Abstract
Background: Cardiovascular disease (CVD) is common among women and is a leading cause of death in the United States. This study assessed the impact of CVD on compliance with the US Preventive Services Task Force guidelines for cervical and breast cancer screening among U.S. adult women.
Methods: A cross-sectional study was conducted on 17,408 women using data from the National Health Interview Survey 2013. A total of 11,788 respondents (21–65 years old) with complete information on Pap smear and 11,409 women (40+ years old) with complete information on mammography compliance were included. Multivariate logistic regression models were used to assess the impact of CVD on cervical and breast cancer screening practices.
Results: Women with CVD were marginally more likely to have had a mammogram in accordance with guidelines (odds ratio 1.17; 95% confidence interval 1.04–1.31) than those without CVD. However, compliance with Pap tests was similar (80.6% vs 82.3%, p>0.05) between the two groups. Myocardial infarction was associated with reduced odds of Pap smear compliance (odds ratio: 0.30; 95% confidence interval 0.18–0.51).
Conclusions: Women with prior myocardial infarction should be encouraged to continue receiving regular Pap smears. More research is needed to assess whether observed differences in Pap testing between patients with and without a history of myocardial infarction result from lack of provider recommendation or from patient noncompliance with their recommendations.
Introduction
The United States Preventive Services Task Force (USPSTF) now recommends that women undergo mammography at 2-year intervals beginning at 40 years old, and Papanicolaou (Pap) smear at 3-year intervals (or Pap smear+human papilloma virus [HPV] testing at 5-year intervals) between 21 and 65 years of age.1 These tests, however, are not always obtained by all eligible women in the U.S.2 Factors that may affect cancer screening rates include self-reported health status and the presence of comorbidities and disability.3–7 Since patients with chronic disability account for nearly half of the U.S. health burden,8 it is important that they obtain these recommended services. However, it is unclear whether these patients undergo regular cancer screening. One article found that patients with more comorbidities had decreased screening rates.5 In contrast, another reported an association between a higher number of chronic illnesses and increased screening among black and Hispanic women.7
The most prevalent chronic disease and leading cause of death in the United States is cardiovascular disease (CVD).9,10 This condition shares common risk factors such as obesity, poor diet quality, inactivity, and smoking with breast cancer as well as several other cancers.11 Thus, cancer screening in patients with CVD is especially important. However, the association between CVD and cancer screening has not been investigated. The purpose of this study was to examine the impact of CVD on compliance with cervical and breast cancer screening recommendations among adult U.S. women, using data from the 2013 National Health Interview Survey (NHIS).
Materials and Methods
Study population
NHIS data is collected from a representative cross-sectional, noninstitutionalized sample to assess the health status and behaviors of U.S. people. Health information is collected through an annual, in-person household survey and uses a complex, stratified, multistage sample design to provide nationally representative data.12 In 2013, NHIS augmented their sample size to include 32 states and the District of Columbia, increasing the number of states for which reliable estimates could be made. Blacks, Hispanics, and Asians were oversampled to increase the precision of analyses among these groups. Verbal consent for survey participation was provided by each subject.
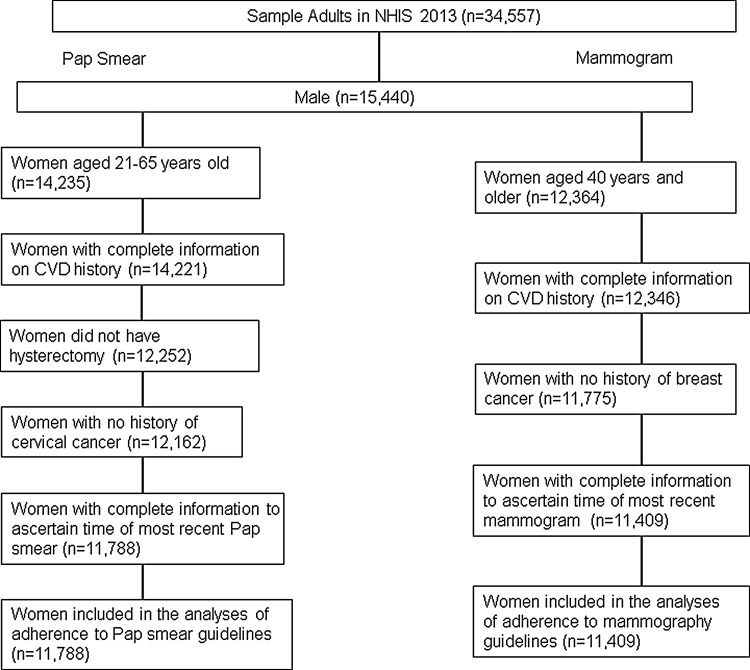
We limited our analyses to age-eligible women who had complete information on CVD. Women with breast cancer or those with missing information about their most recent mammogram were excluded from the analyses on breast cancer screening, while those with a history of cervical cancer, prior hysterectomy, or missing data on most recent Pap smear were excluded from analyses on cervical cancer screening. Out of 19,117 adult women respondents in NHIS 2013, 11,788 women were included in the analyses of Pap smear compliance (Fig. 1) after excluding women with age not in the range of 21–65 years old (n=4,882), missing information on history of CVD (n=14), women having hysterectomy (n=1,969), women with cervical cancer (n=90), and missing information on time of most recent Pap smear (n=374). After excluding women <40 years old (n=6,753), missing information on history of CVD (n=18), women with a history of breast cancer (n=571), and missing information on time of most recent mammogram (n=366), 11,409 women were included in the mammogram compliance analyses. The total sample size included in this study was 17,408.
FIG. 1.
Flowchart of the numbers of participants included in the analyses of cervical and breast cancer screening use in National Health Interview Survey (NHIS) 2013. CVD, cardiovascular diseases.
Outcome measurements
Female participants were asked about ever having had a Pap smear for cervical cancer screening or a mammogram for breast cancer screening. Those who responded yes to either question were further questioned about the timing of their most recent examination. This information was recoded using the NHIS 2000 method by combining information available on month/year, days/weeks/months/years of the exam, the original time interval grouping, and the interview date.13 Compliance with Pap smear recommendations was defined as having had their most recent Pap smear within the past 3 years, and compliance with mammogram recommendations was defined as having had the most recent mammogram within the past 2 years.
Sociodemographics
Sociodemographic measures included in our analyses were age, categorized into five levels (21–29 years, 30–39 years, 40–49 years, 50–65 years, and older than 65 years); race, categorized as non-Hispanic white (NH-white), non-Hispanic black (NH-black), Hispanic, and others; immigration status (born in United States, in U.S. <10 years, and in U.S. 10+years); region resided (Northeast, Midwest, South, and West); marital status (married/living with partner, widowed/divorced/separated, and single); education level (<high school, high school, and >high school); and family income to poverty threshold ratio (<1, 1–3, and >3). Family income to poverty threshold ratio is a ratio of the family's income to the appropriate federal poverty threshold.
Health care access
Health insurance coverage (public, private, and none) and usual place of health care (yes, no) were used to assess access to health care. Those who listed the emergency room as the usual place of health care were considered to have no access to usual source of care. We also included in our analyses whether respondents reported visiting an obstetrician/gynecologist (OB/GYN) in the last year in the analyses.
Cardiovascular disease
NHIS Participants were asked to report their CVD history, including hypertension, previous myocardial infarction, stroke, coronary heart disease, and other heart problems. We evaluated each condition separately, and then combined them to create a category of any type of CVD.
Statistical analysis
Statistical analyses were conducted using SAS software version 9.3 (SAS Institute; Carey, NC). P<0.05 was determined to be statistically significant. All analyses of NHIS data took into account differential probabilities of selection and the complex sample design following NHIS analytic guidelines.12 Sample weights of final annual person weights were incorporated in all analyses to account for nonresponse and post stratification adjustment (age-sex-race/ethnicity adjustment to 2010 Census population Control totals). Standard errors were calculated using Taylor series linearization.
The frequency of women who complied with Pap smear or mammogram recommendations was estimated for this sample by sociodemographic characteristics. Multivariate logistic regression models were used to evaluate screening in women with CVD compared with those without CVD. For the analysis on individual CVD type, women with other types of CVD were not excluded. Variables that were controlled for in Model 1 included age group, race, immigration status, region of residence, marital status, education level, and family income to poverty threshold ratio. We categorized age into several age groups to account for potential nonlinear effects of age on cancer screening. Variables in Model 2 included Model 1 variables as well as health insurance coverage, usual place of care, and visiting an OB/GYN in the past year. We also assessed interactions with insurance coverage, usual place of care, or visiting and OB/GYN in the past year in Model 2, to assess the influence of medical care access and usage on compliance among women both with and without CVD.
Since the outcomes used to assess compliance of cancer screening use are any Pap smear or mammogram in the past 3 years and 2 years, respectively, we conducted a sensitivity analyses on women aged 24–65 years for Pap smear and women aged 42 years and older for mammogram to account for the potential bias.
Results
Among women included in the analyses of Pap smear compliance, 4.7% were recent immigrants (Table 1). Slightly over 10% did not finish high school, 18.8% did not have health insurance, 15.2% had no access to usual care, and 46.7% visited an OB/GYN in the past year. Among women included in the analyses of mammography compliance, 13.5% did not finish high school, 11.0% did not have health insurance, 7.7% had no access to usual care, and 33.5% visited an OB/GYN in the past year.
Table 1.
Sociodemographic Characteristics of Adult Women Eligible for the Analyses of Breast and Cervical Cancer Screening Use, NHIS 2013 (N=17,408)
| Pap smear | Mammogram | |||||
|---|---|---|---|---|---|---|
| Complied (%)b | Complied (%)c | |||||
| Characteristic | n (%)aTotal | No CVD | CVD | n (%)aTotal | No CVD | CVD |
| Total | 11,788 (100) | 82.3 | 80.6 | 11,409 (100) | 67.8 | 69.9 |
| Age group | ||||||
| 21–29 | 2,713 (24.2) | 79.6 | 86.6 | |||
| 30–39 | 3,115 (24.4) | 84.9 | 85.8 | |||
| 40–49 | 2,472 (21.8) | 83.8 | 82.4 | 2,875 (28.2) | 61.9 | 62.7 |
| 50–65 | 3,488 (29.6) | 81.1 | 77.1 | 4,725 (44.0) | 73.8 | 74.6 |
| 66+ | 3,809 (27.7) | 65.5 | 67.7 | |||
| Race/ethnicity | ||||||
| NH-white | 6,318 (61.8) | 85.0 | 79.9 | 7,199 (70.3) | 69.1 | 70.3 |
| NH-black | 1,936 (12.8) | 83.6 | 82.9 | 1,763 (11.5) | 65.7 | 71.9 |
| Hispanic | 2,432 (16.8) | 76.8 | 81.5 | 1,612 (11.5) | 62.2 | 66.1 |
| Other | 1,102 (8.6) | 72.6 | 78.3 | 835 (6.7) | 67.5 | 66.7 |
| Immigration status | ||||||
| Born in United States | 9,278 (79.8) | 84.5 | 80.6 | 9,479 (83.4) | 68.4 | 70.2 |
| In U.S. 10+ years | 1,856 (15.2) | 76.9 | 81.7 | 1,745 (15.0) | 67.8 | 69.7 |
| In U.S. <10 years | 618 (4.7) | 67.4 | 73.1 | 158 (1.4) | 42.3 | 49.4 |
| Missing | 36 (0.3) | 27 (0.2) | ||||
| Region of residence | ||||||
| Northeast | 1,925 (17.8) | 84.3 | 84.4 | 1,977 (18.3) | 72.6 | 71.4 |
| Midwest | 2,263 (21.3) | 83.8 | 81.7 | 2,267 (21.3) | 68.7 | 71.7 |
| South | 4,385 (36.9) | 81.7 | 77.0 | 4,279 (38.0) | 65.4 | 67.3 |
| West | 3,215 (24.0) | 80.4 | 83.0 | 2,886 (22.4) | 66.8 | 71.8 |
| Marital status | ||||||
| Married/living with partner | 6,038 (62.5) | 85.4 | 83.1 | 5,157 (60.2) | 71.9 | 74.6 |
| Widowed/divorced/separated | 2,481 (14.8) | 79.7 | 73.9 | 4,937 (31.1) | 58.9 | 63.6 |
| Single | 3,228 (22.5) | 75.5 | 82.1 | 1,278 (8.4) | 61.4 | 70.5 |
| Missing | 41 (0.3) | 37 (0.3) | ||||
| Education level | ||||||
| <High school | 1,525 (11.0) | 72.1 | 66.0 | 1,850 (13.5) | 52.8 | 56.5 |
| High school | 2,536 (21.4) | 77.0 | 77.6 | 3,135 (27.6) | 63.0 | 67.8 |
| >High school | 7,684 (67.2) | 85.5 | 85.1 | 6,371 (58.4) | 72.2 | 75.9 |
| Missing | 43 (0.4) | 53 (0.5) | ||||
| Family income to poverty threshold ratio | ||||||
| <1 | 2,393 (15.0) | 71.4 | 69.9 | 1,794 (11.3) | 46.1 | 55.5 |
| 1–3 | 4,279 (34.5) | 77.6 | 76.1 | 4,477 (36.4) | 57.9 | 65.4 |
| >3 | 5,116 (50.5) | 88.4 | 88.0 | 5,138 (52.4) | 76.4 | 78.7 |
| Health insurance coverage | ||||||
| None | 2,415 (18.8) | 63.8 | 63.2 | 1,301 (11.0) | 37.5 | 42.0 |
| Private | 7,028 (64.4) | 87.7 | 86.9 | 6,727 (64.0) | 75.2 | 76.9 |
| Public | 2,303 (16.4) | 82.9 | 76.2 | 3,351 (24.8) | 62.0 | 64.9 |
| Missing | 42 (0.4) | 30 (0.3) | ||||
| Having a usual source of care | ||||||
| No | 1,917 (15.2) | 63.7 | 61.6 | 950 (7.7) | 29.5 | 35.7 |
| Yes | 9,869 (84.7) | 86.0 | 82.7 | 10,459 (92.3) | 72.2 | 71.6 |
| Missing | 2 (0.0) | |||||
| Visiting on OB/GYN in the past year | ||||||
| No | 6,498 (53.2) | 69.0 | 67.5 | 7,980 (66.4) | 55.6 | 62.9 |
| Yes | 5,284 (46.7) | 96.9 | 97.4 | 3421 (33.5) | 87.6 | 87.9 |
| Missing | 6 (0.1) | 8 (0.1) | ||||
| Types of CVD in NHIS sample | ||||||
| Any type of CVD | 2,848 (22.6) | 5,694 (45.9) | ||||
| Hypertension | 2,434 (19.0) | 5,145 (40.7) | ||||
| Myocardial infarction | 117 (0.9) | 446 (3.3) | ||||
| Stroke | 141 (1.1) | 484 (3.9) | ||||
Data source: Disease Control and Prevention, National Center for Health Statistics (CDC/NCHS). National Health Interview Survey (NHIS), 2013.
Percentage was weighted using sample weights.
Proportion of women complied with Pap smear recommendation. Compliance with Pap smear recommendation was defined as having Pap smear within 3 years. Proportion was weighted using sample weights.
Proportion of women complied with mammogram recommendation. Compliance with mammogram recommendation was defined as having mammogram within 2 years. Proportion was weighted using sample weights.
CVD, cardiovascular disease; OB/GYN, obstetrician /gynecologist; Pap, Papanicolaou (test).
Among women with CVD, 63.2% of women with no health insurance coverage complied with Pap smear recommendations, while 61.6% without a usual source of care complied. On the other hand, among women with CVD, 42.0% of women with no health insurance coverage complied with mammography recommendations, while 35.7% without a usual source of care complied.
A total of 22.6% of women included in the analyses of compliance with Pap smear recommendations had CVD. The most common type of CVD was hypertension, which included 83.8% of women with CVD. Almost 4% of women with CVD had a history of myocardial infarction. Among women included in the analyses of mammography compliance, 45.9% had CVD, of whom 88.7% had hypertension and 7.1% had a prior myocardial infarction.
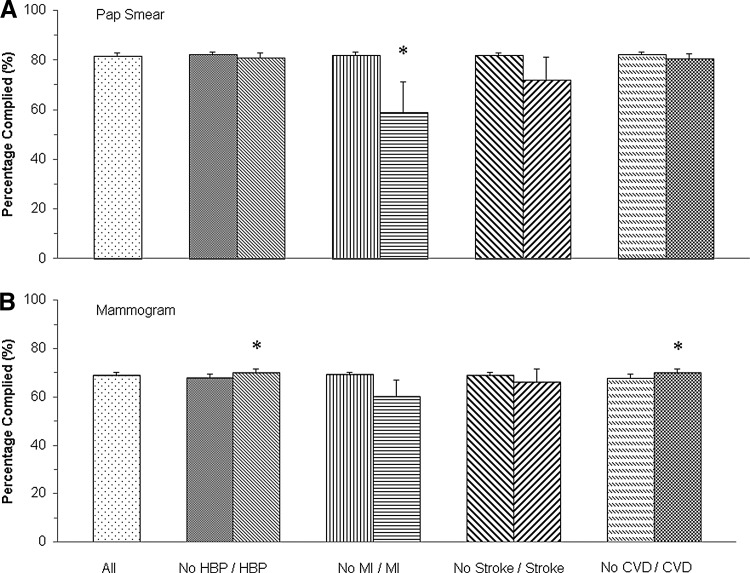
In NHIS 2013, 81.9% of adult women complied with recommended cervical cancer screening (Fig. 2A). Moreover, 80.6% of women with CVD had a Pap smear in the last 2 years, similar to women without CVD (82.3%). Differences in compliance with Pap smear guidelines were only observed between women with and without a history of myocardial infarction (58.8% vs. 82.1%, p<0.001). We examined the referral rate of Pap smears in the past year for women who were eligible for cervical cancer screening (i.e., excluding those who had Pap smears more than 1 year but less than 3 years ago). The referral rate was slightly lower among women with a history of myocardial infarction than those without but did not reach statistical significance (59.2% vs. 62.7%, p=0.63). Among those referred women, 72.2% of those with a history of myocardial infarction received a Pap smear in the past year, which was significantly lower than the rate among those without a myocardial infarction history (92.3%, p<0.001).
FIG. 2.
Compliance with Papanicolaou (Pap) smear and mammogram recommendations by CVD status in U.S. adult women, NHIS 2013. (A) Compliance with Pap smear recommendations; (B) Compliance with mammogram recommendations. Data are presented as proportion of women complied with cervical and breast cancer-screening guidelines. Error bar is 95% confidence interval. Proportion was weighted using sample weights. *Statistical significance for the comparison between people with/without disease, after adjusted for age group, race, immigration status, region of residence, marital status, education level, family income to poverty threshold ratio, health insurance coverage, usual place of care, and visiting an obstetrician/gynecologist in the past year. CVD, any type of cardiovascular diseases; HBP, high blood pressure; MI, myocardial infarction.
Among women ≥40 years old, 68.8% complied with mammography guidelines (Fig. 2B). Women with CVD had slightly better compliance compared with women without CVD (69.9% vs. 67.8%, p=0.01). There were differences in compliance with mammography guidelines between women with and without hypertension (70.1% vs. 67.9%, p=0.01). Other types of CVD did not impact mammography guideline adherence among women 40 years of age and older.
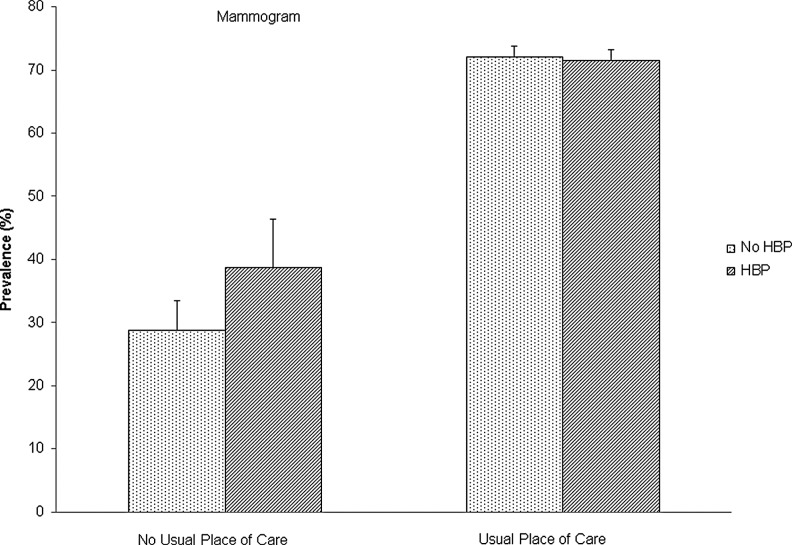
After adjusting for all other variables in Model 2 (Table 2), the multivariate model showed that women with a history of myocardial infarction had markedly decreased odds of complying with Pap smear guidelines as compared with those without (odds ratio 0.30, 95% confidence interval 0.18–0.51). Moreover, women with a history of hypertension had slightly increased odds of complying with mammography guidelines compared with women without hypertension (odds ratio 1.17, 95% confidence interval 1.04–1.31). Inclusion of Model 2 variables did not attenuate the difference in complying with Pap smear guidelines among women with a history of myocardial infarction compared with women without, or the difference in compliance with mammograms among women with hypertension. We also assessed interactions with insurance coverage, usual place of care, or visiting an OB/GYN in the past year in Model 2. However, none of these interaction terms reached the statistically significant level at α=0.1, except for the interaction of hypertension by usual place of care for compliance with mammogram recommendations (p=0.04). Among women with a usual place of care, the compliance rate was similar between those with and without hypertension (71.6% vs. 72.2%, Fig. 3), while among women without a usual place of care, hypertensive women had a significantly higher compliance rate for mammograms than women without hypertension (38.8% vs. 28.8%, p<0.001). This indicated that having a usual place of care was associated with the odds of obtaining a timely mammogram among hypertensive women.
Table 2.
Multivariate Logistic Models for Compliance with Screening Recommendations in U.S. Adult Women by CVD Status
| Odds ratio (95% confidence interval) of disease vs. without disease | ||||
|---|---|---|---|---|
| Pap smear | Mammogram | |||
| Disease/condition | Model 1a | Model 2b | Model 1a | Model 2b |
| Hypertension | 1.10 (0.93–1.31) | 1.00 (0.84–1.20) | 1.23 (1.09–1.37) | 1.17 (1.04–1.31) |
| Myocardial infarction | 0.39 (0.23–0.66) | 0.30 (0.18–0.51) | 0.86 (0.63–1.17) | 0.89 (0.67–1.19) |
| Stroke | 0.71 (0.45–1.14) | 0.70 (0.42–1.15) | 1.10 (0.84–1.43) | 1.04 (0.79–1.36) |
| Any type of CVD | 1.03 (0.89–1.20) | 0.92 (0.79–1.09) | 1.23 (1.10–1.37) | 1.16 (1.04–1.30) |
Data source: CDC/NCHS, National Health Interview Survey, 2013.
Variables in Model 1 included disease status, age group, race, immigration status, region of residence, marital status, education level, and family income to poverty threshold ratio.
Variables in Model 2 further included variables from Model 1 and health insurance coverage, usual place of care, and visiting an OB/GYN in the past year.
FIG. 3.
Compliance with mammogram recommendations by status of hypertension and usual place of care among U.S. adult women, NHIS 2013. Data are presented as proportion of women complied with cervical and breast cancer-screening guidelines. Error bar is 95% confidence interval. Proportion was weighted using sample weights.
For the analyses on individual CVD type, women with other types of CVD were not excluded. However, we conducted a separate analysis excluding women with other types of CVD from the comparison group and obtained similar results (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jwh). We also conducted a sensitivity analysis on women aged 24–65 years for Pap smears and women aged 42 years and older for mammograms. We got similar results regarding the impact of CVD on cancer screening (Supplementary Table S2 and Supplementary Fig. S1).
Discussion
This study showed that women with a history of myocardial infarction were much less likely to be compliant with cervical cancer screening guidelines compared with those without a history of this disease. This is in agreement with prior studies demonstrating that women with comorbidities, disabilities, or shorter life expectancy are less likely to comply with cancer screening guidelines.3–6 For example, Kiefe et al. assessed the effect of index of comorbidity on screening rates in two outpatient clinics, and found that as comorbidity increased, screening rates decreased.5 Moreover, they found that each unit of increase in the comorbidity index corresponded with a 17% decrease in the likelihood of undergoing mammography and a 20% decrease in obtaining a Pap smear.
The incidence of cervical cancer is highest among women aged 35–44 years old and subsequently decreases in age groups of 45–54 years and 55–64 years.14–17 The percentage of cervical cancer deaths is highest among women aged 45–54,14 and national guidelines recommend regular cervical screening until age 65.1 In our study, women with a history of myocardial infarction were 70% less likely to receive a Pap smear in the past 3 years, which is higher than observed in other studies on comorbidities. It is unlikely that this is due to the fact that women with myocardial infarction were older, as we controlled for age in the model. Some have suggested that the reason for this is that the physicians of women with significant health issues do not place a high priority on cancer screening.18,19 However, our findings do not support this theory, as we found that the referral rate of Pap smears was only slightly reduced among women with a history of myocardial infarction, but their rate of obtaining Pap smears was significantly reduced. This suggests that the reduced screening rate in women with prior history of myocardial infarction was more likely due to patient noncompliance than lack of provider recommendation. We were not able to determine the temporal relationship between provider recommendation and Pap smears, which is a limitation of the NHIS. Thus, more research is needed to assess whether the differences we observed result from lack of provider recommendation or patient noncompliance as well as methods to improve screening rates in this population.
Two studies found that women with more comorbidities sometimes undergo increased screening as more doctor visits provide them with more opportunities to be referred for screening.7,20 This could explain why we observed that women with CVD, of which the most common disorder was hypertension, were more likely to have received a mammogram in the past 2 years. We could not determine from the data available, however, why they would be more likely to undergo mammography and not Pap smears. The appropriate decision of whether to undergo cancer screening should depend on the cost-effectiveness of the screening test, subsequent available treatment, and the benefit to risk ratio to the patient.3 The provider and the patient should also consider the patient's life expectancy, values and preferences before making a final decision about screening.1,3 Our sample contained relatively young women (<65 years old) who should receive regular screenings for both cervical and breast cancer, even if they may have a history of a serious condition. Therefore, they should have been counseled to continue receiving both mammograms and Pap smears, as their history of any of the conditions we examined would not be a contraindication to screening.
Strengths of this study include use of data from a large, nationally representative sample with high response rates and the availability of information on sociodemographic characteristics to allow assessment of screening behaviors in different populations. However, our study has several limitations. First, all data from NHIS are self-reported, so we may have overestimated their screening compliance as we did not have medical records to confirm their reports.21–27 Second, we did not include human papillomavirus (HPV) testing in the analyses, as information about HPV DNA testing was not available in the 2013 NHIS dataset. Additionally, NHIS did not have enough information to determine the severity of CVD. Nevertheless, myocardial infarction and stroke are the two most severe cardiovascular conditions, and we found that a history of myocardial infarction had a negative effect on Pap smear use among adult women. Furthermore, NHIS is a cross sectional study and does not include temporal information on CVD diagnoses. Thus, it is possible that some women may have been newly diagnosed with CVD, after they have their cancer screening in the past 2 or 3 years. We also could not ascertain the temporal relationship between provider recommendation and cancer screening tests. More research is needed to ascertain temporal relationships and to evaluate methods to improve cervical cancer screening among women with a previous history of myocardial infarction.
In conclusion, most women with a history of CVD are in compliance with USPSTF guidelines regarding cervical and breast cancer screening. However, those with a history of myocardial infarction may be less likely to receive Pap smears at intervals recommended by the USPSTF. These women may be focusing much of their time visiting healthcare specialists who are not as familiar with those guidelines and thus do not know when to recommend that their patients receive this type of screening. Therefore, educational programs for specialists treating women for chronic conditions may be needed to improve their patients' adherence to Pap smearing guidelines. Moreover, more research needs to be done to help understand what barriers these women may be facing in complying with age-appropriate cancer screening guidelines.
Supplementary Material
Acknowledgments
All data from National Health Interview Survey used in this study were collected by the National Center for Health Statistics (NCHS) for Disease Control and Prevention (CDC).
Dr. Guo is currently a postdoctoral fellow supported by an institutional training grant (National Research Service Award T32HD055163, Berenson, Principal Investigator) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. Dr. Hirth is supported by a research career development award (Building Interdisciplinary Research Careers in Women's Health Program, BIRCWH K12HD052023, Berenson, Principal Investigator) from the Office of Research on Women's Health, the Office of the Director, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of CDC/NCHS, NICHD, or NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.U.S. Preventive Services Task Force. The guide to clinical preventive services, 2014. Available at www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/guide/cpsguide.pdf Accessed August11, 2014
- 2.Hewitt M, Devesa SS, Breen N. Cervical cancer screening among U.S. women: Analyses of the 2000 National Health Interview Survey. Prev Med 2004;39:270–278 [DOI] [PubMed] [Google Scholar]
- 3.Walter LC, Schonberg MA. Screening mammography in older women: A review. JAMA 2014;311:1336–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royce TJ, Hendrix LH, Stokes WA, Allen IM, Chen RC. Cancer screening rates in individuals with different life expectancies. JAMA Intern Med 2014;174:1558–1565 [DOI] [PubMed] [Google Scholar]
- 5.Kiefe CI, Funkhouser E, Fouad MN, May DS. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med 1998;13:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagay CR, Quale C, Smith-Bindman R. Screening mammography in the American elderly. Am J Prev Med 2006;31:142–149 [DOI] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Gold K, O'Malley AS, et al. Breast and cervix cancer screening among multiethnic women: role of age, health, and source of care. Prev Med 1999;28:418–425 [DOI] [PubMed] [Google Scholar]
- 8.Collaborators UBoD. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. Natl Vital Stat Rep 2013;61:1–117 [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215 [DOI] [PubMed] [Google Scholar]
- 11.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004;109:3244–3255 [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics (NCHS). Survey description, National Health Interview Survey, 2013. Hyattsville, MD: NCHS, 2014. [Google Scholar]
- 13.National Center for Health Statistics. Survey description, National Health Interview Survey, 2000. Hyattsville, MD: NCHS, 2002. [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER). SEER stat fact sheets: Cervix uteri cancer. Available at http://seer.cancer.gov/statfacts/html/cervix.html Accessed January16, 2015
- 15.Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep 2012;61:258–261 [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29 [DOI] [PubMed] [Google Scholar]
- 17.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29 [DOI] [PubMed] [Google Scholar]
- 18.Glasgow RE, Bull SS, Piette JD, Steiner JF. Interactive behavior change technology. A partial solution to the competing demands of primary care. Am J Prev Med 2004;27(2 Suppl):80–87 [DOI] [PubMed] [Google Scholar]
- 19.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract 2001;14:352–361 [PubMed] [Google Scholar]
- 20.Bostick RM, Sprafka JM, Virnig BA, Potter JD. Predictors of cancer prevention attitudes and participation in cancer screening examinations. Prev Med 1994;23:816–826 [DOI] [PubMed] [Google Scholar]
- 21.Bowman JA, Sanson-Fisher R, Redman S. The accuracy of self-reported Pap smear utilisation. Soc Sci Med 1997;44:969–976 [DOI] [PubMed] [Google Scholar]
- 22.Montaño DE, Phillips WR. Cancer screening by primary care physicians: a comparison of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health 1995;85:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez L, Goldman DA, Weiss NS. Validity of Pap smear and mammogram self-reports in a low-income Hispanic population. Am J Prev Med 1995;11:94–98 [PubMed] [Google Scholar]
- 24.Vernon SW, Briss PA, Tiro JA, Warnecke RB. Some methodologic lessons learned from cancer screening research. Cancer 2004;101(5 Suppl):1131–1145 [DOI] [PubMed] [Google Scholar]
- 25.Johnson CS, Archer J, Campos-Outcalt D. Accuracy of Pap smear and mammogram self-reports in a southwestern Native American tribe. Am J Prev Med 1995;11:360–363 [PubMed] [Google Scholar]
- 26.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst 1993;85:566–570 [DOI] [PubMed] [Google Scholar]
- 27.Caplan LS, McQueen DV, Qualters JR, Leff M, Garrett C, Calonge N. Validity of women's self-reports of cancer screening test utilization in a managed care population. Cancer Epidemiol Biomarkers Prev 2003;12:1182–1187 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.