Abstract
The aim of this study was to determine the effects of previous administration of metyrapone (met) on the acute lung injury (ALI) induced by caecal ligation and puncture (CLP) and to explore met’s relationship with endogenous glucocorticoids (GCs) as measured by inflammatory, oxidative and functional parameters. One hundred and thirty-five Wistar rats were divided into three main groups: Control (Naïve), Sham and CLP. The animals received pretreatment one hour before surgery. The Naïve group did not undergo any procedure or pretreatment. The Sham group only had the caecum exposed and was pretreated with saline. The CLP group was divided into three pretreatments: metyrapone (CLP met 50 mg/kg i.p.), dexamethasone (CLP dex 0.5 mg/kg i.p.) or saline (CLP sal equivalent volume of 0.9% NaCl). Analyses were performed after 6 and 24 h of sepsis. Previous administration of met significantly increased inflammatory cells, as well as myeloperoxidase (MPO) activity in the lung tissue and alveolar collapsed area, with consequent impairment of respiratory mechanics being observed compared to Sham and Naïve; CLP sal exhibited similar results to those of met. The met reduced corticosterone (CCT) levels and dramatically increased hydrogen peroxide (H2O2) levels in the lung tissue compared to CLP sal. Our results suggest that previous administration of met may have contributed to increased pulmonary oxidative stress and increased mortality by mechanisms dependent of endogenous GC.
Keywords: acute lung injury/acute respiratory distress syndrome, glucocorticoids, metyrapone, sepsis
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are defined as a syndrome of acute respiratory insufficiency. The ARDS is considered a more severe manifestation of the disease with a more severe degree of hypoxaemia (Murray et al. 1988; Bernard et al. 1994). ALI/ARDS is an inflammation syndrome associated with a collection of clinical, radiological and physiological abnormalities (Bernard et al. 1994) characterized by rapid-onset respiratory failure following a variety of direct (pulmonary) and indirect (extrapulmonary) insults to the parenchyma or vasculature of the lungs respectively (Raghavendran & Napolitano 2011). Alveolar epithelium and pulmonary capillary endothelium are destroyed by activated neutrophils and macrophages, which release inflammatory mediators, such as cytokine tumour necrosis factor (TNF-α), interleukins (ILs) and oxygen metabolites (Puneet et al. 2005). Sepsis is one of the primary risk factors for ALI/ARDS (Hudson et al. 1995). Patients with sepsis-induced ALI had greater illness severity and organ dysfunction (Sevransky et al. 2009). Despite extensive investigation over many years, the impact of ARDS is still a problem for the public and healthcare administrations. Glucocorticoids (GC) have been proposed as a treatment for ALI/ARDS (Annane 2007; Meduri et al. 2008) because of their anti-inflammatory properties (Rhen & Cidlowski 2005; Marik et al. 2011). However, the results have been controversial (Mesotten et al. 2008; Peter et al. 2008).
Metyrapone (met), an 11-beta steroid hydroxylation inhibitor, is a powerful and selective inhibitor of GC synthesis in animals and humans (Roozendal et al. 1996). This agent affects the conversion of deoxycorticosterone, the corticosterone (CCT) precursor, in the rat adrenal cortex and prevents the synthesis and subsequent release of CCT, which is the predominant GC in the bloodstream (Breivik et al. 2000). There is concern that a reduction in tissue-generated cortisol by met might decrease feedback to the hypothalamic–pituitary–adrenal (HPA) axis, resulting in an upregulation of cortisol production (Maric & Adzic 2013). The effects of met on surfactant, oxidative stress (Sosenko et al. 1986) and pro-inflammatory cytokines (Peruzzo et al. 2010) have been analysed. Current studies have indicated the potential of met to block deleterious effects on the lung (Baitharu et al. 2012; Paek et al. 2014) through neuroprotective action (Baitharu et al. 2012).
Met has not been studied in an animal model of sepsis, such as caecal ligation and puncture (CLP), to document the effects on the lung injury that is known to be part of the systemic sepsis. Met was used to demonstrate the effects of endogenous GC in the pathogenesis of lung injury. Therefore, we examined the consequences of ALI and investigated previous doses of the GC synthesis inhibitor (met) on the interaction between CCT levels, inflammation (cells and MPO activity), oxidative stress (H2O2 levels), alveolar morphology and lung function in a relevant model of sepsis.
Materials and methods
Animals
One hundred and thirty-five adult male rats (Wistar, 250 ± 50 g) were housed in a 12-h daylight cycle and fed with standard chow and water ad libitum in the animal care facility of the Laboratory of Physiology, Federal University of Alfenas. The experiments were conducted in accordance with the Declaration of Helsinki for the welfare of experimental animals, and the Ethics Committee of the Federal University of Alfenas approved the experimental methods (protocol number 341/11).
Experimental outline
First, rats were randomized into three main groups: CLP, Sham and Control (Naïve). One hour before CLP surgery, the CLP group was divided into subgroups, which received metyrapone (CLP met, 50 mg/kg, i.p.; Sigma, St Louis, MO, USA), dexamethasone (CLP dex; 0.5 mg/kg, i.p.; Merck, Sharp & Dohme, Brazil) or an equivalent volume of saline intraperitoneally (CLP sal; 0.9% NaCl). The Naïve group did not undergo surgery or receive any drugs. The Sham group received an equivalent volume of saline intraperitoneally (Sham; 0.9% NaCl). To prepare for surgical procedures, animals were anesthetized with tribromoethanol (TBE) 250 mg/kg. A laparotomy was performed (1 cm longitudinal incision, lower abdomen), and the caecum was exteriorized and ligated just distally to the ileocaecal valve to avoid any intestinal obstruction and subsequently punctured with a 19-gauge needle. A small amount of the bowel contents were then extruded through the puncture site. The caecum was subsequently replaced into the peritoneal cavity, and the incision was closed with surgical staples. In Sham animals, the caecum was only exteriorized before being returned and closing the abdomen. Fluid resuscitation was with sterile saline (100 mg/kg i.p.). Animals were recovered from anaesthesia in a warm environment and were monitored for 6 or 24 h until the beginning of experimental procedures, which were as follows: blood and tissues samples for biochemical measures (N = 8 per group), respiratory mechanical and tissue samples for histological procedures (N = 5–7 per group).
Corticosterone
Early-morning baseline samples of blood for hormone measurements were collected in Eppendorf tubes containing 20 μl of 0.05 M EDTA. Blood was immediately placed on ice and centrifuged, and the plasma was collected. The CCT level was performed by radioimmunoassay using a standard and specific antibody (Sigma). Before performing the radioimmunoassay, plasma samples were placed into individual tubes, and ethanol was added. The tubes were centrifuged for 15 min at 259 g under refrigeration (4°C), and the supernatants were subsequently transferred to other tubes for lyophilization. Dry material was stored at −20°C until the completion of radioimmunoassay. The results are expressed in ng/ml.
Myeloperoxidase (MPO) activity
Lung samples weighing between 310 and 360 mg were homogenized in buffer containing 50 mM potassium phosphate (pH 6.0). The mixture was centrifuged at 12,000 g for 30 min, and the resulting pellet was resuspended in phosphate buffer containing 5% wt/vol hexadecyltrimethyl ammonium bromide (Sigma). The suspension was subjected to three cycles of freezing and further disrupted by sonication for 40 s. The sample was then centrifuged at 7,200 g for 5 min at 4°C, and the supernatants were used for the MPO assay. The Amplex Red peroxidase assay kit (Molecular Probes, Invitrogen, Eugene, OR, USA) was used to determine MPO activity in the lung homogenates according to the manufacturer’s instructions. The absorbance of each sample was measured using a microplate reader equipped for excitation in the range of 560 nm (Synergy H1, hybrid reader; BioTek; Winooski, VT, USA). The results from lungs are expressed in mU/g.
Hydrogen peroxide (H2O2) measurement
Lung samples weighing between 190 and 230 mg were homogenized in 140 mM/l NaCl, 10 mM/l potassium phosphate buffer and 5 mM/l dextrose (pH 7.0). The mixture was centrifuged at 580 g for 10 min at 4°C, and the supernatant was used for the H2O2 assay. The Amplex Red peroxide assay kit (Molecular Probes, Invitrogen) was used to determine H2O2 measured in the lung homogenates according to the manufacturer’s instructions. The absorbance of each sample was measured using a microplate reader equipped for excitation in the range of 560 nm (Synergy H1, hybrid reader; Biotek). The results from lungs are expressed in μM/g.
Respiratory mechanical
Rats (6 or 24 h after CLP, Sham surgery or Naïve) were anesthetized (xylazine 8 mg/kg, pentobarbital 40 mg/kg) and received an analgesic (Tramal 30 mg/kg i.p.); next, a tracheotomy tube was inserted, and ventilation was started (tidal volume 6 ml/kg; positive end-expiratory pressure, 3.0 cm H2O; respiratory rate 110/min) with a computer-controlled ventilator (SCIREQ, Flexivent, Montreal, QC, Canada). The animals were paralysed with pancuronium bromide (0.5 ml/kg, i.p.) and kept warm using a heating pad. The respiratory system input impedance (Zrs) was measured by applying 3 s oscillatory volume perturbations to the tracheal cannula that was connected to the airway opening. By fitting the constant phase model to the obtained data, the mechanical parameters airway resistance (Raw), tissue damping (Gtis), tissue elastance (Htis) and hysteresivity (ŋ) were estimated. This technique was specially designed to measure the input Zrs of small animals (Gomes et al. 2000) and has been described in detail previously (Hantos et al. 1992; Peták et al. 1997). The experiments were conducted with the chest wall preserved.
Histology
After mechanical ventilation, the trachea was clamped at the end of expiration, heparin (1000 UI) was intravenously injected into the vena cava, the aorta was cut (leading to a massive haemorrhage and killing the animals quickly), and the right lung was fixed in 10% paraformaldehyde and embedded in paraffin. Slides were to cut to 4 μm thickness and stained with haematoxylin–eosin. The lung morphology was analysed in ten random areas in non-coincident microscopic fields with an integrating eyepiece on a coherent system made up of a 100 point grid consisting of 50 lines of known length and coupled to a conventional light microscope (Nikon, Tokyo, Japan). The volume fractions of the collapsed and normal pulmonary areas were determined using the point-counting technique (Weibel & Gomez 1962) at a magnification of 200×. Neutrophils, mononuclear cells (MN) and lung tissue were evaluated at 1000× magnification. Points falling on neutrophil and MN cells were counted and divided by the total number of points falling on tissue area in each microscopic field.
Statistical analysis
All data, except for mortality percentage, are expressed as the mean ± standard error of the mean (SEM). Statistical significance was assessed by parametric methods using one-way analysis of variance followed by Tukey test (anova) in GraphPad Prism (version 5.0; San Diego, CA, USA). The analysis of mortality percentage was assessed by analysis and adjustment of logistic regression models (binomial) using the glm function of Statistical Computing System R 3.0.2 software (R Foundation for Statistical Computing, Vienna, Austria). For each analysis, P < 0.05 was considered to be statistically significant.
Results
Corticosterone plasma levels at 6 (F4,30 = 9.84) and 24 h (F4,28 = 6.06) (Figure1a and b respectively) show a significantly elevated, nearly 2.5-fold increase in the CLP sal group in comparison with the Naïve (P < 0.001) and Sham (P < 0.01) groups. CLP met and CLP dex groups are different from CLP sal (P < 0.05; P < 0.001 respectively) at 6 h. After 24 h, the CCT levels exhibited the same profile, except for the CLP met group, which exhibited similar values to the Naïve and Sham groups.
Figure 1.

Plasma levels of corticosterone (CCT) (a) 6 and (b) 24 h after surgery. Values are mean ± SEM. ##P < 0.01 and ###P < 0.001 compared to Naïve group; *P < 0.05 and **P < 0.01 compared to Sham group; +P < 0.05, ++P < 0.01 and +++P < 0.001 compared to CLP sal group. N = 8.
The biochemical measurements of lung tissue at 6 h in the CLP groups were not significantly different from the Naïve and Sham groups (data not shown). The values observed at 24 h (Figure2a,b) for MPO activity (F4,27 = 15.50), a marker of inflammation, in the CLP sal and CLP met groups showed a significant increase compared to the Naïve (P < 0.001) and Sham (P < 0.001) groups. The MPO values of the CLP dex group were similar to the Naïve and Sham groups demonstrating a reduced activity in comparison with the CLP sal (P < 0.001) and CLP met (P < 0.01) groups. The H2O2 levels (F4,27 = 55.42), an oxidative stress indicator, were elevated in the CLP sal and CLP met groups compared to the Naïve (P < 0.01; P < 0.001 respectively) and Sham (P < 0.001) groups. The H2O2 values for the CLP met group were higher than all the other groups (P < 0.001). The H2O2 values of the CLP dex group were similar to the Naïve and Sham groups exhibiting a reduced level in comparison with the CLP sal (P < 0.05) and CLP met (P < 0.001) groups.
Figure 2.
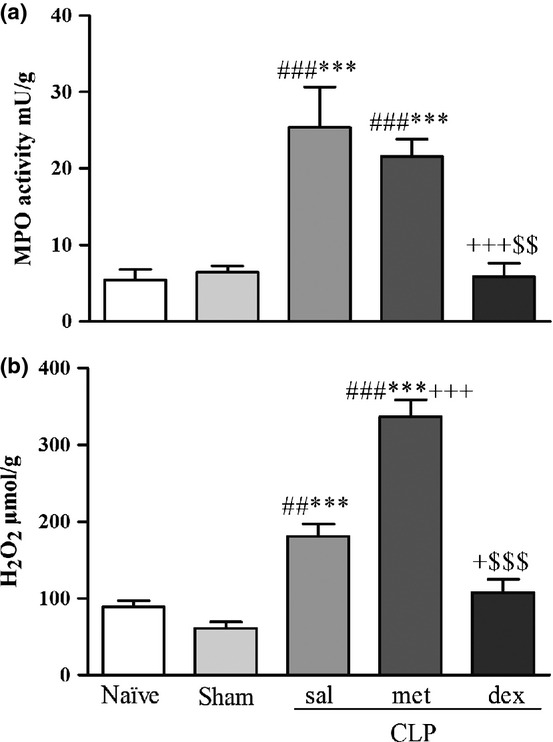
(a) Myeloperoxidase (MPO) activity and (b) hydrogen peroxide (H2O2) levels in lung tissue 24 h after surgery. Values are mean ± SEM. ##P < 0.01 and ###P < 0.001 compared to Naïve group; ***P < 0.001 compared to Sham group; +P < 0.05 and +++P < 0.001 compared to CLP sal group; and $$P < 0.01 and $$$P < 0.001 compared to CLP met group. N = 8.
All respiratory mechanical results at 6 h, as well as Raw and Htis results at 24 h, were not significantly different from the Naïve or Sham groups (data not shown). The Gtis (F4,27 = 15.69) and η (F4,27 = 4.93) values at 24 h are shown in Figure3a and b respectively. The CLP sal and CLP met groups showed significantly increased Gtis compared to Naïve (P < 0.001) and dex groups (P < 0.01; P < 0.001 respectively). The Gtis values of the CLP met group were significantly different from the Sham group (P < 0.01). The ŋ values of the CLP sal and CLP met groups were significantly different from the Sham group (P < 0.05).
Figure 3.
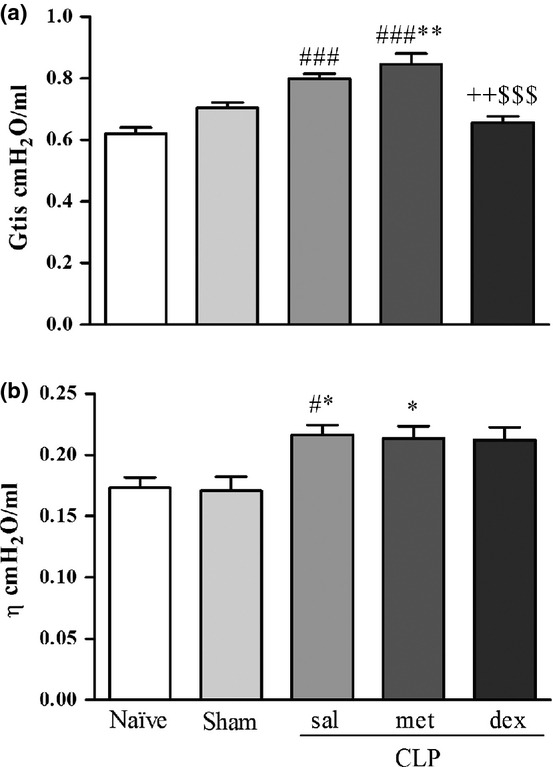
(a) Tissue damping (Gtis) and (b) hysteresivity (η) 24 h after surgery. Values are mean ± SEM. #P < 0.05 and ###P < 0.001 compared to Naïve group; *P < 0.05 and **P < 0.01 compared to Sham group; ++P < 0.01 compared to CLP sal group; and $$$P < 0.001 compared to CLP met group. N = 5–7.
The CLP sal and CLP met groups were characterized by a significant reduction in fractions of alveolar normal area (F4,24 = 159.1) and increased alveolar collapsed area (F4,24 = 166.1) compared to Naïve (P < 0.001) and Sham (P < 0.001) groups (Table1). CLP dex showed increased alveolar normal area and decreased alveolar collapsed area compared to the CLP sal (P < 0.01) and CLP met groups (P < 0.05). The CLP sal group showed an increased percentage of Neut (F4,20 = 11.88) and MN (F4,20 = 13.71) cells at 24 h compared to the Naïve (P < 0.01; P < 0.001 respectively) and Sham groups (P < 0.001). The CLP met group showed an increased percentage of Neut and MN cells at 24 h compared to the Naïve (P < 0.05; P < 0.001 respectively) and Sham groups (P < 0.01; P < 0.001 respectively). The CLP dex group showed a significant decrease in Neut and MN fractions compared to the CLP sal (P < 0.01) and CLP met groups (P < 0.01; P < 0.05 respectively).
Table 1.
Morphometric parameters at 24 h
| Normal Alveoli (%) | Collapsed Alveoli (%) | Neut† (%) | MN‡ (%) | |
|---|---|---|---|---|
| Naïve | 95.40 ± 0.52 | 4.61 ± 0.52 | 1.63 ± 0.21 | 0.73 ± 0.08 |
| Sham | 93.57 ± 0.72 | 5.10 ± 0.50 | 1.12 ± 0.15 | 0.77 ± 0.19 |
| CLP sal | 68.69 ± 4.00###*** | 28.97 ± 3.77###*** | 4.81 ± 0.99##*** | 2.40 ± 0.27###*** |
| CLP met | 73.47 ± 1.97###*** | 24.48 ± 1.37###*** | 4.23 ± 0.26#** | 2.63 ± 0.35###*** |
| CLP dex | 86.25 ± 4.30++$ | 12.68 ± 4.11++$ | 1.28 ± 0.42++$$ | 1.39 ± 0.23$ |
Normal alveoli, collapsed alveoli, neutrophils† and mononuclear‡ cells percentage. Values are mean ± SEM. All values were computed in 10 random, non-coincident fields per rat.
P < 0.05
P < 0.01 and
P < 0.001 compared to Naïve group
P < 0.01 and
P < 0.001 compared to Sham group
P < 0.01 compared to CLP sal group
P < 0.05 and
P < 0.01 compared to CLP met group; N = 5.
After 24 h of CLP-induced sepsis, the mortality percentage varied according to the previously administered drug. The results were the following: 0% Naïve and Sham, 13% CLP dex, 38% CLP sal and 75% CLP met. As observed, the CLP met group mortality percentage was significantly different from the Naïve, Sham, CLP sal and CLP dex groups. The death risk of the CLP met group was nine times greater compared to the Naïve group.
Discussion
In the present study, using a previous dose of the GC antagonist met, we observed increased pulmonary inflammation and a higher alveolar collapsed area with consequent impairment in respiratory mechanics, similar to the CLP sal group. However, met reduced CCT levels with drastically increased pulmonary oxidative stress compared to the CLP sal group. When we observed rats submitted to sepsis that had received a previous single low dose of GC agonist (dex) at 24 h (demonstrating a short-time effect), a beneficial effect on lung protection was demonstrated with a reduction in CCT levels, lung inflammation and oxidative stress. Moreover, the alveolar collapsed area decreased and the physiological parameters of respiratory mechanics were improved compared to the CLP sal group.
There are many controversies regarding lung protection mediated by plasma levels of endogenous GC in sepsis-induced ALI because endogenous GCs are not always effective in suppressing systemic inflammation (Liu et al. 1993; Meduri et al. 2005), even though the degree of cortisolaemia frequently correlates with severity of illness and mortality rates (Annane et al. 2000; Mesotten et al. 2008; Thompson 2010). In the present study, rats from the CLP sal group were compared to Naïve and Sham groups until 24 h and showed exacerbated adrenal activity as evidenced by increased CCT levels, which were associated with an increase in lung inflammation. This finding suggests a failure in suppressing inflammation, which could be due to inadequacy and/or tissue resistance to concentrations and durations of endogenous GC elevations (Meduri et al. 2002). This result suggests an endogenous GC hypofunction caused by reduced GC receptor-binding capacity in the lung, which may accelerate the pathological response of ALI (Liu et al. 1993). In this study, failure of high levels of CCT to suppress inflammation increased the percentage of inflammatory cells, the MPO activity and oxidative stress, with a consequent increased alveolar collapsed area and impairment of respiratory mechanics in CLP sal animals compared to Sham and Naïve animals.
Therapeutic interventions have been sought to improve and/or prevent lung injuries observed in patients with ALI/ARDS with the aim of enhancing survival. In the present study, the animals from the CLP met and CLP dex groups had basal levels of CCT at 6 and 24 h, showing that the met and dex doses were efficient to block synthesis and the HPA axis, respectively, in those animals. However, met significantly increased the mortality compared to Naïve animals as evidenced by a ninefold greater death risk.
Met is an inhibitor of the final enzyme in the pathway for CCT synthesis (Sosenko et al. 1986; Roozendal et al. 1996). In the present study, met administration decreased CCT levels and drastically increased pulmonary oxidative stress, as demonstrated by increased H2O2 levels compared to the CLP sal group. This result showed that the decreased lung anti-oxidative capacity by met is dependent on endogenous GC levels. These findings conform to another study that demonstrated that met promoted a decrease in antioxidant enzymes, as well as superoxide dismutase, catalase and glutathione peroxidase in rat lung with low GC levels, and affected the rates of rat survival, suggesting that effect could be attributable to met (Sosenko et al. 1986).
The CLP met rats showed increased MPO activity and a greater percentage of neutrophil cells in the lung, similar to the animals of CLP sal group. MPO from neutrophils correlates with lung inflammation and the degree of pulmonary dysfunction (Aaron et al. 2001). It was demonstrated that in inflammatory disease, met increased expression of pro-inflammatory cytokines, such as TNF-α, IL1-β and IL-6, regardless of its effect on plasma CCT levels (Peruzzo et al. 2010). In the present study, the inflammatory process in the CLP met group was similar to the CLP sal group, but there were differences in the levels of CCT; thus, we can say that the increase of MPO and inflammatory cells in the lung is independent of the levels of endogenous GC.
The ratio of Gtis/Htis, which is known as η, has been shown to increase in association with regional heterogeneities developed throughout the lung (Rocco et al. 2001; Bates & Allen 2006), suggesting that parenchymal mechanical dysfunction plays an important role in the pathophysiology of ALI. Increase in η could indicate the diffused character of pulmonary deterioration (Bates & Allen 2006). Alterations in Gtis and η were also observed in our CLP met and CLP sal animals. These findings were due to histological alterations because these animals exhibited a higher alveolar collapsed area. This study showed that pulmonary mechanics were significantly affected by sepsis and that these effects were not enhanced by met pretreatment in rats. This finding suggests that endogenous GC in CLP-induced ALI models does not play an important role in protecting the lung during sepsis. In contrast, in a porcine model for endotoxin-induced ALI, met worsened respiratory function by blocking endogenous cortisol, resulting in greatly enhanced endotoxin-induced ALI and shock (Middelveld et al. 1999).
If inadequate secretion of CCT fails to suppress inflammation, hormonal administration should promote GC anti-inflammatory function by decreasing the production of inflammatory cytokines, cytokine-driven HPA axis activity and cytokine-driven organ dysfunction (Meduri 1999). The effects of dex are repeatedly demonstrated, and the results of the present study are in agreement with the literature regarding the prevention of lung inflammation (Meduri et al. 2002; Wang et al. 2008), oxidative stress and MPO activity (Sun et al. 2009), decreased alveolar collapsed area and improvement in the physiological parameters of respiratory mechanics (Meduri et al. 2002; Rocco et al. 2003) with a consequent increase in survival (Cicarelli et al. 2006, 2007).
In conclusion, previously administered metyrapone did not prevent lung inflammation and did not improve the morphology and lung function in CLP-induced ALI/ARDS rats after 24 h of sepsis. Moreover, met administered before injury drastically increased pulmonary oxidative stress and increased mortality by mechanisms dependent on endogenous GC. Therefore, met pretreatment in ALI/ARDS should be performed with caution. Thus, we suggest further studies on the effects of met in different models of critical illness which involve disorders in endogenous GC levels.
Conflict of interest
The authors declare no conflict of interest.
Funding source
This work was supported by FAPEMIG process # APQ-02534/10 and APQ-01887/13.
Acknowledgments
The authors thank Dr. Estela Regina de Oliveira for her expert assistance, Ms. Marina de Fátima Venâncio for providing technical assistance and Dr. Luiz Alberto Beijo for statistical assistance.
References
- Aaron SD, Angel JB, Lunau M, et al. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163:349–355. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- Annane D. Glucocorticoids for ARDS: Just Do It! Chest. 2007;131:945–946. doi: 10.1378/chest.06-3005. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- Baitharu I, Deep SN, Jain V, et al. Corticosterone synthesis inhibitor metyrapone ameliorates chronic hypobaric hypoxia induced memory impairment in rat. Behav. Brain Res. 2012;228:53–65. doi: 10.1016/j.bbr.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Bates JH. Allen GB. The estimation of lung mechanics parameters in the presence of pathology: a theoretical analysis. Ann. Biomed. Eng. 2006;34:384–392. doi: 10.1007/s10439-005-9056-6. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee (Review) J. Crit. Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Breivik T, Sluyter F, Holf M, Cools A. Differential susceptibility to periodontitis in genetically selected Wistar rat lines that differ in their behavioral and endocrinological response to stressor. Behav. Genet. 2000;30:123–130. doi: 10.1023/a:1001903221046. [DOI] [PubMed] [Google Scholar]
- Cicarelli DD, Benseñor FE, Vieira JE. Effects of single dose of dexamethasone on patients with systemic inflammatory response. Sao Paulo Med J. 2006;124:90–95. doi: 10.1590/S1516-31802006000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicarelli DD, Vieira JE, Benseñor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007;125:237–241. doi: 10.1590/S1516-31802007000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J. Appl. Physiol. 2000;89:908–916. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J. Appl. Physiol. 1992;72:168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Liu LY, Sun B, Tian Y, Lu BZ, Wang J. Changes of pulmonary glucocorticoid receptor and phospholipase A2 in sheep with acute lung injury after high dose endotoxin infusion. Am. Rev. Respir. Dis. 1993;148:878–881. doi: 10.1164/ajrccm/148.4_Pt_1.878. [DOI] [PubMed] [Google Scholar]
- Maric NP. Adzic M. Pharmacological modulation of HPA axis in depression - new avenues for potential therapeutic benefits. Psychiatr. Danub. 2013;25:299–305. [PubMed] [Google Scholar]
- Marik PE, Meduri GU, Rocco PR, Annane D. Glucocorticoid treatment in acute lung injury and acute respiratory distress syndrome. Crit. Care Clin. 2011;27:589–607. doi: 10.1016/j.ccc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Meduri GU. An historical review of glucocorticoid treatment in sepsis: disease pathophysiology and the design of treatment investigation. Sepsis. 1999;3:21–38. [Google Scholar]
- Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am. J. Respir. Crit. Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. Am. J. Respir. Crit. Care Med188Erratum in: . 2013; : 1477. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. NeuroImmunoModulation. 2005;12:321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- Mesotten D, Vanhorebeek I, Van den Berghe G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:496–505. doi: 10.1038/ncpendmet0921. [DOI] [PubMed] [Google Scholar]
- Middelveld RJ, Wanecek M, Bergman D, Weitzberg E, Alving K. Effect of cortisol-synthesis inhibition on endotoxin-induced porcine acute lung injury, shock, and nitric oxide production. Shock. 1999;12:382–390. doi: 10.1097/00024382-199911000-00007. [DOI] [PubMed] [Google Scholar]
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Paek DS, Sakurai R, Saraswat A, et al. Metyrapone alleviates deleterious effects of maternal food restriction on lung development and growth of rat offspring. Reprod. Sci. 2014;xx:1–16. doi: 10.1177/1933719114537712. Pii: 1933719114537712. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzo DC, Benatti BB, Andersen ML, Tufik S, Casati MZ, Nociti FH., Jr Evidence that metyrapone in the presence of inflammation modulates cytokine mRNA expression. Cytokine. 2010;52:184–189. doi: 10.1016/j.cyto.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Peták F, Hayden MJ, Hantos Z, Sly PD. Volume dependence of respiratory impedance in infants. Am. J. Respir. Crit. Care Med. 1997;156:1172–1177. doi: 10.1164/ajrccm.156.4.9701049. [DOI] [PubMed] [Google Scholar]
- Peter J, John P, Graham P, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome (Review) Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:3–15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K. Napolitano LM. Definition of ALI/ARDS. Crit. Care Clin. 2011;27:429–437. doi: 10.1016/j.ccc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T. Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rocco PR, Negri EM, Kurtz PM, et al. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am. J. Respir. Crit. Care Med. 2001;164:1067–1071. doi: 10.1164/ajrccm.164.6.2007062. [DOI] [PubMed] [Google Scholar]
- Rocco PR, Souza AB, Faffe DS, et al. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am. J. Respir. Crit. Care Med. 2003;168:677–684. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- Roozendal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Sevransky JE, Martin GS, Shanholtz C, et al. Mortality in sepsis versus non-sepsis induced acute lung injury. Crit. Care. 2009;13:R150. doi: 10.1186/cc8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosenko IR, Lewis PL, Frank L. Metyrapone delays surfactant and antioxidant enzyme maturation in developing rat lung. Pediatr. Res. 1986;20:672–675. doi: 10.1203/00006450-198607000-00019. [DOI] [PubMed] [Google Scholar]
- Sun J, Yang D, Li S, Xu Z, Wang X, Bai C. Effects of curcumin or dexamethasone on lung ischaemia-reperfusion injury in rats. Eur. Respir. J. 2009;33:398–404. doi: 10.1183/09031936.00142407. [DOI] [PubMed] [Google Scholar]
- Thompson BT. Corticosteroids for ARDS (Review) Minerva Anestesiol. 2010;76:441–447. [PubMed] [Google Scholar]
- Wang XQ, Zhou X, Zhou Y, Rong L, Gao L, Xu W. Low-dose dexamethasone alleviates lipopolysaccharide-induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology. 2008;13:772–780. doi: 10.1111/j.1440-1843.2008.01344.x. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Gomez DM. A principle for counting tissue structures on random sections. J. Appl. Physiol. 1962;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]


