Abstract
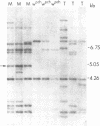
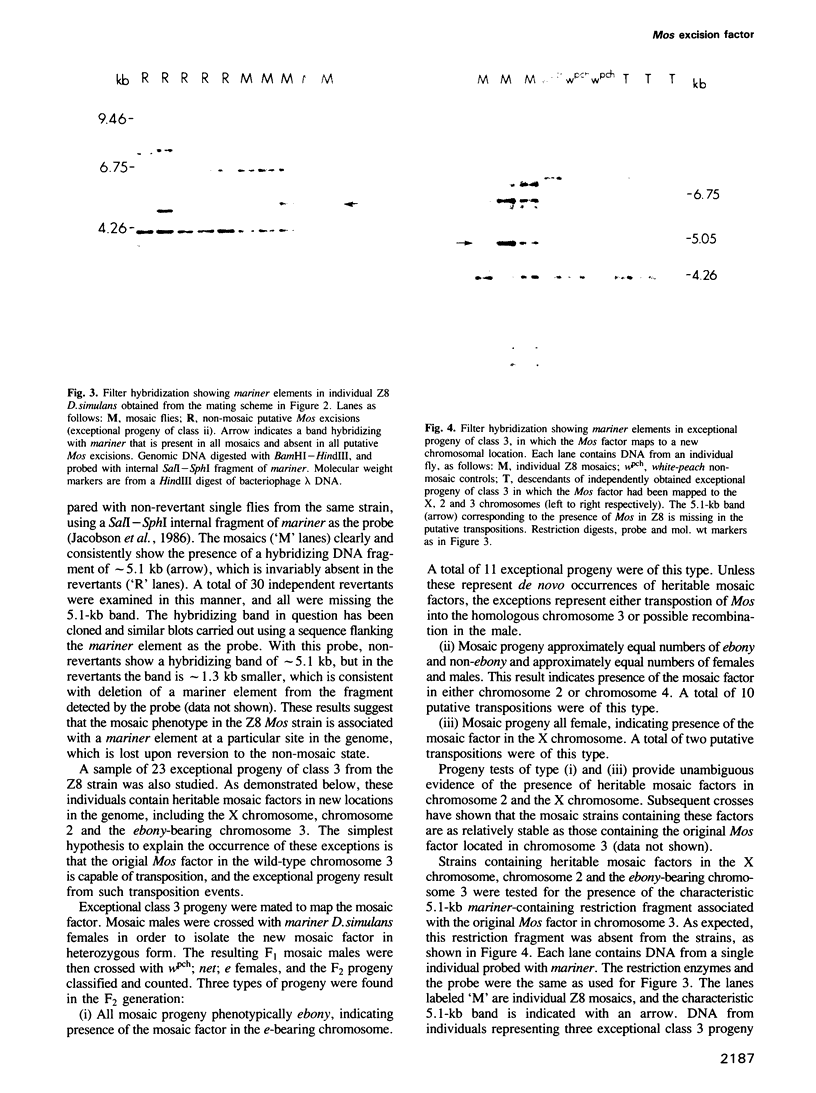
Genetic and molecular evidence presented in this paper demonstrate that the Mos factor for inherited mosaicism is a special copy of the transposable element mariner. Mosaicism observed in the presence of the Mos (Mosaic) factor results from a high frequency of excision of the mariner element from an insertion site near the white-eye gene in Drosophila mauritiana. The Mos factor promotes the excision of mariner elements from genomic insertion sites other than the site in wpch, and it also promotes its own loss from the genome. Putative transpositions of Mos to new genomic sites have also been observed. A copy of mariner present at a particular site in a Mos strain has been shown to be missing in derived strains in which the Mos factor has been lost, and in strains with putative transpositions. We propose that this copy of mariner is identical to the Mos factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan G. J., Jacobson J. W., Hartl D. L. Heritable somatic excision of a Drosophila transposon. Science. 1987 Mar 27;235(4796):1636–1638. doi: 10.1126/science.3029874. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Haymer D. S., Marsh J. L. Germ line and somatic instability of a white mutation in Drosophila mauritiana due to a transposable genetic element. Dev Genet. 1986;6(4):281–291. doi: 10.1002/dvg.1020060406. [DOI] [PubMed] [Google Scholar]
- Jacobson J. W., Hartl D. L. Coupled instability of two X-linked genes in Drosophila mauritiana: germinal and somatic mutability. Genetics. 1985 Sep;111(1):57–65. doi: 10.1093/genetics/111.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J. W., Medhora M. M., Hartl D. L. Molecular structure of a somatically unstable transposable element in Drosophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8684–8688. doi: 10.1073/pnas.83.22.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Nakanishi M., Ali Z., Drees B., Gustavson E., Theis J., Kauvar L., Kornberg T., O'Farrell P. H. Molecular cloning of engrailed: a gene involved in the development of pattern in Drosophila melanogaster. Cell. 1985 Aug;42(1):309–316. doi: 10.1016/s0092-8674(85)80126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986 Jan 17;44(1):7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Peterson P A. The Pale Green Mutable System in Maize. Genetics. 1960 Jan;45(1):115–133. doi: 10.1093/genetics/45.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]