite female runners. Med. Sci. Sports Exerc. 24, 483–489 [PubMed] [Google Scholar]
- 22.Blanksby B.A., and Reidy P.W. (1988). Heart rate and estimated energy expenditure during ballroom dancing. Br. J. Sports Med. 22, 57–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonnessen E., Hem E., Leirstein S., Haugen T., and Seiler S. (2013). Maximal aerobic power characteristics of male professional soccer players, 1989–2012. Int. J. Sports Physiol. Perform. 8, 323–329 [DOI] [PubMed] [Google Scholar]
- 24.Bowie C.R., and Harvey P.D. (2006). Administration and interpretation of the Trail Making Test. Nat. Protoc. 1, 2277–2281 [DOI] [PubMed] [Google Scholar]
- 25.Shin M.S., Park S.Y., Park S.R., Seol S.H., and Kwon J.S. (2006). Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat. Protoc. 1, 892–899 [DOI] [PubMed] [Google Scholar]
- 26.Bell D.R., Guskiewicz K.M., Clark M.A., and Padua D.A. (2011). Systematic review of the balance error scoring system. Sports Health 3, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin A.P., Ramadan S., Stern R.A., Box H., Nowinski C.J., Ross B.D., and Mountford C.E. (2015). Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimers Res. Ther. 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross B.D., Ernst T., Kreis R., Haseler L.J., Bayer S., Danielsen E., Bluml S., Shonk T., Mandigo J.C., Caton W., Clark C., Jensen S.W., Lehman N.L., Arcinue E., Pudenz R., and Shelden C.H. (1998). 1H MRS in acute traumatic brain injury. J. Magn. Reson. Imaging 8, 829–840 [DOI] [PubMed] [Google Scholar]
- 29.Nakashima T., Nakayama N., Miwa K., Okumura A., Soeda A., and Iwama T. (2007). Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. AJNR. Am. J. Neuroradiol. 28, 236–242 [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., and Sharp D.J. (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 31, 13442–13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin A., Tran T., Bluml S., Merugumala S., Liao H.J., and Ross B.D. (2012). Guidelines for acquiring and reporting clinical neurospectroscopy. Semin. Neurol. 32, 432–453 [DOI] [PubMed] [Google Scholar]
- 32.Lin A., Ross B.D., Harris K., and Wong W. (2005). Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2, 197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garnett M.R., Corkill R.G., Blamire A.M., Rajagopalan B., Manners D.N., Young J.D., Styles P., and Cadoux-Hudson T.A. (2001). Altered cellular metabolism following traumatic brain injury: a magnetic resonance spectroscopy study. J. Neurotrauma 18, 231–240 [DOI] [PubMed] [Google Scholar]
- 34.Brooks W.M., Stidley C.A., Petropoulos H., Jung R.E., Weers D.C., Friedman S.D., Barlow M.A., Sibbitt W.L., Jr., and Yeo R.A. (2000). Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J. Neurotrauma 17, 629–640 [DOI] [PubMed] [Google Scholar]
- 35.Ashwal S., Holshouser B., Tong K., Serna T., Osterdock R., Gross M., and Kido D. (2004). Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr. Res. 56, 630–638 [DOI] [PubMed] [Google Scholar]
- 36.Kierans A.S., Kirov I.I., Gonen O., Haemer G., Nisenbaum E., Babb J.S., Grossman R.I., and Lui Y.W. (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology 82, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy S.L., Lagopoulos J., Hickie I.B., Diamond K., Graeber M.B., Lewis S.J., and Naismith S.L. (2014). Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers Dement. 10, 67–75 [DOI] [PubMed] [Google Scholar]
- 38.Kreisl W.C., Lyoo C.H., McGwier M., Snow J., Jenko K.J., Kimura N., Corona W., Morse C.L., Zoghbi S.S., Pike V.W., McMahon F.J., Turner R.S., Innis R.B., and Biomarkers Consortium P.E.T.R.P.T. (2013). In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain 136, 2228–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashendorf L., Jefferson A.L., O'Connor M.K., Chaisson C., Green R.C., and Stern R.A. (2008). Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 23, 129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chio A., Calvo A., Dossena M., Ghiglione P., Mutani R., and Mora G. (2009). ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph. Lateral Scler. : 10, 205–209 [DOI] [PubMed] [Google Scholar]
- 41.Johnson B.D., Zhang K., Gay M., Neuberger T., Hallett M., Horovitz S., Sebastianelli W., and Slobounov S. (2012). The use of magnetic resonance spectroscopy (1H-MRS) in the sub-acute evaluation of athletes recovering from single and multiple MTBI. J. Neurotrauma 29, 2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole V.N., Abbas K., Shenk T.E., Breedlove E.L., Breedlove K.M., Robinson M.E., Leverenz L.J., Nauman E.A., Talavage T.M., and Dydak U. (2014). MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev. Neuropsychol. 39, 459–473 [DOI] [PubMed] [Google Scholar]