Abstract
Prostate physiology is highly dependent on oestrogenic and androgenic homeostasis. Interferences in this equilibrium, especially in early periods of life, may disrupt the prostate and increase the susceptibility to the development of diseases with ageing. Taking this into account, and considering the increase of environmental chemicals with endocrine-disrupting potential such as bisphenol-A (BPA), this study aimed to evaluate the prostates of adult female gerbils exposed to BPA and BPA plus testosterone from pubertal to adult periods. Morphological, stereological and chemical analyses revealed that long-term BPA exposure, even in environmental dosages, increases the proliferative status of the prostate, increases the number of ERα-positive stromal cells and elicits the development of prostatic hyperplasia in adult female gerbils. Moreover, we also observed that the association with testosterone did not increase the proliferative status of the gland, which shows that low levels of BPA are enough to cause an oestrogenic disruption of the prostate in young adults. This evidence suggests that this oestrogenic endocrine disruptor may increase the susceptibility to prostatic disorders with ageing.
Keywords: bisphenol-A, endocrine-disrupting chemicals, female prostate, gerbil, oestrogen receptor-alpha
Bisphenol-A is an endocrine disruptor largely used as a monomer for the production of polycarbonate, which is employed in plastic bottles for babies, as a component of epoxy resin in coating food cans and in dental sealants (Prins 2008; Vogel 2009; Prins et al. 2011). Its release from plastic occurs from increased temperatures, changes in pH or due to repeated washings. Thus, BPA can be found in human plasma in high concentrations, in addition to being found both in the placenta and in foetal tissues (Schonfelder et al. 2002; Prins et al. 2008).
The extensive human exposure to BPA and its clinical potential has considerably attracted the attention of scientists, governments and the population in general. Several studies have reported the adverse effects associated with exposure, even at low concentrations of BPA. An exposure of 50 μg/kg/day is the dose of daily intake acceptable and commonly considered ‘safe’ by the U.S. Food and Drug Administration (FDA) and the U.S. Environmental Protection Agency (Hunt et al. 2009). However, studies show that this chemical is a potential disruptor even at lower concentrations, because it is a toxic substance that elicits indirect effects on the tissues of the reproductive tract (Markey et al. 2005; Ho et al. 2006).
The female gerbil prostate is similar to the ventral male prostate and presents a mature and differentiated glandular epithelium composed mainly of basal and secretory cells with high synthetic activity (Santos et al. 2003; Custódio et al. 2004). Prostate physiology is regulated by androgens, which are essential for cellular differentiation and prostate development, as well as for the growth and maintenance of the secretory activity of the gland. Furthermore, prostate metabolism is influenced by other steroids, especially oestrogens, which act by modulating the effects of androgens (Marker et al. 2003).
Studies with rodents showed that synthetic oestrogens permanently disrupt prostatic growth and differentiation of the gland, resulting in injuries with ageing (Prins 1992; Perez et al. 2011). In this sense, recently published data suggest an important role for oestrogen in prostate pathogenesis through multiple mechanisms, including genotoxicity, epigenotoxicity, chronic inflammation and events mediated by oestrogen receptors (Nelles et al. 2011).
In this context, biochemical studies have examined the kinetics of BPA with the oestrogen receptors (ERs) and determined that it binds to both the ERα and ERβ (Gould et al. 1998). Recent evidence regarding these receptors in studies employing adult mice showed that BPA exposure affects the expression of the aromatase enzyme in the prostate, thereby increasing the levels of estradiol (Castro et al. 2013). These interferences resulting from exposure to oestrogenic disruptors increase the susceptibility to neoplastic lesions, predisposing the gland to develop diseases in adulthood (Prins et al. 2008).
Thus, our hypothesis is that BPA has hazardous effects on the prostate of female gerbil, increasing the susceptibility to the development of lesions throughout one’s lifespan. Therefore, the aim of this study was to evaluate the effects of long-term BPA exposure, from pubertal to adult life, on the prostate of female gerbils.
Methods
The animals were provided by the Federal University of Goiás (UFG) (Goiânia-GO). They were maintained in polyethylene cages under controlled conditions of light and temperature and were provided with filtered water and rodent food ad libitum. Animal handling and experiments were performed according to the ethical guidelines of the Federal University of Goiás (UFG) (ethical committee number 052/11 CEP) and in keeping with the Guide for Care and Use of Laboratory Animals (The National Academies Press, 2011, Washington, D.C., USA). During all experiments, we provided filtered water in glass bottles to avoid exposing the animals to additional endocrine-disrupting chemicals such as BPA from plastic bottles.
In this experiment, we used 25 one month-old females. These animals were separated from their parents after weaning (at 30 days) and divided into five groups: control (C) – five females were maintained under standard conditions until they reached 4 months of age; low bisphenol-A (LBPA) – five females received water with BPA at a concentration of 40 μg/kg/day until 4 months of age; low bisphenol-A plus testosterone (LBPA + T) – five females received water with BPA at a concentration of 40 μg/kg/day up to 4 months of age. At 3 months and 7 days, these animals were treated with subcutaneous injections of testosterone (1 mg/kg) diluted in 100 ml of mineral oil (nujol – Mantecorp) once a week for 21 days; high bisphenol-A (HBPA) – five females received water with BPA at a concentration of 4 mg/kg/day until 4 months of age; and high bisphenol-A plus testosterone (HBPA + T) – five females received water with BPA at a concentration of 4 mg/kg/day up to 4 months of age. At 3 months and 7 days, these animals were treated with subcutaneous injections of testosterone (1 mg/kg) diluted in 100 ml of mineral oil (Nujol - Mantecorp) once a week for 21 days until 4 months of age, when they were killed. The average water consumption and weight of the animals per cage were measured daily for the purpose of calculating the dilution of BPA in water.
All animals were killed by CO2 inhalation followed by decapitation. The body and prostatic complex (PrC – urethra, vagina and prostate structures) were weighed. These fragments were dissected out using a Leica stereoscopic microscope (Leica, Germany) to remove adipose tissues and isolate the urethral segment plus the associated prostatic tissue.
Light microscopy
PrC from female gerbils were fixed by immersion in 4% paraformaldehyde (buffered in 0.1 M phosphate, pH 7.2) or in methacarn (proportions: methanol 60%, chloroform 30% and acetic acid 10%) for 3 h. After fixation, the tissues were washed in water, dehydrated in ethanol, clarified in xylene and embedded in paraffin (Histosec, Merck, Darmstadt, Germany). All tissue fragments employed in this study were serially sectioned into 5-μm slices with an automatic rotator microtome (Leica RM2155, Nussloch, Germany). The sections were stained with haematoxylin–eosin (HE) for general morphological analysis. The specimens were analysed with an Olympus BX60 light microscope (Olympus, Tokyo, Japan), and the images were digitalized using DP-BSW software v3.1 (Olympus) and a virtual slide system BX 61VS (Olympus).
Ethical approval
The research was approved by the Federal University of Goiás Ethical Committee for Animal Research under the Protocol Code Nr. 052/11.
Stereology
The stereological analyses were carried out using Weibel’s multipurpose graticulate with 130 points and 10 test lines (Weibel 1978) to compare the relative proportion (relative frequency) of each component of prostatic tissue (epithelium, lumen, stroma), as described by Huttunen et al. (1981). We chose thirty microscopic fields at random from each experimental group (six fields per animal; n = 5). Briefly, we determined the relative values by counting the coincident points in the test grid and dividing them by the total number of points. Stereological analysis was performed using Image-Pro Plus software v6.1 for Windows (Media Cybernetics Inc., Silver Spring, MD, USA).
Immunohistochemistry
Tissue sections were subjected to immunohistochemistry for the detection of the androgen receptor (AR), as described in protocols applied to the prostate (Cordeiro et al. 2008), oestrogen receptor-alpha (ERα) and PCNA. Primary antibodies reactive to AR (rabbit polyclonal IgG, N-20, sc-816, Santa Cruz Biotechnology, Santa Cruz, CA, USA), ERα (rabbit polyclonal IgG, MC-20, sc-542, Santa Cruz Biotechnology) and PCNA (mouse monoclonal IgG2a, SC 56, Santa Cruz Biotechnology) were employed at a dilution of 1:100. Polymers (Post Primary Block and Polymer, Novocastra™, RE7260-K, Newcastle Upon Tyne, UK; DAKO Envisiontm + Dual link system-HRP, K4061; DAKO, North America, Inc., Carpinteria, CA, USA) were used as secondary antibodies according to the procedures described by the manufacturers. The sections were stained with diaminobenzidine and counterstained with Harris’s haematoxylin. The histological sections were analysed using an Olympus BX60 light microscope (Olympus).
ERα and PCNA quantification
For ERα quantification, thirty microscopic fields (magnification of 400x) were used for each experimental group. In each field, the total number of positive stromal cells was obtained as a relative frequency (%) in relation to the total number of negative stromal cells. Between positive and negative cells, we counted a mean of 5300 stromal cells for each experimental group.
Regarding PCNA quantification, we employed thirty microscopic fields (magnification of 400x) for each experimental group. In each field, the total number of positive epithelial cells was obtained as a relative frequency (%) in relation to the total number of epithelial cells of the acini. The same procedure was followed for the positive stromal cells. Between positive and negative cells, we counted a mean of 4000 epithelial cells and 2000 stromal cells for each experimental group. All these analyses were performed using the image analysis system previously described.
Statistical analyses
The hypothesis tests employed to determine statistical significance were the Kruskal–Wallis test for nonparametric distributions and ANOVA for parametric distributions. Further determination of the statistically significant differences between experimental groups was performed using Dunn’s test for nonparametric distributions and Tukey’s test for parametric distributions. The data were analysed using Statistica 6.0 (StarSoft, Inc., Tulsa, OK, USA) and BioEstat 5.0 (free statistical program) software. The level of significance was set at 5% (P ≤ 0.05). Values are presented as mean ± standard error of mean (SEM).
Results
Biometry
Biometric analysis of adult female gerbils indicated that there were no significant differences in body weight, prostatic complex and the relative weight of all groups measured (Table1).
Table 1.
Biometric and stereological parameters (mean ± standard error) in control and BPA-treated Mongolian gerbils
| Groups | |||||
|---|---|---|---|---|---|
| Control | LBPA | LBPA + T | HBPA | HBPA + T | |
| †Biometry | |||||
| Bodyweight (g) | 56.8 ± 1.4 | 58.7 ± 8.4 | 59.7 ± 3.0 | 51.3 ± 2.0 | 51.1 ± 2.2 |
| Female prostate complex (g) | 0.15 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.03 | 0.1 ± 0.01 | 0.13 ± 0.01 |
| Female prostate relative weight (×10−3) | 2.0 ± 0.1 | 2.0 ± 0.4 | 2.0 ± 0.5 | 1.9 ± 0.1 | 2.6 ± 0.1 |
| ‡Stereology (%) | |||||
| Epithelium* | 11.7 ± 0.9a | 26.6 ± 1.8b | 25.6 ± 1.5b | 27 ± 1.8b | 26.3 ± 1.3b |
| Lumen* | 58.0 ± 1.8a | 40.2 ± 2.7b | 36.6 ± 1.6b | 38.4 ± 2.0b | 37.4 ± 2.0b |
| Stroma* | 30.3 ± 1.8a | 33.2 ± 2.3a | 37.8 ± 2.5b | 34.6 ± 2.9a | 36.3 ± 2.5b |
Body, female prostate and relative weight in C and BPA-treated females (n = 5/group). Relative weight corresponds to the ratio between the weight of the female prostate complex and the whole body. Values are means ± standard error of the means.
Stereological data obtained for the female prostate during BPA treatments (mean ± standard error of mean; n = 30 fields in 5 animals/group).
Statistically significant differences between control and treatments (P ≤ 0.05). Superscript letters (a,b) represent statistically significant differences between the experimental groups.
Morphology and stereology
Analysis of the prostate of adult female gerbils demonstrated that BPA exposure caused morphological changes in epithelial and stromal compartments of the gland (Figure1). The groups exposed to low environmental levels of BPA (LBPA: Figure1d, e, f and LBPA + T: Figure1g, h, i) and to high dosages (HBPA: Figure1j, k, l and HBPA + T: Figure1m, n, o) showed the same pattern of morphological changes, mainly characterized by epithelial increase (Figure1e, h, k, n), epithelial and stromal hyperplasia (Figure1f, i, l, o) and inflammatory foci (Figure1e, l, n). However, these changes were more evident and constant in groups exposed to BPA and associated with the administration of androgens (LBPA + T and T + HBPA).
Figure 1.
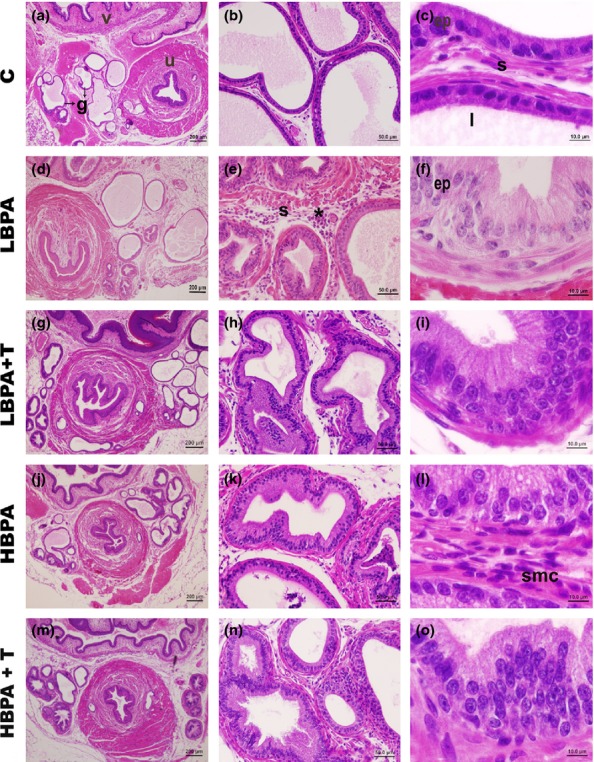
Histological sections of the female gerbil prostates stained with haematoxylin–eosin demonstrating general morphology of the gland. (a–c) Normal aspects of the C group, characterized by a wide lumen (l), with simple cubic or cylindrical epithelium (ep) and stromal compartment (s) with cells, fibres and smooth muscle. (d–o) In all groups exposed to BPA, the female prostate presented epithelial and stromal features of intense development, accompanied by proportional luminal narrowing. Several hyperplastic and inflammatory foci were observed throughout the gland. There is intense epithelial stratification in the glands of the females exposed to BPA (e, f, h, i, k, l, n, o). Urethra (u), vagina (v), alveoli (g), epithelium (ep), lumen (l), inflammatory foci (*), control (C); low bisphenol-A (LBPA); bisphenol-A plus testosterone (LBPA + T); high bisphenol-A (HBPA); high bisphenol-A plus testosterone (HBPA + T). (Scale bar: 200 μm – Figure1a, d, g, j, m; scale bar: 50 μm – Figure1b, e, h, k, n; scale bar: 10 μm – Figure1c, f, i, l, o).
The prostatic epithelium, ranging from simple cuboidal to cylindrical in control animals (Figure1a–c), became very proliferative and developed in all experimental groups (Figure1d–o). The majority of prostatic hyperplastic alveoli assumed an appearance characterized by several areas of stratification (Figure1h, i, k, o). This epithelial growth was confirmed by stereological analysis, which showed that all groups exposed to BPA had an increase from 2 to 2.3 times in the relative frequency of the epithelial compartment (P ≤ 0.05, Table1).
The BPA exposure also caused changes related to the increase in the stromal smooth muscle cells surrounding the alveoli and inflammatory cells in the interalveolar stroma (Figure1 e, h, l, n). These changes were also confirmed by stereological analysis showing a significant increase in the relative frequency of the stromal compartment of all groups treated with BPA (P ≤ 0.05, Table1).
Immunohistochemical analyses
ER Alpha
Immunostaining for ERα was detected in prostate stromal cells of the female gerbils (Figure2). The immunolabelled cells were more numerous in all the groups subjected to BPA treatment (Figure2d–n). By counting cells (Figure3), we detected that the treatment with BPA stimulated a significant increase in the frequency of ERα-positive cells in the stromal compartment of all analysed females (P ≤ 0.05).
Figure 2.
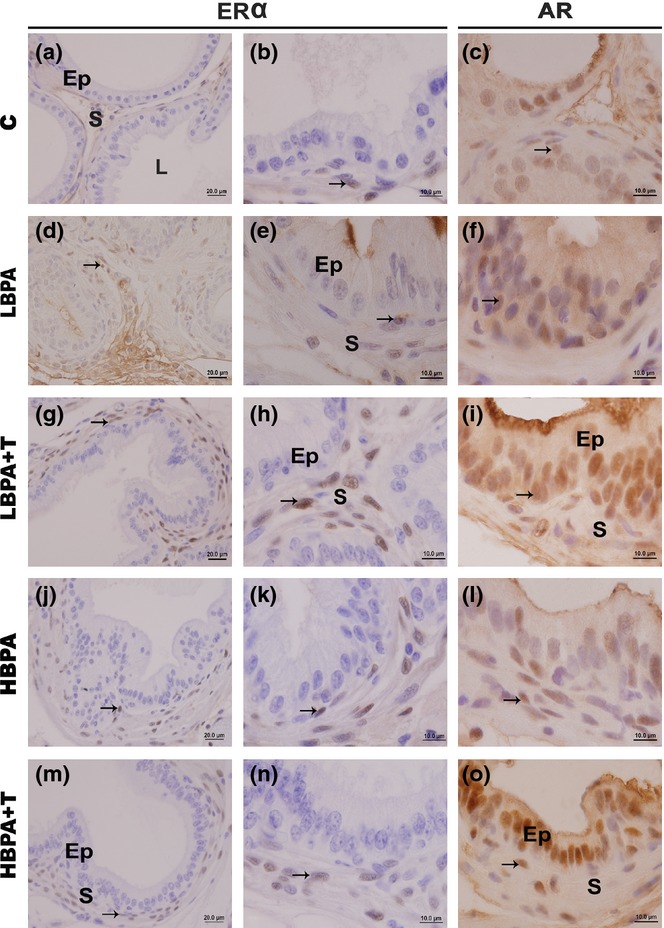
Immunohistochemistry for oestrogen receptor-alpha (ERα) and androgen receptor (AR) in female gerbil prostates. (a–n) The immunohistochemical analysis for ERα showed a pattern of nuclear staining in the stromal cells (S) of all examined groups (arrows). It is noted, however, that in all treated groups, the immunolabelling was more frequent and evident. (c–o) The immunolabelling for AR was observed in the nucleus of the secretory epithelial cells (Ep) and in the nucleus of fibroblasts and smooth muscle cells in the stroma (S) of all experimental groups (arrows). (Scale bar: 20 μm – Figure 2a, d, g, j, m; scale bar: 10 μm – Figure 2b, e, h, k, n; scale bar: 10 μm – Figure 2c, f, i, l, o).
Figure 3.
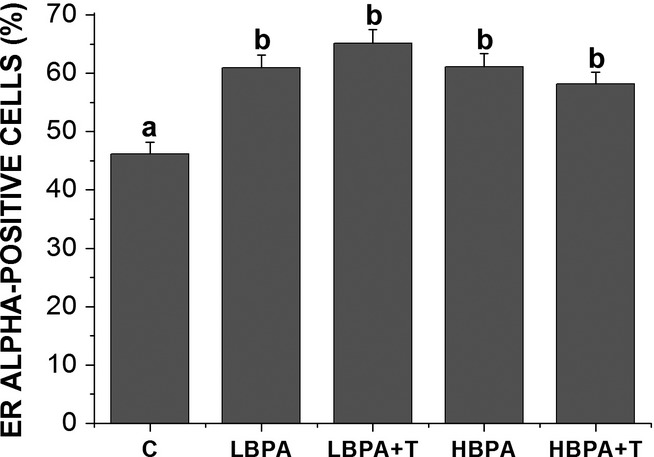
The frequency of ERα-positive cells in the prostate of adult females subjected to different forms of BPA exposure. Values are means ± standard error of the means. Superscript letters (a,b) represent statistically significant differences between the experimental groups (P ≤ 0.05); n = 30 fields in 5 animals/group.
Ar
AR-positive cells were observed in the epithelial and stromal compartments of the female gerbil prostates of all experimental groups (Figure2c, f, i, l, o). The immunolabelling for AR was similar in all groups, although it was more prominent in secretory epithelial cells. In the stroma, the imunolabelling of this receptor occurred in fibroblasts and smooth muscle cells.
Pcna
PCNA-positive cells were observed in all experimental groups (Figure4). However, these markings were more common in the prostate glands of BPA-treated groups, especially in areas of hyperplasia and epithelial stratification (Figure4c and g). Employing PCNA quantification, the analysis showed a statistically significant increase of PCNA-positive cells in both epithelial and stromal compartments of all treated groups in comparison with the control group (Figure5).
Figure 4.
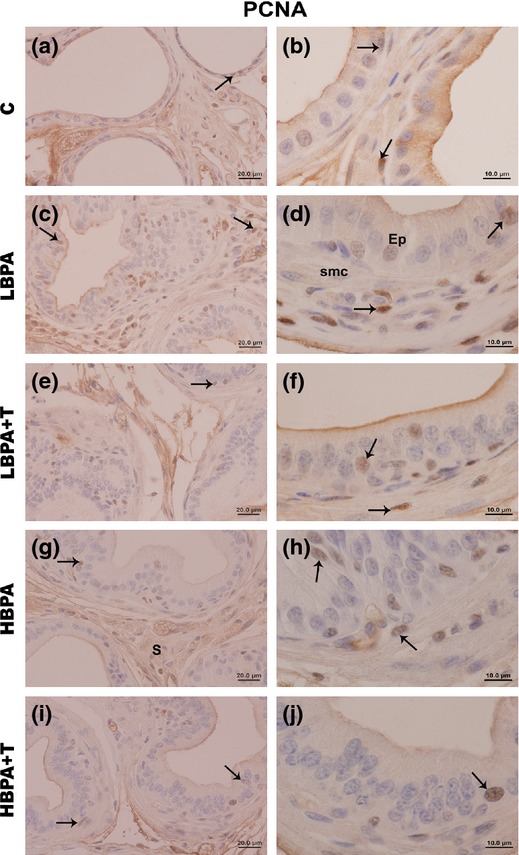
Immunohistochemistry for proliferation (PCNA) in the female prostate gland of all experimental groups. Immunolabelling for PCNA (arrows) is present in epithelial and stromal cells, particularly in the regions of stratification in the prostate of the LBPA, LBPA + T, HBPA and HBPA + T groups. (Scale bar: 20 μm – Figure 4a, c, e, g, i; scale bar: 10 μm – Figure 4b, d, f, h, j).
Figure 5.
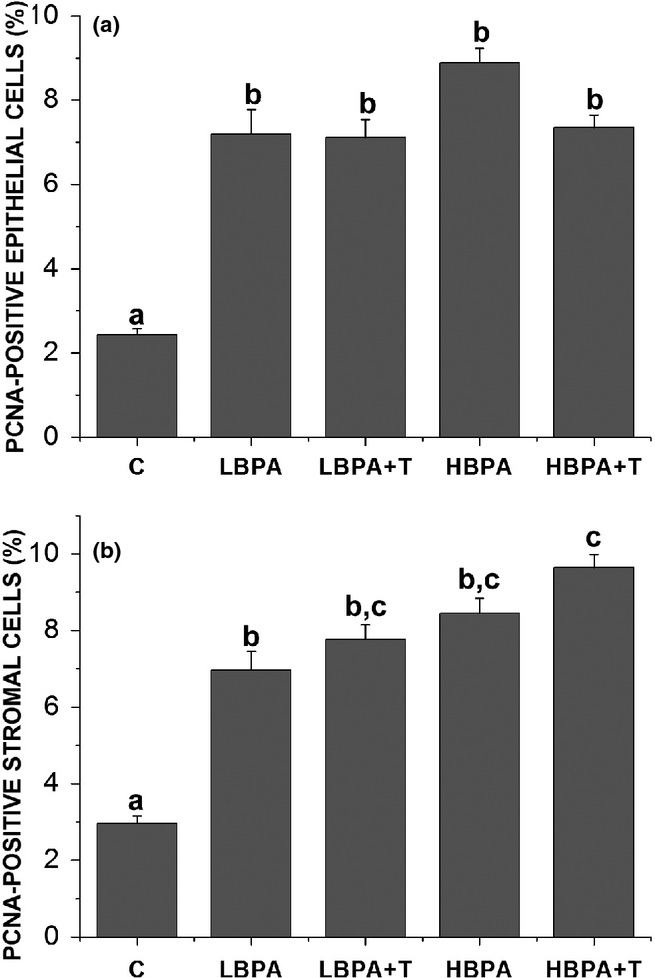
The frequency of PCNA-positive cells in the prostate of adult females subjected to different forms of BPA exposure. Observe the increased number of PCNA-positive cells either in epithelial (a) or in stromal (b) compartments. Values are means ± standard error of the means. Superscript letters (a,b,c) represent statistically significant differences between the experimental groups (P ≤ 0.05); n = 30 fields in 5 animals/group.
Discussion
This study demonstrated that long-term BPA exposure, even in environmental dosages, increases the proliferative index of the prostate, promotes ERα upregulation and favours the development of prostatic hyperplastic foci in adult female gerbils. These results, in a nutshell, demonstrate that the low levels of BPA are enough to cause an oestrogenic interference on the female prostate. Moreover, considering that the evidence of this study was obtained from adult gerbils, these data show that, even in early adult age, BPA has a potential of increasing the susceptibility to prostatic disorders such as hyperplasia.
With respect to BPA levels employed in this study, according to Reagan-Shaw et al. (2007), the human equivalent dosage is around six times less than in rats and 12 times less than in mice. Therefore, the low dosage of 40 μg/kg/day administered in the gerbil is expected to be translated into a much lower human equivalent dose, implying that the BPA effects would be very potent in humans. Recent studies have shown the influence of oestrogenic chemicals such as BPA on the reproductive organs (Timms et al. 2005; Prins et al. 2008). These studies demonstrated that exposure to oestrogenic compounds such as BPA is associated with an increase in the prostatic volume, urethral malformations and cell proliferation (Timms et al. 2005), in addition to being associated with a high susceptibility to carcinogenesis (Prins et al. 2008).
Stereological analysis has shown a statistically significant increase in the epithelial compartment in all treated groups. Although not statistically significant for all groups, we observed an increase in the stromal compartment, which was more evident in the HBPA group. These stereological data confirm a general increase in both epithelial and stromal compartments observed through morphological analysis. Moreover, the most notable finding of our study was the significant increase in ERα-positive stromal cells in the prostate gland of all treated groups. Studies have shown that BPA is an oestrogen receptor agonist, and the binding of BPA to ERα promotes cell proliferation in the prostate (Luccio-Camelo & Prins 2011; Taylor et al. 2011). Based on these evidences, our results suggest that BPA, even in low dosages, acted as an agonist of ERα, promoting a proliferative status of the gerbil female prostate.
Moreover, according to the quantification of the ERα-positive stromal cells, we did not observe any statistically significant difference between the treated groups, even when comparing the BPA plus testosterone-treated groups. Castro et al. (2013) demonstrated that adult exposure at low dose of BPA (25 μg/kg/day) increases the plasma estradiol /testosterone ratio and aromatase mRNA levels in the prostate of adult rats. In this way, considering that part of exogenous testosterone can be aromatized into estradiol by the increased aromatase levels, new approaches are necessary to evaluate the behaviour of this enzyme in these experimental conditions.
Recent studies have shown that oestrogen receptor subtypes (ERα and ERβ) have opposing roles in the prostate (Morani et al. 2008; Ellem & Risbridger 2009). According to the literature, ERα is directly associated with aberrant proliferation and inflammation and acts as an inducer of premalignant lesions (Ellem & Risbridger 2009). On the other hand, ERβ is critical in regulating antiproliferative activity in the prostate (Ellem & Risbridger 2009), besides being involved in differentiation, extracellular matrix organization and stromal–epithelial communication (Morani et al. 2008).
In addition to these aspects of oestrogen receptors, it has long been known that prostatic stromal cells are determinants for prostate epithelial physiology from early developmental periods to late phases during senescence (Prins & Putz 2008; Thomson 2008). The main mechanisms of this physiology are mediated by mesenchymal–epithelial interactions, which drive the development of the gland and are directly responsible for the fate of the organ’s health (Cunha 2008; Prins & Putz 2008; Thomson 2008). Between these mesenchymal–epithelial interactions, the steroid receptors such as ERs and ARs play a fundamental role in normal prostate morphogenesis (McPherson et al. 2008; Thomson 2008).
A literature review by Cunha et al. (2003) reported the importance of stromal–epithelial interactions in the development of prostate lesions. According to the report, the presence of stromal cells expressing ERα is a determinant for eliciting prostate carcinogenesis in mice treated with testosterone plus estradiol (T + E2), showing the critical influence of the stromal microenvironment in benign versus malignant growth. Although we did not observe the presence of malignant lesions in any treated group, the findings may be an indicator of a precursor status which may lead to a malignant condition with ageing.
Thus, considering all this evidence, and based on our findings regarding ERα-positive stromal cells, we believe that BPA directly influences ERα in the stromal cells. Indeed, the increase of the proliferative index and the presence of several proliferative foci in the prostate of treated groups suggest that BPA exposure may disrupt normal prostate physiology and increase the susceptibility to the development of lesions.
Regarding the proliferation analysis, we observed a statistically significant increase in the proliferative index in all treated groups, as confirmed by quantification of PCNA-positive epithelial cells. These data are in accordance with the findings regarding morphological, stereological and ERα quantification. As ERα influences the proliferation of the epithelial compartment, the increase in PCNA-positive epithelial cells corroborates the evidence of increased proliferation in the prostates of all treated groups.
The present study is the first to show the effects of BPA exposure on the prostate of female rodents. Because the concern about the female prostate either in humans or in rodents has been increasing lately (Zaviačič 1999; Santos & Taboga 2006; Perez et al. 2011; Reis et al. 2011; Biancardi et al. 2012), the present study opens new frontiers in research of the effects of oestrogenic chemicals on female prostate glands. These results have clinical relevance, as several studies have demonstrated the occurrence of Skene’s paraurethral glands lesions in young and senile women (Sloboda et al. 1998; Kazakov et al. 2010; Kelly et al. 2011). However, although the present data provide evidence of the effects of BPA on the prostate of adult female gerbils, new studies are necessary to evaluate old animals subjected to the same treatments, as old age is critical in terms of prostate pathophysiology.
Moreover, these findings show the importance of early periods of prostate development for the fate of the gland throughout life. There is more documentation in the scientific literature of the importance of early periods of prostate development and its association with the pathogenesis of prostatic diseases throughout life (Lee & Peehl 2004; Cunha & Ricke 2011). Considering that human beings are exposed to several EDCs, new studies of these substances are extremely important to improve our knowledge underlying the mechanisms of action of these compounds.
Acknowledgments
We are very grateful to Luiz Roberto Falleiros Júnior as well as other researchers of the Laboratory of Microscopy and Microanalysis for their technical assistance. This paper was supported by a grant from the Brazilian agency CNPq (Brazilian National Research and Development Council, Procs. Nr. 301596/2011-5; 475148/2012-6) and FAPEG (Goiás Research Foundation, Procs Nr. 05/2012).
Conflict of interest
No conflict of interests declared.
References
- Biancardi MF, Perez APS, Góes RM, Santos FCA, Vilamaior PSL. Taboga SR. Prenatal testosterone exposure as a model for the study of endocrine-disrupting chemicals on the gerbil prostate. Exp. Biol. Med. 2012;237:1298–1309. doi: 10.1258/ebm.2012.012051. [DOI] [PubMed] [Google Scholar]
- Castro B, Sanchez P, Torres JM, Preda O, del Moral RG. Ortega E. Bisphenol A exposure during adulthood alters expression of aromatase and 5α- reductase isozymes in rat prostate. PLoS ONE. 2013;8:e55905. doi: 10.1371/journal.pone.0055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro RS, Scarano WR, Campos SG, et al. Androgen receptor in the Mongolian gerbil ventral prostate: evaluation during different phases of postnatal development and following androgen blockage. Micron. 2008;39:1312–1324. doi: 10.1016/j.micron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Ricke WA. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation. 2011;82:168–172. doi: 10.1016/j.diff.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ. Ricke W. Role of the stromal microenvironment in carcinogenesis of the prostate. Int. J. Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Custódio AMG, Góes RM. Taboga SR. Acid phos-phatase activity in gerbil prostate: comparative study in male and female during postnatal development. Cell Biol. Int. 2004;28:335–344. doi: 10.1016/j.cellbi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ellem SJ. Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann. N. Y. Acad. Sci. 2009;1155:174–186. doi: 10.1111/j.1749-6632.2009.04360.x. [DOI] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, et al. Bisphenol A interacts with the estrogen receptor in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998;142:203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J. Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C. Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen E, Romppanen T. Helminen HJ. A histoquantitative study on the effects of castration on the rat ventral prostate lobe. J. Anat. 1981;3:357–370. [PMC free article] [PubMed] [Google Scholar]
- Kazakov DV, Stewart CJ, Kacerovska D, et al. Prostatic-type tissue in the lower female genital tract: a morphologic spectrum, including vaginal tubulosquamous polyp, adenomyomatous hyperplasia of paraurethral Skene glands (female prostate), and ectopic lesion in the vulva. Am. J. Surg. Pathol. 2010;34:950–955. doi: 10.1097/PAS.0b013e3181e0f371. [DOI] [PubMed] [Google Scholar]
- Kelly P, McBride HA, Kennedy K, Connolly LE. McCluggage WG. Misplaced Skene’s glands: glandular elements in the lower female genital tract that are variably immunoreactive with prostate markers and that encompass vaginal tubulosquamous polyp and cervical ectopic prostatic tissue. Int. J. Gynecol. Pathol. 2011;30:605–612. doi: 10.1097/PGP.0b013e31821713b6. [DOI] [PubMed] [Google Scholar]
- Lee KL. Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J. Urol. 2004;172:1784–1791. doi: 10.1097/01.ju.0000133655.71782.14. [DOI] [PubMed] [Google Scholar]
- Luccio-Camelo DC. Prins GS. Disruption of androgen receptor signaling in males by environmental chemicals. J. Steroid Biochem. Mol. Biol. 2011;127:74–82. doi: 10.1016/j.jsbmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R. Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C. Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol. Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Ellem SJ. Risbridger GP. Estrogen-regulated development and differentiation of the prostate. Differentiation. 2008;76:660–670. doi: 10.1111/j.1432-0436.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- Morani A, Warner M. Gustafsson JÅ. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J. Intern. Med. 2008;264:128–142. doi: 10.1111/j.1365-2796.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- Nelles JL, Hu WY. Prins GS. Estrogen action and prostate cancer. Expert. Rev. Endocrinol. Metab. 2011;6:437–451. doi: 10.1586/eem.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AP, Biancardi MF, Goes RM, dos Santos FA. Taboga SR. Exposure to ethinylestradiol during prenatal development and postnatal supplementation with testosterone causes morphophysiological alterations in the prostate of male and female adult gerbils. Int. J. Exp. Pathol. 2011;92:121–130. doi: 10.1111/j.1365-2613.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer. 2008;15:649–656. doi: 10.1677/ERC-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS. Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Tang WL, Belmonte J. Ho SM. Perinatal Exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2008;102:134–138. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Ye SH, Birch L, Ho SM. Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod. Toxicol. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M. Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reis LO, Billis A, Ferreira FT, Ikari LY, Stellini RF. Ferreira U. Female urethral carcinoma: evidences to origin from Skene’s glands. Urol. Oncol. 2011;29:218–223. doi: 10.1016/j.urolonc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Santos FCA. Taboga SR. Female prostate: a review about the biological repercussions of this gland in humans and rodents. Anim. Reprod. 2006;3:3–18. [Google Scholar]
- Santos FCA, Carvalho HF, Góes RM. Taboga SR. Structure, histochemistry and ultrastructure of the epithelium and stroma in the gerbil (Meriones unguiculatus) female prostate. Tissue Cell. 2003;35:447–457. doi: 10.1016/s0040-8166(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M. Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda J, Zaviacic M, Jakubovský J, Hammar E. Johnsen J. Metastasizing adenocarcinoma of the female prostate (Skene’s paraurethral glands). Histological and immunohistochemical prostate markers studies and first ultrastructural observation. Pathol. Res. Pract. 1998;194:129–136. doi: 10.1016/S0344-0338(98)80080-0. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Richter CA, Ruhlen RL. vom Saal FS. Estrogenic environmental chemicals and drugs: mechanisms for effects on the developing male urogenital system. J. Steroid Biochem. Mol. Biol. 2011;127:83–95. doi: 10.1016/j.jsbmb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AA. Mesenchymal mechanisms in prostate organogenesis. Differentiation. 2008;76:587–598. doi: 10.1111/j.1432-0436.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- Timms BG, Kembra LH, Barton L, Bradley S, Richter CA. vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl Acad. Sci. USA. 2005;61:200–208. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SA. The politics of plastics: the making and unmaking of bisphenol A “safety”. Am. J. Public Health. 2009;99:S559–S566. doi: 10.2105/AJPH.2008.159228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 1978;12:131–155. [PubMed] [Google Scholar]
- Zaviačič M. The Female Prostate: From vestigial Skene’s parauretral glands and ducts to woman’s functional prostate. 1st edn. Bratislava, Slovakia: Slovack Academic Press; 1999. pp. 22–68. [Google Scholar]


