Abstract
Pediatric human immunodeficiency virus (HIV-1) infection remains a global health crisis. Children are much more susceptible to HIV-1 neurological impairments than adults, which can be exacerbated by coinfections. Neurological characteristics of pediatric HIV-1 infection suggest dysfunction in the frontal cortex as well as the hippocampus; limited MRI data indicate global cerebral atrophy, and pathological data suggest accelerated neuronal apoptosis in the cortex. An obstacle to pediatric HIV-1 research is a human representative model system. Host-species specificity of HIV-1 limits the ability to model neurological consequences of pediatric HIV-1 infection in animals. Several models have been proposed including neonatal intracranial injections of HIV-1 viral proteins in rats and perinatal simian immunodeficiency virus (SIV) infection of infant macaques. Nonhuman primate models recapitulate the complexity of pediatric HIV-1, neuropathogenesis while rodent models are able to elucidate the role specific viral proteins exert on neurodevelopment. Nonhuman primate models show similar behavioral and neuropathological characteristics to pediatric HIV-1 infection and offer a stage to investigate early viral mechanisms, latency reservoirs, and therapeutic interventions. Here we review the relative strengths and limitations of pediatric HIV-1 model systems.
Keywords: Pediatric, human immunodeficiency virus-1, simian immunodeficiency virus, animal models, neurological impairments, myelin, hippocampus, stereology
As part of the United Nations Millennium Declaration, the global community took the initiative to halt and reverse the worldwide spread of HIV/AIDS by the year 2015.1 Through the collective effort of international governments, organizations, local communities and advancements in scientific discovery, the number of new HIV-1 infections continues to decline.2 Although there has been a decline in mother-to-child HIV-1 transmission over the past decade, an estimated 650 children under the age of 15 years become infected with HIV-1 each day,3–6 with approximately 50% of these children7 being perinatally infected through mother-to-child transmission (MTCT) via breast milk.8–10 The achievement in reducing perinatal infection rates has been accompanied by increased survival rates of HIV-1 infected children due, in part, to advances and access to antiretroviral therapy (ART),11 both in North America and worldwide.2,12–15 However, of the estimated 2.5 million children under the age of 14 living with HIV-1, only about 25% receive antiretroviral therapy.2 In 2012, over 200 000 children died from AIDS-related causes16 with resource-poor areas such as Sub-Saharan Africa accounting for the majority of children under the age of 14 years living with HIV-1 and new infections worldwide.4 Perinatal infection rates in North America are less than 2%, mainly due to interventions such as routine HIV-1 screening of pregnant women, use of antiretroviral drugs, avoidance of breastfeeding, and elective cesarean delivery.3,17–19 Currently, there are an estimated 10 000 perinatally infected HIV-1 children/adolescents in the United States, who are disproportionately distributed among Black/African-American and Hispanic/Latino populations.19
Long-term survival poses a set of unique management challenges as perinatally HIV-1 infected children transition to adolescence and adulthood.13,20,21 Early adolescence marks a period whereby adolescents begin to take charge of their own health management and lifestyles (life-long habits). There is a general paucity of data concerning the health outcomes of perinatally HIV-1 infected children and adolescents.22 Existing data suggest poor adherence to antiretroviral medications21,23,24 and marked obesity rates leading to physical complications such as cardiovascular disorders.14,22,25–29 This is further complicated by a high prevalence of neurodevelopmental and cognitive deficits,30–36 as well as increased frequency of reported psychiatric disorders within the perinatally HIV-1 infected adolescent population.37
NEURODEVELOPMENTAL DISORDERS
Perinatally HIV-1 infected individuals are disproportionately affected by HIV-1 related neurological impairments in comparison to adult infected patients.38,39 Children will often display neurobehavioral deficits prior to significant immunosuppression.40 Neurocognitive impairment is associated with a greater risk for disease progression and poorer morbidity, even in the advent of ART.41 In 2013, only 1 of 4 HIV-1 infected children needing access to ART, received it, and thus, the remainder were potentially susceptible to severe neurological damage.42 Early ART intervention partially ameliorates the neurological consequences of perinatal HIV-1 infection; however, deficits persist even with successful viral suppression.38,43–46
In the presence of ART, HIV-1 infected infants present a range of neurodevelopmental delays and impairments,47 which include fine and gross motor impairments, cognitive delays,48,49 verbal comprehension deficits,50,51 executive function impairments,40,41 working memory deficits, impaired visual-spatial integration,40 abnormal muscle tone, and spasticity.30,32,33,38,40,43,52–56 A higher prevalence of seizure activity57 and multiple-sclerosis-like illness have also been reported in perinatally infected infants.58 The variety of neurological complications that affect perinatally infected children results in impaired emotional and social skills, hyperactivity, and anxiety.51,59 The neurobehavioral deficits noted in childhood persist through adolescence, with an increased incidence of anxiety and depression that typically require psychotropic medication.37,44 As with children, the neurocognitive impairments and psychiatric disorders in adolescence interfere with performance in social and schooling situations36 and negatively affect overall health care management. The impact of HIV-1 viral load on neuropsychological impairment may be related to host chemokine receptor expression including CCR2,60 as well as viral factors.61
HIV-1 infection worldwide is attributed to different clades (A–K), which are unequally distributed by geographical region. Although clade C is predominant worldwide, namely, in India and throughout Africa,62,63 clade B has been more extensively studied as it is the prevalent form in North America and Europe.64–66 Clade-specific differences in disease transmission, including viral replication and progression, have been reported in adult populations.64,67,68 Clade-specific induction and severity of neuropathogensis have been reported.64,67–69 Specifically, clade B has a higher neurotoxic potential than clade C and may account for the higher incidence of HIV-1 associated neurological disorders in adults in Western countries.67–70 The scarce reports of neurological manifestations of HIV-1 infection in children of developing countries suggest a similar neurological profile to infected children in Western countries where clade B HIV-1 is prevalent.44,71–76 However, clade-specific neuropathological manifestations within the pediatric population has not been clearly established. Although, there is a positive correlation between neurological impairment and plasma HIV-1 viral load,43,77 the direct relationship between specific neuronal lesions and viral load, which is critical for our understanding of disease progression, has not been established.41,61,78 Similarly, it has also been shown that low neuropsychological function is related to disease progression.41 The longitudinal neurobehavioral trajectory of vertically infected adolescents remains grossly understudied36 but is of critical importance, as ART has reduced mortality and perinatally HIV-1 infected infants now survive into adolescence and adulthood.
NEUROIMAGING
Early neuroimaging studies commonly found global cerebral atrophy and basal ganglia calcifications.79–81 Imaging studies from HIV-1 infected children under the influence of ART have shown ventricular enlargement, sulcal widening white matter lesions,43,82 and altered metabolite concentrations in the frontal cortex and hippocampus.40,56,83,84 A recent MRI study in HIV-1 positive children under the age of 6 years found white matter signal abnormalities predominantly in the frontal and parietal lobes. Children in this study began ART by 8 weeks of life, suggesting that the white matter abnormalities manifest early during the infection, possibly due to early entry of HIV-1 into the central nervous system (CNS).85 There is also an increased prevalence of cerebrovascular disease.86 Diffusion tensor imaging also indicates reduced radial diffusivity, suggesting demyelination,56 which is consistent with sparse reports of multiple-sclerosis-like disorders in HIV-1 infected children.58 White matter abnormalities may occur very early in the infection, possibly within 8 weeks of life.85
NEUROPATHOLOGY AND NEUROTOXICOLOGY OF HIV-1
The scarce pathology reports from HIV-1 infected children indicate that neurological damage is an indirect consequence of perivascular inflammatory cell infiltrates containing HIV-1 infected macrophages and multinucleated cells, leading to infection of astrocytes and activation of a neurotoxic cascade87,88 including apoptosis within the cerebral cortex.88,89 There is also evidence suggesting a possible direct neuronal infection in infants.90 Pathology reports confirm imaging data suggesting ventricular enlargement, myelin pallor, cerebral atrophy, and basal ganglia calcification.91 Proinflammatory cytokines related to abdominal obesity have also been associated with progressive neurocognitive impairment in HIV-1 patients.92 Uninfected children (HIV-seroreverter) born to seropositive HIV-1 mothers also display neurobehavioral deficits,81,93,94 albeit not as severe or prevalent as vertically infected children.40,48,95 This could be due to inflammatory-mediated damage related to maternal HIV-1 infection or possibly mitochondrial toxicities related to maternal ART.96–99
The pathophysiology of pediatric and adult HIV-1 infection appears to share key features. HIV-1 primarily targets CD4+ T cells by the binding of the HIV-1 envelope glycoprotein 120 (gp120) to the CD4 cellular receptor and a chemokine coreceptor, mainly CCR5 and CXCR4. Binding to these receptors causes conformational changes in the gp120 proteins that lead to fusion of the HIV-1 particle with the host cell. The capsid of the virus disintegrates and it releases HIV-1 RNA, reverse transcriptase, integrase, ribonuclease, and protease into the cell. Then, the single stranded RNA of the virus is reverse transcribed to double-stranded DNA, which is incorporated into the host cell genome and then replicated.11,100,101 The virus can kill infected cells directly, via immune-mediated mechanisms, or can cause apoptosis in uninfected cells.
Neuronal damage within the CNS is mediated through viral proteins Tat (trans-activator of transcription), Nef, Vpr (viral protein R), and gp120. Indeed, both Tat and gp120 have been shown to directly induce neuronal apoptosis, while Nef and Vpr are key regulators of apoptosis of infected cells.11,102 Indirect neurotoxicity may result from the release of cytokines, metalloproteinases, gp120, and Tat from HIV-1 infected macrophages and microglia.11,103 HIV-1 associated proteins have been shown to directly impact the viability and function of the neurons.11 They also affect neurotoxicity by affecting the formation of ion channels that trigger excitatory responses in hippocampal neurons.11 HIV-1 pathogenesis in infants goes beyond gp120 and Tat toxicity, as elevated levels of proinflammatory cytokines (TNF-α, INF-γ, IL-12) are negatively correlated to neurocognitive function in HIV-1 positive children.34 Neural progenitor cell proliferation is also adversely affected by HIV-1 infection and related viral proteins.11,104,105 Moreover, postmortem studies of brain specimens from patients with HIV-1 associated neurological disorders showed a greater decrease in neural progenitor cells in the dentate gyrus, compared to HIV-1 negative controls and HIV-1 positive individuals that did not possess the same neurocognitive deficits.106
The ability of HIV-1 infected monocytes or T cells to progress into the brain relies on the ability of the virus to transverse the blood-brain barrier (BBB),107 to replicate within the brain, and to initiate the cascade of neuroinflammation. The BBB functions as a selective barrier between the CNS and the bloodstream. The BBB also adjusts inflammatory and immune responses by reducing the passage of toxins and pathogens into the CNS from the bloodstream. Like the immune system, the perinatal brain BBB is in an immature state108 and it is hypothesized that HIV-1 infection interferes with the formation of the BBB by reducing the population of pericytes, a significant constituent of the BBB.109,110 Neuroinflammation and breakdown of the BBB have been implicated as mechanisms contributing to HIV-1 related neurological disorders.87,111–115 Once the virus transverses the BBB in cell-free or cell-associated form, the ability of HIV-1 to infect new cells within the brain depends on the presence of CCR5 and CXCR4, which are known to be transiently expressed during early development of the CNS in primates.116 Expression of the main HIV-1/SIV receptor CD4 and of the chemokine coreceptors CCR5 and CXCR4 in the brain are critical in the neuropathogenesis of HIV-1,116 and signaling through these receptors might alter the balance between survival and proinflammatory neuronal death.117 These receptors are expressed on neural progenitor cells and have been proposed to play a role in HIV-1 induced reductions in neurogenesis.11,103,118,119 The ability of HIV-1 to induce neuroinflammatory and neuroapoptotic cascades appears to be pathway specific.118–120 For example, cathepsin B, which is secreted from activated macrophages, has been linked to HIV-1 induced neuroapoptosis120 and activation of the p38 mitogen-activated protein kinase is involved with HIV-1-induced deterioration of the BBB.111
ANIMAL MODEL SYSTEMS
As evidenced from the scarcity of neuroimaging and pathological reports, a main and obvious obstacle in pediatric HIV-1 research is sample access. The developing immune system is clearly more susceptible than the adult to adverse viral infections;121 therefore, it is critical to design and test potential intervention therapies in pediatric animal model systems.122 There are relatively few research groups investigating the pathogenesis and prevention of pediatric HIV-1 infection in animal models.123–129 Here we will examine recent neurological findings in both rodent and primate models of pediatric HIV-1 that will guide the discussion on validity and choice of model systems.
Rodent Models
Small animal model systems such as mice and rats provide an efficient and accessible method of investigating neuropathogenic mechanisms of pediatric HIV-1. There are, however, a number of limitations of rodent models. Foremost, mice and rats are not the natural hosts of HIV-1 and are not susceptible to HIV-1 infection and therefore do not develop disease.130 To date, there are no known HIV-1 rodent homologues. Alternate strategies to study HIV-1 pathogenesis in rodents131 include intracranial administration of Tat/gp120 proteins,132,133 inducible Tat/gp120 transgenic mouse models,104,134 and humanized mouse models.135,136 Although rodent models are the most common non-tissue-culture means of investigating HIV-1 neuropathogenesis, only the Tat/gp120 intracranial administration has been used to model the neurological consequences of pediatric HIV-1 infection.132,133
Rodent models have demonstrated that Tat1–72 and gp120 are involved in the neuropathophysiology of HIV-1 infection, with the hippocampus being particularly susceptible to the neurotoxic cascade of HIV-1 proteins.124,132,137 Bilateral intrahippocampal administration of Tat1–72 on postnatal day 1 (PND1; third trimester equivalent) in rats has been shown to alter prepulse inhibition that lasted from adolescence into adulthood (PND 30 and 60 for males; PND 30, 60, and 90 for females), suggesting impaired sensorimotor gating, which is a reflection of cognitive processing.138 Tat1–72 administration also impairs spatial memory in adolescence.132 In contrast, neonatal intrahippocampal gp120 administration transiently alters sensory-motor function (deficit at PND 3 but not PND 8);139,140 however, it may alter dopaminergic activity, leading to long-term sensorimotor gating deficits (assessed at PND90–120).141 Combined gp120/Tat1–72 affects eye opening and negative geotaxis (examined at PND 14–16 and PND 3–4, respectively).132 Neonatal administration of the Tat1–86 protein, encoding for exons 1 and 2, results in altered reflex development, increased response latency for negative geotaxis, and failure to habituate in a locomotor activity chamber.133 In this model, design-based stereology revealed that neonatal intrahippocampal gp120 and Tat1–72 administration results in differential and regionally selective cell loss within the hippocampus. gp120 reduces the neuronal population within the of the cornu ammonis subfields 2/3 (CA2/3). In contrast, neonatal intrahippocampal Tat administration reduced the neuronal population in the CA2/3 subfields and the hilus of the dentate gyrus (DGH), elevated the astrocyte population in the DGH and subiculum, and elevated the oligodendrocyte population in the DGH (~PND 200).137 The postnatal timing of intrahippocampal Tat1–72 administration is related to the toxicity of this viral protein. When Tat1–72 administration is delayed to PND10, still in the rodent third trimester equivalent, neuronal numbers within the hippocampus are not altered. However, glial cells and astrocytes are increased in the DGH and subiculum and oligodentrocytes are increased in the DGH, similar to the effects following PND1 Tat1–72 administration.124 Neither intrahippocampal neonatal Tat1–86 or gp120 administration was able to induce inflammatory proteins such as IL-1β, or transcription factors NF-kβ and I-Kβ.133 The effect on cell number in the DGH was indicative of the spatial memory alterations observed in adulthood.132 These results support the hypothesis that Tat plays a significant role in pediatric HIV-1 neuropathogenesis and the development of psychological impairments that are found in HIV-1 infected children.124
The main limitation with neonatal intracranial viral protein administration is that it is not an infection model. The humanized mouse model has the potential to overcome this limitation. Within the past two decades, humanized rodent models have been utilized to study HIV-1 infection.142,143 HIV-1 infection in the humanized rodent model leads to persistent HIV-1 infection and immunopathogenesis, including immune-activation and depletion of human CD4 T cells.144 In recent years, improvements in the ability to engraft human cells and tissues into immunodeficient mice have led to successful infection by various strains of HIV-1, namely, by “knockout” or “knockin” host innate immune system.145 Adult HIV-1 infection in the NOD/scid-IL-2Rγcnull humanized mouse model leads to an influx of CD8+ cells in the brain, microglia activation, neuronal reductions, compromised oligodendrocyte numbers, and meningitis.135,136,142 Behaviorally, these mice have a lack of habituation to the open field, memory loss, and anxiety-like behavior,142 similar to that seen in neonatal gp120/ Tat intracranial injections.133 The humanized mouse model has the potential to unravel the complex neuropathogenesis of HIV-1 infection and be a vessel for testing therapeutic approaches, however this model system has not yet been employed to investigate pediatric HIV-1 infection. Hypothetically, it is possible to humanize mice at a young age, but it does require surgery as well as a 12 week latency between transplantation and reconstitution thereby limiting its value as a potential model of neonatal and pediatric HIV-1 infection.146
The EcoHIV mouse also holds potential as a pediatric model as it takes advantage of a murine retrovirus, ecotropic murine leukemia virus, to recapitulate HIV-1 infection. EcoHIV can be injected systemically with minimal invasiveness to the immunocompetent host.147 EcoHIV has been shown to infect the liver, lung, and brain with an accompanying elevation of IL-6 and TNFα expression, suggesting systemic inflammation after 3–4 weeks of infection of adult mice.148,149 The EcoHIV model presents a relatively inexpensive and accessible model in which to investigate pediatric HIV-1 infection. However, immune and brain development in neonatal rodents differs substantially from human neonates, suggesting limited use of the humanized mouse model to answer questions related to pediatric HIV-1 induced neuropathogenesis.122,150
Nonhuman Primate Models
The complex neuropathogenesis of HIV-1 infection is not readily recapitulated in rodents, necessitating the need for alternative models. Simian immunodeficiency virus (SIV) infection in macaques is a valid alternative, because SIV and HIV-1 have similar pathogenesis, including routes of transmission, infection of CD4+T cells and macrophages, immune suppression, disease progression and neurological complications in juvenile and adult primates.151 Moreover, mother-to-child transmission (MTCT) can occur by the same routes in both monkeys and humans.122 In addition, infant macaques show similar immune and neurodevelopment to human infants.122,152,153
There are several reported models investigating the neuropathogenesis of pediatric SIV infection. In the pigtailed macaque (Macaca nemestrina) model, vertical infection was induced by intravenous inoculation of the dam during the third trimester with HIV-2287, with 58% of the infants being infected at birth. These infants displayed significant cognitive and motor delays in the Well and Screen Task used to test object permanence and the Fine Motor Task used to evaluate motor capabilities. Deficits in motor and cognitive development were correlated with CD4+ lymphocyte cell counts at birth.154 Another cohort of pigtailed macaques that received perinatal intravenous or intrathecal HIV-2287 on PND36 displayed similar behavioral manifestations to those infected in utero. Viral RNA was detectable in the cerebrospinal fluid (CSF) within one week postinoculation and peaked within 2–3 weeks followed by a decline. The concentration of quinolinic acid, an associated marker of neuronal death, was elevated 4–8-fold within 5 weeks postinoculation. Histopathologically, these infected animals displayed evidence of periventricular white matter loss, microgliosis, perivascular lymphocyte infiltration, and neuronal degeneration.154,155 The extent and type of cell loss, however, has not been reported in this model.
In the rhesus macaque (Macaca mulatta) model, three isolates of SIV (SIVmac239, SIVmac239/316, and SIVmac251), known to penetrate the CNS, have been used to investigate pediatric HIV-1. In one study comparing the three isolates, subjects (n = 18) were intravenously inoculated within 24 h of birth with approximately 103 50% tissue culture infectious doses/kg with one of the isolates.128 Histological lesions of the CNS included perivascular lymphocyte infiltration with in the basal ganglia and cortical white and gray matter. Only one subject had detectable gp120 protein by immunohistochemistry in the CNS. In order to detect the virus in the CNS, a more sensitive PCR-based probe had to be used. This method detected viral DNA as early as 3 days postinoculation mainly in the cortical gray matter and basal ganglia. Viral RNA was detectable in the CSF of all subjects within 14 days of inoculation.128
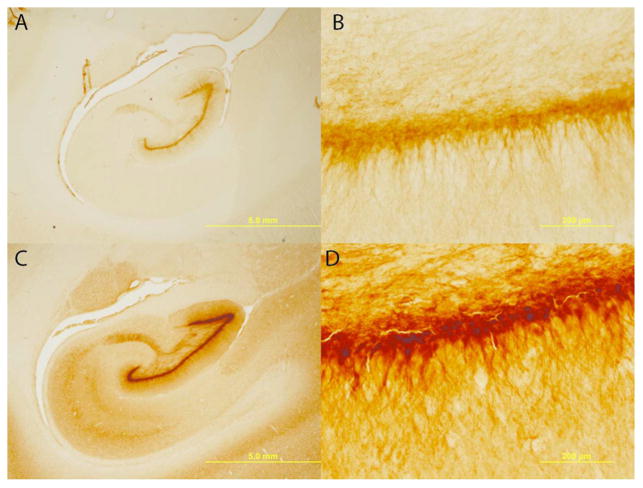
As described extensively, SIVmac251 infected newborn rhesus macaques infected intravenously or orally with virulent, uncloned SIVmac251 show persistently high viremia and rapid immunosuppression, with the majority of animals developing clinical disease and meeting the criteria for euthanasia (often including neurological signs) within 6 months of infection.156 In one study, newborn rhesus macaques received 100 tissue culture doses of 50% (TCID50) of SIVmac251 within 72 h by the intravenous route to ensure a 100% infection rate.122 Animals were sacrificed when they met clinical criteria for euthanasia of retrovirus-infected animals, as early as 7–10 weeks post-infection. Brains were extracted and prepared for histological analysis.157 Each brain was serially sectioned, with each hemisphere yielding approximately 1400 sections and banked in antigen preserve. This method of serial sectioning and brain banking maximizes the utility for design-based stereological analysis and immunohistochemistry.158–160 Design-based stereology is a mechanism for quantitatively estimating cell populations within a given brain region while reducing the bias of cell shape, size, orientation, and distribution.158 Data from this model indicates that, within two months of infection, SIV significantly reduces the hippocampal neuronal population (Figure 1) in the pyramidal layer of the CA1, CA2, and CA3 subregions. Immature neurons within the dentate gyrus also experience a significant loss (Figure 2).161 This is congruent with adolescent and adult rodent models that have also demonstrated attenuated neurogenesis. The loss of immature neurons and pyramidal neurons may explain the neuropathogenesis and long-term neurological consequences observed in HIV-1 positive children.104,121,162–164 Potentially exacerbating the neurological consequences of pediatric HIV-1 infection is potential demyelination. In humans, myelination is developmentally protracted throughout childhood with adult levels not being obtained until sexual maturity.165 The effects of HIV-1 infection on this prolonged myelination are still unclear; however, clinical data suggests multiple sclerosis type behavior58 and reduced radial diffusivity, an indicator of demyelination, in diffusion tensor imaging56 have been reported. There are also reductions in hippocampal myelination in our perinatally SIV-infected subjects166 (Figure 3), which may provide the anatomical basis for clinical reports. Data from this model do not preclude deficits in other brain areas. The brain banking of histological sections provides versatility for exploring the extent and type of neuronal loss in other brain areas and potential mechanisms of neurotoxicity through immunohistochemistry.
Figure 1.
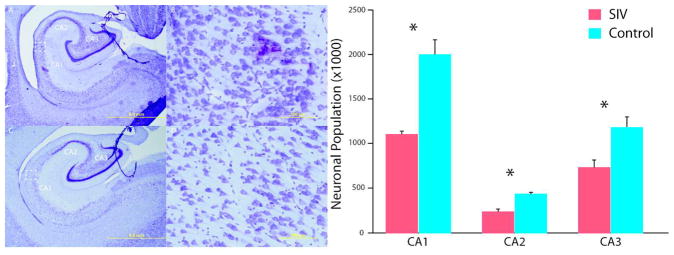
Hippocampal neuronal loss. SIV infected infants have apparent enlarged ventricles and thinned pyramidal neuronal layers (A, B) compared to control subjects (C, D). In these cresyl violet stained sections, neurons can be differentiated from glia based on a clearly visible nucleolus surrounded by cytoplasm. The CA fields were delineated on the basis of cyto- and chemoarchitecture, and equidistant sections were evaluated throughout the entire length of the hippocampus. Design-based stereology of the hippocampal CA subregions found an overall 42% neuronal reduction. There were no overall volume differences in hippocampus. Magnifications of (A, C) 1.25× and (B, D) 20×; scale bars = 5 mm and 200 μm, respectively. Figure adapted from Curtis et al., 2014.157
Figure 2.
Immature neuronal loss. Serial sections from the entire extent of the hippocampus were immunostained with doublecortin, a putative marker for immature neurons.193 At this developmental period, immature neurons densely populate the dentate gyrus as evidenced in the control subjects (C, D). In the SIV subjects (A, B), however, individual neurons can be detected throughout the dentate gyrus, suggesting an apparent lack of double cortin positive neurons. Magnifications of (A, C) 1.25× and (B, D) 20×; scale bars = 5 mm and 200 μm, respectively. Figure adapted from Curtis et al., 2014.157
Figure 3.
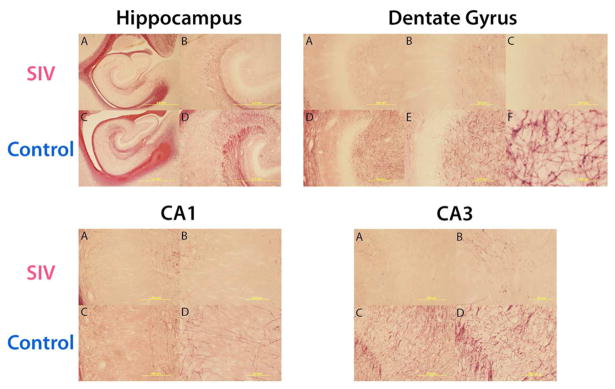
Myelination. Matched sections throughout the hippocampus were stained with gold chloride, a putative marker for myelin.194 Reductions in myelination are apparent in each region of the hippocampus of SIV infected subjects as compared to control subjects. It is not clear if the reduction of myelination is due to decreased neurons, axonal degeneration, or demyelination of axons. These reductions of myelination further validate the clinical relevance of this model. Magnifications: hippocampus 1.25× (A, C), 4× (B, D); dentate gyrus 10× (A, D), 20× (B, E), and 100× (C, F); CA1 and CA3 10× (A, C), 20× (B, D).
Can Animal Models Be Utilized to Study Neurological Consequences of Pediatric HIV-1 Infection?
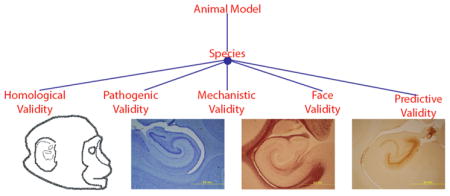
Cell culture systems have been used extensively to elucidate the cytotoxic roles of HIV-1 genes (gag, pol, env, tat, rev, vif, vpu, vpr, and nef), but are limited in their ability to address dynamic physiological interplay between cell types, proteins and organ systems.167 Animal models are critical for investigating the complex neuropathogenesis of HIV-1 during each phase of infection. The development and choice of animal model depends on the question being proposed. The seminal work of Willner168 set forth a convention of criterion to evaluate animal model validity that included face, predictive and construct validity. These criteria have further been expanded to include homological, pathogenic, mechanistic, face, and predictive validity.169
Homological validity is used to assess species and strain in relation to the research question.
Pathogenic validity addresses disease process similarities (i.e., transformation into a pathological condition).
Mechanistic validity refers to the ability of the model to assess proposed mechanisms of action in the human condition by producing similar behaivoural/cognitive signs and biological markers that are reactive to human therapeutic agents.
Face validity refers to the similarity of observable disease features between the animal and human condition, including disease-induced behavioral and biomarker alterations.
Predictive validity concentrates on the ability of the model to make predictions about the efficacy of pharmacological interventions aimed at reducing disease related signs in the model system as well as the relationship between disease induction and its observable effects on the organism.169
These five criteria will be used in this Review to assess the relative strengths and limitations of both rodent and nonhuman primate animal models that investigate the neurological consequences pediatric HIV-1 infection (Table 1).
Table 1.
Animal Models of Pediatric HIV-1a
| species | method | model task | homological validity |
pathogenic validity |
mechanistic validity
|
face validity
|
predictive validity
|
ref | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| behavioral/ cognitive |
biological markers |
behavioral/ cognitive |
biological markers |
ART | nonART | ||||||
| rat | neonatal intrahippocampal viral protein injection | effect of viral proteins | strength | limited | strength | strength | strength | strength | NA* | NA* | 124, 133, 134, 138–142 |
| neonatal intrahippocampal viral protein injection | infection model and CNS outcome | limited | limited | limited | limited | limited | limited | limited | limited | 124, 133, 134, 138–142 | |
| humanized mouse | immunodeficient mice engrafted with human cells | effect of viral proteins | NA* | NA* | NA* | NA* | NA* | NA* | NA* | NA* | 134, 136, 137, 143–145 |
| immunodeficient mice engrafted wih human cells | infection model and CNS outcome | NA* | NA* | NA* | NA* | NA* | NA* | NA* | NA* | 134, 136, 137, 143–145 | |
| EcoHIV mouse | immnucompetent host injected systemically with EcoHIV | effect of viral proteins | NA* | NA* | NA* | NA* | NA* | NA* | NA* | NA* | 148–150 |
| immnucompetent host injected systemically with EcoHIV | infection model and CNS outcome | NA* | NA* | NA* | NA* | NA* | NA* | NA* | NA* | 148–150 | |
| Macaca nemestrina | IV inoculation during the third trimester with HIV-2287 | infection model and CNS outcome | strength | strength | strength | strength | strength | strength | NA* | NA* | 155 |
| Macaca nemestrina | perinatal IV or IT HIV-2287 inoculation on PND36 | infection model and CNS outcome | strength | strength | strength | strength | strength | strength | NA* | NA* | 155 |
| Macaca mulatta | IV inoculated within 24 h of birth with SIVmac239, SIVmac239/316, or SIVmac251 | infection model and CNS outcome | strength | strength | NA* | Strength | NA* | strength | NA* | NA* | 128 |
| Macaca mulatta | IV inoculation of SIVmac25i within 72 h of birth | infection model and CNS outcome | strength | strength | strength | strength | strength | strength | strength | NA* | 157 158 162 167, 181–184, |
| Macaca mulatta | OralSIV | Infection model and CNS outcome | strength | strength | NA* | NA* | NA* | NA* | strength | NA* | 122 126, |
Compared to juvenile and adult studies, there are relatively few animal models that specifically investigate the neurological consequences of perinatal HIV-1 infection. While no single animal model fully recapitulates pediatric HIV-1 infection, each model possesses its own unique strengths and limitations. Here we summarize the relative strengths and limitations of pediatric HIV-1 models according to validity criteria.169 The relative strength of each model in the various categories depends on the proposed research question and results obtained in published reports. Within predictive validity, these models have not yet tested specific pharmacological treatments aimed at reducing the neurological burden of HIV-1 infection. With that caveat, each of the presented models possesses the potential to test novel non-cART therapies aimed at reducing the neurological consequences of pediatric HIV-1. NA*: Published data for these categories are not available in these specific animal models; however, these models do hold potential for these categories.
Homological Validity
On the surface, it would seem that nonhuman primate model systems would provide the highest degree of homological validity considering the similarities with humans in overall fetal and infant development, including neurodevelopment,150 homologous brain areas,170,171 immune systems,122,172 and the homologous nature of SIV to HIV-1.122,151 The degree of homological validity depends on the investigative question, which is interrelated to mechanistic validity. For example, to study the roles of specific HIV-1 proteins on the developing dopamine system, intracranial injection rodent models would provide homologic validity.173 Alternatively, if the aim of the investigation is to examine the host–virus interaction to include systemic infection and related inflammatory cytokine actions in the pediatric setting, then the humanized mouse, EcoHIV mouse, or SIV primate models could also provide the homologous validity.
Pathogenic Validity
Although neonatal intracranial injection of viral proteins results in long-term behavioral and anatomical alterations, it is not pathogenic and neuroinflammatory cytokine activity is not elevated38,132,133,137,138,140 and therefore is limited in pathogenic validity. The humanized mouse and EcoHIV mouse models have the potential to overcome this limitation124,148,149,174 but have not yet been used in the pediatric setting. Infant macaques infected with SIVmac251 will generally progress into simian AIDS within 6 months.122 Neonatally, SIV can be delivered either orally or intravenously, rapidly disseminate within 1 week, and cause elevated systemic proinflammatory cytokine activity and monocyte infection.122,126,128,154 Neonatally SIV-infected animals also show CNS penetration of the virus.128 Given that the humanized and EcoHIV mouse models have not yet been tested in neonatal mice, the pediatric SIV models reviewed here offer a superior pathogenic validity.
Mechanistic Validity
Neonatal rodent and primate models offer unique strengths in terms of mechanistic validity. First the neonatal rodent intracranial injection model allows for the in vivo analysis of specific HIV-1 proteins on neurodevelopment. In this manner, it is possible to dissociate the mechanistic actions of each of the HIV-1 related proteins.117,111,102,116 The limitation of this approach is that CNS and systemic inflammatory proteins, which are thought to participate in the pathogenesis of HIV-1,87,111–115 are not activated. Pediatric SIV infection overcomes this limitation, but then suffers from the inability to delineate specific roles of viral proteins.
Face Validity
Similar to mechanistic validity, both neonatal rodent and primate models parallel the observable disease features of the human condition. Impaired sensorimotor gating, cognitive processing,138 spatial memory,132 and sensory-motor function139,140 along with elevated numbers of glial cells and astrocytes124 and differential decreases in hippocampal neuronal populations137 have all been reported in neonatal rodent models. Likewise, in the several models of pediatric SIV infection, subjects have shown cognitive and motor delays,129 elevated CSF markers of neuronal death, periventricular white matter loss, microgliosis,130 perivascular lymphocyte infiltration,106,130 hippocampal neuronal loss, immature neuronal loss, and demyelination.155 Results from both rodent and nonhuman primate models support face validity based on similar behavioral/cognitive signs and biological markers to those reported in HIV-1 infected children.124
Predictive Validity
Since the advent of ART, the prevalence of severe neurological impairment has declined in both the pediatric38,43–50 and adult175 clinical settings. Despite ART therapy success in partially ameliorating the neurological consequences and increasing the life expectancy of infected individuals, there remains controversy in its use in both pediatric and adult HIV-1 patients.175–178 In particular, there is potential for chronic ART to contribute to CNS and peripheral nervous system (PNS) neurotoxicity through oxidative stress mechanisms.175,176,179 The majority of therapeutic interventions have concentrated on controlling systemic viral infection and its neurological consequences through ART.175,176 There is robust literature reviewing the efficacy of ART in adult nonhuman primate and rodent models,175–177,179 so the evaluation of predictive validity here will concentrate on the pediatric primate model system.
The SIVmac251 pediatric animal model of SIV-infected newborn macaques has been used to test the efficacy of antiviral drugs. Early studies demonstrated that pre- or early postexposure zidovudine treatment led to reduced viremia, delayed disease progression with improved CNS function in SIVmac251- or SIVsmm/B670-infected newborn macaques.180–182 In a later study, treatment of SIVmac251-infected infant macaques with the more potent drug tenofovir (PMPA) was the first demonstration of in vivo efficacy of this c gtompound against established SIV infection.183 Some tenofovir-treated animals survived for 7–14 years, without any significant lesions observed on routine brain histopathology.183,184 This high efficacy of tenofovir was translated into clinical practice, as tenofovir has become a widely used drug to treat adult and pediatric HIV-1 infection. Despite the limited data in SIV-infected newborn macaques, several antiretroviral drug studies in SIV-infected juvenile or adult macaques included more detailed evaluation of neurological function, histopathology or virus levels in the CNS. In an established model of neuropathogenesis in which animals are infected with a combination of neurovirulent SIV/17E-fr and the immunosuppressive strain SIV/Deltab670, relative early therapy (12–24 days after infection, i.e., acute viremia) with maraviroc, quadruple antiretroviral therapy, or with the antibiotic minocycline had neuroprotective effects based on viral RNA levels in CSF and brain, markers of inflammation and immune activation, and amyloid precursor protein levels.185,186 Simian immunodeficiency virus infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA.186,187 Few studies investigated the effect of drug treatment during chronic infection. Fox et al.188 demonstrated that tenofovir treatment of SIV-infected adult macaques during the chronic stage of infection normalized neurophysiological abnormalities, but not movement abnormalities.
Limiting the effectiveness of ART to treat neurological consequences of HIV-1 infection is the ability of the various antiretrovirals to penetrate the CNS. The CNS penetration effectiveness (CPE) ranks provides a scale based on the pharmocodynamic properties of the antiretroviral drugs to penetrate the CNS and reduce CSF viral loads with higher scores correlating to lower detectable CSF viral loads. For example, tenofovir has a CPE score of 1 with few reports of adverse CNS effects, while nevirapine has a rank of 4 but patients have reported psychotic symptoms.178
A recent trend is to develop and test non-ART pharmacological agents to treat HIV-1 related neuropathology. In both adult and neonatal HIV-1 models, neurogenesis and proliferation are altered by viral infection103,161,164,189 which can be a prime target for intervention.104,105,190 Like most other areas of HIV-1 research, the focus has been on adult models, but pediatric rodent and nonhuman primate models presented here offer a unique platform to test therapeutic intervention aimed at ameliorating the negative consequences of HIV-1 in the CNS.
In summary, the use of SIV-infected nonhuman primates as a model for pediatric neuropathogenesis has been limited, possibly due to cost, accessibility, or institutional infrastructure. Available data suggests that perinatally infected primates share a similar neuropathophysiology to their human counterparts, and that antiretroviral treatment, especially if initiated early, has beneficial effects. Pediatric SIV models evaluating CNS involvement are scarce, but could be utilized effectively in future studies to close gaps in our knowledge, such as mechanisms of early infection, the effects of ART on neurodevelopment, the potential of the CNS as reservoir for latent virus, the long-term behavioral implications, and the development of new strategies to reduce mechanisms of neurological dysfunction that are not resolved by antiretrovirals.
CONCLUSIONS
Early ART intervention partially ameliorates the neurological consequences of perinatal HIV-1 infection; however, deficits persist even with successful viral suppression.38,43–46 Despite the neurologic improvement with ART, there is evidence to suggest that some ART regimens may act synergistically with HIV-1 to induce neuronal damage in the CNS46,179,191 and thus remain controversial. Thus, given the persistent high rate of HIV-1 infection in infants in resource-poor countries, and increased life expectancy of HIV-1 infected children receiving an overall health benefit ART, there is an urgent need to assess the impact of HIV-1 infection and ART therapy on neurodevelopment, with the goal of optimizing ART regimens. Neonatal rodent HIV-1 and perinatal SIV models support clinical evidence that the neurons of the hippocampus, as well as hippocampal neurogenesis, are particularly susceptible to the neurotoxic cascade of HIV-1 proteins.132,133,137,140,161 Although the immature neuronal population is susceptible to perinatal HIV-1 infection, it may also be the key to therapeutic intervention aimed at reducing the impact of HIV-1 induced neurological impairment.39,104,121,192 The extent of HIV-1 infection of specific cell types, neuronal loss, and its relationship to viral loads, and the CNS as a potential reservoir for latent HIV-1 in infants is currently unknown. This limits the ability to develop and evaluate therapeutic paradigms to minimize the neurological impairments as a result of HIV-1 infection. While each animal model presents its own limitations, rodent and nonhuman primate models are poised to address specific mechanistic and therapeutic questions.
Acknowledgments
Funding
National Science Foundation and the WBHR-LSAMP Program (NSF HRD-100286) to M.S. and J.L. District of Columbia Developmental Center for AIDS Research (P30AI087714), Latham Trust Foundation Grant, and R03MH107261 to M.W.B.; and 1R01DE019064 (NIH/NIDCR) and 1R01DE022285 (NIH/NIDCR) to K.D.P.; and P51OD011107 (Office of Research Infrastructure Programs/ OD) to CNPRC.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.United Nations. United Nations Millennium Declaration. United Nations General Assembly; New York: 2000. [Google Scholar]
- 2.UNAIDS. Global Report UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS (UNAIDS); Geneva: 2013. pp. 1–198. [Google Scholar]
- 3.WHO. Taking stock: HIV in children. World Health Organization; Geneva: 2006. p. 12. [Google Scholar]
- 4.UNAIDS. It takes a village: Ending mother-to-child transmission a partnership uniting the Millennium Villages Project and UNAIDS. 2010 http://search.unaids.org/search.asp?lg=en&search=ittakesavillage.
- 5.UNAIDS; UNICEF/UNAIDS/WHO/UNFPA, editor Children and AIDS fifth Stocktaking report, 2010. 2010 http://www.unaids.org/en/targetsandcommitments/eliminatingnewhivinfectionamongchildren/
- 6.UNAIDS. UNAIDS Fact Sheet 2014. UNAIDS; Geneva: 2014. [Google Scholar]
- 7.Lane JH, Tarantal AF, Pauley D, Marthas M, Miller CJ, Lackner AA. Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am J Pathol. 1996;149:1097–1104. [PMC free article] [PubMed] [Google Scholar]
- 8.Luzuriaga K, Newell ML, Dabis F, Excler JL, Sullivan JL. Vaccines to prevent transmission of HIV-1 via breastmilk: Scientific and logistical priorities. Lancet. 2006;368:511–521. doi: 10.1016/S0140-6736(06)69159-9. [DOI] [PubMed] [Google Scholar]
- 9.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, Essex M, Ekouevi DK, Jackson D, Coutsoudis A, Kilewo C, Leroy V, Wiktor SZ, Nduati R, Msellati P, Zaba B, Ghys PD, Newell ML. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: A meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect. 2012;88(Suppl 2):i44–51. doi: 10.1136/sextrans-2012-050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaul M. HIV’s double strike at the brain: Neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, Van Dyke RB. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquired Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mofenson LM, Cotton MF. The challenges of success: Adolescents with perinatal HIV infection. J Int AIDS Soc. 2013;16:18650. doi: 10.7448/IAS.16.1.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai N, Mullen P, Mathur M. Lipodystrophy in pediatric HIV. Indian J Pediatr. 2008;75:351–354. doi: 10.1007/s12098-008-0037-2. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Smit C, van Rossum AM, Fraaij PL, Wolfs TF, Geelen SP, Scholvinck EH, Warris A, Scherpbier HJ, Pajkrt D. Long-term response to combination antiretroviral therapy in HIV-infected children in the Netherlands registered from 1996 to 2012. AIDS. 2013;27:2567–2575. doi: 10.1097/01.aids.0000432451.75980.1b. [DOI] [PubMed] [Google Scholar]
- 16.Nations U. In: The Millennium Development Goals Report. Too-Kong T, editor. United Nations; New York: 2014. [Google Scholar]
- 17.CDC. Achievements in public health. Reduction in perinatal transmission of HIV infection—United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 18.Lu D, Liu J, Samson L, Bitnun A, Seigel S, Brophy J, Leonard L, Remis RS. Factors responsible for mother-to-child HIV transmission in Ontario, Canada, 1996–2008. Can J Public Health. 2014;105:e47–52. doi: 10.17269/cjph.105.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. HIV Among Pregnant Women, Infants, and Children. 2014 http://www.cdc.gov/hiv/risk/gender/pregnantwomen/facts/index.html.
- 20.Dowshen N, D’Angelo L. Health care transition for youth living with HIV/AIDS. Pediatrics. 2011;128:762–771. doi: 10.1542/peds.2011-0068. [DOI] [PubMed] [Google Scholar]
- 21.Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. doi: 10.7448/IAS.16.1.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidari S, Mofenson LM, Hobbs CV, Cotton MF, Marlink R, Katabira E. Unresolved antiretroviral treatment management issues in HIV-infected children. J Acquired Immune Defic Syndr. 2012;59:161–169. doi: 10.1097/QAI.0b013e3182427029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: Children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 24.MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav. 2013;17:86–93. doi: 10.1007/s10461-012-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somarriba G, Lopez-Mitnik G, Ludwig DA, Neri D, Schaefer N, Lipshultz SE, Scott GB, Miller TL. Physical fitness in children infected with the human immunodeficiency virus: Associations with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29:112–120. doi: 10.1089/aid.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel K, Wang J, Jacobson DL, Lipshultz SE, Landy DC, Geffner ME, Dimeglio LA, Seage GR, 3rd, Williams PL, Van Dyke RB, Siberry GK, Shearer WT, Young L, Scott GB, Wilkinson JD, Fisher SD, Starc TJ, Miller TL. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation. 2014;129:1204–1212. doi: 10.1161/CIRCULATIONAHA.113.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipshultz SE, Miller TL, Wilkinson JD, Scott GB, Somarriba G, Cochran TR, Fisher SD. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597. doi: 10.7448/IAS.16.1.18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipshultz SE, Williams PL, Wilkinson JD, Leister EC, Van Dyke RB, Shearer WT, Rich KC, Hazra R, Kaltman JR, Jacobson DL, Dooley LB, Scott GB, Rabideau N, Colan SD. Cardiac status of children infected with human immunodeficiency virus who are receiving long-term combination antiretroviral therapy: Results from the Adolescent Master Protocol of the Multicenter Pediatric HIV/AIDS Cohort Study. JAMA Pediatr. 2013;167:520–527. doi: 10.1001/jamapediatrics.2013.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16:18600. doi: 10.7448/IAS.16.1.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisiacchi PS, Suppiej A, Laverda A. Neuropsychological evaluation of neurologically asymptomatic HIV-infected children. Brain Cognit. 2000;43:49–52. [PubMed] [Google Scholar]
- 31.Smith RJ. Adherence to antiretroviral HIV drugs: How many doses can you miss before resistance emerges? Proc Biol Sci. 2006;273:617–624. doi: 10.1098/rspb.2005.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith R, Malee K, Leighty R, Brouwers P, Mellins C, Hittelman J, Chase C, Blasini I. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006;117:851–862. doi: 10.1542/peds.2005-0804. [DOI] [PubMed] [Google Scholar]
- 33.Kapetanovic S, Leister E, Nichols S, Miller T, Tassiopoulos K, Hazra R, Gelbard HA, Malee KM, Kammerer B, Mendez AJ, Williams PL. Relationships between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS. 2010;24:1481–1491. doi: 10.1097/QAD.0b013e32833a241b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster SB, Lu M, Glaze DG, Reuben JM, Harris LL, Cohen EN, Lee BN, Zhao E, Paul ME, Schwarzwald H, McMullen-Jackson C, Clark C, Armstrong FD, Brouwers PY, Miller TL, Colin AA, Scott GB, Shahzeidi S, Willen EJ, Asthana D, Lipshultz SE, Thompson BW, Shearer WT. Associations of cytokines, sleep patterns, and neurocognitive function in youth with HIV infection. Clin Immunol. 2012;144:13–23. doi: 10.1016/j.clim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker SY, Pierre RB, Christie CD, Chang SM. Neurocognitive function in HIV-positive children in a developing country. Int J Infect Dis. 2013;17:e862–867. doi: 10.1016/j.ijid.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadow KD, Angelidou K, Chernoff M, Williams PL, Heston J, Hodge J, Nachman S. Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr. 2012;33:456–468. doi: 10.1097/DBP.0b013e31825b8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govender R, Eley B, Walker K, Petersen R, Wilmshurst JM. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. J Child Neurol. 2011;26:1355–1364. doi: 10.1177/0883073811405203. [DOI] [PubMed] [Google Scholar]
- 39.Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: Pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Pearson DA, McGrath NM, Nozyce M, Nichols SL, Raskino C, Brouwers P, Lifschitz MC, Baker CJ, Englund JA. Predicting HIV disease progression in children using measures of neuropsychological and neurological functioning. Pediatric AIDS clinical trials 152 study team. Pediatrics. 2000;106:E76. doi: 10.1542/peds.106.6.e76. [DOI] [PubMed] [Google Scholar]
- 42.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS; Geneva: 2013. [Google Scholar]
- 43.van Arnhem LA, Bunders MJ, Scherpbier HJ, Majoie CB, Reneman L, Frinking O, Poll-The BT, Kuijpers TW, Pajkrt D. Neurologic abnormalities in HIV-1 infected children in the era of combination antiretroviral therapy. PLoS One. 2013;8:e64398. doi: 10.1371/journal.pone.0064398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: implications for clinical practice and management challenges in the African setting. Semin Pediatr Neurol. 2014;21:3–11. doi: 10.1016/j.spen.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Foster CJ, Biggs RL, Melvin D, Walters MD, Tudor-Williams G, Lyall EG. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. 2006;48:677–682. doi: 10.1017/S0012162206001423. [DOI] [PubMed] [Google Scholar]
- 46.Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:316–331. doi: 10.1002/rmv.1793. [DOI] [PubMed] [Google Scholar]
- 47.Lowick S, Sawry S, Meyers T. Neuro-developmental delay among HIV-infected preschool children receiving antiretroviral therapy and healthy preschool children in Soweto, South Africa. Psychol Health Med. 2012;17:599–610. doi: 10.1080/13548506.2011.648201. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26:497–504. doi: 10.1080/09540121.2013.841828. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Ter Stege JA, Geurtsen GJ, Scherpbier HJ, Kuijpers TW, Reiss P, Schmand B, Pajkrt D. Poorer cognitive performance in perinatally HIV-infected children as compared to healthy socioeconomically matched controls. Clin Infect Dis. 2015;60:1111–1119. doi: 10.1093/cid/ciu1144. [DOI] [PubMed] [Google Scholar]
- 50.Ferguson G, Jelsma J. The prevalence of motor delay among HIV infected children living in Cape Town, South Africa. Int J Rehabil Res. 2009;32:108–114. doi: 10.1097/MRR.0b013e3283013b34. [DOI] [PubMed] [Google Scholar]
- 51.Thomaidis L, Bertou G, Critselis E, Spoulou V, Kafetzis DA, Theodoridou M. Cognitive and psychosocial development of HIV pediatric patients receiving highly active anti-retroviral therapy: a case-control study. BMC Pediatr. 2010;10:99. doi: 10.1186/1471-2431-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol. 2002;21:223–241. doi: 10.1207/S15326942DN2103_1. [DOI] [PubMed] [Google Scholar]
- 53.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, Kerr SJ, Kanjanavanit S, Ngampiyaskul C, Wongsawat J, Luesomboon W, Vibol U, Pruksakaew K, Suwarnlerk T, Apornpong T, Ratanadilok K, Paul R, Mofenson LM, Fox L, Valcour V, Brouwers P, Ruxrungtham K. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013;32:501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puthanakit T, Aurpibul L, Louthrenoo O, Tapanya P, Nadsasarn R, Insee-ard S, Sirisanthana V. Poor cognitive functioning of school-aged children in thailand with perinatally acquired HIV infection taking antiretroviral therapy. AIDS Patient Care STDS. 2010;24:141–146. doi: 10.1089/apc.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoare J, Fouche JP, Spottiswoode B, Donald K, Philipps N, Bezuidenhout H, Mulligan C, Webster V, Oduro C, Schrieff L, Paul R, Zar H, Thomas K, Stein D. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive “slow progressors”. J Neurovirol. 2012;18:205–212. doi: 10.1007/s13365-012-0099-9. [DOI] [PubMed] [Google Scholar]
- 57.Samia P, Petersen R, Walker KG, Eley B, Wilmshurst JM. Prevalence of seizures in children infected with human immunodeficiency virus. J Child Neurol. 2013;28:297–302. doi: 10.1177/0883073812446161. [DOI] [PubMed] [Google Scholar]
- 58.Facchini SA, Harding SA, Waldron RL. Human immunodeficiency virus-1 infection and multiple sclerosis-like illness in a child. Pediatr Neurol. 2002;26:231–235. doi: 10.1016/s0887-8994(01)00378-2. [DOI] [PubMed] [Google Scholar]
- 59.Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, Yogev R, McIntosh K, Stanley K, Pelton S. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 60.Singh KK, Ellis RJ, Marquie-Beck J, Letendre S, Heaton RK, Grant I, Spector SA. CCR2 polymorphisms affect neuropsychological impairment in HIV-1-infected adults. J Neuroimmunol. 2004;157:185–192. doi: 10.1016/j.jneuroim.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 61.Fraser C, Lythgoe K, Leventhal GE, Shirreff G, Hollingsworth TD, Alizon S, Bonhoeffer S. Virulence and pathogenesis of HIV-1 infection: an evolutionary perspective. Science. 2014;343:1243727. doi: 10.1126/science.1243727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geretti AM. HIV-1 subtypes: epidemiology and significance for HIV management. Curr Opin Infect Dis. 2006;19:1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 63.Siddappa NB, Dash PK, Mahadevan A, Jayasuryan N, Hu F, Dice B, Keefe R, Satish KS, Satish B, Sreekanthan K, Chatterjee R, Venu K, Satishchandra P, Ravi V, Shankar SK, Shankarappa R, Ranga U. Identification of subtype C human immunodeficiency virus type 1 by subtype-specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol. 2004;42:2742–2751. doi: 10.1128/JCM.42.6.2742-2751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell GR, Loret EP, Spector SA. HIV-1 clade B Tat, but not clade C Tat, increases X4 HIV-1 entry into resting but not activated CD4+ T cells. J Biol Chem. 2010;285:1681–1691. doi: 10.1074/jbc.M109.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra M, Vetrivel S, Siddappa NB, Ranga U, Seth P. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol. 2008;63:366–376. doi: 10.1002/ana.21292. [DOI] [PubMed] [Google Scholar]
- 66.Shankar SK, Mahadevan A, Satishchandra P, Kumar RU, Yasha TC, Santosh V, Chandramuki A, Ravi V, Nath A. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res. 2005;121:468–488. [PubMed] [Google Scholar]
- 67.Mishra V, Assche SB, Greener R, Vaessen M, Hong R, Ghys PD, Boerma JT, Van Assche A, Khan S, Rutstein S. HIV infection does not disproportionately affect the poorer in sub-Saharan Africa. AIDS. 2007;21(Suppl 7):S17–28. doi: 10.1097/01.aids.0000300532.51860.2a. [DOI] [PubMed] [Google Scholar]
- 68.Rao VR, Sas AR, Eugenin EA, Siddappa NB, Bimonte-Nelson H, Berman JW, Ranga U, Tyor WR, Prasad VR. HIV-1 clade-specific differences in the induction of neuropathogenesis. J Neurosci. 2008;28:10010–10016. doi: 10.1523/JNEUROSCI.2955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong JK, Campbell GR, Spector SA. Differential induction of interleukin-10 in monocytes by HIV-1 clade B and clade C Tat proteins. J Biol Chem. 2010;285:18319–18325. doi: 10.1074/jbc.M110.120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell GR, Watkins JD, Loret EP, Spector SA. Differential induction of rat neuronal excitotoxic cell death by human immunodeficiency virus type 1 clade B and C tat proteins. AIDS Res Hum Retroviruses. 2011;27:647–654. doi: 10.1089/aid.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Msellati P, Lepage P, Hitimana DG, Van Goethem C, Van de Perre P, Dabis F. Neurodevelopmental testing of children born to human immunodeficiency virus type 1 seropositive and seronegative mothers: a prospective cohort study in Kigali, Rwanda. Pediatrics. 1993;92:843–848. [PubMed] [Google Scholar]
- 72.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 73.Bagenda D, Nassali A, Kalyesubula I, Sherman B, Drotar D, Boivin MJ, Olness K. Health, neurologic, and cognitive status of HIV-infected, long-surviving, and antiretroviral-naive Ugandan children. Pediatrics. 2006;117:729–740. doi: 10.1542/peds.2004-2699. [DOI] [PubMed] [Google Scholar]
- 74.McGrath N, Bellinger D, Robins J, Msamanga GI, Tronick E, Fawzi WW. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics. 2006;117:e216–225. doi: 10.1542/peds.2004-1668. [DOI] [PubMed] [Google Scholar]
- 75.Drotar D, Olness K, Wiznitzer M, Schatschneider C, Marum L, Guay L, Fagan J, Hom D, Svilar G, Ndugwa C, Mayengo RK. Neurodevelopmental outcomes of Ugandan infants with HIV infection: An application of growth curve analysis. Health Psychol. 1999;18:114–121. doi: 10.1037//0278-6133.18.2.114. [DOI] [PubMed] [Google Scholar]
- 76.Wilmshurst JM, Donald KA, Eley B. Update on the key developments of the neurologic complications in children infected with HIV. Curr Opin HIV AIDS. 2014;9:533–538. doi: 10.1097/COH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 77.Jeremy RJ, Kim S, Nozyce M, Nachman S, McIntosh K, Pelton SI, Yogev R, Wiznia A, Johnson GM, Krogstad P, Stanley K. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- 78.Zink WE, Zheng J, Persidsky Y, Poluektova L, Gendelman HE. The neuropathogenesis of HIV-1 infection. FEMS Immunol Med Microbiol. 1999;26:233–241. doi: 10.1111/j.1574-695X.1999.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 79.Johann-Liang R, Lin K, Cervia J, Stavola J, Noel G. Neuroimaging findings in children perinatally infected with the human immunodeficiency virus. Pediatr Infect Dis J. 1998;17:753–754. doi: 10.1097/00006454-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 80.Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART) J Pediatr. 2005;146:402–407. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 81.Tahan TT, Bruck I, Burger M, Cruz CR. Neurological profile and neurodevelopment of 88 children infected with HIV and 84 seroreverter children followed from 1995 to 2002. Braz J Infect Dis. 2006;10:322–326. doi: 10.1590/s1413-86702006000500004. [DOI] [PubMed] [Google Scholar]
- 82.Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, Deville J, Church JA, Thomas MA. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage: Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagarajan R, Sarma MK, Thomas MA, Chang L, Natha U, Wright M, Hayes J, Nielsen-Saines K, Michalik DE, Deville J, Church JA, Mason K, Critton-Mastandrea T, Nazarian S, Jing J, Keller MA. Neuropsychological function and cerebral metabolites in HIV-infected youth. J Neuroimmune Pharmacol. 2012;7:981–990. doi: 10.1007/s11481-012-9407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ackermann C, Andronikou S, Laughton B, Kidd M, Dobbels E, Innes S, van Toorn R, Cotton M. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. Pediatr Infect Dis J. 2014;33:e207–212. doi: 10.1097/INF.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patsalides AD, Wood LV, Atac GK, Sandifer E, Butman JA, Patronas NJ. Cerebrovascular disease in HIV-infected pediatric patients: neuroimaging findings. AJR Am J Roentgenol. 2002;179:999–1003. doi: 10.2214/ajr.179.4.1790999. [DOI] [PubMed] [Google Scholar]
- 87.Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32:253–267. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 88.Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 89.Gelbard HA, James HJ, Sharer LR, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 90.Canto-Nogues C, Sanchez-Ramon S, Alvarez S, Lacruz C, Munoz-Fernandez MA. HIV-1 infection of neurons might account for progressive HIV-1-associated encephalopathy in children. J Mol Neurosci. 2005;27:79–89. doi: 10.1385/JMN:27:1:079. [DOI] [PubMed] [Google Scholar]
- 91.George R, Andronikou S, du Plessis J, du Plessis AM, Van Toorn R, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatr Radiol. 2009;39:575–585. doi: 10.1007/s00247-009-1170-4. [DOI] [PubMed] [Google Scholar]
- 92.Sattler F, He J, Letendre S, Wilson C, Sanders C, Heaton RK, Ellis RJ, Franklin D, Grant I, McCutchan JA. Abdominal obesity, inflammation, immune activation and neurocognitive impairment. CROI; March 3–6, 2014; Boston, MA. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piazza F, Astori MG, Maccabruni A, Caselli D, Bossi G, Lanzi G. Neuropsychological development of children born to HIV-positive mothers. Pediatr Med Chir. 1995;17:331–333. [PubMed] [Google Scholar]
- 94.Bruck I, Tahan TT, Cruz CR, Martins LT, Antoniuk SA, Rodrigues M, Souza SM, Bruyn LR. Developmental milestones of vertically HIV infected and seroreverters children: follow up of 83 children. Arq Neuro-Psiquiatr. 2001;59:691–695. doi: 10.1590/s0004-282x2001000500007. [DOI] [PubMed] [Google Scholar]
- 95.Blanchette N, Smith ML, Fernandes-Penney A, King S, Read S. Cognitive and motor development in children with vertically transmitted HIV infection. Brain Cogn. 2001;46:50–53. doi: 10.1016/s0278-2626(01)80032-4. [DOI] [PubMed] [Google Scholar]
- 96.Poirier MC, Gibbons AT, Rugeles MT, Andre-Schmutz I, Blanche S. Fetal consequences of maternal antiretroviral nucleoside reverse transcriptase inhibitor use in human and nonhuman primate pregnancy. Curr Opin Pediatr. 2015;27:233–239. doi: 10.1097/MOP.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tardieu M, Brunelle F, Raybaud C, Ball W, Barret B, Pautard B, Lachassine E, Mayaux MJ, Blanche S. Cerebral MR imaging in uninfected children born to HIV-seropositive mothers and perinatally exposed to zidovudine. AJNR Am J Neuroradiol. 2005;26:695–701. [PMC free article] [PubMed] [Google Scholar]
- 98.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, Dollfus C, Mayaux MJ, Blanche S French Perinatal Cohort Study G. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 99.Selvam A, Buhimschi I, Makin J, Pattinson R, Anderson R, Forsyth B. Hyperferritinemia and markers of inflammation and oxidative stress in the cord blood of HIV-exposed, uninfected (HEU) infants. HIV Med. 2015 doi: 10.1111/hiv.12214. [DOI] [PubMed] [Google Scholar]
- 100.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 101.Nischang M, Gers-Huber G, Audige A, Akkina R, Speck RF. Modeling HIV infection and therapies in humanized mice. Swiss Med Wkly. 2012;142:w13618. doi: 10.4414/smw.2012.13618. [DOI] [PubMed] [Google Scholar]
- 102.Bagashev A, Sawaya BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotoxic Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis. 2011;41:678–687. doi: 10.1016/j.nbd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, Nath A. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19:418–431. doi: 10.1007/s13365-013-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- 107.Irish BP, Khan ZK, Jain P, Nonnemacher MR, Pirrone V, Rahman S, Rajagopalan N, Suchitra JB, Mostoller K, Wigdahl B. Molecular Mechanisms of Neurodegenerative Diseases Induced by Human Retroviruses: A Review. Am J Infect Dis. 2009;5:231–258. doi: 10.3844/ajidsp.2009.231.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355:687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18:3653–3662. doi: 10.2174/138161212802002706. [DOI] [PubMed] [Google Scholar]
- 110.Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 111.Khan NA, Di Cello F, Stins M, Kim KS. Gp120-mediated cytotoxicity of human brain microvascular endothelial cells is dependent on p38 mitogen-activated protein kinase activation. J Neurovirol. 2007;13:242–251. doi: 10.1080/13550280701286531. [DOI] [PubMed] [Google Scholar]
- 112.Khan NA, Di Cello F, Nath A, Kim KS. Human immunodeficiency virus type 1 tat-mediated cytotoxicity of human brain microvascular endothelial cells. J Neurovirol. 2003;9:584–593. doi: 10.1080/13550280390218760. [DOI] [PubMed] [Google Scholar]
- 113.Shiu C, Barbier E, Di Cello F, Choi HJ, Stins M. HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation: involvement of reactive oxygen species. Alcohol: Clin Exp Res. 2007;31:130–137. doi: 10.1111/j.1530-0277.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- 114.Stins MF, Pearce D, Choi H, Di Cello F, Pardo CA, Kim KS. CD4 and chemokine receptors on human brain microvascular endothelial cells, implications for human immunodeficiency virus type 1 pathogenesis. Endothelium. 2004;11:275–284. doi: 10.1080/10623320490904179. [DOI] [PubMed] [Google Scholar]
- 115.Wang H, Sun J, Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol. 2008;82:7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Westmoreland SV, Alvarez X, deBakker C, Aye P, Wilson ML, Williams KC, Lackner AA. Developmental expression patterns of CCR5 and CXCR4 in the rhesus macaque brain. J Neuroimmunol. 2002;122:146–158. doi: 10.1016/s0165-5728(01)00457-x. [DOI] [PubMed] [Google Scholar]
- 117.Shepherd AJ, Loo L, Gupte RP, Mickle AD, Mohapatra DP. Distinct modifications in Kv2.1 channel via chemokine receptor CXCR4 regulate neuronal survival-death dynamics. J Neurosci. 2012;32:17725–17739. doi: 10.1523/JNEUROSCI.3029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22:315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medders KE, Kaul M. Mitogen-activated protein kinase p38 in HIV infection and associated brain injury. J Neuroimmune Pharmacol. 2011;6:202–215. doi: 10.1007/s11481-011-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodriguez-Franco EJ, Cantres-Rosario YM, Plaud-Valentin M, Romeu R, Rodriguez Y, Skolasky R, Melendez V, Cadilla CL, Melendez LM. Dysregulation of macrophage-secreted cathepsin B contributes to HIV-1-linked neuronal apoptosis. PLoS One. 2012;7:e36571. doi: 10.1371/journal.pone.0036571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Das S, Basu A. Viral infection and neural stem/ progenitor cell’s fate: Implications in brain development and neurological disorders. Neurochem Int. 2011;59:357–366. doi: 10.1016/j.neuint.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 122.Abel K. The rhesus macaque pediatric SIV infection model—A valuable tool in understanding infant HIV-1 pathogenesis and for designing pediatric HIV-1 prevention strategies. Curr HIV Res. 2009;7:2–11. doi: 10.2174/157016209787048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McClure HM, Anderson DC, Ansari AA, Klumpp SA. The simian immunodeficiency virus infected macaque: a model for pediatric AIDS. Pathol Biol. 1992;40:694–700. [PubMed] [Google Scholar]
- 124.Fitting S, Booze RM, Hasselrot U, Mactutus CF. Dose-dependent long-term effects of Tat in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2010;20:469–480. doi: 10.1002/hipo.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Rompay KK, Kearney BP, Sexton JJ, Colon R, Lawson JR, Blackwood EJ, Lee WA, Bischofberger N, Marthas ML. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J Acquired Immune Defic Syndr. 2006;43:6–14. doi: 10.1097/01.qai.0000224972.60339.7c. [DOI] [PubMed] [Google Scholar]
- 126.Abel K, Pahar B, Van Rompay KK, Fritts L, Sin C, Schmidt K, Colon R, McChesney M, Marthas ML. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol. 2006;80:6357–6367. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ranganathan UD, Larsen MH, Kim J, Porcelli SA, Jacobs WR, Jr, Fennelly GJ. Recombinant pro-apoptotic Mycobacterium tuberculosis generates CD8+ T cell responses against human immunodeficiency virus type 1 Env and M. tuberculosis in neonatal mice. Vaccine. 2009;28:152–161. doi: 10.1016/j.vaccine.2009.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westmoreland SV, Williams KC, Simon MA, Bahn ME, Rullkoetter AE, Elliott MW, deBakker CD, Knight HL, Lackner AA. Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol. 1999;155:1217–1228. doi: 10.1016/S0002-9440(10)65224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 130.Bieniasz PD, Cullen BR. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol. 2000;74:9868–9877. doi: 10.1128/jvi.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burdo TH, Miller AD. Animal models of HIV peripheral neuropathy. Future Virol. 2014;9:465–474. doi: 10.2217/fvl.14.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp120 and Tat: differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moran LM, Fitting S, Booze RM, Webb KM, Mactutus CF. Neonatal intrahippocampal HIV-1 protein Tat1–86 injection: neurobehavioral alterations in the absence of increased inflammatory cytokine activation. Int J Dev Neurosci. 2014;38:195–203. doi: 10.1016/j.ijdevneu.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, Epstein AA, Gelbard HA, Boska MD, Poluektova LY. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gorantla S, Makarov E, Finke-Dwyer J, Castanedo A, Holguin A, Gebhart CL, Gendelman HE, Poluektova L. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177:2938–2949. doi: 10.2353/ajpath.2010.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: A design-based stereological study. Hippocampus. 2008;18:135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hill JM, Mervis RF, Avidor R, Moody TW, Brenneman DE. HIV envelope protein-induced neuronal damage and retardation of behavioral development in rat neonates. Brain Res. 1993;603:222–233. doi: 10.1016/0006-8993(93)91241-j. [DOI] [PubMed] [Google Scholar]
- 140.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007;28:101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: The role of dopaminergic alterations in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- 142.Boska MD, Dash PK, Knibbe J, Epstein AA, Akhter SP, Fields N, High R, Makarov E, Bonasera S, Gelbard HA, Poluektova LY, Gendelman HE, Gorantla S. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice. Mol Neurodegener. 2014;9:58. doi: 10.1186/1750-1326-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Denton PW, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011;13:135–148. [PMC free article] [PubMed] [Google Scholar]
- 144.Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, Liu YJ, Su L, Zhang L. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 2014;10:e1004291. doi: 10.1371/journal.ppat.1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berges BK, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marsden MD, Zack JA. Studies of retroviral infection in humanized mice. Virology. 2015;479–480C:297–309. doi: 10.1016/j.virol.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sindberg GM, Sharma U, Banerjee S, Anand V, Dutta R, Gu CJ, Volsky DJ, Roy S. An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol. 2015;10:74–87. doi: 10.1007/s11481-014-9574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kelschenbach JL, Saini M, Hadas E, Gu CJ, Chao W, Bentsman G, Hong JP, Hanke T, Sharer LR, Potash MJ, Volsky DJ. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: a model of protective immunity to HIV. J Neuroimmune Pharmacol. 2012;7:380–387. doi: 10.1007/s11481-011-9316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 151.Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Nowakowski RS, Rakic P. The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:129–154. doi: 10.1002/cne.901960110. [DOI] [PubMed] [Google Scholar]
- 154.Worlein JM, Leigh J, Larsen K, Kinman L, Schmidt A, Ochs H, Ho RJ. Cognitive and motor deficits associated with HIV-2(287) infection in infant pigtailed macaques: a nonhuman primate model of pediatric neuro-AIDS. J Neurovirol. 2005;11:34–45. doi: 10.1080/13550280590901732. [DOI] [PubMed] [Google Scholar]
- 155.Kinman LM, Worlein JM, Leigh J, Bielefeldt-Ohmann H, Anderson DM, Hu SL, Morton WR, Anderson BD, Ho RJ. HIV in central nervous system and behavioral development: an HIV-2287 macaque model of AIDS. AIDS. 2004;18:1363–1370. doi: 10.1097/01.aids.0000131307.62828.a1. [DOI] [PubMed] [Google Scholar]
- 156.Marthas ML, van Rompay KK, Otsyula M, Miller CJ, Canfield DR, Pedersen NC, McChesney MB. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1995;69:4198–4205. doi: 10.1128/jvi.69.7.4198-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Curtis K, Rollins M, Carryl H, Kimberley B, Van Rompay KK, Abel K, Burke MW. Reduction of pyramidal and immature hippocampal neurons in pediatric simian immunodeficiency virus infection. NeuroReport. 2014;25:973–978. doi: 10.1097/WNR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burke M, Zangenehpour S, Mouton PR, Ptito M. Knowing what counts: unbiased stereology in the non-human primate brain. J Vis Exp. 2009 doi: 10.3791/1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burke MW, Zangenehpour S, Ptito M. Brain banking: Making the most of your research specimens. J Vis Exp. 2009 doi: 10.3791/1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zangenehpour S, Burke MW, Chaudhuri A, Ptito M. Batch immunostaining for large-scale protein detection in the whole monkey brain. J Vis Exp. 2009 doi: 10.3791/1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Curtis K, Rollins M, Carryl H, Bradshaw K, Van Rompay K, Abel K, Burke M. Reduction of pyramidal and immature hippocampal neurons in pediatric simian immunodeficiency virus infection. NeuroReport. 2014;25:973–978. doi: 10.1097/WNR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13:274–283. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- 163.Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- 164.Mishra M, Taneja M, Malik S, Khalique H, Seth P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neurovirol. 2010;16:355–367. doi: 10.3109/13550284.2010.513028. [DOI] [PubMed] [Google Scholar]
- 165.Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kamboj H, Curtis K, Carryl H, Agyemang H, Van Rompay K, Abel K, Burke M. Central nervous system demylenation in pediatric SIV infection. Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA. 2014. [Google Scholar]
- 167.Yamada E, Yoshikawa R, Nakano Y, Misawa N, Koyanagi Y, Sato K. Impacts of humanized mouse models on the investigation of HIV-1 infection: illuminating the roles of viral accessory proteins in vivo. Viruses. 2015;7:1373–1390. doi: 10.3390/v7031373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Willner P. The validity of animal models of depression. Psychopharmacology (Berlin, Ger) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 169.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1:9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fuster JM. The Prefrontal Cortex. 4. Academic Press; London: 2008. [Google Scholar]
- 171.Amaral DG, Lavenex P. Hippocampal neuroanatomy. Oxford University Press; Oxford: 2007. [Google Scholar]
- 172.Makori N, Tarantal AF, Lu FX, Rourke T, Marthas ML, McChesney MB, Hendrickx AG, Miller CJ. Functional and morphological development of lymphoid tissues and immune regulatory and effector function in rhesus monkeys: Cytokine-secreting cells, immunoglobulin-secreting cells, and CD5(+) B-1 cells appear early in fetal development. Clin Diagn Lab Immunol. 2003;10:140–153. doi: 10.1128/CDLI.10.1.140-153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fitting S, Booze RM, Mactutus CF. HIV-1 Proteins, Tat and gp120, Target the Developing Dopamine System. Curr HIV Res. 2015;13:21–42. doi: 10.2174/1570162x13666150121110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brehm MA, Wiles MV, Greiner DL, Shultz LD. Generation of improved humanized mouse models for human infectious diseases. J Immunol Methods. 2014;410:3–17. doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kranick SM, Nath A. Neurologic complications of HIV-1 infection and its treatment in the era of antiretroviral therapy. Continuum (Minneapolis, MN) 2012;18:1319–1337. doi: 10.1212/01.CON.0000423849.24900.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Demir M, Laywell ED. Neurotoxic effects of AZT on developing and adult neurogenesis. Front Neurosci. 2015;9:93. doi: 10.3389/fnins.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 178.Etherton MR, Lyons JL, Ard KL. HIV-associated Neurocognitive Disorders and Antiretroviral Therapy: Current Concepts and Controversies. Curr Infect Dis Rep. 2015;17:485. doi: 10.1007/s11908-015-0485-6. [DOI] [PubMed] [Google Scholar]
- 179.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, Gannon PJ, Mankowski J, Dorsey JL, Buch AM, Cross SA, Cook DR, Pena MM, Andersen ES, Christofidou-Solomidou M, Lindl KA, Zink MC, Clements J, Pierce RC, Kolson DL, Jordan-Sciutto KL. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20:39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van Rompay KK, Otsyula MG, Marthas ML, Miller CJ, McChesney MB, Pedersen NC. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob Agents Chemother. 1995;39:125–131. doi: 10.1128/aac.39.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rausch DM, Heyes M, Eiden LE. Effects of chronic zidovudine administration on CNS function and virus burden after perinatal SIV infection in rhesus monkeys. Adv Neuroimmunol. 1994;4:233–237. doi: 10.1016/s0960-5428(06)80261-5. [DOI] [PubMed] [Google Scholar]
- 182.Rausch DM, Heyes MP, Murray EA, Eiden LE. Zidovudine treatment prolongs survival and decreases virus load in the central nervous system of rhesus macaques infected perinatally with simian immunodeficiency virus. J Infect Dis. 1995;172:59–69. doi: 10.1093/infdis/172.1.59. [DOI] [PubMed] [Google Scholar]
- 183.Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses. 2012;28:16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- 184.Van Rompay KK, Brignolo LL, Meyer DJ, Jerome C, Tarara R, Spinner A, Hamilton M, Hirst LL, Bennett DR, Canfield DR, Dearman TG, Von Morgenland W, Allen PC, Valverde C, Castillo AB, Martin RB, Samii VF, Bendele R, Desjardins J, Marthas ML, Pedersen NC, Bischofberger N. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother. 2004;48:1469–1487. doi: 10.1128/AAC.48.5.1469-1487.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kelly KM, Beck SE, Metcalf Pate KA, Queen SE, Dorsey JL, Adams RJ, Avery LB, Hubbard W, Tarwater PM, Mankowski JL. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. 2013;27:F21–28. doi: 10.1097/QAD.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, Graham DR, Tarwater PM, Mankowski JL, Clements JE. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA, J Am Med Assoc. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- 188.Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ. Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest. 2000;106:37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meulendyke KA, Queen SE, Engle EL, Shirk EN, Liu J, Steiner JP, Nath A, Tarwater PM, Graham DR, Mankowski JL, Zink MC. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J Neurovirol. 2014;20:591–602. doi: 10.1007/s13365-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 2014;28:131–145. doi: 10.1007/s40263-013-0132-4. [DOI] [PubMed] [Google Scholar]
- 192.Burke M, Ptito M, Ervin F, Palmour R. Effects of fluoxetine on neurogenesis in fetal-ethanol exposed and sucrose-control vervet monkeys. Research Society on Alcoholism; San Antonio, Texas: 2010. [Google Scholar]
- 193.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 194.Schmued LC. A rapid, sensitive histochemical stain for myelin in frozen brain sections. J Histochem Cytochem. 1990;38:717–720. doi: 10.1177/38.5.1692056. [DOI] [PubMed] [Google Scholar]