Abstract
The aim of the study was to investigate the influence of flaxseed and lactobacilli supplementation to the diet of piglets during the time period between 10 days before and 21 days after weaning. The morphometry of the jejunal mucosa and proliferative ratio of both epithelial and lamina propria cells were compared with those found in a group of piglets fed with the usual diet added with sunflower oil during the same time period. The addition of flaxseed oil to the diet significantly increased the crypt depth in comparison with both groups supplemented with sunflower (P < 0.05 and 0.001 respectively) on the weaning day. Moreover, the flaxseed addition caused a significant decrease in villus height (P < 0.01) and crypt depth (P < 0.01) 21 days postweaning in comparison with the sunflower group. The proliferative ratio of the epithelial cells in the sunflower group on the weaning day was significantly higher than in both flaxseed groups (P < 0.01). Paradoxically, significantly higher proliferative activity in the mucosal connective tissue in the group with flaxseed supplementation in comparison with the sunflower group was observed on the day of weaning, as well as 3 days later (P < 0.05 both). A combination of flaxseed with lactobacilli showed significantly lower proliferative activity in the connective tissue cells from weaning up to 7 days after weaning (P < 0.05 all) in comparison with the flaxseed group.
Keywords: cell proliferation, crypt depth, flaxseed, lactobacilli, mucosal thickness, villus height
At weaning piglets undergo many stressful circumstances associated with change of their environment, separation from the mothers and general replacement of fluid milk nutrition by more abrasive solid food with different composition and nutrient value. As a consequence, weaning is very often accompanied by disorders in feed intake and increased susceptibility to intestinal dysfunction, including reduction of digestive and absorptive capacities (Pluske et al. 1997) and breakdown of intestinal barriers that, in turn, leads to increased permeability and mucosal inflammation (Moeser et al. 2007; Smith et al. 2010). The intestinal and immune system alterations result in reduced health and frequent diarrhoea. To lower the economic burden of such intestinal dysfunctions, the antibiotic growth promoters were included in the diet of the weaning piglets a few years ago (Aarestrup 2000). Their effect depends on reduction of the bacterial growth in the intestinal tract leading to protection of nutrients against bacterial metabolism, decrease of toxin production by intestinal bacteria and reduction in the incidence of subclinical intestinal infections (Butaye et al. 2003). However, the present European legislation prohibits antibiotic growth promoter incorporation into animal diets, endeavouring to reduce antibiotic resistance phenomena in humans. Thus, the European breeders need to replace them using different biological active substances, for example probiotics, prebiotics, non-specific substrates, plant extracts, organic acids or polyunsaturated fatty acids (PUFA; Bomba et al. 2006).
Weaning is also associated with marked changes in the morphology and biochemistry of the small intestine, such as villous atrophy and crypt hyperplasia, which cause decreased digestive and absorptive capacity while contributing to postweaning (PW) diarrhoea. It has been also shown that in this condition, villous atrophy is caused by either an increased rate of cell loss or a reduced rate of cell renewal (Pluske et al. 1997; Adamkov et al. 2014).
The immunomodulatory and anti-inflammatory properties of ω-3 PUFA suggest their possible use as a substitute for antibiotics. A combination of PUFA with probiotic has proved of benefit in the metabolism of fatty acids and synthesis of prostaglandins (Kastel et al. 2007), while favouring lactobacilli adhesion to the intestinal mucosa, and inhibiting Escherichia coli K88 infection in germ-free piglets (Nemcova et al. 2012). Moreover, the influence of dietary supplementation with flaxseed and lactobacilli on the innate immune cells in the jejunal mucosa of piglets PW has been described in a previous study from our group (Toth et al. 2015).
The objective of this study was to investigate the effects of active feed additives on the intestinal mucosa of weaned pigs using probiotics (lactobacilli) and flaxseed oil (source of ω-3 PUFA), in terms of changes of both morphology and proliferative rate of epithelial and lamina propria cell populations. These changes were evaluated at different time points from the day of weaning to 21 days after weaning. The results were compared to the groups of piglets receiving sunflower oil as source of ω-6 PUFA.
Materials and methods
Probiotic bacteria
The Lactobacillus probiotic strains were isolated at the University of Veterinary Medicine and Pharmacy, Košice, Slovakia: namely the Lactobacillus plantarum – Biocenol™ LP96 (CCM 7512) – strain was selected from the gut contents of healthy suckling piglets and Lactobacillus fermentum – Biocenol™ LF99 (CCM 7514) – was isolated from the gastrointestinal tract of adult chickens. Cheddar cheese was applied as vehicle for probiotic strains at 109 CFU/g of cheese. The cheese without probiotic strains was used as control.
Animals, housing and diets
The study was carried out on 88 healthy piglets (cross-breds – Yorkshire × Pietrain). The piglets were housed in typical indoor pens and divided into four groups: control group S (control cheese + sunflower oil) 19 piglets; group SL (probiotic cheeses + sunflower oil) 23 piglets; group F (control cheese + whole crushed flaxseed) 23 piglets; and group FL (probiotic cheeses + whole crushed flaxseed) 23 piglets.
The animals were fed with a meal formula of basal diet (Table1) supplemented with whole crushed flaxseed cultivar Flanders (Agrola, Kožušice, Czech Republic) for F and FL groups and control sunflower oil for S and SL groups both at a concentration of 10% (Table2).
Table 1.
Ingredient (%) and chemical composition (g/kg DM) of the basal diet
| Ingredient % | |
|---|---|
| Wheat | 27.60 |
| Soya bean, extr. | 22.00 |
| Maize (8.4% CP) | 19.70 |
| Barley | 17.00 |
| Oat (11.2% CP) | 5.00 |
| Powdered whey | 5.00 |
| Calcium carbonate | 1.10 |
| Mono-calcium phosphate | 1.00 |
| Sodium chloride | 0.40 |
| Vitamin–mineral premix 0.5% | 0.40 |
| L-Lysine HCl | 0.35 |
| L-Threonine | 0.35 |
| DL-Methionine | 0.10 |
| Chemical composition (g/kg DM) | |
|---|---|
| CP (g) | 187.9 |
| ME (MJ) | 12.8 |
| Fibre (g) | 38.3 |
| Lysine (g) | 11.6 |
| Methionine and cysteine (g) | 6.4 |
| Threonine (g) | 7.6 |
| Tryptophan (g) | 2.3 |
| Choline (mg) | 1352 |
| Vitamin A (IU) | 11,530 |
| Vitamin D3 (IU) | 1500 |
| Vitamin E (mg) | 68.7 |
| Vitamin B2 (mg) | 7.1 |
| Vitamin B12 (μg) | 26.4 |
| Ca (g) | 7.5 |
| P (g) | 6.2 |
| Na (g) | 1.9 |
| Cu (mg) | 10.0 |
| Fe (mg) | 163.4 |
| Zn (mg) | 125.8 |
| Mn (mg) | 72.7 |
DM, dry matter; CP, crude protein; ME, metabolizable energy.
Table 2.
Fatty acid profile (percentage)
| Fatty acid | Flaxseed oil | Sunflower oil | Basal diet |
|---|---|---|---|
| Lipids (DM basis) | 45.8 | ND | 2.2 |
| Palmitic, C16:0 | 5.1 | 6.3 | 17.4 |
| Stearic, C18:0 | 3.7 | 3.2 | 2.2 |
| Oleic, C18:1 | 18.4 | 22.6 | 24.7 |
| Linoleic, C18:2 | 16.1 | 67.9 | 51.9 |
| Linolenic, C18:3 | 56.8 | 0 | 3.8 |
ND, not detected.
The piglets were weaned at the age of 28 days. During the period of time between 10 days before and 21 days PW, the piglets in the groups SL and FL were supplied with probiotic cheeses (4 g/animal/day for each cheese, in total 8 g), while those in the groups S and F were supplied with control cheese (8 g/animal/day). The probiotic and control cheese were supplied to piglets once a day (individually in the morning) in the form of a grated cheese deposited on the surface of the meal. The animals felt the cheese and consumed it instantaneously. During the same period, the animals had ad libitum access to water and feed supplemented with whole crushed flaxseed or sunflower oil.
Biological samples
All pigs were sacrificed using T-61 euthanasia solution (active ingredients: embutramide, mebezonium iodide, tetracaine hydrochloride; Intervet International B.V. Boxmeer, The Netherlands, doses: 0.3 ml/kg body weight) injected intracardially on the day of weaning (28-day-old piglets) and days 3, 7 and 21 PW. The abdominal wall was immediately open, and four rings of 1–2 cm width were harvested from the first jejunal loop 10 cm downstream of the ligament of Treitz. The tissues were immediately fixed in 4% paraformaldehyde and embedded in paraffin. Serial 4- to 5-μm-thick sections were cut, mounted on electrostatic slides (Super Frost Plus; Menzel-Glaser, Braunschweig, Germany) and dried overnight. After dewaxing and rehydration, sections were processed for both haematoxylin–eosin (HE) staining and immunohistochemistry.
Histopathological evaluation
The histopathological injury index (HII) was examined blind by an expert pathologist and graded according to the Park/Chiu scoring system adapted by Quaedackers et al. (2000) with a scale ranging from 0 to 8, as follows: 0 = normal mucosa; 1 = subepithelial space at villus tip, often with capillary congestion; 2 = more extended subepithelial space with moderate epithelial lifting; 3 = epithelial lifting along villus sides; 4 = denuded villi; 5 = loss of villous tissue; 6 = crypt layer destruction; 7 = transmucosal infarction; 8 = transmural infarction.
Morphometrical analysis
Morphometry was performed using an Olympus BX50 light microscope with Olympus camera SP350 (Olympus, Tokyo, Japan) and QuickPHOTO Industrial 2.3 image analyzer software (Promicra, Prague, Czech Republic). Villus height (i.e. the distance from the villus base to the tip) and crypt depth (i.e. the distance between the villus base and the muscularis mucosae) were measured in 12 axially oriented villi in at least three different quadrants of each intestinal sample. Specifically, the crypt/villous junction was defined as the position at which epithelial cell nuclei changed from being central and with a more basophilic staining to being smaller and basally located, and where the cytoplasm developed mucin vacuoles (Holt et al. 1984). The intestinal mucosa thickness was measured as the sum of villus height and crypt depth. All measurements were taken using magnification ×200.
Immunohistochemical analysis
After blocking endogenous peroxidase activity with 3% H2O2 and methanol, histological sections underwent two cycles of pretreatment in a microwave oven at 600 W for 15 min each in 0.01 M citrate buffer at pH 6.0. After that, a primary Ki67 polyclonal antibody, clone MIB-5 (Millipore Bioscience Research Reagents, Billerica, MA, USA), which stains the nucleus of actively proliferating cells, was applied at 1:100 dilution with an overnight incubation at 4°C. Then, a biotinylated secondary anti-goat anti-mouse antibody IgG (H+L) (Millipore Bioscience Research Reagents) was used followed by application of the IHC Select® Immunoperoxidase Secondary Detection System (Millipore Bioscience Research Reagents). Positive cells were visualized with diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO, USA), and the nuclei were counterstained using Mayer’s haematoxylin. Omitting the primary antibody on a serial section was considered as the negative control. The tissue sections were examined in a blind fashion by two independent histologists and photographed using an Olympus BX50 light microscope with an Olympus SP350 camera (Olympus). Counts were performed at a constant magnification (×200) and the results expressed as the percentage of cells with the nucleus stained brown.
Statistical analysis
The statistical analysis was performed using graphpad instat version 3.01 (GraphPad Software, San Diego, CA, USA). The quantitative results were determined using one-way anova with a multiple-comparison Tukey–Kramer post hoc test. The results are expressed as mean (M) ± standard error of the mean (SEM). P values less than 0.05 were considered significant.
Ethical Approval
The experimental protocol (number 2108/07-221) was approved by the State Veterinary and Food Administration of the Slovak Republic, and the animals were handled and sacrificed in accordance with the guidelines established by the respective commission.
Results
As shown in Figure1, no relevant histopathological changes were found in the intestinal mucosa of all the piglet groups examined at any time point, except for a mild subepithelial swelling at some villus tips in the groups F (0.92 ± 0.27) and FL (1.17 ± 0.38) on the weaning day.
Figure 1.
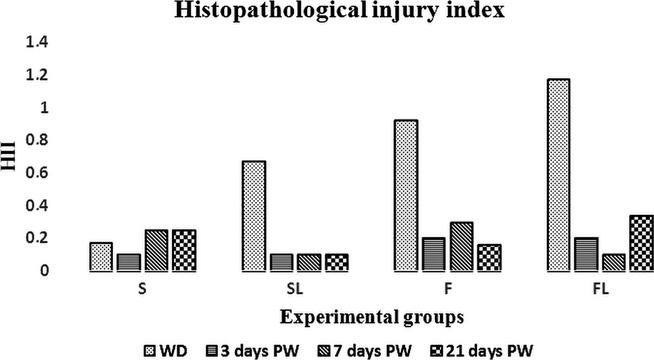
Histopathological injury index (HII). Values are mean ± SEM, n = 19–23.
The morphometric analysis showed a trend towards shortening of the villi in all piglet groups (Table3) after weaning. More precisely, the decrease in villus height was most prominent in F and FL groups, where the villi at 21 days PW were nearly 30% shorter than those at WD, while in both S and SL groups, after a transient reduction, the villus height was approximately the same, thus reaching the statistical significance in the S group in comparison with the F group (P < 0.01). As far as the crypt depth is regarded (Table4), a robust increase in the group S was observed 21 days PW in comparison with the previous time periods (vs. weaning day P < 0.05; vs. 3 days PW P < 0.01 and vs. 7 days PW P < 0.001). Conversely, a significant decrease of the crypt depth was found in the F group (all time periods vs. 21 days PW P < 0.05), where it was almost 50% lower than that in S group (P < 0.01) at the end of the experiment. Similarly, deeper crypts were evident even in the FL group 21 days PW with respect to the F one (vs. F P < 0.01). However, the lowest crypt depth was observed in the SL group at the WD, and the difference was significant in comparison with the other groups (P < 0.001 for all). When considering the villus height/crypt depth ratio, no differences were observed in all the experimental groups, except for a significant increase in the SL group (2.13 ± 0.12) in comparison with the other groups at the weaning day (P < 0.05 for all), that underwent normalization at day 7 PW (0.98 ± 0.09; P < 0.01) and remained stable during the following time points (Figure2).
Table 3.
Morphometrical assessment of villus height
| Days postweaning | P value | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 21 | AGE | DIET | |
| S | 315.2 ± 24.4 | 203.5 ± 12.3 | 181.1 ± 14.5a | 320.1 ± 51.9a,b | <0.05a | <0.01b |
| SL | 273.3 ± 20.8 | 236.2 ± 23.0 | 206.5 ± 30.6 | 265.3 ± 25.3 | ns | ns |
| F | 343.3 ± 94.4 | 239.1 ± 28.2 | 280.3 ± 45.3 | 236.0 ± 6.5b | ns | <0.01b |
| FL | 320.0 ± 26.7 | 252.4 ± 11.1 | 282.6 ± 12.0 | 246.9 ± 14.1 | ns | ns |
Values are expressed in μm and results given as mean ± SEM, n = 19–23; AGE, differences within the animals of different age at a same diet (rows); DIET, differences within the animals at the same age with different diet (columns)
values in the same row with the same letters differ significantly
values in the same column with the same letters differ significantly; ns, not significant.
Table 4.
Morphometrical assessment of crypt depth
| Days postweaning | P value | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 21 | AGE | DIET | |
| S | 256.4 ± 15.9a,d,e,g | 214.2 ± 5.6b | 187.5 ± 16.1c | 329.7 ± 12.2a,b,c,h | <0.05a | <0.001d |
| <0.01b | <0.001e | |||||
| <0.001c | <0.05g | |||||
| SL | 127.8 ± 4.2d,e,f | 170.8 ± 14.6 | 213.5 ± 35.7 | 236.4 ± 39.0 | ns | <0.001e |
| <0.001f | ||||||
| F | 315.5 ± 5.2a,e,g | 247.0 ± 14.8a,b, | 243.3 ± 21.0a,c | 168.3 ± 14.8a,b,c,h,i | <0.05a,b,c | <0.001e |
| <0.05g | ||||||
| <0.001h | ||||||
| <0.001i | ||||||
| FL | 287.8 ± 17.0a,d,f | 180.3 ± 28.1a,b | 234.6 ± 4.2 | 271.3 ± 4.8b,i | <0.01a,b | <0.001d |
| <0.001f | ||||||
| <0.01i | ||||||
Values are expressed in μm and results given as mean ± SEM, n = 19–23; CD, crypt depth; WD, weaning day; AGE, differences within the animals of different age at a same diet (rows); DIET, differences within the animals at the same age with different diet (columns)
values in the same row with the same letters differ significantly
values in the same column with the same letters differ significantly; ns, not significant.
Figure 2.
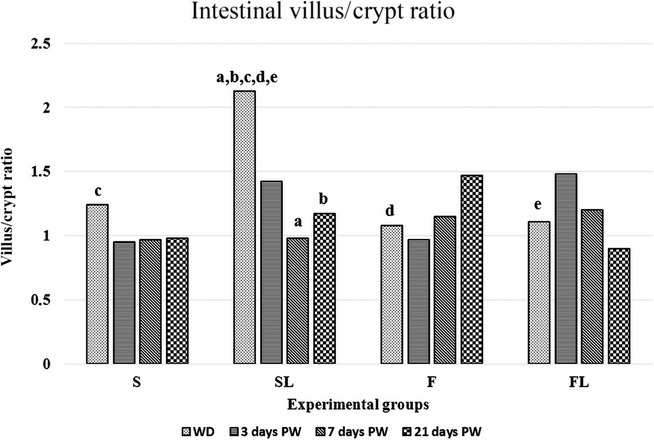
Intestinal villus/crypt ratio. Values are mean ± SEM, n = 19–23, a,b,c,d,evalues with the same letters differ significantly; aP<0.01, b,c,d,eP<0.05.
A time-dependent decrease of the intestinal mucosal thickness (Table5) was found in the flaxseed oil piglet groups (both F and FL) at day 21 PW in comparison with the weaning day (F P < 0.05; FL P < 0.05), with the FL group showing a statistically significant reduction also at day 3 PW vs. WD (P < 0.01). Furthermore, the mucosa appeared thinner in both the L (SL and FL) groups than in both groups without lactobacilli (S and F) on WD.
Table 5.
Morphometrical analysis of mucosal thickness
| Mucosa thickness (μm) | ||||||
|---|---|---|---|---|---|---|
| Day postweaning | P value | |||||
| 0 | 3 | 7 | 21 | AGE | DIET | |
| S | 602.0 ± 49.2 | 481.8 ± 11.4 | 446.6 ± 87.9 | 652.3 ± 26.4 | ns | ns |
| SL | 426.8 ± 27.2c,d | 444.7 ± 4.3d | 484.7 ± 91.9 | 610.3 ± 52.7 | ns | <0.05d |
| <0.01c | ||||||
| F | 779.1 ± 75.7a,c | 615.0 ± 40.6d | 616.3 ± 57.5 | 453.1 ± 22.5a | <0.05a | <0.05d |
| <0.01c | ||||||
| FL | 704.0 ± 21.1a,b,d | 478.3 ± 44.2b | 577.0 ± 8.5 | 530.5 ± 40.0a | <0.05a | <0.05d |
| <0.01b | ||||||
Values are mean ± SEM, n = 19–23, AGE, differences within the animals of different age at a same diet (rows); DIET, differences within the animals at the same age with different diet (columns)
values in the same row with the same letters differ significantly
values in the same column with the same letters differ significantly; ns, not significant.
When the proliferative rate of the epithelial cell population was evaluated, the highest mitotic activity was observed in the S group at WD (Figure3), followed by a decrease at 3 and 7 days PW, and a recovery at 21 days PW (Table6). Even when comparing the amount of proliferating cells (Ki67-positive) with the values found in all the other groups at the weaning day, the S group displayed a significantly higher rate (vs. SL P < 0.001; vs. F and FL P < 0.01 for both). By contrast, in the F group an opposite pattern was found, with a progressive increase in the number of Ki67-positive cells that reached statistical significance at day 21 PW as compared with the WD (P < 0.05). When comparing the data at 21 days PW in both F and FL groups with those found at 3 days PW, again a significantly higher proliferation rate was clearly evident (P < 0.05 for both). Finally, in the F group the number of proliferating cells was higher at all time points from the day 3 PW onwards than those found in the SL group (P < 0.05 for all).
Figure 3.
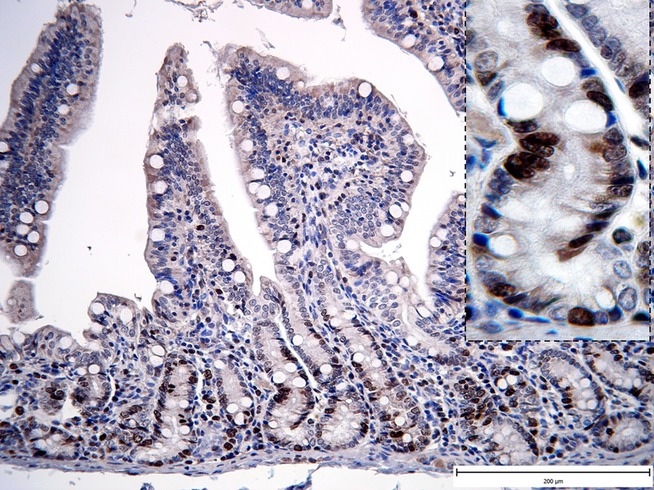
Representative photomicrographs illustrating the proliferative activity of the cells in the jejunal biopsy (S group – WD). Ki67 immunohistochemical staining. Significant increase in Ki67 immunoreactivity in lamina epithelialis mucosae (Fig.1, ×200; detail ×1000).
Table 6.
Epithelial cell proliferative rate
| Proliferative activity of cells in lamina epithelialis | ||||||
|---|---|---|---|---|---|---|
| Day postweaning | P value | |||||
| 0 | 3 | 7 | 21 | AGE | DIET | |
| S | 22.3 ± 1.7e,f,g | 12.9 ± 1.8a | 11.7 ± 0.7 | 17.1 ± 1.6 a | <0.05a | <0.001e |
| <0.01f,g | ||||||
| SL | 11.1 ± 0.6e | 10.4 ± 0.6h | 11.7 ± 0.3i,j | 12.9 ± 1.7k | ns | <0.05h,g |
| F | 15.7 ± 1.0b,f | 16.1 ± 1.0c,h | 17.5 ± 1.7i | 22.7 ± 1.8b,c,k | <0.05b,c | <0.05i,k |
| FL | 14.6 ± 0.4g | 10.7 ± 1.0d | 14.5 ± 1.3j | 16.3 ± 1.4d | <0.05d | <0.05j |
Values are mean ± SEM, n = 19–23; AGE, differences within the animals of different age at a same diet (rows); DIET, differences within the animals at the same age with different diet (columns)
values in the same row with the same letters differ significantly
values in the same column with the same letters differ significantly; ns, not significant.
When considering the proliferative rate of the lamina propria cells (Table7), the highest number of Ki67-positive cells was found in the F group at the WD with respect the S and FL groups (Figure4; P < 0.05 for both) and at 3 days PW with respect to all the other groups (P < 0.05 vs. S; P < 0.01 vs. both SL and FL). A high level of proliferative activity was maintained in the F group until the day 21 PW in comparison with the SL and FL groups. A lower number of proliferating cells were found in both lactobacilli groups reaching statistically significant differences when comparing the mean value of the FL and F groups at days 3 (P < 0.01) and 7 PW (P < 0.05). Moreover, both the lactobacilli groups showed the lower proliferative activity from the WD up to 21 days PW as compared to the flaxseed group.
Table 7.
Lamina propria cell proliferative rate
| Lamina propria proliferative rate | ||||||
|---|---|---|---|---|---|---|
| Day postweaning | P value | |||||
| 0 | 3 | 7 | 21 | AGE | DIET | |
| S | 15.4 ± 0.8b | 13.3 ± 0.8a,d | 18.2 ± 0.4h | 19.9 ± 2.0a,i | <0.05a | <0.05b,c,i |
| SL | 15.9 ± 1.9 | 10.9 ± 1.6e | 14.0 ± 1.3 | 11.3 ± 0.5 | ns | <0.01e |
| F | 22.1 ± 2.0b,c | 23.3 ± 2.4d,e,f | 17.7 ± 1.6g | 22.1 ± 1.8j | ns | <0.05d,j |
| <0.01f | ||||||
| FL | 14.7 ± 0.5c | 10.7 ± 1.3f | 12.1 ± 1.0g,h | 14.5 ± 2.3 | ns | <0.05g,h |
Values are mean ± SEM, n = 19–23; AGE, differences within the animals of different age at a same diet (rows); DIET, differences within the animals at the same age with different diet (columns)
values in the same row with the same letters differ significantly
values in the same column with the same letters differ significantly; ns, not significant.
Figure 4.
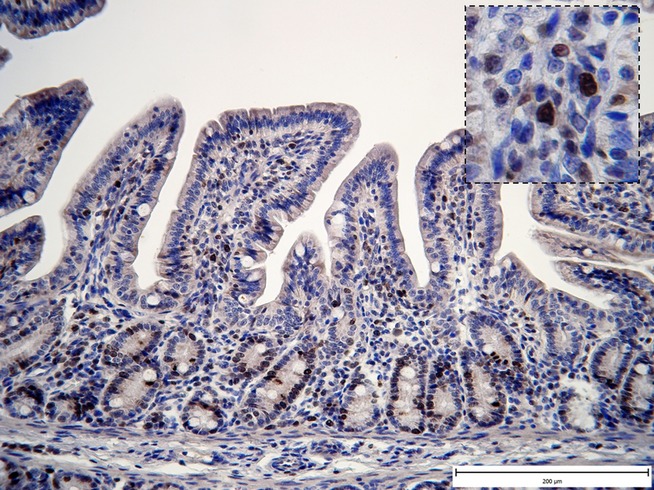
Representative photomicrographs illustrating the proliferative activity of the cells in the jejunal biopsy (F group – WD). Ki67 immunohistochemical staining. Significant increase in Ki67 immunoreactivity in lamina propria mucosae (Fig.1, ×200; detail ×1000).
Discussion
The intestinal tract of pigs undergoes morphological and functional modification in response to dietary changes. The intestinal villi are covered by highly differentiated epithelial cells, the enterocytes, whose brush border includes a number of specific channels and transporters devoted to absorption of a variety of nutrients. Moreover, digestive enzymes, such as disaccharidases and peptidases, are expressed on the brush border to complete the breakdown and then facilitate nutrient absorption. Villus atrophy therefore impairs pig growth performance by reduction of nutrient absorption. The presence of a positive correlation between villous height and disaccharidase activities (lactase, sucrase, maltase) was already established through all the growth stages of piglets (Tsukahara et al. 2013). The maintenance of villus structure to retain disaccharidase activity and carbohydrate absorption plays a pivotal role in preventing diarrhoea and ensuring an efficient growth of the piglets in the PW stage (Pluske 2001); thus, many nutritional strategies have been proposed to maintain PW villous height. According to our experimental study, only slight damage to the intestinal mucosa in both flaxseed piglet groups was found on WD, while the morphometric analysis showed a tendency to villus shortening during the PW times in all experimental groups. Both groups receiving sunflower oil (S and SL) that is rich in linoleic acid (ω-6 PUFA) showed approximately the same villus height 21 days PW as that on WD. When this was examined in more depth, following an initial decrease by 40% in the S group, a critical increase was observed from day 7 to day 21 PW. An opposite pattern was evident in the groups supplemented with flaxseed (F and FL) that is rich in linolenic acid (ω-3 PUFA). At time of 7 days PW the decrease in villus height in both flaxseed groups was lower than that observed in the S groups, although in the F group significantly shorter villi than those in S group were found at day 21 PW. At the same time, the crypt depth decreased significantly in the F group in comparison with the S one. Our findings are extremely important if considering that the marked changes in the morphology of the small intestine associated with the weaning, such as villous atrophy and crypt hyperplasia, are responsible for decreased digestive and absorptive capacities and contribute to PW diarrhoea (Pluske et al. 1997). Probiotic bacteria are often applied to prevent or cure a wide range of intestinal disorders, as elegantly shown by Yoshida et al. (2009) who found that small intestinal villous atrophy can be alleviated by the administration of probiotic lactobacilli which stimulate a peculiar array of cytokines responsible for mucosal recovery. The improvement in villous atrophy was still observed an oral preparation of heat-killed and dried cells was administered (Tsukahara et al. 2011). Moreover, the adhesion of bacteria to the mucosa is pivotal for the beneficial effects of living probiotics, and the administration of PUFA positively affects the adhesion of lactobacilli to the jejunal mucosa as assessed in gnotobiotic piglets (Bomba et al. 2003) and germ-free piglets (Nemcova et al. 2012). However, at variance with these latter studies, our results did not confirm the restorative effect of lactobacilli addition to the piglet diet, as no significant differences in villous height were found in both lactobacilli groups (SL and FL) in comparison with the S groups. Significantly lower crypt depth than in S, F and FL groups was found only on WD in SL group, which led to a significant increase in the intestinal villus/crypt ratio. Conversely, the thickness of the jejunal mucosa was significantly lower in the FL group than in the F group on WD and 3 days later, as a result of reduction of both villus height and crypt depth. Similarly, no effect of ω-3 PUFA on intestinal microbial communities and performance of broiler chickens was found (Geier et al. 2009). The significant decrease in crypt depth in both flaxseed groups during the first week PW could contribute to reduction of the PW diarrhoea incidence (Popper et al. 2014). The low crypt deep could mirror a low enterocyte apoptotic rate on the villous surface, so the reduced incidence of the diarrhoea could be attributed to the anatomo-functional integrity of the whole epithelium.
In the current study we observed different effects of ω-6 and ω-3 PUFA on the proliferative activity of both the epithelial and lamina propria cells of the intestinal mucosa. The highest proliferative activity of epithelial cells in the group fed with ω-6 PUFA (S) was observed on WD, followed by a decrease 3 days later. Conversely, the highest proliferative activity in lamina propria cells in the animals with ω-3 PUFA in the diet (F) was found on WD and 3 days later. Linoleic acid and linolenic acid are two of the main representative compounds, known as dietary essential fatty acids because they prevent deficiency symptoms and cannot be synthesized by humans (Benatti et al. 2004). According to Viegas et al. (2012), PUFA regulate cell proliferation and gene expression, but cellular responses to fatty acids may depend on the differentiation status of the cell and the origin of the cell lines as well. The positive effect of ω-6 PUFA on epithelial restitution has been demonstrated in previous studies. For instance, Blikslager et al. (1997, 1999) showed that arachidonic acid 6-derived prostaglandins stimulate rapid recovery of gut barrier function after injury. Moreover, Hess et al. (2008) observed a dose-dependent relationship between dietary arachidonic acid concentration and arachidonic acid content of mucosal phospholipids, while only a negligible effect on gross villus/crypt morphology was observed. Finally, the prostanoids produced from arachidonate (via cyclooxygenase enzymes) have been implicated in accelerating recovery of gut barrier function following ischaemic injury, a mechanism thought to play a role in the pathogenesis of necrotizing enterocolitis (Blikslager et al. 2007).
An adequate content of essential fatty acids in the diet is necessary as their biologically active metabolites regulate gene expression and enzyme activity, dampen inflammation, modulate immune response, and affect gluconeogenesis by direct and indirect pathways, as agonists of a number of G-protein-coupled receptors (Das 2011). After ingestion, essential fatty acids undergo homogenous distribution to virtually every cell in the body, thus affecting membrane composition and cellular function. They serve in the plasma membranes as substrates for the enzymes cyclooxygenase and lipooxygenase which convert them into a number of important, very active, short-lived, hormone-like compounds referred to as ‘eicosanoids’. These latter elicit changes in gene expression that precede changes in membrane composition by directly governing the activity of nuclear transcription factors (Benatti et al. 2004). In this regard, it should be pointed out that both the linoleic and linolenic acids compete for the same 6-desaturase in the metabolic cascade and linolenic acid is a strong suppressor of ω-6 fatty acid metabolism. These effects may not only be mediated by direct competitive mechanisms, but very likely also via regulation of the activity or abundance of desaturation and elongation enzymes at the level of expression of the corresponding gene (Jump & Clarke 1999). Fat nutrients therefore may regulate cellular functions by affecting the expression or activity of genes in the signal transduction pathway related to the control of cell growth and apoptosis. The finding that oleic acid and ω-3 PUFA block the desaturase reaction, the first step from linoleic acid to eicosanoids, may also partially explain their inhibitory effects on cell proliferation (Benatti et al. 2004).
In conclusion, we suggest that the inhibitory effect on proliferative activity of the intestinal epithelium could be demonstrated on WD in both flaxseed groups. Three weeks PW, the villus height and crypt depth were decreased significantly in comparison with the piglets receiving ω-6 PUFA in the diet. The higher proliferative activity of the lamina propria cells in comparison with sunflower and lactobacilli groups with ω-3 PUFA, which was found both on WD and 3 days later in the intestinal mucosa of flaxseed-fed piglets, was probably caused by different sensitivity of the two cellular populations. This suggestion confirms published results (Viegas et al. 2012) about different effects of PUFA on the cells of various origin and maturation stage by regulation of cell proliferation and gene expression. The cellular response to the fatty acids could be effected by the lactobacilli presence, as the proliferative activity of connective tissue cells in FL group was lower significantly than that in F group from WD till 7 days PW.
Acknowledgments
This study was supported by the grant of the European Regional Development Fund – Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123), Grant project SK0021, co-financing through the European Economic area financial mechanism, the Norwegian financial mechanism and the state budget of the Slovak Republic.
Conflict of interest
Authors declare no conflict of interest.
References
- Aarestrup FM. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS Suppl. 2000;101:1–48. [PubMed] [Google Scholar]
- Adamkov M, Furjelová M, Horáček J, Benčat M. Kruzliak P. Relationship of mismatch repair proteins and survivin in colon polyps and carcinomas. Acta Histochem. 2014;116:1007–1014. doi: 10.1016/j.acthis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Benatti P, Peluso G, Nicolai R. Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- Blikslager AT, Roberts MC, Rhoads JM. Argenzio RA. Prostaglandins I-2 and E-2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J. Clin. Invest. 1997;100:1928–1933. doi: 10.1172/JCI119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikslager AT, Roberts MC. Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am. J. Physiol. 1999;276:G28–G36. doi: 10.1152/ajpgi.1999.276.1.G28. [DOI] [PubMed] [Google Scholar]
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL. Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- Bomba A, Nemcová R, Gancarcíková S, et al. The influence of omega-3 polyunsaturated fatty acids (omega-3 PUFA) on lactobacilli adhesion to the intestinal mucosa and on immunity in gnotobiotic piglets. Berl. Munch. Tierarztl. Wochenschr. 2003;116:312–316. [PubMed] [Google Scholar]
- Bomba A, Jonecová Z, Koščová J, et al. The improvement of probiotics efficacy by synergistically acting components of natural origin: a review. Biologia. 2006;61:729–734. [Google Scholar]
- Butaye P, Devriese LA. Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Influence of polyunsaturated fatty acids and their metabolites on stem cell biology. Review. Nutrition. 2011;27:21–25. doi: 10.1016/j.nut.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Geier MS, Torok VA, Allison GE, et al. Dietary omega-3 polyunsaturated fatty acid does not influence the intestinal microbial communities of broiler chickens. Poult. Sci. 2009;88:2399–2405. doi: 10.3382/ps.2009-00126. [DOI] [PubMed] [Google Scholar]
- Hess HA, Corl BA, Lin X, et al. Enrichment of intestinal mucosal phospholipids with arachidonic and eicosapentaenoic acids fed to suckling piglets is dose and time dependent. J. Nutr. 2008;138:2164–2171. doi: 10.3945/jn.108.094136. [DOI] [PubMed] [Google Scholar]
- Holt PR, Pascal RR. Kotler DP. Effect of ageing upon small intestinal structure in the Fischer rat. J. Gerontol. 1984;39:642–647. doi: 10.1093/geronj/39.6.642. [DOI] [PubMed] [Google Scholar]
- Jump DB. Clarke SD. Regulation of gene expression by dietary fat. Annu. Rev. Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- Kastel R, Bomba A, Vasko L, Trebunova A. Mach P. The effect of probiotics potentiated with polyunsaturated fatty acids on the digestive tract of germ-free piglets. Vet. Med. 2007;52:63–68. [Google Scholar]
- Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G413–G421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- Nemcova R, Borovska D, Koscova J, et al. The effect of supplementation of flax-seed oil on interaction of Lactobacillus plantarum – Biocenol™ LP96 and Escherichia coli O8:K88ab:H9 in the gut of germ-free piglets. Res. Vet. Sci. 2012;93:39–41. doi: 10.1016/j.rvsc.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Pluske JR. Morphological and functional changes in the small intestine of the newly-weaned pig. In: Piva A, Bach Knudsen KE, Lindberg E J, editors. Gut Environment of Pigs. Nottingham: Nottingham University Press; 2001. pp. 1–27. [Google Scholar]
- Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 1997;4:215–236. [Google Scholar]
- Popper M, Gancarcikova S, Scirankova L, et al. 2014. Synergic effect of Lactobacillus plantarum L81, Lactobacillus fermentum and flax-seed in prevention of postweaning diarrhea in weaned pigs; pp. 83–86. Proceedings of scientific contributions and abstracts at 5th International Scientific Conference, Infectious and Parasitic Diseases of Animals, Košice. [Google Scholar]
- Quaedackers JS, Beuk RJ, Bennet L, et al. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant. Proc. 2000;32:1307–1310. doi: 10.1016/s0041-1345(00)01238-0. [DOI] [PubMed] [Google Scholar]
- Smith FI, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G352–G363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth S, Jonecova Z, Kruzliak P, Ciccocioppo R. Nemcova R. Influence of dietary supplementation with flaxseed and lactobacilli on the cells of local innate immunity response in the jejunal mucosa in piglets after weaning. Acta Histochem. 2015;117:185–195. doi: 10.1016/j.acthis.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Yoshida Z, Tsushima T, et al. Evaluation of the heat-killed and dried cell preparation of Enterococcus faecalis against villous atrophy in early-weaned mice and pigs. Anim. Sci. J. 2011;82:302–306. doi: 10.1111/j.1740-0929.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Kishino E, Inoue R, et al. Correlation between villous height and the disaccharidase activity in the small intestine in piglets from nursing to growing. Anim. Sci. J. 2013;84:54–59. doi: 10.1111/j.1740-0929.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- Viegas MN, Dias J, Cancela ML. Laize V. Polyunsaturated fatty acids regulate cell proliferation, extracellular matrix mineralization and gene expression in a gilthead seabream skeletal cell line. J. Appl. Ichthyol. 2012;28:427–432. [Google Scholar]
- Yoshida Y, Tsukahara T. Ushida K. Oral administration of Lactobacillus plantarum Lq80 and Megasphaera elsdenii iNP-001 induces efficient recovery from mucosal atrophy in the small and the large intestines of weaning piglets. Anim. Sci. J. 2009;80:709–715. doi: 10.1111/j.1740-0929.2009.00692.x. [DOI] [PubMed] [Google Scholar]


