SUMMARY
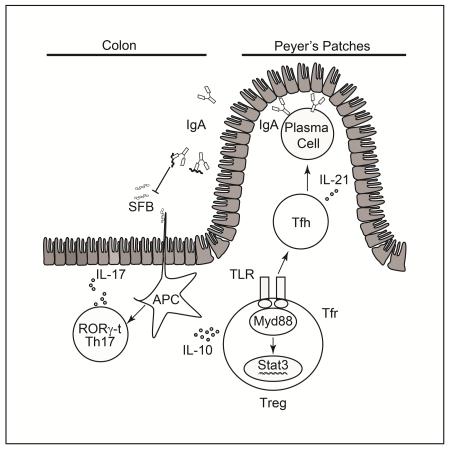
Commensal microbiota promote mucosal tolerance in part by engaging regulatory T (Treg) cells via Toll like receptors (TLR). We report that Treg cell-specific deletion of the TLR adaptor MyD88 resulted in deficiency of intestinal Treg cells, a reciprocal increase in T helper-17 (Th17) cells and heightened interleukin-17 (IL-17)-dependent inflammation in experimental colitis. It also precipitated dysbiosis with overgrowth of segmented filamentous bacteria (SFB) and increased microbial loads in deep tissues. The Th17 cell dysregulation and bacterial dysbiosis were linked to impaired anti-microbial intestinal IgA responses, related to defective MyD88 adaptor- and Stat3 transcription factor-dependent T follicular regulatory and helper cell differentiation in the Peyer’s patches. These findings establish an essential role for MyD88-dependent microbial sensing by Treg cells in enforcing mucosal tolerance and maintaining commensalism by promoting intestinal Treg cell formation and anti-commensal IgA-responses.
INTRODUCTION
The gastrointestinal commensal microbiota play a critical role in shaping host immune and metabolic responses (Backhed et al., 2005; Chu and Mazmanian, 2013; Lee and Mazmanian, 2010; Round and Mazmanian, 2009). Whereas pathogenic bacteria trigger inflammation and symbiotic bacteria promote tolerance, both sets of responses involve the activation of host pattern recognition receptors (PRR), including toll-like receptors (TLRs) (Hooper et al., 2012; Palm and Medzhitov, 2009). In the case of commensal bacteria, PRR signaling in the absence of tissue damage channels the immune response towards tolerance [reviewed in (Chu and Mazmanian, 2013)]. T regulatory (TR) cells expressing the transcription factor Foxp3 play a critical role in this process (Josefowicz et al., 2012; Nutsch and Hsieh, 2012; Round and Mazmanian, 2009). How Treg cells sense microbial signals and translate them into a tolerogenic response remains incompletely understood.
Both natural (nTreg) and induced (iTreg) cells contribute to gastrointestinal tolerance (Haribhai et al., 2009; Haribhai et al., 2011). The former are a distinct thymus-derived lineage that express a T cell antigen receptor (TCR) repertoire biased towards self antigens (Hsieh et al., 2004). The latter are induced de novo from conventional CD4+Foxp3− T cells upon encountering antigens in presence of transforming growth factor-β (TGF-β), interleukin-2 (IL-2) and retinoic acid (Coombes et al., 2007; Mucida et al., 2005; Mucida et al., 2007; Sun et al., 2007). iTreg cells carry a distinct TCR repertoire that is biased towards recognition of foreign antigens including the microbiota, reflective of their derivation from conventional T (Tconv) cells (Haribhai et al., 2011; Lathrop et al., 2011; Lathrop et al., 2008). Both nTreg and iTreg cells are required for optimal peripheral tolerance and prevention of intestinal inflammation (Haribhai et al., 2009; Haribhai et al., 2011). In their absence, the microbiota drive intestinal inflammation in a TLR and MyD88-dependent manner (Izcue et al., 2009; Rivas et al., 2012).
Commensal bacteria favor iTreg cell differentiation in the gut (Atarashi et al., 2011; Geuking et al., 2011; Lathrop et al., 2011; Round et al., 2011; Round and Mazmanian, 2010). Promotion by the gut microbiota of Treg cell generation involves TLR signaling, evidenced by the failure to expand colonic lamina propria (cLP) Treg cells in germ free (GF) mice doubly deficient in the TLR adaptor molecules MyD88 and Trif when colonized with altered Schaedler flora (Geuking et al., 2011). TLR2 and TLR4 signaling promotes Treg cell proliferation and survival (Caramalho et al., 2003; Chen et al., 2009; Liu et al., 2006; Sutmuller et al., 2006). Polysaccharide A of Bacteroides fragilis signals directly via TLR2 receptors on T cells to promote iTreg cell differentiation and IL-10 and TGF-β production, suppress Th17 cell differentiation and establish colonization of Bacteroides fragilis at the mucosal interface (Round et al., 2011; Wang et al., 2006). Collectively, these studies indicate that Treg cells may directly respond to microbial signals, and that this response is important for tolerance acquisition.
To further elucidate the role TLR-MyD88 signaling in Treg cells in promoting mucosal tolerance, we examined the consequences of Treg cell lineage-specific Myd88 deletion. We identified an essential role for MyD88 in the induction and stability of mucosal Treg cells and the differentiation of T follicular regulatory (Tfr) and helper (Tfh) cells in the Peyer’s patches (PP). Furthermore, MyD88 signaling in Treg cells acts via a Stat3-dependent mechanism to promote healthy commensalism by supporting anti-microbial IgA antibody responses, thus suppressing overgrowth of segmented filamentous bacteria (SFB), and restraining Th17 cell responses.
RESULTS
Treg cell-specific MyD88 deletion results in Treg cell deficiency and Th17 cell dysregulation in the gut mucosa
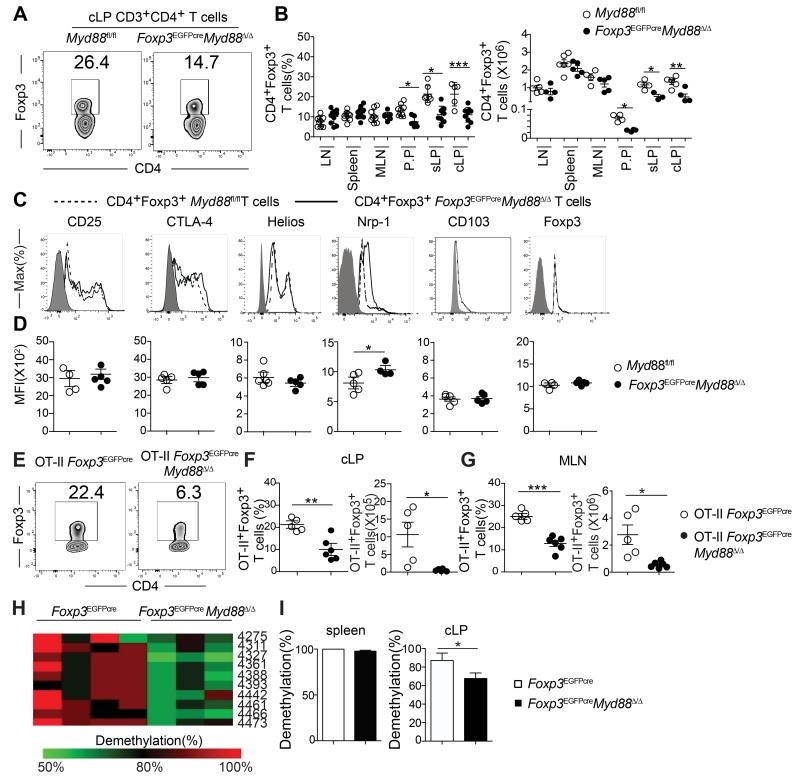
To analyze the role of TLR signaling in Treg cells in maintaining peripheral tolerance, we generated mice with Treg cell-specific MyD88 deficiency by crossing mice harboring a Cre recombinase and an EGFP reporter under the control of the Foxp3 promoter (Foxp3EGFPcre) with others, that carried a floxed Myd88 allele (Figure S1A) (Hou et al., 2011; Zhou et al., 2009). The resultant mice, termed Foxp3EGFPcreMyd88Δ/Δ, expressed MyD88 in different tissues examined and in immune cells, including conventional CD4+ T cells, with the exception of CD4+Foxp3+ Treg cells, where transcripts encoding MyD88 were absent in the face of normal expression of Foxp3 transcripts (Figure S1B). These results indicate that Foxp3EGFPcre was fully effective in specifically deleting Myd88 in Treg cells while sparing conventional CD4+ T (Tconv) cells. Mice with the MyD88 lineage-specific deletion were no different in weight and gross phenotype from their MyD88 sufficient littermates (data not shown). The frequency and absolute numbers of Treg cells of MyD88-sufficient (MyD88fl/fl) and deficient (Foxp3EGFPCreMyD88Δ/Δ) littermate mice were similar across most lymphoid tissues, including the thymus, spleen and peripheral lymph nodes. However, the Foxp3EGFPcreMyd88Δ/Δ mice exhibited a selective decrease in the Treg cells found in the small and large intestinal lamina propria (sLP and cLP, respectively) as well as those in the PP (Figures 1A and 1B). This decrease was not reproduced by deletion of MyD88-coupled cytokine receptor pathways, including IL-1R1 and IL-18R. It was however recapitulated by antibiotic-treatment of MyD88-sufficient mice (Figures S1C and S1D). Comparison of MyD88-sufficient and deficient cLP Treg cells revealed that expression of most key Treg cell markers, including Foxp3, CD25, CTLA-4, CD103 and Helios, was not different between the two populations, and that their in vitro suppressive capacity was similar (Figures 1C and 1D and data not shown). However, expression of Nrp1, associated with nTreg cells, was increased in the cLP of Foxp3EGFPcreMyd88Δ/Δ mice, suggesting a deficiency in iTreg cells.
Figure 1. Treg cell-specific MyD88 deletion results in gut mucosa Treg cell deficiency.
A. Flow cytometric analysis of CD4+Foxp3+ Treg cells in the cLP of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. B. Frequencies and absolute numbers of Treg cells in different tissues of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. C, D. Flow cytometric analysis (C) and mean fluorescence intensity (MFI) (D) of CD25, CTLA-4, Helios, Nrp1, CD103 and Foxp3 cLP Treg cells of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. E. Flow cytometric analysis of Foxp3 expression in cLP CD45.2+CD4+ T cells in CD45.1+ WT mice that received naïve CD45.2+CD4+Foxp3− T cells from OT-II+ Foxp3EGFPcre or OT-II+ Foxp3EGFPcreMyd88Δ/Δ mice and were then fed OVA in the drinking water for 1 week. F. Frequencies (left panel) and absolute numbers (right panel) of donor CD45.2+CD4+EGFP+ iTreg in the cLP of recipient mice from panel (E). G. Absolute numbers of donor CD45.2+CD4+Foxp3EGFP+ iTreg in the MLN of recipient mice. H. Methylation status of individual CpG motifs within the Foxp3 CNS2 in cLP Treg cells of Foxp3EGFPcre and Foxp3EGFPcreMyd88Δ/Δ mice. Individual CpG motifs are numbered with reference to the transcription initiation site of Foxp3. I. Global methylation status of Foxp3 CNS2 in splenic and cLP Treg cells shown in H. Results are representative of 4 independent experiments. N=3-8 mice/group. *p<0.05; **p<0.01; ***p<0.001 by Student’s unpaired two tailed t test and One-way ANOVA.
To establish the presence of a defect in iTreg cell formation in Foxp3EGFPcreMyd88Δ/Δ mice, we compared the capacity of naïve CD4+ T cells from Foxp3EGFPCre and Foxp3EGFPcreMyd88Δ/Δ to differentiate into antigen-specific iTreg cells in vivo. Naïve CD45.2+CD4+ T cells expressing the OT-II TCR transgene, specific for the OVA323-339 peptide in the context of I-Ab, were isolated from either Foxp3EGFPcre or Foxp3EGFPcreMyd88Δ/Δ and transferred into CD45.1+ recipient mice. The latter were subsequently exposed to OVA via the drinking water and examined for the presence of CD45.2+CD4+EGFP+ Treg cells in the cLP. Treg cell-specific MyD88 deficiency resulted in a profound reduction of OVA-induced iTreg cell formation in cLP and mesenteric lymph nodes (MLN), indicating that decreased iTreg cell formation is a key contributor to Treg cell deficiency in intestinal mucosa of Foxp3EGFPcreMyd88Δ/Δ mice (Figures 1E-G). This deficiency could not be attributed to diminution of intestinal Treg cell proliferation, as revealed by staining with the proliferation marker Ki-67 or by labeling with 5-bromodeoxyuridine (BrdU), nor to increased apoptosis as detected by AnnexinV staining (Figures S1E-G). Also, MyD88-deficient T cells retained the capacity to differentiate to iTreg cells in vitro in response to treatment with anti-CD3 and CD28 mAb and TGF-β (Figure S1H). Analysis of the conserved non-coding sequence 2 (CNS2) at the Foxp3 locus, whose epigenetic demethylation is necessary for stable Foxp3 expression, revealed decreased CNS2 demethylation in Foxp3EGFPcreMyd88Δ/Δ Treg cells (Figures 1H and 1I). Together, these results indicate that MyD88 signaling plays a critical role in mucosal iTreg cell induction and epigenetic stability.
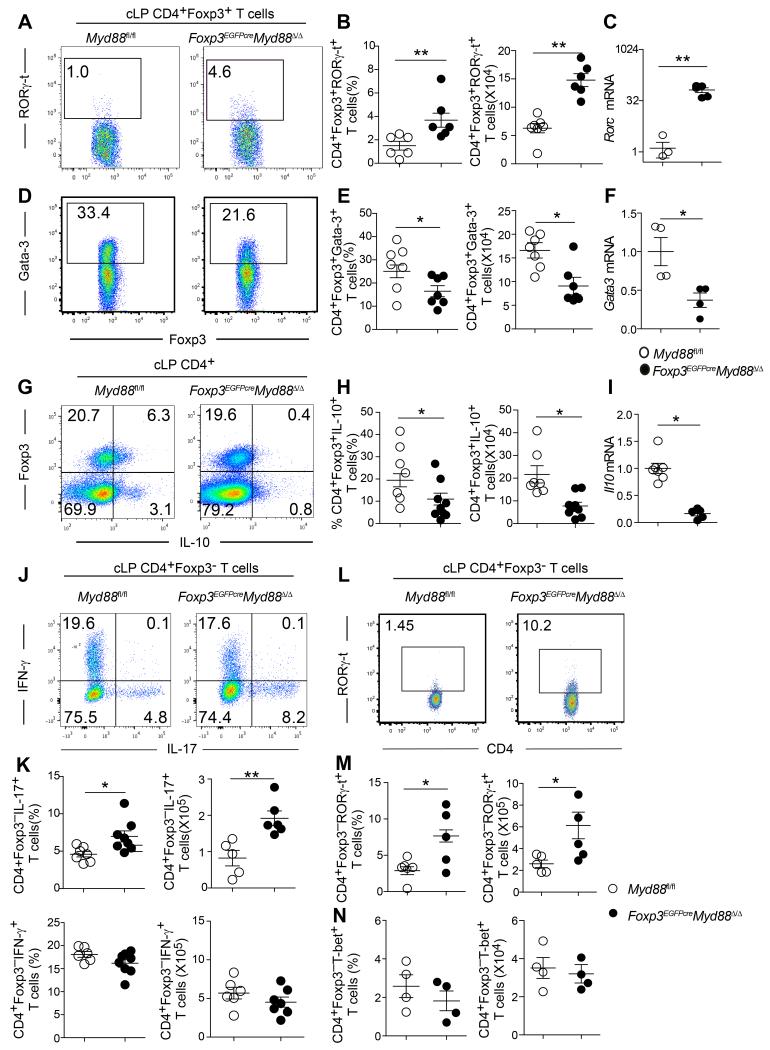
Analysis of cLP Treg cells of Foxp3EGFPcreMyd88Δ/Δ mice uncovered several abnormalities. Flow cytometric and real-time PCR analysis revealed that MyD88-deficient cLP Treg cells exhibited increased expression of the transcription factor RORγ-t (Figures 2A-C). In contrast, expression of the transcription factor GATA-3, implicated in Treg cell stability, was decreased (Figures 2D-F) (Wang et al., 2011; Wohlfert et al., 2011). IL-10 expression and Il10 transcripts were decreased in MyD88-deficient as compared to MyD88-sufficient cLP Treg cells (Figures 2G-I).
Figure 2. Treg cell-specific MyD88 deletion results in colonic Th17 skewing.
A, B. Flow cytometric analysis (A) and enumerated cell frequencies and numbers (B) of cLP RORγ-t+CD4+Foxp3+Treg cells in Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. C. Real time PCR analysis of Rorc expression in cell-sorted cLP CD4+Foxp3+ Treg cells. D-I. Analysis of GATA3 expression and Gata3 mRNA (D-F) and IL-10 expression and Il10 mRNA (G-I) in cLP CD4+Foxp3+ Treg cells of the respective mouse strain, performed as in A-C. J, K. Expression of IL-17 and IFN-γ in cLP CD4+Foxp3− T cells of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. Flow cytometric analysis (J), frequencies and absolute numbers (K) of cytokine expressing cells. L, M. Flow cytometric analysis (L), and frequencies and absolute numbers (M) of cLP CD4+Foxp3−RORγ-t+ T cells of the respective mouse strain. N. Frequencies (left panel) and total cell number (right panel) of cLP CD4+Foxp3−T-bet+ of Foxp3EGFPcreMyd88Δ/Δ mice compared with Myd88fl/fl control mice. Results are representative of 4 independent experiments. N=4-7 mice/group. *p<0.05; **p<0.01; ***p<0.001 by Student’s unpaired two tailed t test.
In parallel with the contraction of the cLP Treg cell population in Foxp3EGFPcreMyd88Δ/Δ mice, there was expansion of IL-17-expressing cLP CD4+ Tconv cells (Figures 2J and 2K). The frequency and numbers of cLP CD4+ T cells expressing RORγ-t was also increased (Figures 2L and 2M). In contrast, interferon-γ (IFN-γ) secretion (Figures 2J and 2K) and expression of the T helper-1 (Th1) master regulator T-bet by cLP CD4+ Tconv cells was unchanged (Figure 2N). Similarly, the frequency of IL-22-expressing cLP CD4+ Tconv cells was unchanged, as was the expression of Il22 transcripts in colonic tissues (data not shown).
Treg cell-specific MyD88 deficiency aggravates the severity of experimental colitis
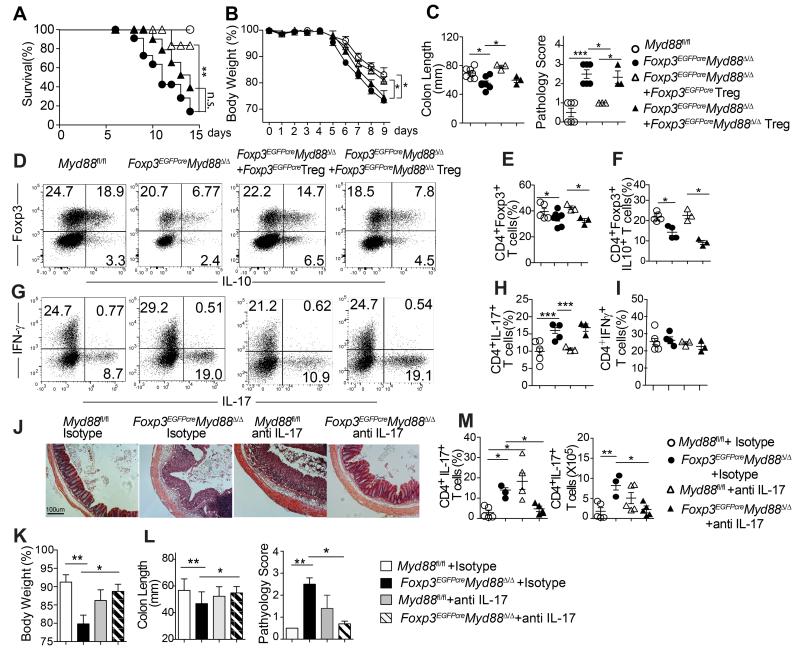
To determine the functional significance of MyD88 deficiency in Treg cells, we examined the outcome of dextran sodium sulfate (DSS)-induced colitis in Foxp3EGFPcreMyd88Δ/Δ mice as compared to control littermate Myd88fl/fl mice. Mice were exposed to DSS at 3.5-5% in drinking water for 5 days then placed on regular water and followed up for another week. Treg cell-specific MyD88 deficiency resulted in aggravated DSS-induced weight loss and mortality associated with increased mucositis as revealed by colon length and histological scoring (Figures 3A-C). This phenotype was rescued by treatment with MyD88-sufficient but not deficient Treg cells, consistent with impaired function of the latter as the underlying mechanism of disease severity. MyD88 deficiency was associated with decreased IL-10 production by Treg cells and increased IL-17 but not IFN-γ production by CD4+ Tconv cells (Figures 3D-I).
Figure 3. Treg cell-specific MyD88 deficiency aggravates the severity of experimental colitis.
A. Survival curves of the following mouse groups treated with 5% DSS for 5 days then followed thereafter: 1) Myd88fl/fl, 2) Foxp3EGFPcreMyd88Δ/Δ and 3, 4) Foxp3EGFPcreMyd88Δ/Δ mice that were given cell-sorted CD4+EGFP+ splenic Treg cells from either Foxp3EGFPcre (MyD88-sufficient) or Foxp3EGFPcreMyd88Δ/Δ (MyD88-defcient) mice. B. Body weight loss following treatment of mouse groups as in panel A with 3.5% DSS for 5 days. C. Colon length and histological scores of the respective mouse groups in (B) at day 9 post DSS treatment. D-I. Flow cytometric analysis and frequencies of total and IL-10+ CD4+Foxp3+ Treg cells (D-F) and IFN-γ and IL-17 secreting CD4+Foxp3− Tconv cells (G-I) isolated from the cLP of the respective mouse groups in (B, C) at day 9 post DSS treatment. J-M. Groups of Foxp3EGFPcre and Foxp3EGFPcreMyd88Δ/Δ mice were subjected to DSS-induced colitis and treated with either isotype control or neutralizing anti-IL-17A mAbs at days 0, 2, and 4 of DSS treatment. J. Colon histology (Hematoxylin and eosin staining; 40× magnification). Body weight loss (K), colon length and clinical score (L) of the respective mouse groups. M. Frequencies and absolute numbers of IL-17 and IFN-γ secreting cLP CD4+Foxp3− Tconv cells of the respective mouse groups. Results are representative of 3 independent experiments. N =9-10 mice/group for panel A; **p<0.01 by log rank test. N= 4-7 mice/group for panels B-M; *p<0.05; **p<0.01; ***p<0.001 by One-way ANOVA.
To analyze the role of dysregulated IL-17 production in disease severity, we examined the impact of treatment with a neutralizing IL-17 antibody on disease pathogenesis. Results revealed that the anti-IL-17 antibody treatment rescued disease severity in Foxp3EGFPcreMyd88Δ/Δ but not Myd88fl/fl control mice (Figures 3J-M). This rescue was associated with decreased frequency and cell number of Th17 but not Th1 cells in Foxp3EGFPcreMyd88Δ/Δ (Figure 3M).
Defective function of MyD88-deficient Treg cells in suppressing inflammation was further verified in a second lymphopenia-induced model of colitis. MyD88-deficient Treg cells were ineffective in controlling the colitic response as evidenced by the weight loss, pathological response and dysregulated Th17 and Th1 effector cell responses (Figures S2A-E). Importantly, MyD88-deficient donor Treg cells were prone to lose Foxp3 expression and become pathogenic ex-Treg cells that secreted IFN-γ and IL-17, indicative of increased instability (Figures S2F and S2G).
TR-cell specific MyD88 deficiency impairs intestinal IgA production and increases commensal bacterial translocation to deep tissues
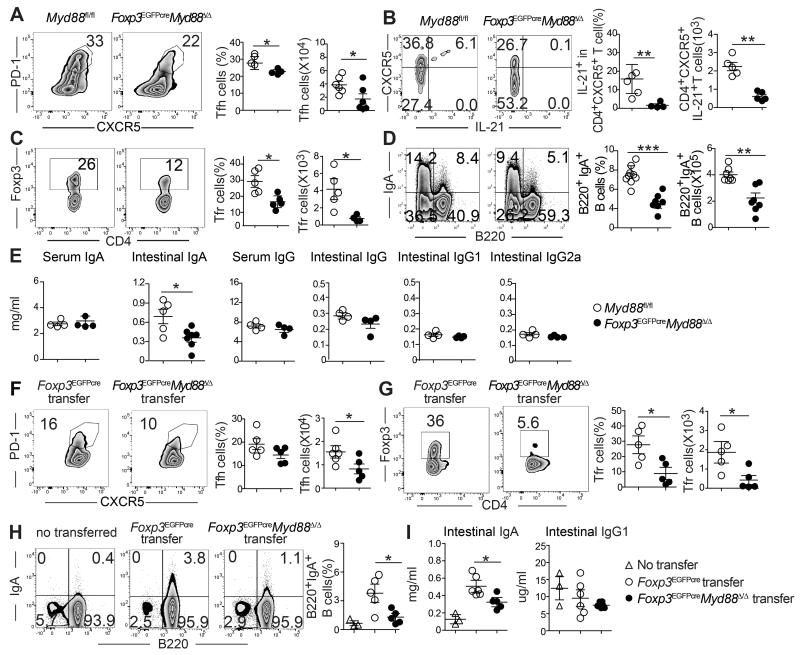
As noted earlier, the defect in mucosal Treg cell populations extended to the PP (Figure 1B). PP Tfr cells have previously been shown to promote luminal IgA production either directly and/or through differentiation into Tfh cells (Cong et al., 2009; Kawamoto et al., 2014; Tsuji et al., 2009). As compared to WT Tfh cells, those of Foxp3EGFPcreMyd88Δ/Δ mice were decreased in frequencies and numbers and exhibited decreased expression of the cytokine IL-21, important for B cell germinal center response (Figures 4A and 4B). Tfr cells were more severely compromised (Figures 4C), and they exhibited decreased expression of canonical Tfr cell markers including BCL-6, ICOS, and CD40L (Figures S3A and S3B). In contrast, both cell populations were not altered in the mesenteric lymph nodes (data not shown). We examined Foxp3EGFPcreMyd88Δ/Δ and control Myd88fl/fl littermate mice for their frequencies of IgA positive B cells in the PP. Results revealed decreased frequencies of switched IgA+B220+ B cells and IgA+B220− cells (the latter composed mostly of CD138+ plasma cells) in the PP of Foxp3EGFPcreMyd88Δ/Δ mice (Figure 4D and data not shown). The intestinal luminal, but not serum, IgA concentrations were decreased also in Foxp3EGFPcreMyd88Δ/Δ as compared to Myd88fl/fl mice, and it remained so in the context of DSS-induced colitis mice (Figure 4E). In contrast, serum and intestinal luminal concentrations of IgG, and of IgG1 and IgG2a subclasses, were similar in the two mouse strains (Figure 4E). Antibiotic treatment of WT mice reproduced the effects of Treg cell-specific MyD88 deficiency in terms of decreased Tfh and Tfr frequencies in the PP and depressed luminal but not serum IgA concentrations, indicating that the adverse effects of MyD88 deficiency on gut IgA responses resulted from the interruption of microbial sensing by Treg cells (Figure S3C-E).
Figure 4. Treg cell-specific MyD88 deficiency impairs PP Tfr and Tfh cell differentiation and intestinal IgA production.
A-D. Flow cytometric analysis, frequencies and absolute numbers of Tfh cells (CD4+CXCR5+PD-1high) (A), CXCR5+IL-21+ cells within the Tfh populations (B), Tfr cells (CD4+Foxp3+CXCR5+PD-1high) (C) and IgA+B220+ B cells (D) in PP of Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice. E. Serum and intestinal lavage fluid concentrations of IgA (I) and IgG, IgG1 and IgG2a (J) in Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice. F-H. Flow cytometric analysis, and frequencies and absolute numbers of Tfh (F), Tfr (G) and IgA+B220+ (H) cells isolated from the PP of Tcra−/− mice 2 weeks following the transfer of CD4+EGFP+Treg cells isolated from either Foxp3EGFPcre or Foxp3EGFPcreMyd88Δ/Δ. I. Luminal concentration of IgA and IgG1 in the mouse groups from panel (H). Results are representative of 3 independent experiments. N=3-5 mice/group. *p<0.05; **p<0.01; by Student’s unpaired two tailed t test.
We further investigated the impact of MyD88 Treg cell deficiency on the differentiation of antigen-specific Tfr and Tfh cells in the PP by means of adoptive transfer experiments. First, we examined the capacity of sorted splenic Foxp3EGFPcre and Foxp3EGFPcreMyd88Δ/Δ Treg cells transferred into Tcra−/− mice to differentiate into Tfr and Tfh cells in the PP and support luminal IgA production. Results showed that whereas the total numbers of T cells in the PP were similar in the two transfer groups (data not shown), the frequencies and absolute numbers of Foxp3EGFPcreMyd88Δ/Δ-derived PP Tfh cells were decreased as compared to Foxp3EGFPcre-derived cells (Figure 4F). In particular, Foxp3EGFPcreMyd88Δ/Δ Tfr cells were profoundly decreased both in frequencies and cell numbers as compared to those derived from Foxp3EGFPcre cells (Figure 4G). In contrast, the frequencies and numbers of Foxp3EGFPcreMyd88Δ/Δ Treg cells in the spleens of recipient mice were similar to those of transferred Foxp3EGFPCre Treg cells (data not shown). The percentage of IgA+B220+ and the concentrations of luminal IgA were all markedly decreased in Tcra−/− mice receiving Foxp3EGFPcreMyd88Δ/Δ as compared to Foxp3EGFPcre Treg cells. In contrast, luminal IgG1 concentrations were similar between the two groups (Figures 4H and 4I).
To rule out a role for changes in Treg cell TCR repertoire induced by MyD88 deficiency in the observed results, we carried out transfer studies using OT-II+ TCR transgenic cells, specific for OVA323-339 peptide. Naïve CD4+Foxp3− T cells isolated from OT-II+ Foxp3EGFPcre or OT-II+ Foxp3EGFPcreMyd88Δ/Δ mice were transferred into Tcra−/− mice. The recipients were fed OVA in the drinking water for one week then examined for the differentiation of the donor T cells in the PP into Tfr and Tfh cells. Results revealed that there was no difference in the frequency of donor cells in the spleens of recipient mice (data not shown). In the PP, the frequencies of OT-II+ Tfh and Tfr cells were markedly reduced in Tcra−/− recipients of naïve CD4+ T cells from Foxp3EGFPcreMyd88Δ/Δ as compared to Foxp3EGFPcre mice (Figures S4A and S4B). The frequencies of PP IgA+B220+ B cells, concentrations of luminal IgA and titers of luminal OVA-specific IgA antibodies were similarly reduced (Figures S4C and S4D). The reduced capacity of naïve OT-II+CD4+ T cells of OT-II+ Foxp3EGFPcreMyd88Δ/Δ mice to support Tfh and Tfr cell differentiation in PP, luminal IgA production and luminal OVA-specific IgA responses was reproduced when OT-II+ Foxp3EGFPcreMyd88Δ/Δ Treg cells were isolated and transferred into Tcra−/− (Figures S4E-H). These results indicate that the antigen-driven differentiation of Tfr and Tfh cells in the PP from both naïve CD4+ T cell precursors and preformed Treg cells was dependent on Treg cell-intrinsic MyD88 signaling.
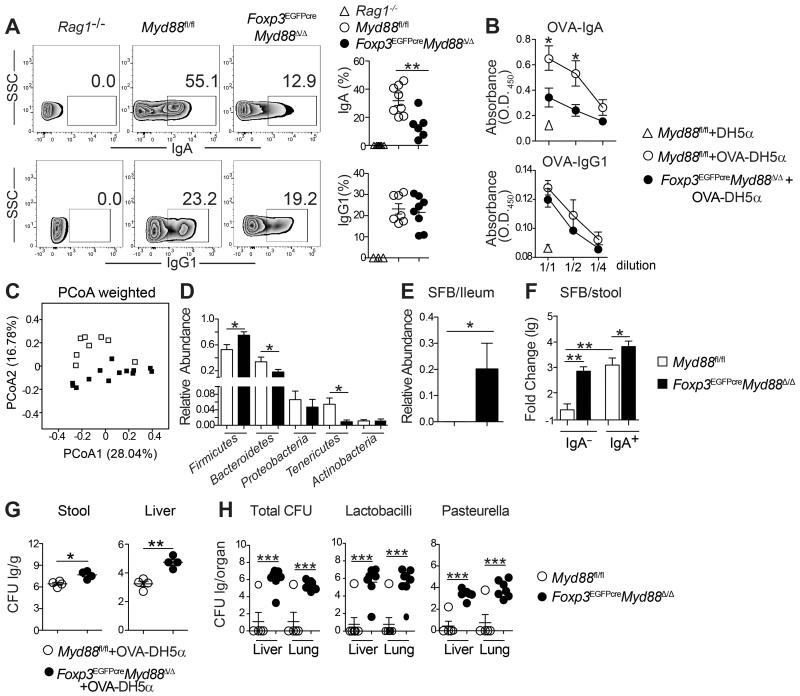
Intestinal IgA production controls pathobionts and maintains commensalism (Cong et al., 2009; Fagarasan et al., 2010; Peterson et al., 2007). Significantly, analysis revealed decreased IgA bound to bacteria in fecal pellets of Foxp3EGFPcreMyd88Δ/Δ as compared to Myd88fl/fl mice, indicative of decreased IgA anti-bacterial responses (Figure 5A). The functional implications of luminal IgA deficiency in Foxp3EGFPcreMyd88Δ/Δ mice were further examined using a non-pathogenic E. coli (DH5α strain) that expressed a chicken Ovalbumin (OVA) protein fragment (amino acids 139–386). Oral gavage with OVA-DH5α E. coli revealed profoundly decreased luminal OVA-specific IgA, but not IgG1, consistent with impaired bacteria-specific IgA antibody responses (Figure 5B).
Figure 5. TR-cell specific MyD88 deficiency promotes dysbiosis and and bacterial translocation to deep tissues.
A. Flow cytometric analysis and frequencies of IgA- and IgG1-coated bacteria in the fecal pellets of Rag1−/−, Foxp3EGFPcreMyd88Δ/Δ and MyD88fl/fl mice. B. OVA-specific luminal IgA and IgG1 concentrations in mice (panel A) gavaged with OVA-DH5α bacteria. C. Weighted Unifrac plot of ileal mucosa-associated microbial communities, visualized with principal coordinate analysis (PCoA), in Foxp3EGFPcreMyd88Δ/Δ versus Myd88fl/fl mice (p-value = 0.001 by AMOVA). D. Relative abundance of different microbial phyla in ileal mucosa-associated microbiota in Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice (*false discovery rate<0.05). E. Relative abundance of SFB taxa in illeal mucosa-associated microbiota in Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice. F. Real time PCR analysis of SFB 16S rRNA in IgA+ and IgA− fecal bacterial fractions of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. G. Stool (left panel) and liver (right panel) OVA-DH5α bacterial load [expressed as log colony forming units (lg CFU)/g] in Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice treated with the OVA-DH5α bacteria by oral gavage. H. Loads of total bacteria, Lactobacilli and Pasteurella (all expressed as CFU/g tissue) in the lung and liver of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice. Results are representative of 3 independent experiments. Results representative of 3 independent experiments. N=5 mice/group. *p<0.05; **p<0.01; by Student’s unpaired two tailed t test.
To determine whether impaired gut anti-microbial IgA responses were associated with altered small intestinal microbiota communities, we performed direct highly parallel pyrosequencing of 16S ribosomal DNA (rDNA) amplicons derived from illeal tissues of Foxp3EGFPcreMyd88Δ/Δ and Myd88fl/fl mice (see Supplemental Experimental Procedures section for description of the sequencing methods and bioinformatic analysis). Analysis of microbiota diversity using the Shanon measure revealed no differences between Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice (data not shown). However, Unifrac analysis showed significant differences in the microbial community structure between Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice (Figure 5C). Statistical testing at the level of bacterial phyla revealed increased relative abundance of Firmicutes and decreases in Bacteroidetes and Tenericutes phyla in Foxp3EGFPcreMyd88Δ/Δ as compared to Myd88fl/fl mice (Figure 5D). Analysis of taxa that discriminated between the two groups of mice identified 6 taxa at the genus level, encompassing 16 Operational Taxonomic Units (OTUs) (Tables S1 and S2). The most abundant of the latter, OTU0006, was detected in Foxp3EGFPcreMyd88Δ/Δ but not Myd88fl/fl samples (Figure 5E and Table S1). It was identified by phylogenetic placement and sequence alignment as SFB, consistent with the role of IgA in immunity to SFB (Figure S5) (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009; Lecuyer et al., 2014; Suzuki et al., 2004). Magnetic cell sorting of IgA-bound fecal bacteria followed by real time PCR analysis revealed that SFB sharply segregated into the IgA+ fraction in Myd88fl/fl mice, in agreement with recent studies (Palm et al., 2014). In contrast, it was increased in both the IgA+ and IgA− fractions in Foxp3EGFPcreMyd88Δ/Δ mice (Figure 5F). These studies suggest that the SFB dysbiosis in Foxp3EGFPcreMyd88Δ/Δ mice was related to the defective anti-bacterial IgA response.
Dysbiosis in Foxp3EGFPcreMyd88Δ/Δ mice was associated with increased bacterial translocation into deep tissues. Oral gavage of OVA-DH5α E. coli resulted in its increased recovery in the fecal pellets and livers of Foxp3EGFPcreMyd88Δ/Δ mice as compared to Myd88fl/fl controls (Figure 5G). More broadly, the livers and lungs of Foxp3EGFPcreMyd88Δ/Δ had increased bacterial loads as compared to those of Myd88fl/fl mice, with increased recovery of Lactobacilli and Pasteurella species in both organs (Figure 5H). Overall, these results indicate that MyD88 expression in Treg cells enable optimal intestinal IgA antibody responses to commensal bacteria and protect against dysbiosis and unrestrained gut bacterial translocation to deep tissue.
Rescue with IgA+ PP B cells restores luminal IgA and corrects the skewed intestinal Th17 cell response
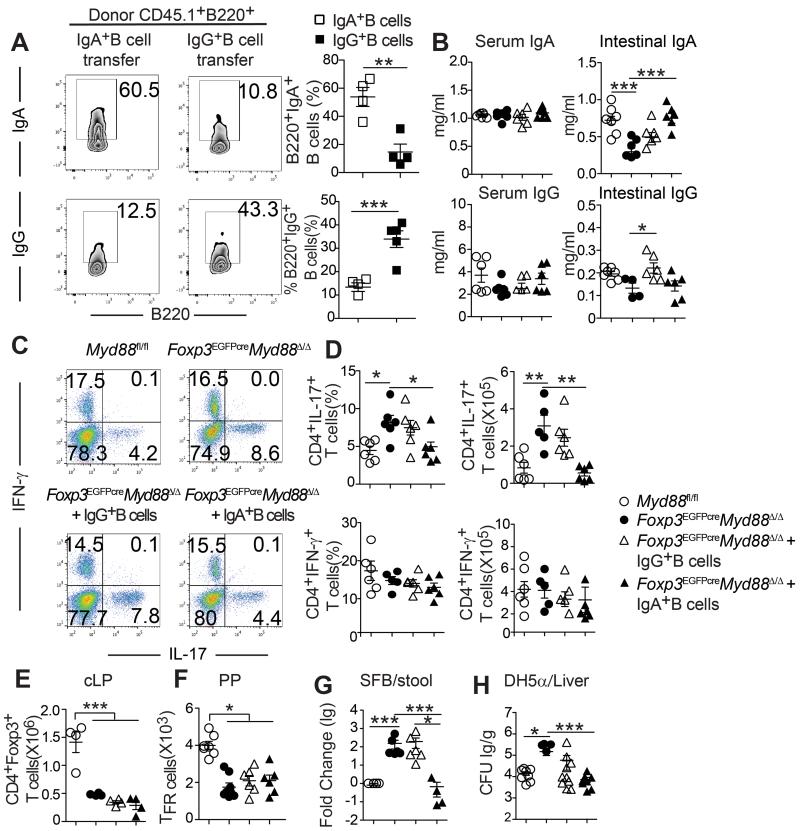
To determine the relationship of the deficient luminal IgA response to the commensal bacteria and the dysregulated intestinal Th17 cell response, we conducted studies in which IgA+ or IgG1+ B cells derived from the PP of WT CD45.1+ C57BL/6 mice were isolated by cell sorting and transferred into CD45.2+ Foxp3EGFPcreMyd88Δ/Δ mice (Figure 6A). The recipient mice were examined for luminal IgA concentrations and intestinal Treg and Th17 cell populations. Transfer of IgA+ but not IgG1+ PP B cells corrected the luminal IgA deficiency without impacting the luminal IgG or serum IgA or IgG concentrations (Figure 6B). Importantly, transfer of IgA+ but not IgG+ PP B cells reversed the Th17 cell skewing in the intestinal cLP without impacting Th1 cells, indicating that the increase in LP Th17 cells in Foxp3EGFPcreMyd88Δ/Δ mice was a consequence of deficient intestinal luminal IgA (Figures 6C and 6D). In contrast, the deficiency in LP Treg and PP Tfr cells was not corrected, indicating an intrinsic function for MyD88 signaling in promoting intestinal Treg cell responses (Figures 6E and 6F).
Figure 6. Adoptive transfer of IgA+ PP B cells restores intestinal IgA production and inhibits Th17 skewing in Foxp3EGFPcreMyd88Δ/Δ recipients.
A. Flow cytometric analysis and frequencies of CD45.1+B220+IgA+ and CD45.1+B220+IgG+ B cells found in the PP of Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ recipients 2 weeks following their adoptive transfer. B. Serum and intestinal IgA (upper panels) and IgG1 (lower panels) concentrations in sham treated Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ mice and in Foxp3EGFPcreMyd88Δ/Δ mice that received CD45.1+B220+IgA+ and CD45.1+B220+IgG+ B cells. C, D. Flow cytometric analysis (C), frequencies and absolute numbers (D) of IL-17 and IFN-γ -expressing cLP CD4+Foxp3− Tconv cells in the mouse groups described in (B). E, F. Absolute numbers of Treg cells in the cLP (E) and Tfr cells in the PP (F) in mouse groups outlined in (B). G. Real time PCR analysis of SFB 16S rRNA in the fecal pellets of the respective mouse groups. H. Translocation of gavaged DH5α bacteria into livers of mice of the respective mouse groups. Results representative of 3 independent experiments. N=3-5 mice/group. *p<0.05; **p<0.01; ***p<0.001 by Student’s unpaired two tailed t test and one-way ANOVA.
IgA regulates the abundance of segmented filamentous bacteria (SFB), instigators of Th17 cell differentiation in the gut (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009; Lecuyer et al., 2014; Suzuki et al., 2004). Real time PCR Analysis of SFB 16S rRNA in the ileal tissues and in the stool revealed their elevation in Foxp3EGFPcreMyd88Δ/Δ mice as compared to WT controls. Rescue with PP IgA+ but not IgG+ B cells suppressed the increase in SFB rDNA content in the fecal pellets of Foxp3EGFPcreMyd88Δ/Δ mice (Figure 6G). Transfer of PP IgA+, and to a lesser extent IgG+ B cells also suppressed the translocation of DH5α E. coli bacteria into the liver (Figure 6H). Overall, these results indicate that the expansion in Th17 cells in Foxp3EGFPcreMyd88Δ/Δ mice and the increased bacterial translocation into deep tissues were consequences of the failure luminal IgA immunity in suppressing SFB and commensal invasiveness, respectively.
IL-10 production by Treg cells has been implicated in the regulation of Th17 cell differentiation (Rubtsov et al., 2008). Because MyD88-deficient Treg cells expressed reduced amounts of IL-10 (Figures 2G and 2H), we examined whether IL-10 deficient Treg cells impacted gut IgA production. Transfer studies in Tcra−/− mice revealed that IL-10 sufficient or deficient Treg cells were equally effective in supporting PP Tfh and Tfr cell differentiation, IgA+B220+ B cell expansion, luminal IgA production and the IgA anti-microbial response (Figures S6A-E). These results indicate that the effects of MyD88 deficiency on PP Tfh and Tfr cell differentiation and gut IgA production are not related to their decreased IL-10 production.
Treg cell specific MyD88 signaling regulates the luminal IgA-SFB-Th17 cell axis by a Stat3-dependent mechanism
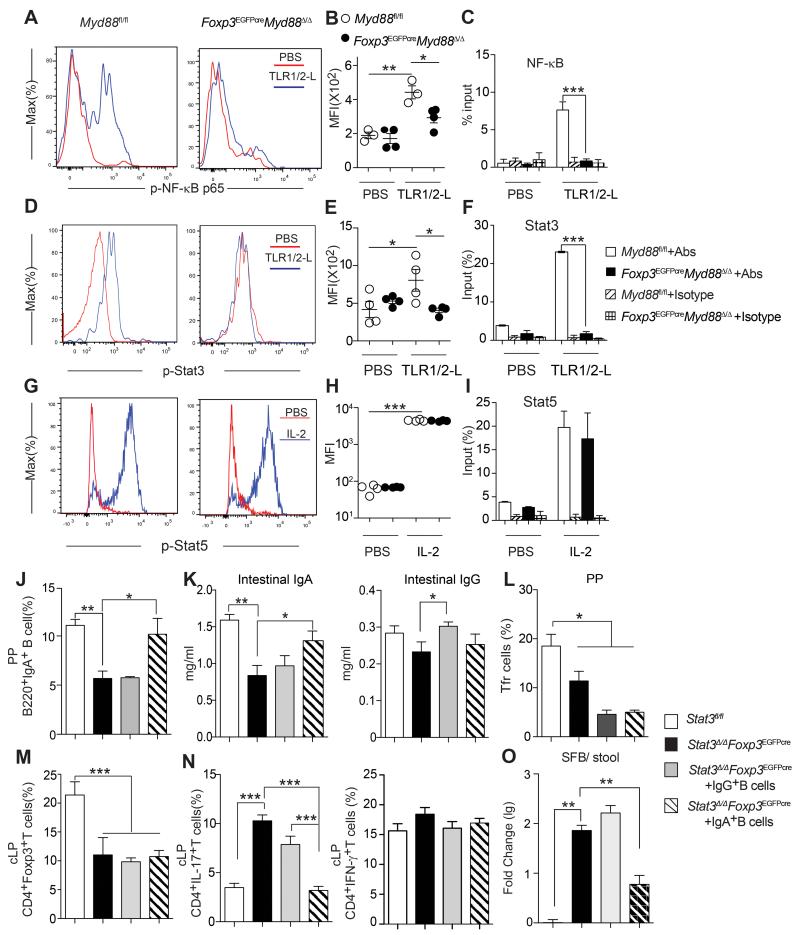
To elucidate the mechanism by which MyD88 signaling in Treg cells regulates mucosal tolerance, we examined the activation by TLR signaling of the down-stream transcription factors NF-κB and Stat3. Both transcription factors were phosphorylated in response to a common TLR1-2 ligand in MyD88-sufficient but not - deficient cLP Treg cells (Figures 7A and 7B; Figures 7D and 7E). Chromatin immunoprecipitation assays revealed that both factors bound the Foxp3 CNS2 following TLR1-2 stimulation of MyD88-sufficient but not deficient cLP Treg cells (Figures 7C and 7F). In contrast, IL-2 stimulation resulted in Stat5 phosphorylation and its translocation to the CNS region in both MyD88-sufficient and deficient cLP Treg cells (Figures 7 G-I). Also, IL-10 treatment induced Stat3 phosphorylation equally well in MyD88-sufficient and deficient Treg cells, indicating that MyD88-related signaling defects were TLR-stimulation specific (Figures S7A and S7B).
Figure 7. Treg cell-specific MyD88 signaling activates Stat3 and its deficiency is phenocopied by TR-cell specific Stat3 deficiency.
A-I. TLR1-2-mediated activation of p65-NF-κB, Stat3 and Stat5 in Myd88fl/fl and Foxp3EGFPcreMyd88Δ/Δ cLP CD4+Foxp3+Treg cells. A, D, G. Flow cytometric analysis of p-p65-NF-κB (A), pStat3 (D) and pStat5 (G) in cLP CD4+Foxp3+Treg cells treated with either PBS or the TLR1-2 ligand (TLR1-2-L) Pam3SCK4. (B, E, H) MFI of the respective phosphoprotein. (C, F, I). Chromatin immunoprecipitation (ChIP) assay of NF-κB p65, Stat3 and Stat5 bound to the Foxp3-CNS2 in the respective cLP CD4+Foxp3+Treg cells treated with either PBS, TLR1-2-L (for p65-NF-kB and Stat3 ChIP) or IL-2 (for Stat5 ChIP). J-O. WT and Foxp3EGFPcreStat3Δ/Δ mice were either untreated or received adoptively transferred CD45.1+B220+IgA+ or CD45.1+B220+IgG+ PP B cell derived from CD45.1+ WT donor mice. Analysis was carried out 2 weeks following the B cell transfer. J. Frequencies of total B220+IgA+ in the PP. K. Intestinal lavage fluid IgA and IgG concentrations. L, M. Frequencies of PP Tfr and cLP Treg cells, respectively. N. Frequencies of CD4+IL-17+ and CD4+IFN-γ+ Tconv cells. O. Real time PCR analysis of SFB 16S rDNA in the stool. Similar results were obtained in 2 independent experiments. N= 4-7 /group *P<0.05; **P<0.01, ***P<0.001 by One-way ANOVA.
TLR-MyD88 signaling classically activates the transcription factor NF-κB (Kawai and Akira, 2007). We therefore examined the capacity of augmented NF-κB activation in Treg cells to rescue IgA deficiency in Foxp3EGFPcreMyd88Δ/Δ mice by using a Foxp3EGFPcre-regulated transgene encoding a gain of function Inhibitor of Kappa B kinase Beta mutant (R26IIkbkb) (Sasaki et al., 2006). Expression of the R26IIkbkb transgene upregulated the phosphorylation of p65 subunit of NF-κB in Treg cells both at rest and following stimulation with a TLR1-2 ligand (Figures S7C-F). Transgene expression in Treg cells failed to correct the deficit in luminal IgA concentrations in Foxp3EGFPcreMyd88Δ/Δ mice, while leaving those of Foxp3EGFPCre mice unaffected. It also did not affect the luminal total IgG concentrations in both mouse strains (Figure S7G). Significantly, transgene expression decreased PP Tfr and cLP Treg cells and increased the cLP Th17 cells, indicating that it promoted Treg cell conversion in to Th17 cells (Figure S7H, I). Transgene expression did not significantly affect SFB abundance in Foxp3EGFPcre mice, but compounded the SFB expansion in Foxp3EGFPcreMyd88Δ/Δ mice (Figure S7J). Overall, these data indicate that the deficit in IgA production in Foxp3EGFPcreMyd88Δ/Δ mice cannot be rescued by augmented activation of NF-κB in Treg cells.
Stat3 has been implicated in Tfh differentiation (Ma et al., 2012). Treg cell-intrinsic Stat3 signaling also promotes iTreg cell generation and is required for the regulation of Th17 cells (Chaudhry et al., 2009; Laurence et al., 2012). Foxp3EGFPcreStat3Δ/Δ mice, with Treg cell-specific Stat3 deficiency, recapitulated key phenotypes of Foxp3EGFPcreMyD88Δ/Δ mice. They exhibited reduced PP IgA+, but not IgG+, B cells and decreased intestinal IgA, but not IgG, antibody concentrations. Both deficiencies were rescued by the transfer of PP IgA+ but not IgG+ B cells (Figures 7J and 7K). Similar to Foxp3EGFPcreMyd88Δ/Δ mice, Foxp3EGFPcreStat3Δ/Δ mice exhibited reduced cLP Treg cells and PP Tfr cells that were not restored by the transfer of either PP IgA+ or IgG+ B cells (Figures 7L and 7M). Importantly, transfer of PP IgA+, but not IgG+ B cells to Foxp3EGFPCreStat3Δ/Δ mice completely suppressed the dysregulated cLP Th17 cell response previously described in these mice while leaving unaffected the cLP Th1 cell response (Chaudhry et al., 2009) (Figure 7N). The dysregulated cLP Th17 cell response of Foxp3EGFPcre Stat3Δ/Δ mice was associated with overgrowth of SFB, which was also effectively suppressed by the transfer of PP IgA+ but not IgG+ B cells (Figures 7O). Overall, these results implicate Stat3 activation as the critical mechanism by which by MyD88 signaling regulates IgA production, SFB commensalism and Th17 cell responses.
Discussion
Our studies revealed MyD88-dependent microbial sensing by Treg cells as serving to coordinately regulate gut mucosal cellular and humoral adaptive immune responses in favor of tolerance. MyD88 signaling promoted the generation of antigen-specific iTreg cells at the mucosal interface, including LP and PP. Its deficiency in Treg cells impaired the regulation of inflammatory responses in two different models of microbial flora-dependent gut inflammation, including DSS and lymphopenia-induced colitis. A second cardinal function of MyD88 signaling in Treg cells is its promotion of IgA immunity to the commensal flora by virtue of its regulation of Tfr and Tfr-derived Tfh cell differentiation in the PP. Together, these functions of MyD88 signaling in Treg cells enable the maintenance of healthy commensalism and restrain microbiota-driven dysregulated mucosal immune responses.
TR-specific MyD88 deficiency compromised the LP and PP Treg cell populations. The mucosal Treg cell deficit reflected impairment of in vivo induction of iTreg cells in the face of normal development of nTreg cells in the thymus, consistent with enrichment of gut tissues with microbiota-specific iTreg cells. In contrast, splenic Treg cells, which are predominantly thymus derived, were unaffected. MyD88 deficiency also resulted in Treg cell instability, with increased generation of Foxp3− ex-Treg cells in the context of mucosal inflammation, such as lymphopenia-induced colitis, and poor epigenetic demethylation of Foxp3 CNS2.
A major finding of our studies is that Treg cell-specific MyD88 signaling regulates the gut commensal flora. Its deficiency altered gut microbial community structure and composition. It resulted in dysbiotic expansion of SFB, a pathobiont that drives Th17 cell differentiation, accounting for the dysregulated expansion of the latter in Foxp3EGFPcreMyd88Δ/Δ mice. It also promoted commensal bacterial invasiveness, with increased bacterial translocation in to deeper tissues. A key mechanism by which Treg cell-specific MyD88 signaling influences the gut microbiota is through its promotion of anti-commensal IgA responses by virtue of its regulation of Tfh and IgA+ B cell differentiation in the PP. Transfer of IgA+ B cells completely suppressed the SFB expansion and reversed the Th17 cell dysregulation in Foxp3EGFPcreMyd88Δ/Δ mice, thus linking the failure of IgA immunity with Th17 cell-mediated immune dysregulation. Transfer of IgA+ B cells did not ameliorate the Treg cell deficit in the gut, reflecting a requirement for MyD88 signaling in differentiating Treg cells. Our results support a key role for MyD88 in directing Tfr to Tfh cell differentiation in PP, a differentiation step previously proposed by Fagarasan et al (Kawamoto et al., 2014; Tsuji et al., 2009). Th17 cells have also been proposed to promote serum IgA responses when transferred into Tcra−/− mice (Hirota et al., 2013). However, serum IgA responses were unchanged in Foxp3EGFPcreMyd88Δ/Δ mice despite increased mucosal Th17 cells.
Mechanisms by which MyD88 promoted mucosal Treg cell generation and directed luminal IgA responses appeared to involve Stat3, whose activation by TLR may proceed via a MyD88-DOCK8 signaling cascade (Jabara et al., 2012). Consistent with such a mechanism, Stat3 activation in Treg cells treated with TLR ligands was abrogated by MyD88 deficiency. TR-cell specific Stat3 deficiency, which has been previously shown to precipitate Th17 cell dysregulation, recapitulated the other key phenotypes of TR-cell specific MyD88 deficiency including decreased mucosal Treg cells, impaired PP IgA+ B cell differentiation and luminal IgA production, and SFB dysbiosis (Chaudhry et al., 2009). In both cases, the IgA deficit, SFB dysbiosis and Th17 cell dysregulation but not the mucosal Treg cell deficiency were reversed by the transfer of PP IgA+ B cells. Whereas Treg cell-specific Stat3 deficiency induces spontaneous age-dependent gut inflammation in affected mice, the phenotype of Treg cell-specific MyD88 deficiency is milder, with its disease promoting function becoming pronounced in the context of an inflammatory stimulus (DSS or lymphopenia-induced colitis) (Chaudhry et al., 2009). This difference may relate to the fact that MyD88 deficiency does not disrupt non-TLR-dependent Stat3 activation in Treg cells, such as that induced by IL-10, which may provide an additional layer of immunoregulation at steady state (Chaudhry et al., 2011).
While NF-κB promotes thymic Treg cell development and is activated by MyD88, Treg cell-specific expression of a gain of function IKK beta kinase mutant failed to rescue the phenotype of IgA efficiency in Foxp3EGFPcreMyd88Δ/Δ mice (Long et al., 2009). Instead, it induced decreased mucosal Treg cells and increased Th17 cells in otherwise MyD88 sufficient mice, suggesting that sustained NF-κB activation in mucosal Treg cells, such as in the context of an inflammatory environment, may favor their switching into Th17 cells. Accordingly, it can be postulated that the outcome of MyD88 signaling in promoting iTreg cell differentiation may well depend on a balanced Stat3: NF-κB signaling and the interaction of the respective pathways with Foxp3.
In summary, MyD88 signaling in Treg cells integrates cues from the gut microbiota to augment the mucosal Treg cell compartment and to enforce commensalism by directing anti-microbial IgA responses. It thus provides an unusual example of a Treg cell pathway that promotes, rather than suppresses, immune responses in the service of tolerance induction.
Materials and Methods
Mice
CD45.1+, CD45.2+OT-II+, Foxp3EGFPcre, Foxp3EGFP, Myd88fl/fl, Stat3fl/fl, R26IIkbkb, Il18r−/−, Il1r1−/−, Il10−/−, Rag1−/− and Tcra−/− mice, all on C57BL6/J background, were obtained from the Jax Lab (Haribhai et al., 2007; Murtaugh et al., 2003; Sasaki et al., 2006; Yang et al., 2004; Zhou et al., 2009). Myd88−/− mice were a gift from S. Akira (Osaka University, Osaka, Japan) and were crossed with Foxp3EGFP mice (Rivas et al., 2012). All mutant mouse strains and their respective crosses were backcrossed 8-10 generations on C57BL/6 background. The mice were housed under specific pathogen-free conditions and used according to the guidelines of the institutional Animal Research Committees at the Boston Children’s Hospital.
16S rRNA pyrosequencing and data analysis
Methods describing bacterial rRNA sequencing and analysis are detailed in the Supplemental Information section.
DSS and lymphopenia-induce colitis
DSS-induced colitis was initiated by treating mice with 3.5-5% DSS (MP Biomedicals) in the drinking water ad libitum for 5 days, as indicated (Geuking et al., 2011). The DSS solution was then replaced by normal water for the indicated periods, following which the mice were euthanized. Subgroups of mice were injected i.p. with 300 μ g of neutralizing IL-17A mouse antibody (17F3) or IgG1 isotype control antibodies(MOPC21) (Bio × Cell) on days 0, 2, and 4 of the DSS protocol. Body weights were monitored daily. Histological assessments of colitis and severity scores were carried out in a blinded manner after hematoxylin and eosin (H&E) staining.
For Lymphopenia-induced colitis, Rag1−/− mice were injected i.p. with 5 × 105 congenic CD45.1+CD4+CD45RBhigh T cells (Haribhai et al., 2009). Some mice were injected i.p with 2.5 × 105 CD45.2+CD4+Foxp3+ Treg cells either from Foxp3EGPFCre or Foxp3EGFPCreMyD88Δ/Δ mice at the same time. Mice were observed daily and weighed weekly. Mice developed clinical signs of colitis around week 4 post transferred.
Mouse colonization with OVA139-386DH5α bacteria
Chicken OVA139-386-expressing E coli (DH5α E. coli strain) was a kind gift of Dr. Rick Blumberg (Beth Israel Deaconess Hospital, Boston) (Yoshida et al., 2006). Control DH5α E. Coli bacteria, harboring the parent low-copy plasmid pACYC184 (chloramphenicol selection), was obtained from New England Biolabs. Bacteria were grown under chloramphenicol selection, harvested by centrifugation and orally gavaged at 1010 Colon form units (CFU)/ 500μl PBS/per mouse (Hapfelmeier et al., 2010). The mice were then sensitized with OVA ad libitum for 1 week. Stool, lung and liver were isolated from the inoculated mice and analyzed for chloramphenicol-resistant bacteria by plating serial dilutions and counting CFUs.
Adoptive cell transfers
For in vivo iTreg cells conversion experiments, naïve OT-II (Vα2+) CD4+Foxp3−(EGFP−) Tconv cells were isolated from spleens of OT-II+ Foxp3EGFPcre or OT-II+ Foxp3EGFPcreMyd88Δ/Δ mice by cell sorting using BD FACSAria III (BD Biosciences) (purity >95%). The cells were labeled with Violet CellTrace Dye (Invitrogen) and adoptively transferred (1×106) by retro-orbital injection into congenic CD45.1 mice. Recipient mice were then administered OVA in drinking water ad libitum for 1 week. The different organs were thereafter collected and analyzed by flow cytometry for Violet CellTrace Dye, Vα2 and EGFP.
Adoptive transfer of splenic Treg cells from Foxp3EGFPcre and Foxp3EGFPcreMyd88Δ/Δ - mice into Tcrα−/− mice was carried out as above. Recipient mice were analyzed two weeks later for T and B cell phenotypes and numbers in the PP and other immune tissues.
Adoptive transfer of PP B cells
PP cells of CD45.1 congenic donor mice were isolated and stained with PE-labeled anti-IgA, APC-labeled anti-IgG and PB-labeled anti-B220 IgG mAbs. B220+IgG+ and B220+IgA+ B cells were isolated to 95-98% purity by cell sorting using BD FACSAria III (BD Biosciences). After isolation, 2-5 × 105 cells of the indicated cell types were immediately transfered i.p. into CD45.2 recipient mice. Two weeks later, the recipient mice were euthanized for analysis.
Statistical analysis
Statistical significance between groups was determined by the unpaired, two-tailed student’s t test, One-way and two-way ANOVA using Prism software (GraphPad). Statistical analyses of gut bacterial 16S rRNA studies were carried out as detailed in the Supplemental Information section. Differences at P < 0.05 were considered significant. Data are presented either as means or means ± SEM.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants R01AI085090 (T.A.C.), R01HD061916 (L.B.) and P30DK034854 (L.B. and G.G.), and by the Brigham and Women’s Department of Pathology (G.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION. Supplemental information includes seven Figures, two Tables, and Supplemental Experimental Procedures and can be found with this article online.
REFERENCES
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J Immunol. 2009;183:4458–4466. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li SH, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A. 2011;108:278–283. doi: 10.1073/pnas.1011549108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011 doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O’Shea JJ, Fowler DH. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, Arkwright PD, Kreins AY, Averbuch D, Engelhard D, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Current opinion in immunology. 2012;24:385–391. doi: 10.1016/j.coi.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Rivas MN, Koh YT, Chen A, Nguyen A, Lee YH, Lawson G, Chatila TA. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122:1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.