Abstract
Charcot-Marie-Tooth (CMT) disease is a clinically and genetically heterogeneous distal symmetric polyneuropathy. Whole-exome sequencing (WES) of 40 individuals from 37 unrelated families with CMT-like peripheral neuropathy refractory to molecular diagnosis identified apparent causal mutations in ~45% (17/37) of families. Three candidate disease genes are proposed, supported by a combination of genetic and in vivo studies. Aggregate analysis of mutation data revealed a significantly increased number of rare variants across 58 neuropathy associated genes in subjects versus controls; confirmed in a second ethnically discrete neuropathy cohort, suggesting mutation burden potentially contributes to phenotypic variability. Neuropathy genes shown to have highly penetrant Mendelizing variants (HMPVs) and implicated by burden in families were shown to interact genetically in a zebrafish assay exacerbating the phenotype established by the suppression of single genes. Our findings suggest that the combinatorial effect of rare variants contributes to disease burden and variable expressivity.
Introduction
Charcot-Marie-Tooth (CMT) disease, first described clinically in 1886 [Charcot and Marie, 1886; Tooth, 1886], is a common hereditary peripheral neuropathy with an estimated prevalence of 1/1200 [Braathen, 2012] to 1/2500 [Skre, 1974] individuals. The disease is characterized by distal symmetric polyneuropathy (DSP) with progressive muscle weakness and atrophy, and sensory loss. Two major clinical types are distinguished by electrophysiologic and neuropathologic studies and the type of cells (glia or neurons) primarily affected. CMT1 affects the glia-forming Schwann cells and presents with nerve conduction velocities (NCV) of <38 m/s; CMT2 affects the axons of neurons and usually presents with NCVs of >38m/s or slightly reduced motor NCVs but with diminished amplitudes. Other forms of CMT with additional clinical features have been described, including an intermediate form with overlapping demyelinating and axonal CMT features [Nicholson et al., 2006] and one in which CMT occurs in conjunction with glomerulonephritis [Boyer et al., 2011].
Observed inheritance patterns include: autosomal dominant, autosomal recessive and X-linked (dominant and recessive) forms [Allan, 1939; Rossor et al., 2012]. Nevertheless, most patients present with apparent sporadic disease, attributable partially to the extreme clinical variability and age dependent penetrance of the phenotype. New mutation, however, is often the cause of sporadic CMT, with the de novo CMT1A duplication of 17p11.2 being responsible for 76–90% of sporadic cases [Raeymaekers, et al., 1991; Lupski et al., 1991; Hoogendijk et al., 1992; Nelis et al., 1996]. Locus-specific screening for mutations in known CMT genes concludes a molecular diagnosis for approximately 70–80% of patients [Szigeti and Lupski, 2009; DiVincenzo et al., 2014]. More than 40 genes are known to be causative, but it has been estimated that 30–50 ‘CMT genes’ remain to be discovered [Braathen, 2012; Timmerman et al., 2014].
CMT1A [MIM #118220] is caused by a recurrent 1.4 Mb duplication that encompasses the dosage sensitive myelin gene PMP22 [Lupski et al, 1991; Hoogendijk, 1992; Patel et al., 1992; Lupski et al., 1992], an essential component of compact PNS myelin [Li et al, 2012]. The reciprocal deletion of the identical 17p11.2 region causes hereditary neuropathy with liability to pressure palsies (HNPP) [MIM #162500] [Chance et al, 1993; Chance et al., 1994]. A recent study of 17,000 patients with neuropathy established a molecular diagnosis in 18.5% of these; ~80% of molecular diagnoses were either duplication or deletion CNV of PMP22 [DiVincenzo, et al. 2014]. Point mutations and indels in PMP22 have also been found in patients with CMT1A or HNPP without duplication or deletion [Roa et al., 1993 (a); Nicholson et al., 1994], and in the more severe early-onset phenotype of hypertrophic neuropathy of Dejerine-Sottas [MIM #145900] [Dejerine and Sottas, 1893; Roa et al., 1993 (a); Roa et al., 1993 (b); Li et al., 2012]. Additionally, non-recurrent and complex rearrangements can account for the missing heritability in CMT1A and HNPP, including upstream CNVs that do not include PMP22 coding sequence [Zhang et al., 2010; Weterman et al., 2010].
The second most common form of CMT is CMTX1 [MIM #302800] caused primarily by point mutations that occur in almost every amino acid of GJB1/connexin32 [Kleopa et al., 2006; Scherer et al., 2012]; gene deletions have also been described [Gonzaga-Jauregui et al., 2010]. GJB1 encodes a gap junction protein involved in the formation of connexon hemichannels that facilitate the communication and exchange of ions and other small molecules between Schwann cells and axons [Scherer et al., 2012].
The third most common cause of CMT, and the most common form of CMT2, are heterozygous mutations in MFN2 (CMT2A; [MIM #609260]) [Ben Othmane et al., 1993; Züchner et al., 2004; Verhoeven et al., 2006], essential for mitochondrial fusion and function [Kijima et al., 2005] and maintenance of mitochondrial morphology. Mutations in MFN2 lead to mitochondrial dysfunction due to mtDNA depletion [Vielhaber et al., 2013]. Mutations in GDAP1 cause a recessive form of CMT, which can be either demyelinating (CMT4A; [MIM #214400]) [Cuesta et al., 2002], axonal (CMT2K; [MIM #607831]) [Nelis et al., 2002] or intermediate (CMTRIA; [MIM #608340]) [Senderek et al., 2003] and have been reported to affect mitochondrial fission in Schwann cells and neurons [Niemann et al., 2005].
Known CMT genes encode proteins that span a wide range of functions, from GTPases (RAB7, DNM2), lipid phosphatases (FIG4, MTMR2), to structural myelin proteins (MPZ, PMP22) and gap junction channel components (GJB1). Cellular functions include myelin assembly (PMP22, MPZ, PRX, Cx32), membrane and endocytic trafficking (MTMR2, SBF2, FIG4, SH3TC2) and mitochondrial dynamics (MFN2, GDAP1) [Niemann et al., 2005; Azzedine et al., 2012]. Another predominant contributing gene group is that of aminoacyl-tRNA synthetases, an essential class of enzymes that ligate amino acids onto cognate tRNA molecules [reviewed in Wallen and Antonellis, 2013].
Other complex forms of CMT2 (e.g. spinocerebellar ataxia with axonal neuropathy, SCAN1) have been associated with mutations in TDP1, important for DNA single strand break repair (SSBR) [McKinnon et al., 2007; Caldecott, 2008]. Mutations in SETX, a helicase involved in transcriptional termination and RNA maturation, cause recessive ataxia ocular motor apraxia type 2 (AOA2; #606002) [Moreira et al., 2004] possibly due to transcriptional/translational defects [Anhelm et al., 2012], also disturbing DNA SSBR [Caldecott, 2008]. SETX mutations have been associated with familial amyotrophic lateral sclerosis (ALS), susceptibility that recently was also associated with heterozygous FIG4 mutation carrier states [Chow et al., 2009].
Substantial genetic and clinical heterogeneity of CMT neuropathy makes it challenging for molecular diagnosis by single gene and gene panel testing; the diagnostic utility of genome-wide sequencing approaches has been demonstrated [Lupski et al., 2010; Montenegro et al., 2011; Choi et al., 2012; Lupski et al, 2013]. We performed whole exome sequencing (WES) in a cohort of 40 patients with peripheral neuropathy from 37 unrelated families in whom extensive genetic evaluation had failed to identify a causative mutation or establish a molecular diagnosis (Table 1). Analysis of WES data was performed in two stages: a first-pass analysis that focused on known or novel variants in known CMT and related neuropathy genes, and a second stage analysis to search for rare variants in likely novel candidate genes (Supplementary Figure 1). Our rare variant analyses revealed potential neuropathy candidate ‘disease genes’. Surprisingly, we uncovered evidence for a mutational burden in affected individuals versus a large sample of unrelated control individuals. We show experimentally that genetic interactions implicated by burden contribute to phenotypic variability and potentially to susceptibility to common neuropathies beyond the well characterized Mendelian forms.
| Proband BAB# |
Clinical diagnosis | Gender | Onset (y) | Foot deformities (pes cavus/ hammer toes) |
Weakness or atrophy of distal extremities |
Sensory loss |
Addition clinical findings | DTRs | Median nerve motor NCV (m/sec) |
Median nerve distal CMAP (mV) |
Nerve biopsy | Candidate HPMV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Known variants | 668 | CMT2 | F | 31 | nr | + | + | DM, lumbar disc disease | − | 63 (ulnar) | 7 (ulnar) | NA | MFN2 |
| 710 | CMT2 | F | 10 | + | + | + | nr | − | 36.3 | 0.6 | NA | MED25 | |
| 1405 | CMT1 | M | 3 | nr | + | + | nr | − | 20 | 0.7 | NA | MFN2 | |
| 1564 | CMT2 | M | 3 | nr | + | + | nr | − | 48.2 | 2.6 | axonal and hypomyelinating neuropathy |
MFN2 | |
| 3656 (T) | CMT2 | M | 11–20y | + | NA | NA | − | NA | 50 | 14 | NA | AARS | |
| 3662 (T) | CMT2 | M | 11–20y | + | + | + | − | NA | 48 | 3.2 | NA | MFN2 | |
| 3663 (T) | CMT1 | M | 2–10y | + | + | + | − | NA | 15 | 4 | NA | MPZ | |
| Known genes, novel variants | 1080 | CMT | F | 7 | + | + | + | nr | − | decreased | NA | NA | MFN2 |
| 1280 | CMT2 | F | 9 | nr | + | + | − | 50 | 10 | NA | ARHGEF10 | ||
| 1500 | Dejerine-Sottas | M | infancy | + | + | + | demyelinating CMT, hammer toes | − | 16 | 2.9 | NA | SH3TC2 (hom) | |
| 1955 | CMT2 | M | NA | nr | + | nr | Sotos syndrome; intellectual disability | − | normal | normal | axonal loss, Schwann cell atrophy |
AIFM1 | |
| 3646 | CMT intermediate | F | 16 | + | + | nr | nr | − | NA | NA | NA | YARS | |
| 3657 (T) | CMT1 | M | 21–40y | + | + | + | − | NA | 30 | NA | NA | GJB1 | |
| 3660 (T) | CMT2 | M | 21–40y | + | + | NA | − | NA | 51.6 | 3.6 | NA | MFN2 | |
| 3672 (T) | CMT2 | F | 18mo | + | + | NA | − | NA | 55.6 | 3.05 | NA | MFN2 | |
| 4119 (T)* | CMT2 | M | infancy | + | + | NA | vocal cord paralysis | − | 19 | 0.1 | NA | TRIM2* | |
| Phenotypic re-assessment | 996 | Dejerine-Sottas/ congenital hypomyelinating neuropathy |
M | infancy | nr | + | + | ataxia, tremor, macrocephaly, early onset myopia, genital vitiligo |
− | 12 | 1.5 | NA | ITPR1 |
| 1038 | Dejerine-Sottas | F | infancy | nr | nr | nr | subacute deterioration, saccades, recurrent metabolic acidosis, ataxia/titubation, hypertrichosis |
− | 28 | 2.9 | chronic demyelinating neuropathy with mild axonal loss and early onion bulb formation |
SURF1 | |
| 1163 | Hypotonia, hypomyelinating neuropathy |
M | birth | + | + | + | vocal cord paralysis, feeding difficulties | − | 19 | NA | thinly myelinated axons and diffuse loss of large axons |
ADCY6 (hom) | |
| 1522 | axonal neuropathy and neurosensory deafness |
M | 12 | + | nr | − | thin corpus callosum on MRI; abnormal mitochondria variation, early axonal denervation on muscle biopsy |
− | 61.1 | 6 | mild loss of myelinated fibers | MYH14 | |
| 1566 | congenital hypotonia | F | birth | + | general hypotonia |
nr | respiratory involvement, dysmorphic features |
− | NA | NA | neurogenic atrophy, extensive denervation suggestive of anterior horn disease |
IGHMBP2 (comp het) |
|
| 1581 | congenital hypomyelinating neuropathy |
M | birth | + | general hypotonia |
nr | respiratory involvement w/congenital elevated hemidiaphragm, dysmorphic features |
− | NR | NR | congenital hypomyelinating neuropathy, no onion bulb formation |
IGHMBP2 (comp het) |
|
| 1680 | neuropathy, ataxia, cataracts, hearing loss |
M | NA | nr | nr | nr | ataxia, cataracts, hearing loss | NA | decreased | NA | NA | ABHD12 | |
| 2447 | progressive neurodegenerative disorder w/ peripheral neuropathy |
M | 16 | nr | + | + | oculomotor apraxia, ataxia | NA | NA | NA | NA | SETX (hom) | |
| 3664 (T) | CMT2 | M | NA | NA | NA | NA | pyramidal signs | NA | 41 | NA | NA | AIMP1 | |
| 3669 (T) | CMT2 | M | 21–40y | + | + | NA | − | neurogenic signs |
59.8 | 9.3 | NA | DNAJB2 | |
| 3729 (T) | CMT2 | F | 36 | NA | proximal | + | − | − | 57 | 3.1 | NA | TFG | |
| 3730 (T) | CMT2 | M | 11–20y | + | NA | NA | hypotonia, ptosis | axon loss | 49 | 7.6 | NA | DNAJB2 | |
| Novel genes | 124 | nonspecific myopathy/neuropathy |
F | 20 | nr | nr | nr | nr | NA | 52.4 | 6 | NA | DNAJB5 |
| 1468 | CMT1 | M | 6 | + | + | + | nr | − | 18 (tibial) | reduced | demyelinating neuropathy; onion bulb formation |
PMP2 | |
| 1631 | CMT | M | 30’s | + | + | + | bulbar involvement | − | 52 | 10.9 | NA | SPTLC3 |
abbreviations: CMT - Charcot-Marie-Tooth disease; DM - diabetes mellitus; DTRs - deep tendon reflexes; NCV - nerve conduction velocity; (−) = decreased/absent; NA - not available; NR - no response; nr - not reported; (T) - proband from Turkish cohort. Samples from the Turkish cohort are shaded in blue.
previously published [Pehlivan et al., 2015]
Results
Known alleles in known neuropathy genes
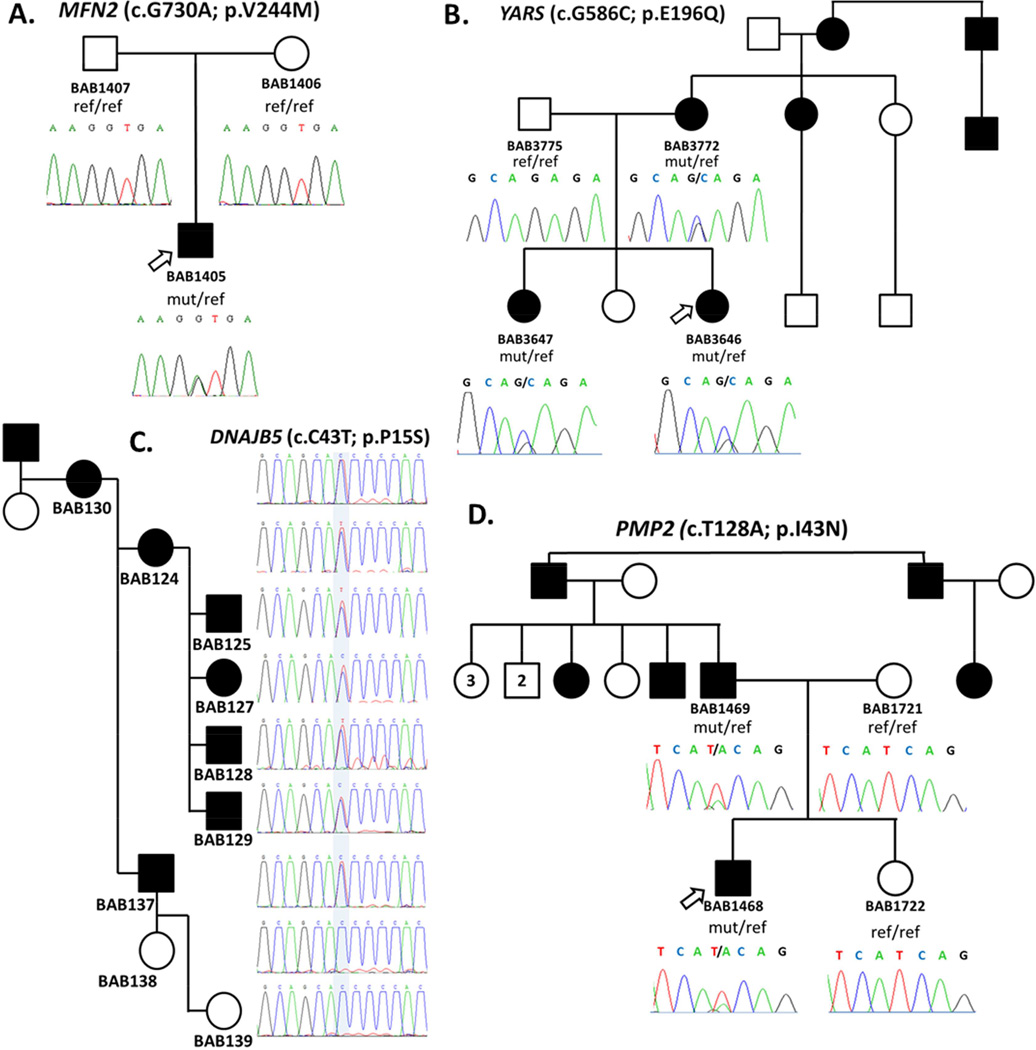
We identified known disease-causing alleles in six of the 37 index patients (see Supplementary Information for detailed clinical information). Two represented phenotypic expansions of CMT2 caused by mutations in MFN2 (Figure 1A), where the clinical presentation made screening for MFN2 unlikely. One family showed two separate segregating causes of CMT [Verny et al., 2004], one X-linked and the other caused by compound heterozygous mutations in MED25. A novel, likely disease causing allele was found in trans with the only known disease causing allele in this gene [Leal et al., 2001; Leal et al., 2009]. In a proband with autosomal dominant neurosensory deafness and axonal neuropathy we found a recently reported mutation in MYH14 [Choi et al., 2011]. Finally, in a consanguineous family, we detected a 14kb homozygous deletion CNV encompassing exon 1 of ABHD12 segregating with the complex neuropathy phenotype observed in the proband and affected siblings [Fiskerstrand et al., 2010] (Supplementary Figure 2). An additional homozygous GDAP1 novel variant was also identified in some affected individuals of this family posing the possibility of an additive contribution from intragenic deletion CNV plus SNV variation.
Figure 1.
Pedigrees of CMT/neuropathy patients and segregation of causative mutations. A. Pedigree showing de novo occurrence of the known p.V244M MFN2 mutation in proband. B. Dominant pedigree of a dominant intermediate form of CMT and segregation of the identified novel variant p.E196Q in YARS. Mutation was inherited to the affected proband and affected sister from the affected mother. C. Pedigree of a dominant form of CMT and segregation of the mutation in candidate gene PMP2 (p.I43N). The affected proband inherited the mutation from his affected father, while both unaffected mother and sister do not carry the mutation. D. Pedigree of a dominantly inherited myopathy-neuropathy phenotype in a family with multiple affected individuals where a novel variant in DNAJB5 (p.P15S) was identified.
Novel alleles in known neuropathy genes
Rare non-synonymous, frameshifting, or splicing variants were identified in known CMT/neuropathy disease genes, illustrating the complexity that can underscore ‘simple’ mendelian conditions (see Supplementary Information for detailed clinical information). We identified a patient with mutations in both MFN2 and GDAP1, both of which are involved in mitochondrial dynamics. Concurrent mutations in these genes have been reported, suggesting the possibility of epistasis or modifying effects [Cassereau et al., 2011; Vital et al., 2012]. In a family with three generations affected by autosomal dominant intermediate CMT, we sequenced two individuals and identified a novel variant in YARS affecting a residue previously reported to be mutated in disease [Jordanova et al., 2006] (Figure 1B). Functional analyses revealed that the identified YARS allele is a functional hypomorph, unable to complement fully deletion of the endogenous yeast gene, TYS1, in growth complementation assays (Supplementary Figure 3), supporting a pathogenic role for this mutation in CMT. A male patient with Sotos syndrome [MIM #117550] due to NSD1 deletion, plus clinical neuropathy was found to carry several predicted deleterious variants in different CMT genes in addition to a novel potentially pathogenic variant in the X-linked AIFM1 gene [Rinaldi et al., 2012]. Compound heterozygous truncating mutations in SURF1 were identified in a proband with demyelinating CMT. Loss of function mutations in SURF1 were recently described in patients with autosomal recessive severe demyelinating neuropathy of childhood onset [Echaniz-Laguna, 2013], consistent with this patient’s clinical and molecular findings.
Genetic and functional evidence for potential candidate CMT genes
We identified variant alleles implicating three potential new candidate neuropathy genes, PMP2, SPTLC3, and DNAJB5, in 3 different families. In a family with a clinical diagnosis of autosomal dominant demyelinating CMT1 neuropathy, we found a candidate missense variant in myelin protein P2, PMP2 (c.T128A; p.I43N) as the most likely disease causing variant. We confirmed this variant in the proband and his affected father, and its absence in both unaffected mother and sister (Figure 1D). PMP2 is a major stabilizing component of the myelin sheath that insulates the axons in the PNS [Majava et al., 2010], but to date has not been associated with any genetic peripheral neuropathy. PMP2 is predominantly expressed in myelinating Schwann cells, with specific expression in sciatic nerve endoneurium and dorsal root ganglia [Zenker et al, 2014]. Homozygous knockout (Pmp2−/−) mice have significantly reduced temporal motor nerve conduction velocities, although no major structural changes in the myelin sheath and peripheral nerves were observed [Zenker et al, 2014].
In vivo modeling experiments interrogated the potential impact of PMP2 loss of function and of this specific novel variant. Two orthologues exist in zebrafish; suppression of either using morpholino (MO) knockdown led to a motor neuron phenotype, including failure of the motor neuron axons to extend from the notochord, as well as pathfinding errors where the axons failed to innervate the myotomes appropriately (Figure 2A–B). These phenotypes could be rescued by co-injection of the MO with wild-type human PMP2; however contrary to wild-type, human mRNA carrying the variant identified in our proband failed to restore the MO induced phenotype (Figure 2A–D and E). Upon overexpression, wild-type human mRNA induced a phenotype similar to the one observed with MO alone in >50% of injected embryos, suggestive of a dosage-sensitive transcript, similar to PMP22. Overexpression of human mutant (p.I43N) PMP2 mRNA exacerbated the phenotype significantly (~20% increase; p=0.0003 Figure 2E–F); consistent with a dominant-negative mechanism of pathogenesis for this allele.
Figure 2.
Suppression of pmp2 and sptlc3 in zebrafish causes defects in motor axon pathfinding and outgrowth. A–F. Lateral views of a control embryo, an embryo injected with pmp2 morpholino (MO) and embryos injected with pmp2 MO+PMP2_WT and pmp2 MO+PMP2_I43N, PMP2_WT and PMP2_I43N cocktails, respectively, at 2dpf (days post fertilization). Controls showed even spacing and normal branching of the motor neuron axons (A). In the pmp2 MO injected embryos the spacing of neuronal axons is perturbed by exiting the periphery but failing to extend (asterisks) or presenting pathfinding errors (arrows; B). Co-injection of pmp2 MO with human PMP2_WT resulted in restoration of the normal neuronal phenotype (C), but PMP2_I43N did not (D). Overexpression of human PMP2_WT causes mild pathfinding errors (E), suggesting dose sensitivity for PMP2. However, the human PMP2 mutant p.I43N, was significantly more severe than PMP2_WT when overexpressed (F) and had similar effects to suppression of pmp2 by MO knockdown. G. Percentage of normal versus abnormal embryos under the conditions being evaluated above. H–K. Wild type embryos (H) and sptlc3 morphants (I) in which secondary axons fail to migrate appropriately (white arrows). The phenotype induced by suppression of sptlc3 could be rescued by co-injection with SPTLC3_WT (J) but not SPTLC3_R150W (K). L. Quantification of normal embryos vs. embryos with motor neuron axon defects. For statistical analyses χ2 -tests were performed.
Of note, antibodies against PMP2 fragments were identified initially in experimental allergic neuritis, an autoimmune peripheral neuropathy in animals like rats and rabbits, and a model for Guillain-Barre syndrome (GBS) [Ishaque et al., 1981; Ishaque et al., 1982]. One of the main characteristics of GBS is the autoimmune attack to the peripheral nerves’ myelin sheath causing demyelination. Antibodies against myelin protein zero (MPZ, P0) and most significantly to myelin protein 2 (PMP2, P2) have been detected in patients with GBS and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) characterized by primary demyelination and lymphocytic infiltration of the peripheral nerve [Inglis et al., 2007]. Thus, discovery of this mutation in a CMT patient suggests a potential mechanistic link between auto-immune neuropathy and inherited neuropathy.
We identified a novel variant in SPTLC3 (c.T448C; p.W150R) changing a highly conserved residue and predicted to be damaging by bioinformatic algorithms in a patient; no parental samples were available. The proband presented with neuropathy with a marked sensory but no apparent autonomic involvement. SPTLC3 is the third subunit of the serine palmitoyltransferase enzyme (SPT) involved in the de novo biosynthesis of sphingolipids [Hornemann et al., 2009]. Heterozygous mutations in subunit 1 of SPT, SPTLC1, were first identified as the cause of hereditary sensory and autonomic neuropathy type 1A [HSAN1A; MIM #162400] [Dawkins et al., 2001]. Both genes encoding the additional subunits of SPT, SPTLC2 and SPTLC3, were screened for mutations in a cohort of typical HSAN patients. Heterozygous missense mutations were identified in SPTLC2 in a fraction of patients but no mutations were found in SPTLC3 [Rotthier et al., 2010]. Consistent with a neuropathy ‘disease gene’, suppression of the sptlc3 orthologue in zebrafish embryos showed motor neuron axon defects that phenocopied suppression of other known CMT genes (Figure 2H–I). The specific phenotype could be rescued by co-injection with SPTLC3 wild-type human mRNA (Figure 2H–L). Injection of human mRNA carrying the variant identified in the proband was unable to rescue the phenotype, supporting the contention that the missense variant represents a hypomorphic or possible loss of function allele (Figure 2H–L).
In a large family with an inheritance pattern consistent with an autosomal dominant myopathy/neuropathy, we identified 10 shared variants in three affected individuals, of which 9 did not segregate with the disease. A novel variant in DNAJB5 (c.C43T; p.P15S) was the only rare variant that co-segregated with the phenotype (Figure 1C). This rare variant was observed in four other independent individuals in our exome database of ~3,000 individuals; however no phenotypic information is available for these individuals. The variant is also present in the heterozygous state in a single individual in the Exome Aggregation Consortium (ExAC) compiled dataset (MAF=0.00004858). This DNAJB5 variant affects a highly conserved amino acid in the DnaJ domain of the protein. A homozygous mutation in DNAJB2 was identified in a large family segregating recessive distal hereditary motor neuropathy of early adulthood onset [Blumen et al., 2012]. Mutations in DNAJB6 have also been implicated in autosomal dominant myopathy [Harms et al., 2012; Sarparanta et al., 2012] and have a dominant negative toxic effect increasing the stability of the cytoplasmic form of the protein and interfering with its chaperone function [Sarparanta et al., 2012]. These three genes encode members of the HSP40/DNAJ family of molecular co-chaperones which protect proteins from irreversible aggregation during protein synthesis or molecular stress. Functional testing of this gene by MO knockdown in zebrafish showed abnormal peripheral nerve axonal architecture supporting a role of this gene in peripheral nerve pathophysiology but had no apparent effect on muscle architecture (Supplementary Figure 4). We propose DNAJB5 as a potential candidate for myopathy/neuropathy based on its relationship with previously reported genes involved in similar phenotypes; HSPB8 (HSP27) and HSPB1 (HSP22) are known genes associated with peripheral neuropathy [Evgrafov et al., 2004; Irobi et al., 2004].
Rare variant contributions to phenotypic manifestations – evidence for a mutation burden
WES of neuropathy patients often identified more than one rare variant in a neuropathy gene within a given personal genome (Table 2). As described above, we identified the predominant highly penetrant Mendelizing variants (HPMV) in multiple patients, as evidenced by co-segregation with disease or de novo appearance in sporadic neuropathy. However, we also identified potential contributing or modifying rare variants in other neuropathy associated genes (Figure 3). These latter rare variants are not likely the mutations predominantly responsible for trait manifestation because they are inherited from an unaffected parent or do not conform to Mendelian expectations (i.e. exceptions to co-segregation with neuropathy in the family). For example, we observed a higher than expected heterozygous carrier frequency of the reported MED25 (p.A335V) mutation in our cohort (10% of patients; MAF = 5.0%) compared to that observed in the NHLBI ESP study sample (65/6498 individuals; MAF=0.5% [P-value=0.001]), a group of 266 controls (2/266 individuals; MAF=0.375% [P-value=0.003]), and the ARIC European-American (ARIC-EA) study participants (80/ 5748 individuals; MAF=0.7%[P-value=0.003]). Although in 3 of 4 cases in our patient cohort there is no ‘second hit’ in MED25 to cause the CMT2B2 phenotype, we cannot discount the possibility of a second pathogenic non-coding variant not captured by WES or the potential contribution of this mutation in a mutational aggregation model to the overall phenotype of these patients.
| BAB# | Clinical DX | Main mutation(s) identified | Additional mutations identified | Inherit | TGP MAF |
ESP MAF |
PhyloP | LRT | SIFT | Pp2 | MT | Molecular DX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Known gene, known variants | 668 | CMT2 |
MFN2 (p.W740S)* g.chr1:12071567(G>C) |
NA | NA | NA | C | D | T | D+ | D | CMT2A2 [#609260] | |
|
MED25 (p.A335V)* g.chr19:50334047 (C>T) |
0.002 | 0.005 | C | N | T | B | P | ||||||
|
IKBKAP (p.N1186K) g.chr9:111641740 (G>C) |
NA | NA | C | D | T | D− | D | ||||||
|
TRPV4 (p.E840K) g.chr12:110221524 (C>T) |
0.004 | 0.007 | C | D | D | B | D | ||||||
| 710 | CMT2 |
MED25 (p.A335V)* g.chr19:50334047(C>T) |
NA | 0.002 | 0.005 | C | D | T | B | P | CMT2B2 [#605589] | ||
|
MED25 (p.P656T)^ g.chr19:50339483(C>A) |
NA | 0.000 | C | D | D | D− | P | ||||||
|
ATP7A (p.V1438I) g.chrX:77301876 (G>A) |
NA | NA | C | D | T | B | P | ||||||
| SH3TC2 (p.A468V) g.chr5:148407892 (G>A) |
0.004 | 0.004 | C | N | T | B | P | ||||||
| 1405 | CMT1 |
MFN2 (p.V244M)* g.chr1:12059066 (G>A) |
de novo | NA | NA | C | D | D | D+ | D | CMT2A2 [#609260] | ||
|
WNK1 (p.T625A) g.chr12:970431 (A>G) |
M | NA | NA | C | D | T | B | P | |||||
| 1564 | CMT2 |
MFN2 (p.V244M)* g.chr1:12059066 (G>A) |
de novo | NA | NA | C | D | D | D+ | D | CMT2A2 [#609260] | ||
|
HSPB1 (p.P182Rfs*19) g.chr7:75933417 (delCA) |
M | NA | NA | NA | NA | NA | NA | D | |||||
| NARS2 (p.N134S) g.chr11:78177004 (T>C) |
P | NA | 0.001 | N | N | T | B | D | |||||
|
TARS (p.I326T) g.chr5:33457501 (T>C) |
M | 0.001 | 0.002 | C | D | D | D− | D | |||||
| 3656 (T) | CMT2 |
AARS (p.R329H)* g.chr16:70302259(C>T) |
P | 0 | 0 | C | N | D | D | D | CMT2N [#613287] | ||
|
SBF2 (p.L1098V) g.chr11:9861208 (G>C) |
NA | 0.01 | 0.02124 | C | N | T | D− | D | |||||
| 3662 (T) | CMT2 |
MFN2 (p.C281S)* | NA | 0 | 0.00019 | C | D | D | D | D | CMT2A2 [#609260] | ||
|
SBF2 (p.S1738Y) g.chr11:9806690 (G>T) |
NA | 0 | 0 | C | D | D | B | D | |||||
| INF2 (p.P528S) | NA | 0.005 | 0.004 | na | na | na | na | na | |||||
|
KIF1B (p.T827I) g.chr1:10384896 (C>T) |
NA | 0.001 | 0.001 | C | N | T | B | N | |||||
| 3663 (T) | CMT1 |
MPZ (p.I135L)* g.chr1:161276543 (T>G) |
NA | 0 | 0 | C | D | D | na | D | CMT1B [#118200] | ||
| Known genes, novel variants | 1080 | CMT |
MFN2 (p.R649P)^ g.chr1:12067183 (G>C) |
NA | 0.000 | NA | C | D | D | D+ | D | CMT2A2 [#609260] | |
|
GDAP1 (p.Q163X)* g.chr8:75274121 (C>T) |
NA | NA | C | D | NA | NA | D | ||||||
| DNMT1 (p.G1007S)^ g.chr19:10254491 (C>T) |
NA | NA | C | D | T | D− | D | ||||||
| 1280 | CMT2 |
ARHGEF10 (p.G132S)^ g.chr8:1808263 (G>A) |
M | NA | NA | C | D | D | D+ | D | AD Slowed nerve conduction velocity [#608236] |
||
|
CARS2 (p.S544R) g.chr13:111293947 (G>T) |
0.003 | 0.002 | N | D | T | B | NA | ||||||
| 1500 | Dejerine Sottas |
SH3TC2 (p.K274X)^ [hom] g.chr5:148418039(insA) |
NA | NA | NA | NA | NA | NA | NA | D | CMT4C [#601596] | ||
|
DNM2 (p.Q301H) g.chr19:10897293 (G>C) |
NA | NA | C | D | D | B | D | ||||||
| 1955 | CMT2 |
AIFM1 (p.R463I)^ g.chrX:129267348 (C>A) |
M | NA | NA | C | D | D | D+ | D | Cowchock syndrome [#310490] |
||
|
AARS (p.N911S)^ g.chr16:70286799 (T>C) |
M | NA | NA | C | N | T | B | D | |||||
|
KIF1B (p.I223T) g.chr1:10328269 (T>C) |
P | NA | NA | C | D | D | D+ | D | |||||
|
WNK1 (p.2099_2102del) g.chr12:1003758 (delCAACTAGTT) |
P | NA | NA | NA | N | D | NA | P | |||||
| 3646 | CMT intermediate |
YARS (p.E196Q)^ g.chr1:33263369 (C>G) |
M | NA | NA | C | D | D | D+ | D | CMTDIC [#608323] | ||
|
DNMT1 (p.I311M) g.chr19:10273370 (A>C) |
P | NA | 0.000 | N | N | T | B | P | |||||
|
DNMT1 (p.Q310P) g.chr19:10273374 (T>G) |
M | 0.001 | 0.000 | C | N | T | D− | D | |||||
|
MARS (p.R301C) g.chr12:57892216 (C>T) |
P | NA | 0.000 | C | D | T | D− | D | |||||
| 3647 | CMT intermediate |
YARS (p.E196Q)^ g.chr1:33263369 (C>G) |
M | NA | NA | C | D | D | D+ | D | CMTDIC [#608323] | ||
|
DNMT1 (p.I311M) g.chr19:10273370 (A>C) |
P | NA | 0.000 | N | N | T | B | P | |||||
|
MARS (p.R301C) g.chr12:57892216 (C>T) |
P | NA | 0.000 | C | D | T | D− | D | |||||
| 3657 (T) | CMT1 |
GJB1 (IVS1-2A>G) g.chrX:70443540 (A>G) |
NA | 0.000 | 0.000 | NA | NA | NA | NA | D | CMTX1 [#302800] | ||
|
LRSAM1 (p.V659M) g.chr9:130263351 (G>A) |
NA | 0.010 | 0.001 | C | N | T | B | D | |||||
|
PRX (p.A1141T) g.chr19:40900838 (C>T) |
NA | 0.000 | 0.000 | C | N | T | B | N | |||||
|
VARS2 (p.L404F) g.chr6:30887580 (C>T) |
NA | NA | NA | N | D | D | D− | D | |||||
| 3660 (T) | CMT2 |
MFN2 (p.V160G)^ g.chr1:12057358 (T>G) |
NA | 0.000 | 0.000 | C | D | D | D | D | CMT2A2 [#609260] | ||
|
SETX (p.G82E) g.chr9:135221791 (C>T) |
NA | 0.000 | 0.000 | N | N | D | B | N | |||||
| 3672 (T) | CMT2 |
MFN2 (p.G176S)^ [hom] g.chr1:12057405(G>A) |
M/P | 0.000 | 0.000 | C | D | T | B | D | CMT2A2 [#609260] | ||
|
LRSAM1 (p.V659M) g.chr9:130263351 (G>A) |
NA | 0.010 | 0.000 | C | N | T | B | D | |||||
| 4119 (T)# | CMT2 |
TRIM2 (p.D667A) [hom] g.chr4:154245278(A>C) |
M/P | NA | NA | C | D | D | D | D | CMT2R [#615490] | ||
| Phenotypic re-assessment | 996 | congenital hypomielinating neuropathy/ataxia |
ITPR1 (p.G2547A)^ g.chr3:4856819 (G>C) |
de novo | NA | NA | NA | NA | D | NA | D | SCA29 [#117360] | |
|
MTMR2 (p.A530G) g.chr11:95568581 (G>C) |
0.010 | NA | C | D | T | B | P | ||||||
|
WNK1 (p.R788C) g.chr12:974498 (C>T) |
P | NA | NA | NA | NA | D | D+ | NA | |||||
| 1038 | CMT/Dejerine-Sotas |
SURF1 (p.Q196X) g.chr9:136219551 (G>A) |
NA | NA | 0.000 | C | D | D | D | D | Leigh Syndrome/ Demyelinating peripheral neuropathy [#256000] |
||
|
SURF1 (p.L105Rfs*11)^ g.chr9:136221515 (del11bp) |
NA | NA | NA | NA | D | NA | D | ||||||
|
PRX (p.R1411C)^ g.chr19:40900028 (G>A) |
NA | NA | C | N | D | D+ | D | ||||||
|
SBF2 (p.V1371L) g.chr11:9834123 (C>G) |
0.001 | 0.001 | C | D | T | B | P | ||||||
|
NEFL (p.A195V) g.chr8:24813446 (G>A) |
0.003 | 0.003 | NA | NA | NA | B | NA | ||||||
| NGF (p.V72M) g.chr1:115829203 (C>T) |
0.010 | 0.006 | C | D | T | D− | D | ||||||
|
MYH14 (p.P681R)^[hom] g.chr19:50758572 (G>C) |
0.030 | NA | NA | NA | D | B | D | ||||||
| 1163 | Hypotonia, hypomyelinating neuropathy |
ADCY6 (p.Y992C)^ [hom] g.chr12:49165569 (T>C) |
M/P | NA | NA | C | D | D | D+ | D | arthrogryposis multiplex congenita with axoglial defects |
||
| 1522 | CMT |
MYH14 (p.R941L)* g.chr19:50771512 (G>T) |
NA | NA | NA | NA | NA | D | D− | D | PNMHH [#614369] | ||
| 1566 | congenital hypotonia |
IGHMBP2 (p.C496X)* g.chr11:68701332 (C>A) |
M | NA | 0.000 | N | D | NA | NA | D | DSMA1/ SMARD1 [#604320] |
||
|
IGHMBP2 (p.M449Sfs*24)^ g.chr11:68700877 (delT) |
P | NA | NA | NA | NA | NA | NA | D | |||||
|
NEFL (p.Q537R) g.chr8:24810345 (T>C) |
M | NA | 0.000 | NA | NA | NA | B | NA | |||||
|
SETX (p.S1366P) g.chr9:135202889 (A>G) |
M | NA | 0.000 | N | NA | D | B | P | |||||
|
SEPT9 (p.R67Q) g.chr17:75471800 (G>A) |
P | NA | 0.000 | NA | NA | D | B | P | |||||
| YARS2 (p.I251V) g.chr12:32908058 (T>C) |
P | NA | 0.000 | C | D | T | D+ | D | |||||
| 1581 | congenital hypomyelinating neuropathy |
IGHMBP2 (p.E514K)* g.chr11:68701934 (G>A) |
NA | NA | 0.000 | C | N | D | D+ | D | DSMA1/ SMARD1 [#604320] |
||
|
IGHMBP2 (p.A256G)^ g.chr11:68682346(C>G) |
0.001 | 0.001 | C | D | T | D− | D | ||||||
|
IGHMBP2 (p.A398E)^ g.chr11:68696783(C>A) |
NA | NA | C | D | D | D+ | D | ||||||
|
SBF2 (p.S1506A) g.chr11:9817432 (A>C) |
NA | NA | C | D | D | D+ | D | ||||||
| FIG4 (p.R244C) g.chr6:110059611 (C>T) |
0.001 | 0.000 | C | N | D | D− | D | ||||||
| NTRK2 (p.D155G) g.chr9:87325587 (A>G) |
NA | NA | C | D | D | D− | D | ||||||
| FARSB (p.P170S) g.chr2:223499208 (G>A) |
NA | NA | C | D | D | D+ | D | ||||||
| 1680 | neuropathy, ataxia cataracts |
ABHD12 (14kb del)* [hom] | M/P | NA | NA | NA | NA | NA | NA | NA | PHARC [#612674] | ||
|
GDAP1 (p.A336S)[hom] g.chr8:75276531(G>T) |
M/P | 0.001 | 0.000 | C | D | T | D+ | D | |||||
|
WNK1 (p.R716H) g.chr12:974283 (G>A) |
P | 0.001 | NA | NA | NA | NA | NA | NA | |||||
| 2447 | progressive neuro- degenerative disorder |
SETX (p.Q2108X)^ [hom] g.chr9:135163625(G>A) |
M/P | NA | NA | NA | NA | NA | NA | D | AOA2/ SCAR1 [#606002] |
||
| 3664 (T) | CMT2 |
AIMP1 (p.Q112X)^ [hom] g.chr4:107249343(C>T) |
P/M | 0.000 | 0.000 | na | na | na | na | na | Hypomyelinating leukodystrophy (HLD3) |
||
| 3669 (T) | CMT2 |
DNAJB2 (c.619-1G>A) [hom] g.chr2:220149353(G>A) |
NA | 0.000 | 0.000 | na | na | na | na | na | DSMA5 [#604139] | ||
|
SETX (p.K611R) g.chr9:135204010 (T>C) |
NA | 0.010 | 0.010 | N | N | D | B | N | |||||
|
IGHMBP2 (p.G676R) g.chr11:68703974 (G>A) |
NA | 0.001 | 0.000 | N | N | T | B | D | |||||
| 3729 (T) | CMT2 |
TFG (p.P285L)* g.chr3:100467026 (C>T) |
NA | 0.000 | 0.000 | C | D | D | P | D | HMSNO [#604484] | ||
| 3730 (T) | CMT2 |
DNAJB2 (p.F103fs)^ [hom] g.chr2:220146739(delC) |
NA | 0.000 | 0.000 | na | na | na | na | na | DSMA5 [#604139] | ||
| Novel genes | 124 | hereditary myoclonus and progressive distal muscular atrophy |
DNAJB5 (p.P15S) g.chr9:34990670 (C>T) |
M | NA | NA | NA | NA | D | NA | P | NA | |
|
GARS (p.T268I) g.chr7:30649268 (C>T) |
M | 0.002 | 0.005 | C | D | D | D+ | D | |||||
|
TRPV4 (p.I545N) g.chr12:110231356 (A>T) |
P | NA | NA | C | D | D | D− | D | |||||
| TRPV4 (p.V562I) | P | 0.010 | 0.007 | C | D | T | B | D | |||||
|
RARS2 (Q491X) g.chr6:88227927 (G>A) |
P | NA | NA | C | N | NA | NA | D | |||||
| 1468 | CMT1 |
PMP2 (p.I43N) g.chr8:82357170 (A>T) |
P | NA | NA | C | D | D | D+ | D | NA | ||
|
PRX (p.E1259K) g.chr19:40900484 (C>T) |
P | NA | NA | N | N | T | B | P | |||||
|
VARS (p.Q1174H) g.chr6:31746948 (C>G) |
P | NA | NA | N | N | T | D− | P | |||||
| 1631 | CMT |
SPTLC3 (p.W150R) g.chr20:13053048(T>C) |
NA | NA | C | D | T | D+ | D | NA | |||
|
MED25 (p.A335V)* g.chr19:50334047 (C>T) |
NA | 0.002 | 0.005 | C | N | T | B | P | |||||
|
SH3TC2 (p.D1229V) g.chr5:148384455 (T>A) |
0.003 | 0.003 | C | D | D | D+ | D | ||||||
|
PRX (p.L275I) g.chr19:40903436 (G>T) |
NA | 0.001 | N | N | T | B | NA |
color coding: green - variants in novel candidate genes; orange - likely disease-causing variants in novel candidate genes. Genomic coordinates are given in GRCh37/hg19
abbreviations: (*) - known mutation; (^) - novel mutation in known gene; M - maternal; P - paternal; NA - not available; In PhyloP column: C (conserved), N (neutral); in LRT column: D (deleterious), N (neutral); in SIFT column: D (deleterious), T (tolerated); in Pp2 (Polyphen 2) column:B (benign), D+ (probably damaging), D− (possibly damaging); in MT column: P (polymorphism), D (disease causing)
previously published [Pehlivan et al., 2015]
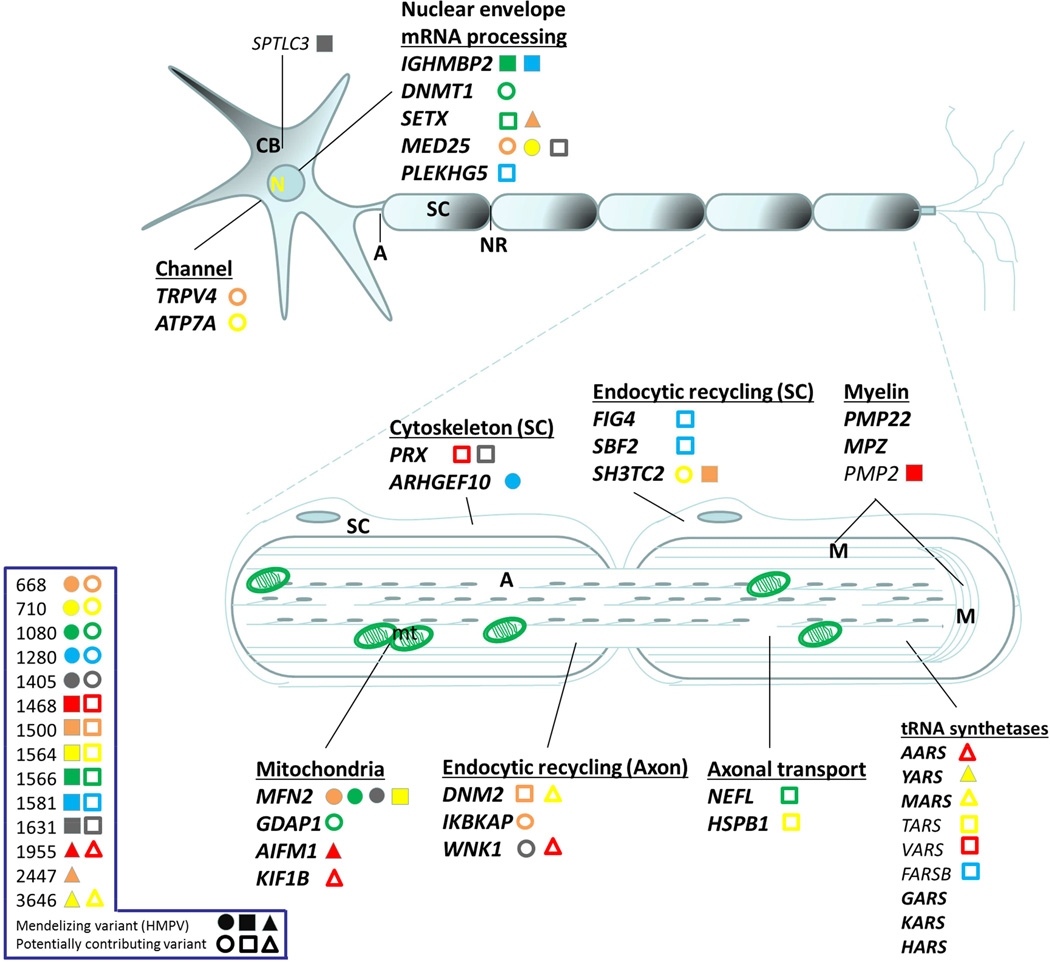
Figure 3.
Neuron schematic of the localization or site of action of the main CMT/ neuropathy gene products. Legend on left shows patient identifier numbers and causative and possibly contributing mutations identified by WES. Full shapes correspond to rare presumed causative mutations deemed Highly Penetrant Mendelizing Variants (HMPVs); while empty shapes correspond to rare variants that may be contributing to the mutation burden in neuropathy patients. Each personal genome is distinguished by a unique color/shape. In bold are some of the canonical CMT genes.
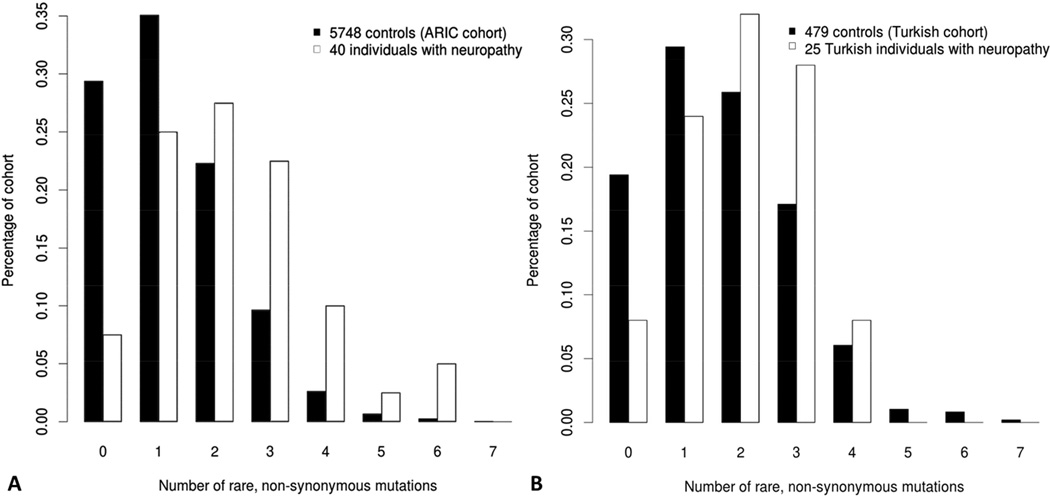
Of note, we identified an average of 2.3 nonsynonymous rare variants per individual in 58 known neuropathy-associated genes in the entire patient cohort (37 samples) versus 1.3 nonsynonymous rare variants in 5748 ARIC-EA control individuals (P < 0.0001; Figure 4A). Cases with a definitive molecular diagnosis had an average of 2.9 variants per individual (including the HPMV) while the undetermined cases had an average of 1.8 variants per individual. After implementing a stringent filter where we subtracted the HPMV of each molecularly defined case, we still found an average of 1.8 variants in the CMT cohort vs. 1.3 in controls (P=0.007), similar to the average of mutations in only the cases without a yet definitive HMPV (Supplementary Figure 5). These data suggest that the mutation burden in CMT genes remains the same between patients with a known versus unknown HMPV and is significantly greater than the background load in unaffected controls.
Figure 4.
Rare variant distribution in studied individuals suggests high carrier frequency for rare alleles in neuropathy genes in exome sequenced neuropathy cohort. A different extended cohort of 5748 Europeans from the ARIC-EA study was observed to have a tendency towards zero or one rare variants in recessive neuropathy genes.
As a further test of this mutational burden observation, we calculated repeatedly the average number of rare, nonsynonymous variants in the 58 neuropathy genes in 40 randomly selected individuals from the BHCMG_EU sample set compared to the 5748 ARIC_EA controls. Upon conclusion of 100 resamplings (with replacement), we only found three instances in which the p-value was lower than the p-value observed in our original US CMT (subtracting the HPMV) vs. ARIC_EA analysis;. These data reinforce the notion that the background mutation load in these 58 neuropathy genes is specific to the population of neuropathy patients.
To further investigate our observation of neuropathy gene mutation burden in neuropathy patients, we analyzed WES data from an independent cohort of 32 patients (30 families) from Turkey with a clinical diagnosis of CMT. When compared to population-matched unrelated Turkish controls, the Turkish neuropathy cohort had a mutation burden of 2.1 vs. 1.6 (P = 0.013) nonsynonymous rare variants per individual, lending further credence to the mutation burden hypothesis (Figure 4B, Supplementary Figure 5). The smaller difference in the number of rare variants per individual may also reflect a greater number of private variants in the Turkish population (particularly recessive alleles) or the contribution of consanguinity in this population.
Functional testing of the mutation burden hypothesis
We hypothesized that the ‘mutation burden’ observed in the CMT cohorts would be reflected in the functional consequences of CMT gene knockdown, and combinations thereof, in a zebrafish model. This functional assay evaluated the integrity and innervation of motor neuron axons along the body axis (Figure 5). A subset of genes was tested for potential genetic interactions and mutation burden effects on phenotype based on our initial cohort’s observed mutation events. Specifically, we suppressed each of mfn2, gdap1, abhd12, med25, hspb1, and wnk1 separately and in pair-wise combinations of sub-effective doses and tested the functional consequences of the genetic interactions between the selected CMT genes. Consistent with our hypothesis, we observed increased severity in the phenotype of aberrant axon extension, branching, pathfinding, and morphology of peripheral neurons in our zebrafish model when we injected pairwise combinations of these genes (Figure 5). In each case, we observed likely multiplicative effects, although the magnitude of interaction was unique for each pairing. For example, sub-effective co-injection of MOs against mfn2 and gdap1, which by themselves gave no phenotype at the dose tested, yielded a milder exacerbated phenotype (Class I/II motor neuron pathology in 80–100% of embryos tested); whereas co-suppression of mfn2 and med25 yielded 80–100% affected embryos, with 1/3 of the embryos affected severely (Class III/IV). These data support the prediction of genetic interaction for loss of function events in bona fide CMT genes. To assess the specificity of our in vivo model we also tested for genetic interaction between GDAP1, a bona fide CMT driver, and 3 genes that have not been associated previously with peripheral neuropathy. Two of those are expressed in the CNS and cause other neuropathologies (SIX6: optic nerve atrophy [Carnes et al., 2014]; RP1L1: retinal degeneration and cerebellar disorganization [Davidson et al., 2013]), and the third is expressed ubiquitously and causes VACTERL (ANKRD6; unpublished data). We injected sub-effective doses of each of the tested genes alone and also in pair-wise combinations (Supplementary Figure 6). Though RP1L1 yields a 20% increase in the percentage of embryos with abnormally formed peripheral neuronal axons when injected alone, we observed no exacerbation of the phenotype when each of those genes was suppressed in combination with GDAP1.
Figure 5.
Functional assessment of mutation burden hypothesis in a zebrafish model. First and second column panels show representative images of acetylated-tubulin (α-AcTub) staining of peripheral neurons in 2-day MO knockdown, single or in pair-wise combinations, zebrafish larvae. Third column panel shows qualitative assessment of morphant fish evaluated as defects in peripheral neuron axon extension, branching or pathfinding according to the scoring system developed. For pair-wise combinations, sub-effective concentrations of each of the gene-specific MOs were injected as shown in the graphs by the number of abnormal larvae in each category. However, when injected together increased severity in the phenotype was observed for all the pair-wise combinations, suggesting in vivo epistatic effects between these pairs of genes as observed in the α-AcTub fluorescence images and quantified in the graphs. Asterisks highlight some evidently affected axons.
The scoring system used for assessing PNS defects in zebrafish was developed ad hoc and implemented here in order to best reflect the observations resulting from our experiments. Class I category refers to single axon defects; Class II category refers to two or more axons exhibiting defects with the presence of some normal axons; Class III category refers to generalized axonal defects; Class IV category refers to complete absence of axonal extension.
Discussion
Whole exome sequencing (WES) allows genome-wide assessment of SNV coding variation in the fraction of the human diploid genome that we can potentially interpret. However, even in genetic conditions with known associated genes, interpretation can be complicated by the presence of novel variants in more than one causative gene [Yang et al., 2013; Yang et al., 2014]. Additionally, the contribution of variants in a multiplicity of genes for a single condition within an individual personal genome and how variation in these can contribute to or modify the phenotype has rarely been assessed.
We identified the apparent HPMV and likely primary disease driver of the neuropathy phenotype in 17/37 (45.9%) families studied and suggest a potential candidate gene for 3 additional families. We discovered a mutational burden of 2.3 damaging variants in CMT patients versus 1.3 in controls for the 58 neuropathy associated genes examined (P < 0.0001). After a highly stringent additional filter consisting of subtraction of the HPMV, neuropathy patients carry a mutation burden consisting of an average of 1.8 rare variants in neuropathy-related genes, as compared to an average of 1.3 rare variants in a control population (P=0.007). A mutation burden (P = 0.013) was replicated in a second, ethnically distinct CMT cohort in comparison to ethnically matched controls. This mutation burden may well influence the phenotype, contributing to the clinical heterogeneity and the spectrum of severity observed in the disease [Haldane, 1941]. We explored this hypothesis in vivo examining phenotypic consequences of genetic interaction between select pairs of neuropathy genes. We observed increased severity of the phenotype in zebrafish consistent with potential additive and positive genetic interactions between neuropathy genes.
Our cohort has an intrinsic bias since individuals had previous extensive clinical and molecular screening for disease causing variation in the most common CMT genes prior to consideration for WES. As anticipated, we found a low frequency of known mutations as these samples were previously screened for such variants. We found variants in known CMT or neuropathy genes in 17 cases; including one (MFN2) showing phenotypic expansion in a CMT1 family. By expanding our candidate list to include additional neuropathy-associated genes, we achieved a 45.9% (17/37) mutation detection rate. Furthermore, we identified likely candidate genes PMP2, SPTLC3, and DNAJB5 in an additional 3 families potentially providing molecular insights into 20/37 (54.1%) of the families. We also provide functional evidence for the pathogenicity of the identified variants in PMP2 and SPTLC3 (Figure 2) and the effect of dnajb5 suppression on motor neurons (Supplementary Figure 4). However, conclusive proof for these genes representing bona fide ‘neuropathy disease genes’ will require the identification of pathogenic variants in additional patients.
Analysis of the WES data from this neuropathy cohort illustrates limitations of clinical phenotyping. Detailed phenotypic information is required for correlating potential disease causing variants to the clinical phenotype of patients. As illustrated in 12 of the study subjects, 8 from the initial cohort and 4 from the Turkish cohort originally referred for a presumptive clinical diagnosis of CMT, after a molecular diagnosis by WES and upon retrospective re-evaluation of clinical records, the broader spectrum of additional clinical features suggested other disorders associated with neuropathy. Moreover, these further refined phenotypes were consistent with the molecular findings from WES in each of the identified genes (Supplementary Table 1). The phenotype driven paradigm for clinical diagnosis is limited by the: i) presentation of the patient at the given time, ii) individual examiner and iii) underlying assumption of a singular unifying diagnosis; the latter potentially not applicable to either a mutation aggregation model or a mutation burden hypothesis.
In 29/40 (72.5%) patients we identified additional ‘carrier status’ mutations in other CMT or neuropathy associated genes besides the apparent HPMV (Table 2). These additional variants might contribute to the variability of expression of the clinical phenotype [Haldane, 1941]. Furthermore, in the cases where specific HPMVs were not identified, novel loci potentially await to be recognized as main disease drivers (Supplementary Table 2), but the mutation burden may still contribute to variable expressivity of the neuropathy phenotype. It is possible that mutation burden and combinatorial effects of rare variants in genes that interact genetically in the same biological pathways, such as those of tRNA biogenesis, endocytic recycling or mitochondrial dynamics, modify the phenotype due to synergistic (exemplified by MFN2 and GDAP1 co-occurring mutations in the same patient) or counteracting effects [Klassen et al., 2011; Davis and Katsanis, 2012]. Alternatively, or additionally, the cumulative mutation burden in genes dispersed across various biological pathways or ‘networks’ might interplay to destabilize or compensate the system and thus modulate the penetrance and/or expressivity of the overall phenotype. Although robust, the capacity of biological networks to buffer perturbations may be limited if various mutational events are coincident in a personal genome. Studies of the human disease network [Goh et al., 2007; Hidalgo et al., 2009] at the genomic scale will likely contribute to our understanding of both disease and homeostatic states in human biology.
Genome-wide approaches have shown that rare variants are more common than previously thought [Coventry et al., 2010; Marth et al., 2011]; a robust observation for both SNV and CNV disease associated alleles [Boone et al., 2013]. The overall phenotype of a given individual may to a greater extent represent contribution of either de novo or more recent and private mutational events with bigger effects on the whole system function, the ‘driver’ genes that occurred in the recent ancestors of the individual or clan [Lupski et al., 2011], rather than more distributed common variants shared in a population or throughout several populations. This mutation burden hypothesis and its role in clan genomics is further illustrated in CMT1A duplication families wherein a phenotypic outlier in the family is recognized when the duplication becomes a triplication [Liu et al., 2014] or a CMT1A duplication is ‘homozygosed’ in a severe neuropathy patient born to heterozygous affected parents [Lupski et al., 1991].
Interestingly, within peripheral neuropathies, several disorders once thought to be mostly caused by environmental factors, have been subsequently shown to have a genetic susceptibility component. A key example is provided by CNV at the PMP22 locus. The reciprocal to the CMT1A duplication, deletion of 17p11.2, causes Hereditary Neuropathy with Liability to Pressure Palsies (HNPP) [Chance et al., 1993]. Trait manifestation is usually associated with an environmental insult, trauma to a specific nerve and often those that come anatomically close to the surface (e.g. the ulnar nerve responsible for the ‘funny bone’ phenomena of numbness and tingling upon hitting the elbow). Locus-specific molecular studies revealed the majority of individuals that carry the HNPP deletion go undiagnosed [Turner et al., 2008] due to phenotypic variability or lack of clinical symptoms [Kumar et al., 1999]. However, association of the deletion carrier status with susceptibility to developing carpal tunnel syndrome (CTS) has been documented [Cruz-Martinez and Arpa, 1998; Potocki et al., 1999; Del Colle et al., 2003]. Additionally, 24 of 51 patients diagnosed with multifocal neuropathies, not considered a genetic disease, were found to carry the HNPP deletion. Moreover, 37% of mutation positive subjects had no family history of neuropathy [Tyson et al., 1996]. Consequently, haploinsufficiency of the dosage sensitive PMP22 gene, either by HNPP deletion (CNV) or loss of function point mutations [Nicholson et al, 1994; Shy et al., 2006], has been associated with susceptibility to milder forms of neuropathy. Furthermore, haploinsufficiency of the CMT SH3TC2 gene can also confer subclinical neuropathy phenotypes in heterozygous carriers, including subclinical axonopathy and median nerve mononeuropathy associated with susceptibility to CTS [Lupski et al., 2010].
From this perspective, our identification of a PMP2 variant, a gene whose product has been linked to experimental autoimmune neuropathy and both Guillain-Barre syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), in one family suggests a potential genetic susceptibility to autoimmune neuropathy. Haploinsufficiency of other CMT or neuropathy genes can also contribute to susceptibility to multifactorial neuropathies. Moreover a recent study to survey possible underlying genetic contribution to developing chemotherapy induced peripheral neuropathy (CIPN) due to allelic variability in known CMT genes identified an association of PRX heterozygous variants in individuals that developed CIPN versus controls similarly exposed [Beutler et al., 2014]. Additionally, three common SNPs in ARHGEF10 were also associated to different outcomes of protection and susceptibility to CIPN in the same cohort [Beutler et al., 2014]. These findings support and highlight one of the main hypotheses from the present study; the mutation burden of carrier status, for neuropathy-associated rare variant recessive alleles, in clinically unaffected individuals can poise the organism to develop other types of complex neuropathies later in life upon gene-environment interactions (GxE). External insults, chemical or mechanical; other pathologic processes like diabetes or infection; or ageing with concomitant prolonged exposures, and/or reduced biological function of cells (e.g. SSBR, gene transcription, protein processing and folding, etc.) or functional units like the neuron can be the critical factor for the system to express the disease later in life. This might also be true for other traits thought to be complex and having a major environmental influence with a reduced genetic component that have been elusive to other approaches. Rather than single locus strong associations across populations, each individual with such a given complex disorder can carry a handful of rare/private variants in a variety of genes in their personal genome that are important for the development of the disease process and that through an oligogenic model confer susceptibility to the individual to develop the disorder upon additional factors such as diet, exposures, ageing, etc.
In summary, our studies of rare genomic variants in neuropathy identify known pathogenic alleles, novel variants in known disease genes, and further document phenotypic expansion for disease gene traits. We identified 3 potential novel candidate neuropathy ‘disease genes’ as supported by both genetic and functional studies. Moreover, we provide evidence that genome-wide studies and molecular diagnosis can further assist interpretation of a clinically based differential diagnosis. Of note, systematic analyses of genes implicated in neuropathy reveal a mutation burden in patients compared with unaffected control populations and zebrafish model organism studies show gene interactions for genes implicated by mutation burden in individual families. This mutation burden is consistent with the concept of clan genomics (Lupski, et al 2011) contributing significantly to both Mendelian and common/complex disease trait manifestation.
Experimental Procedures
Samples
We performed WES through the Baylor-Hopkins Center for Mendelian Genomics (BHCMG). Written informed consent from all participating subjects was obtained for DNA and genetic analyses though a Baylor College of Medicine Institutional Review Board approved protocol, also approved by the BHCMG ELSI committee for inclusion into the BHCMG sequencing project. Some of these samples had been collected and stored over decades; thus, DNA of parents or other family members was not always available for additional testing and co-segregation analyses.
Exome sequencing
We performed whole-exome next-generation sequencing according to previously published methods [Lupski et al., 2013; see Supplementary experimental methods for details], producing an average of 9.25 Gb of raw data per exome and achieving ~93.5x average depth of coverage (median coverage = 97x) per sample with >90% of the captured bases covered at 20x (Supplementary Table 3). Variant data generated will be released and deposited into the NCBI database of Genotypes and Phenotypes (dbGaP: http://www.ncbi.nlm.nih.gov/gap) as part of the Centers for Mendelian Genomics research initiative.
Variant annotation pipeline
Variant calling from the aligned BAM files was performed using the ATLAS [Shen et al., 2010] and SAMtools suites [Li et al., 2009]. Annotation was performed using Sacbe, an in-house developed annotation pipeline [Gonzaga-Jauregui et al., 2013] based on ANNOVAR [Wang et al., 2010] and custom scripts (see Supplementary experimental methods for details).
Data analysis
We performed an initial analysis focusing on a list of 74 CMT and other neuropathy associated genes (Supplementary Table 4). Additionally, we interrogated a list of candidate CMT genes (Supplementary Table 5) based on first degree interactors of known CMT genes and performed a second pass analysis in those cases where we did not identify candidate mutations in CMT genes.
The number of rare (i.e., minor allele frequency of ≤1% in TGP, NHLBI ESP, and the European subset of NHLBI ESP) nonsynonymous variants in 58 well-established CMT genes (Supplementary Table 6) was computed for each sample of the neuropathy cohort and for 5748 Europeans from the ARIC (Atherosclerosis Risk in Communities study) cohort, a large population-based study of cardiovascular disease and its risk factors. The average number of rare nonsynonymous variants was then compared between the neuropathy and ARIC study samples using a non-parametric Mann-Whitney-Wilcoxon test. A permutation procedure with 100,000 iterations was performed to determine statistical significance. For the second CMT cohort of Turkish descent, a set of 472 Turkish controls was used that was sequenced and analyzed using identical protocols, platforms, and standards to those of the cases.
Functional experiments
See Supplementary experimental methods for details.
Supplementary Material
Acknowledgements
This work was supported in part by US National Institute of Neurological Disorders and Stroke (NINDS) grant R01NS058529 to JRL; US National Human Genome Research Institute (NHGRI) grant National Heart, Lung, and Blood Institute (NHLBI) grant U54HG006542 to the Baylor-Hopkins Center for Mendelian Genomics and US National Human Genome Research Institute (NHGRI) grant U54HG003273 to RAG; by seed funding from the Center for Human Disease Modeling, Duke University and by P50 MH094268 to NK. AA is supported by a grant from the Muscular Dystrophy Association (MDA294479). WKW is supported by the Career Development Award K23NS078056 from NINDS. TH is supported by the T32 GM07526-37 Medical Genetics Research Fellowship Program. LBG was supported by the NIH Cellular and Molecular Biology Training Grant (GM007315), the NIH Medical Scientist Training Grant (GM07863), and an NIH F30 NRSA (NS092238). MS is supported by grants from MDA, CMT, NINDS/NCATS (U54NS065712) and from NINDS (R01NS075764).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan W. Relation of hereditary pattern to clinical severity as illustrated by peroneal atrophy. Arch. Intern. Med. 1939;63:1123–1131. [Google Scholar]
- Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N. Engl. J. Med. 2012;366(7):636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- Armstrong L, Biancheri R, Shyr C, Rossi A, Sinclair G, Ross CJ, Tarailo-Graovac M, Wasserman WW, van Karnebeek CD. AIMP1 deficiency presents as a cortical neurodegenerative disease with infantile onset. Neurogenetics. 2014;15(3):157–159. doi: 10.1007/s10048-014-0411-3. [DOI] [PubMed] [Google Scholar]
- Azzedine H, Senderek J, Rivolta C, Chrast R. Molecular genetics of charcot-marie-tooth disease: from genes to genomes. Mol Syndromol. 2012;3(5):204–214. doi: 10.1159/000343487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Othmane K, Middleton LT, Loprest LJ, Wilkinson KM, Lennon F, Rozear MP, Stajich JM, Gaskell PC, Roses AD, Pericak-Vance MA, et al. Localization of a gene (CMT2A) for autosomal dominant Charcot-Marie-Tooth disease type 2 to chromosome 1p and evidence of genetic heterogeneity. Genomics. 1993;17(2):370–375. doi: 10.1006/geno.1993.1334. [DOI] [PubMed] [Google Scholar]
- Beutler AS, Kulkarni AA, Kanwar R, Klein CJ, et al. Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Ann. Neurol. 2014;76(5):727–737. doi: 10.1002/ana.24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen SC, Astord S, Robin V, Vignaud L, Toumi N, Cieslik A, Achiron A, Carasso RL, Gurevich M, Braverman I, et al. A rare recessive distal hereditary motor neuropathy with HSJ1 chaperone mutation. Ann. Neurol. 2012;71(4):509–519. doi: 10.1002/ana.22684. [DOI] [PubMed] [Google Scholar]
- Boone PM, Campbell IM, Baggett BC, Soens ZT, Rao MM, Hixson PM, Patel A, Bi W, Cheung SW, Lalani SR, et al. Deletions of recessive disease genes: CNV contribution to carrier states and disease-causing alleles. Genome Res. 2013;23(9):1383–1394. doi: 10.1101/gr.156075.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Cong EH, Arrondel C, Tête MJ, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med. 2011;365(25):2377–2388. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- Braathen GJ. Genetic epidemiology of Charcot-Marie-Tooth disease. Acta Neurol. Scand. 2012;(Suppl. 193) doi: 10.1111/ane.12013. v-22. [DOI] [PubMed] [Google Scholar]
- Caldecott KW. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9(8):619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- Carnes MU, Liu YP, Allingham RR, Whigham BT, Havens S, Garrett ME, Qiao C NEIGHBORHOOD Consortium Investigators. Katsanis N, Wiggs JL. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS Genet. 2014;10(5):e1004372. doi: 10.1371/journal.pgen.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassereau J, Casasnovas C, Gueguen N, Malinge MC, Guillet V, Reynier P, Bonneau D, Amati-Bonneau P, Banchs I, Volpini V, Procaccio V, Chevrollier A. Simultaneous MFN2 and GDAP1 mutations cause major mitochondrial defects in a patient with CMT. Neurology. 2011;76(17):1524–1526. doi: 10.1212/WNL.0b013e318217e77d. [DOI] [PubMed] [Google Scholar]
- Chance PF, Abbas N, Lensch MW, Pentao L, Roa BB, Patel PI, Lupski JR. Two autosomal dominant neuropathies result from reciprocal DNA duplication/deletion of a region on chromosome 17. Hum. Mol. Genet. 1994;3:223–228. doi: 10.1093/hmg/3.2.223. [DOI] [PubMed] [Google Scholar]
- Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, Swanson PD, Odelberg SJ, Disteche CM, Bird TD. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72:143–151. doi: 10.1016/0092-8674(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Charcot JM, Marie P. Sur une form particulie`re d’atrophie musculaire progressive, souvant familiale, debutant par les pieds et les jambes, et atteignant plus tard les mains. Rev. Med. Paris. 1886;6:97–138. [Google Scholar]
- Choi BO, Kang SH, Hyun YS, Kanwal S, Park SW, Koo H, Kim SB, Choi YC, Yoo JH, Kim JW, Park KD, et al. A complex phenotype of peripheral neuropathy, myopathy, hoarseness, and hearing loss is linked to an autosomal dominant mutation in MYH14. Hum. Mutat. 2011;32(6):669–677. doi: 10.1002/humu.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BO, Koo SK, Park MH, Rhee H, Yang SJ, Choi KG, Jung SC, Kim HS, Hyun YS, Nakhro K, et al. Exome sequencing is an efficient tool for genetic screening of Charcot-Marie-Tooth disease. Hum. Mutat. 2012;33(11):1610–1615. doi: 10.1002/humu.22143. [DOI] [PubMed] [Google Scholar]
- Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, Everett L, Lenk GM, McKenna-Yasek DM, Weisman LS, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 2009;84(1):85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coventry A, Bull-Otterson LM, Liu X, Clark AG, Maxwell TJ, Crosby J, Hixson JE, Rea TJ, Muzny DM, Lewis LR, et al. Deep resequencing reveals excess rare recent variants consistent with explosive population growth. Nat. Commun. 2010;30(1):131. doi: 10.1038/ncomms1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martinez A, Arpa J. Pediatric bilateral carpal tunnel syndrome as first manifestation of hereditary neuropathy with liability to pressure palsies (HNPP) Eur. J. Neurol. 1998;5(3):316–317. doi: 10.1046/j.1468-1331.1998.530316.x. [DOI] [PubMed] [Google Scholar]
- Cuesta A, Pedrola L, Sevilla T, García-Planells J, Chumillas MJ, Mayordomo F, LeGuern E, Marín I, Vílchez JJ, Palau F. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat. Genet. 2002;30(1):22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- Davidson AE, Sergouniotis PI, Mackay DS, Wright GA, Waseem NH, Michaelides M, Holder GE, Robson AG, Moore AT, Plagnol V, Webster AR. Hum. Mutat. 2013;34(3):506–514. doi: 10.1002/humu.22264. [DOI] [PubMed] [Google Scholar]
- Davis EE, Katsanis N. The ciliopathies: a transitional model into systems biology of human genetic disease. Curr. Opin. Genet. Dev. 2012;22(3):290–303. doi: 10.1016/j.gde.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet. 2001;27(3):309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- Dejerine J, Sottas J. Sur la nevrite interstitielle, hypertrophique et progressive de l’enfance. Mem. Soc. Biol. 1893;9:6–96. [Google Scholar]
- Del Colle R, Fabrizi GM, Turazzini M, Cavallaro T, Silvestri M, Rizzuto N. Hereditary neuropathy with liability to pressure palsies: electrophysiological and genetic study of a family with carpal tunnel syndrome as only clinical manifestation. Neurol. Sci. 2003;24(2):57–60. doi: 10.1007/s100720300072. [DOI] [PubMed] [Google Scholar]
- DiVincenzo C, Elzinga CD, Medeiros AC, Karbassi I, Jones JR, Evans MC, Braastad CD, Bishop CM, Jaremko M, Wang Z, et al. The allelic spectrum of Charcot-Marie-Tooth disease in over 17,000 individuals with neuropathy. Mol. Genet. Genomic Med. 2014;2(6):522–529. doi: 10.1002/mgg3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echaniz-Laguna A, Ghezzi D, Chassagne M, Mayençon M, Padet S, Melchionda L, Rouvet I, Lannes B, Bozon D, Latour P, Zeviani M, Mousson de Camaret B. SURF1 deficiency causes demyelinating Charcot-Marie-Tooth disease. Neurology. 2013;81(17):1523–1530. doi: 10.1212/WNL.0b013e3182a4a518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36(6):602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Feinstein M, Markus B, Noyman I, Shalev H, Flusser H, Shelef I, Liani-Leibson K, Shorer Z, Cohen I, Khateeb S, et al. Pelizaeus-Merzbacher-like disease caused by AIMP1/p43 homozygous mutation. Am. J. Hum. Genet. 2010;87(6):820–828. doi: 10.1016/j.ajhg.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskerstrand T, H’mida-Ben Brahim D, Johansson S, M’zahem A, Haukanes BI, Drouot N, Zimmermann J, Cole AJ, Vedeler C, Bredrup C, et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 2010;87(3):410–417. doi: 10.1016/j.ajhg.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL. The human disease network. Proc. Natl. Acad. Sci. USA. 2007;104(21):8685–8690. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Lotze T, Jamal L, et al. Mutations in VRK1 Associated With Complex Motor and Sensory Axonal Neuropathy Plus Microcephaly. JAMA Neurol. 2013;70(12):1491–1498. doi: 10.1001/jamaneurol.2013.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga-Jauregui C, Zhang F, Towne CF, Batish SD, Lupski JR. GJB1/Connexin 32 whole gene deletions in patients with X-linked Charcot-Marie-Tooth disease. Neurogenetics. 2010;11(4):465–470. doi: 10.1007/s10048-010-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JBS. The relative importance of principal and modifying genes in determining some human diseases. J. Genet. 1941;41:149–157. [Google Scholar]
- Harms MB, Sommerville RB, Allred P, Bell S, Ma D, Cooper P, Lopate G, Pestronk A, Weihl CC, Baloh RH. Exome sequencing reveals DNAJB6 mutations in dominantly-inherited myopathy. Ann. Neurol. 2012;71(3):407–416. doi: 10.1002/ana.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo CA, Blumm N, Barabási AL, Christakis NA. A dynamic network approach for the study of human phenotypes. PLoS Comput. Biol. 2009;5(4):e1000353.. doi: 10.1371/journal.pcbi.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk JE, Hensels GW, Gabreëls-Festen AA, Gabreëls FJ, Janssen EA, de Jonghe P, Martin JJ, van Broeckhoven C, Valentijn LJ, Baas F, et al. De-novo mutation in hereditary motor and sensory neuropathy type I. Lancet. 1992;339(8801):1081–1082. doi: 10.1016/0140-6736(92)90668-s. [DOI] [PubMed] [Google Scholar]
- Hornemann T, Penno A, Rütti MF, Ernst D, Kivrak-Pfiffner F, Rohrer L, von Eckardstein A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 2009;284(39):26322–26330. doi: 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis HR, Csurhes PA, McCombe PA. Antibody responses to peptides of peripheral nerve myelin proteins P0 and P2 in patients with inflammatory demyelinating neuropathy. J. Neurol. Neurosurg. Psychiatry. 2007;78(4):419–422. doi: 10.1136/jnnp.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobi J, Van Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, De Vriendt E, Jacobs A, Van Gerwen V, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36(6):597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Hofmann T, Eylar EH. The complete amino acid sequence of the rabbit P2 protein. J. Biol. Chem. 1982;257(2):592–595. [PubMed] [Google Scholar]
- Ishaque A, Szymanska I, Ramwani J, Eylar EH. Allergic neuritis: phospholipid requirement for the disease-inducing conformation of the P2 protein. Biochim. Biophys. Acta. 1981;669(1):28–32. doi: 10.1016/0005-2795(81)90219-1. [DOI] [PubMed] [Google Scholar]
- Ishiura H, Sako W, Yoshida M, et al. The TRK-fused gene is mutated in hereditary motor and sensory neuropathy with proximal dominant involvement. Am. J. Hum. Genet. 2012;91(2):320–329. doi: 10.1016/j.ajhg.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova A, Irobi J, Thomas FP, Van Dijck P, Meerschaert K, Dewil M, Dierick I, Jacobs A, De Vriendt E, Guergueltcheva V, et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat. Genet. 2006;38(2):197–202. doi: 10.1038/ng1727. [DOI] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Izumino H, Umetsu K, Nezu A, Shiiki T, Ogawa M, Ishizaki Y, Kitamura T, Shozawa Y, Hayasaka K. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum. Genet. 2005;116(1–2):23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145(7):1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleopa K, Scherer S. Molecular genetics of X-linked Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:107–122. doi: 10.1385/nmm:8:1-2:107. [DOI] [PubMed] [Google Scholar]
- Kumar N, Cole J, Parry GJ. Variability of presentation in hereditary neuropathy with liability to pressure palsy results in underrecognition. Ann. NY. Acad. Sci. 1999;883:344–350. [PubMed] [Google Scholar]
- Leal A, Huehne K, Bauer F, Sticht H, Berger P, Suter U, Morera B, Del Valle G, Lupski JR, Ekici A, et al. Identification of the variant Ala335Val of MED25 as responsible for CMT2B2: molecular data, functional studies of the SH3 recognition motif and correlation between wild-type MED25 and PMP22 RNA levels in CMT1A animal models. Neurogenetics. 2009;10(4):275–287. doi: 10.1007/s10048-009-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal A, Morera B, Del Valle G, Heuss D, Kayser C, Berghoff M, Villegas R, Hernández E, Méndez M, Hennies HC, et al. A second locus for an axonal form of autosomal recessive Charcot-Marie-Tooth disease maps to chromosome 19q13.3. Am. J. Hum. Genet. 2001;68(1):269–274. doi: 10.1086/316934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 Gene and Its Related Diseases. Mol. Neurobiol. 2012;47(2):673–698. doi: 10.1007/s12035-012-8370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Gelowani V, Zhang F, Drory VE, Ben-Shachar S, Roney E, Medeiros AC, Moore RJ, DiVincenzo C, et al. Mechanism, Prevalence, and More Severe Neuropathy Phenotype of the Charcot-Marie-Tooth Type 1A Triplication. Am. J. Hum. Genet. 2014;94(3):462–469. doi: 10.1016/j.ajhg.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Belmont JW, Boerwinkle E, Gibbs RA. Clan genomics and the complex architecture of human disease. Cell. 2011;147(1):32–43. doi: 10.1016/j.cell.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, de Oca-Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, Trask BJ, Saucedo-Cardenas O, Barker DF, Killian JM, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- Lupski JR, Gonzaga-Jauregui C, Yang Y, Bainbridge MN, Jhangiani S, Buhay CJ, Kovar CL, Wang M, Hawes AC, Reid JG, Eng C, Muzny DM, Gibbs RA. Exome sequencing resolves apparent incidental findings and reveals further complexity of SH3TC2 variant alleles causing Charcot-Marie-Tooth neuropathy. Genome Med. 2013;5(6):57. doi: 10.1186/gm461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Reid JG, Gonzaga-Jauregui C, Rio Deiros D, Chen DC, Nazareth L, Bainbridge M, Dinh H, Jing C, Wheeler DA, et al. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N. Engl. J. Med. 2010;362(13):1181–1191. doi: 10.1056/NEJMoa0908094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Wise CA, Kuwano A, Pentao L, Parke JT, Glaze DG, Ledbetter DH, Greenberg F, Patel PI. Gene dosage is a mechanism for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992;1(1):29–33. doi: 10.1038/ng0492-29. [DOI] [PubMed] [Google Scholar]
- Majava V, Polverini E, Mazzini A, Nanekar R, Knoll W, Peters J, Natali F, Baumgärtel P, Kursula I, Kursula P. Structural and functional characterization of human peripheral nervous system myelin protein P2. PLoS One. 2010;5(4):e10300. doi: 10.1371/journal.pone.0010300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth GT, Yu F, Indap AR, Garimella K, Gravel S, Leong WF, Tyler-Smith C, Bainbridge M, Blackwell T, Zheng-Bradley X, et al. The functional spectrum of low-frequency coding variation. Genome Biol. 2011;12(9):R84. doi: 10.1186/gb-2011-12-9-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon PJ, Caldecott KW. DNA strand break repair and human genetic disease. Annu. Rev. Genomics Hum. Genet. 2007;8:37–55. doi: 10.1146/annurev.genom.7.080505.115648. [DOI] [PubMed] [Google Scholar]
- Montenegro G, Powell E, Huang J, Speziani F, Edwards YJ, Beecham G, Hulme W, Siskind C, Vance J, Shy M, Züchner S. Exome sequencing allows for rapid gene identification in a Charcot-Marie-Tooth family. Ann. Neurol. 2011;69(3):464–470. doi: 10.1002/ana.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MC, Klur S, Watanabe M, Németh AH, Le Ber I, Moniz JC, Tranchant C, Aubourg P, Tazir M, Schöls L, et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia-ocular apraxia 2. Nat Genet. 2004;36(3):225–227. doi: 10.1038/ng1303. [DOI] [PubMed] [Google Scholar]
- Nelis E, Erdem S, Van Den Bergh PY, Belpaire-Dethiou MC, Ceuterick C, Van Gerwen V, Cuesta A, Pedrola L, Palau F, Gabreëls-Festen AA, et al. Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology. 2002;59(12):1865–1872. doi: 10.1212/01.wnl.0000036272.36047.54. [DOI] [PubMed] [Google Scholar]
- Nelis E, Van Broeckhoven C, De Jonghe P, Löfgren A, Vandenberghe A, Latour P, Le Guern E, Brice A, Mostacciuolo ML, Schiavon F, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur. J. Hum. Genet. 1996;4(1):25–33. doi: 10.1159/000472166. [DOI] [PubMed] [Google Scholar]
- Nicholson G, Myers S. Intermediate forms of Charcot-Marie-Tooth neuropathy: a review. Neuromolecular Med. 2006;8(1–2):123–130. doi: 10.1385/nmm:8:1-2:123. [DOI] [PubMed] [Google Scholar]
- Nicholson GA, Valentijn LJ, Cherryson AK, Kennerson ML, Bragg TL, DeKroon RM, Ross DA, Pollard JD, McLeod JG, Bolhuis PA, et al. A frame shift mutation in the PMP22 gene in hereditary neuropathy with liability to pressure palsies. Nat. Genet. 1994;6(3):263–266. doi: 10.1038/ng0394-263. [DOI] [PubMed] [Google Scholar]
- Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell. Biol. 2005;170(7):1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PI, Roa BB, Welcher AA, Schoener-Scott R, Trask BJ, Pentao L, Snipes GJ, Garcia CA, Francke U, Shooter EM, Lupski JR, Suter U. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat. Genet. 1992;1(3):159–165. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- Pehlivan D, Coban Akdemir Z, Karaca E, Bayram Y, Jhangiani S, Yildiz EP, Muzny D, Uluc K, Gibbs RA Baylor-Hopkins Center for Mendelian Genomics. Elcioglu N, Lupski JR, Harel T. Exome sequencing reveals homozygous TRIM2 mutation in a patient with early onset CMT and bilateral vocal cord paralysis. Hum. Genet. 2015;134(6):671–673. doi: 10.1007/s00439-015-1548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Chen KS, Koeuth T, Killian J, Iannaccone ST, Shapira SK, Kashork CD, Spikes AS, Shaffer LG, Lupski JR. DNA rearrangements on both homologues of chromosome 17 in a mildly delayed individual with a family history of autosomal dominant carpal tunnel syndrome. Am. J. Hum. Genet. 1999;64(2):471–478. doi: 10.1086/302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers P, Timmerman V, Nelis E, De Jonghe P, Hoogenduk JE, Baas F, Barker DF, Martin JJ, De Visser M, Bolhuis PA, Van Broeckhoven C. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a) Neuromuscul. Disord. 1991;1:93–97. doi: 10.1016/0960-8966(91)90055-w. [DOI] [PubMed] [Google Scholar]
- Rinaldi C, Grunseich C, Sevrioukova IF, Schindler A, Horkayne-Szakaly I, Lamperti C, Landouré G, Kennerson ML, Burnett BG, Bönnemann C, et al. Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am. J. Hum. Genet. 2012;91(6):1095–1102. doi: 10.1016/j.ajhg.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa BB, Dyck PJ, Marks HG, Chance PF, Lupski JR. Dejerine-Sottas syndrome associated with point mutation in the peripheral myelin protein 22 (PMP22) gene. Nat. Genet. 1993;5(3):269–273. doi: 10.1038/ng1193-269. [DOI] [PubMed] [Google Scholar]
- Roa BB, Garcia CA, Suter U, Kulpa DA, Wise CA, Mueller J, Welcher AA, Snipes GJ, Shooter EM, Patel PI, Lupski JR. Charcot-Marie-Tooth disease type 1A. Association with a spontaneous point mutation in the PMP22 gene. N. Engl. J. Med. 1993;329(2):96–101. doi: 10.1056/NEJM199307083290205. [DOI] [PubMed] [Google Scholar]
- Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. J. Neurol. Neurosurg. Psychiatry. 2012;83(1):6–14. doi: 10.1136/jnnp-2011-300952. [DOI] [PubMed] [Google Scholar]
- Rotthier A, Auer-Grumbach M, Janssens K, Baets J, Penno A, Almeida-Souza L, Van Hoof K, Jacobs A, De Vriendt E, Schlotter-Weigel B, et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 2010;87(4):513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarparanta J, Jonson PH, Golzio C, Sandell S, Luque H, Screen M, McDonald K, Stajich JM, Mahjneh I, et al. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nat. Genet. 2012;44(4):450–455. doi: 10.1038/ng.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Kleopa KA. X-linked Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2012;3:9–13. doi: 10.1111/j.1529-8027.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderek J, Bergmann C, Ramaekers VT, Nelis E, Bernert G, Makowski A, Züchner S, De Jonghe P, Rudnik-Schöneborn S, Zerres K, Schröder JM. Mutations in the ganglioside-induced differentiation-associated protein-1 (GDAP1) gene in intermediate type autosomal recessive Charcot-Marie-Tooth neuropathy. Brain. 2003;126(Pt 3):642–649. doi: 10.1093/brain/awg068. [DOI] [PubMed] [Google Scholar]
- Shen Y, Wan Z, Coarfa C, Drabek R, Chen L, Ostrowski EA, Liu Y, Weinstock GM, Wheeler DA, Gibbs RA, Yu F. A SNP discovery method to assess variant allele probability from next-generation resequencing data. Genome Res. 2010;20(2):273–280. doi: 10.1101/gr.096388.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Scavina MT, Clark A, Krajewski KM, Li J, Kamholz J, Kolodny E, Szigeti K, Fischer RA, Saifi GM, Scherer SS, Lupski JR. T118M PMP22 mutation causes partial loss of function and HNPP-like neuropathy. Ann. Neurol. 2006;59(2):358–364. doi: 10.1002/ana.20777. [DOI] [PubMed] [Google Scholar]
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin. Genet. 1974;6(2):98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Szigeti K, Lupski JR. Charcot-Marie-Tooth disease. Eur. J. Hum. Genet. 2009;17(6):703–710. doi: 10.1038/ejhg.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman V, Strickland AV. Züchner S. Genetics of Charcot-Marie-Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes. 2014;5(1):13–32. doi: 10.3390/genes5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooth HH. The peroneal type of progressive muscular atrophy. London: H. K. Lewis; 1886. [Google Scholar]
- Turner DJ, Miretti M, Rajan D, Fiegler H, Carter NP, Blayney ML, Beck S, Hurles ME. Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet. 2008;40(1):90–95. doi: 10.1038/ng.2007.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson J, Malcolm S, Thomas PK, Harding AE. Deletions of chromosome 17p11.2 in multifocal neuropathies. Ann. Neurol. 1996;39(2):180–186. doi: 10.1002/ana.410390207. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, Claeys KG, Züchner S, Schröder JM, Weis J, Ceuterick C, Jordanova A, Nelis E, De Vriendt E, Van Hul M, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129(Pt 8):2093–2102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- Verny C, Ravisé N, Leutenegger AL, Pouplard F, Dubourg O, Tardieu S, Dubas F, Brice A, Genin E, LeGuern E. Coincidence of two genetic forms of Charcot-Marie-Tooth disease in a single family. Neurology. 2004;63(8):1527–1529. doi: 10.1212/01.wnl.0000142082.65144.ee. [DOI] [PubMed] [Google Scholar]
- Vielhaber S, Debska-Vielhaber G, Peeva V, Schoeler S, Kudin AP, Minin I, Schreiber S, Dengler R, Kollewe K, Zuschratter W, et al. Mitofusin 2 mutations affect mitochondrial function by mitochondrial DNA depletion. Acta Neuropathol. 2013;125(2):245–256. doi: 10.1007/s00401-012-1036-y. [DOI] [PubMed] [Google Scholar]
- Vital A, Latour P, Sole G, Ferrer X, Rouanet M, Tison F, Vital C, Goizet C. A French family with Charcot-Marie-Tooth disease related to simultaneous heterozygous MFN2 and GDAP1 mutations. Neuromuscul. Disord. 2012;22(8):735–741. doi: 10.1016/j.nmd.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Wallen RC, Antonellis A. To charge or not to charge: mechanistic insights into neuropathy-associated tRNA synthetase mutations. Curr Opin Genet Dev. 2013;23(3):302–309. doi: 10.1016/j.gde.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from next-generation sequencing data. Nuc. Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterman MA, van Ruissen F, de Wissel M, Bordewijk L, Samijn JP, van der Pol WL, Meggouh F, Baas F. Copy number variation upstream of PMP22 in Charcot-Marie-Tooth disease. Eur. J. Hum. Genet. 2010;18(4):421–428. doi: 10.1038/ejhg.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013;369(16):1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Xia F, et al. Molecular Findings Among Patients Referred for Clinical Whole-Exome Sequencing. JAMA. 2014;312(18):1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Stettner M, Ruskamo S, Domènech-Estévez E, Baloui H, Médard JJ, Verheijen MH, Brouwers JF, Kursula P, Kieseier BC, et al. A role of peripheral myelin protein 2 in lipid homeostasis of myelinating Schwann cells. Glia. 2014;62(9):1502–1512. doi: 10.1002/glia.22696. [DOI] [PubMed] [Google Scholar]
- Zhang F, Seeman P, Liu P, Weterman MA, Gonzaga-Jauregui C, Towne CF, Batish SD, De Vriendt E, De Jonghe P, Rautenstrauss B, et al. Mechanisms for nonrecurrent genomic rearrangements associated with CMT1A or HNPP: rare CNVs as a cause for missing heritability. Am. J. Hum. Genet. 2010;86(6):892–903. doi: 10.1016/j.ajhg.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36(5):449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.