Abstract
Aflatoxins and fumonisins are important food-borne mycotoxins implicated in human health and have cytotoxic effects. The aims of the current study were to evaluate the protective role of Panax ginseng extract (PGE) against the synergistic effect of subchronic administration of aflatoxin B1 (AFB1) and fumonisin B1 (FB1) on DNA and gene expression in rat. Female Sprague–Dawley rats were divided into eight groups (ten rats/group) and treated for 12 weeks including the control group, the group having received AFB1 (80 µg/kg bw), the group having received FB1 (100 µg/kg bw), the group having received AFB1 plus FB1 and the groups having received PGE (20 mg/kg bw) alone or with AFB1 and/or FB1. At the end of experiment, liver and kidney were collected for the determination of DNA fragmentation, lipid peroxidation (LP), glutathione (GSH) contents and alterations in gene expression. The results indicated that these mycotoxins increased DNA fragmentation, LP and decreased GSH content in liver and kidney and down-regulated gene expression of antioxidants enzymes. The combined treatments with AFB1 and/or FB1 plus PGE suppressed DNA fragmentation only in the liver, normalized LP and increased GSH in the liver and kidney as well as up-regulated the expression of GPx, SOD1 and CAT mRNA. It could be concluded that AFB1 and FB1 have synergistic genotoxic effects. PGE induced protective effects against their oxidative stress and genotoxicity through its antioxidant properties.
Keywords: Aflatoxins, Fumonisin, Panax ginseng, DNA fragmentation, Gene, Expressions, Mycotoxins
Introduction
Mycotoxins are produced by fungi and can contaminate various agricultural commodities either before harvest or under postharvest conditions (FAO 1991). They are of great worldwide concern due to their toxic effects on human and animal health (Ibáñez-Vea et al. 2012). One of the most important mycotoxins produced by the Aspergillusflavus and A. parasiticus are the aflatoxins (AFs), which can occur in a wide range of raw food commodities (CAST 2003). On the other hand, fumonisins (FBs) are the most reported Fusarium toxins produced mainly by F. verticillioides and F. proliferatum (Marasas 1996). The genotoxicity and carcinogenicity of AF have been described and reviewed earlier by EFSA (2007) and Abdel-Wahhab et al. (2010). Aflatoxin B1 (AFB1) is the most carcinogenic mycotoxin known and there is evidence from human studies that AFs are major risk factors for hepatocellular carcinoma, therefore classified in the group 1 by the International Agency for Research on Cancer (IARC 2002). On the other hand, FB1 caused DNA strand breaks in isolated rat liver nuclei (Sahu et al. 1998; Hassan et al. 2010) and the relation with esophageal cancer has been described in the population of the high incidence area of South Africa (Thiel et al. 1992) and China (Chu and Li 1994). Moreover, IARC evaluated FB1, as probably carcinogenic to humans (Group 2B) (IARC 2002). Several reports indicated that FB1 increased lipid peroxidation (Klaric et al. 2007; Stockmann-Juvala et al. 2004) and the production of reactive oxygen species (ROS) in animal models or exposed cells (Galvano et al. 2002a, b; El-Nekeety et al. 2007). Moreover, FB1 was found to modulate sphingolipids (Pinelli et al. 1999; Abdel-Wahhab et al. 2004) and increase concentrations of arachidonic acid metabolites, including prostaglandins, in bronchial epithelial cells after 24 h of incubation (Poux et al. 2000).
Generally, FB1 and AFB1 are the most important mycotoxins due to their prevalence as cereal contaminants and their toxicological potency. They can occur alone or simultaneously in cereals and cereal-based foods (Jestoi 2008), with humans and animals being constantly exposed to low levels of these mycotoxins, either individually or in combination (Theumer et al. 2010). The individual mycotoxicoses occur seasonally on certain areas that hinder an implementation of an effective prophylactic measure (Pfohl-Leszkowicz et al. 2002). However, interactions between given mycotoxins are still unclear. The presence of a mixture of these toxins may present a problem in terms of determining clinical symptoms of an individual mycotoxicosis (Abdel-Wahhab et al. 2012).
Panax ginseng, a traditional multipurpose herb in Asia, has become the World’s most popular herbal supplements in recent years. Ginseng has a variety of beneficial biological processes that include anti-cancer, anti-diabetic and anti-inflammatory effects, as well as cardiovascular and neuro-protection properties (Jung et al. 2005). Most of the pharmacological actions of ginseng are attributed to a variety of ginsenosides, which are phenolic acids, flavonoids and triterpenoid saponins (Huang et al. 2005). It also contains essential oil, peptidoglycans, polysaccharides, nitrogen-containing compounds, fatty acids and phenolic compounds (Lee et al. 2010; Abdel-Wahhab et al. 2010). These properties of ginseng are thought to provide many beneficial effects against organ damages. It was found that ginseng protects from toxic substances (Mannaa et al. 2006; Khalil et al. 2008) and human diseases (Yokozawa and Liu 2000) by several different mechanisms. The aims of the present study was to estimate the synergistic effect of subchronic administration of AF1 and FB1 on DNA and gene expression in rat liver and to evaluate the protective role of PGE against genotoxicity and oxidative stress induced by these mycotoxins.
Materials and methods
Chemicals and kits
Aflatoxin B1 (AFB1) and fumonisin B1 (FB1) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Kits of malondialdehyde (MDA) and catalase (CAT) were purchased from Oxis Research™ Co. (Beverly Hills, CA, USA). Super oxide dismutase (SOD) and glutathione (GSH) were obtained from Randox Laboratories Co (Crumlin, UK). TRIzol reagent was purchased from Molecular Research Center, Inc. (Cincinnati, OH, USA) and RNA Fermentas kit was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of the highest purity commercially available.
The safety measures recommended by WHO (1998) were taken when handling the AFB1 and FB1.
Plant materials
Panax ginseng roots were provided by the Korean Society of Ginseng. The plant was extracted five times with 10 volumes of distilled water at 85 °C for 8 h. The aqueous extracts were combined and concentrated under reduced pressure to give darkish brown syrup (Panaxginseng extract; PGE). The moisture content of PGE was 37.21 %. The ginsenoside content in the PGE was determined as follows: briefly, an aliquot of PGE dissolved in distilled water was passed through Sep-Pak C18 cartridge, and the cartridge was washed with distilled water. Subsequently, ginsenosides were eluted with 90 % methanol and then analyzed by a Hitachi high performance liquid chromatography (Hitachi Technologies, Atlanta, GA, USA). The mobile phase was a binary gradient of acetonitrile (A) and water, at a flow rate of 1.3 mL/min, as follows: 0–15 min, 21 % A; 16–38 min, 30 % A; 39–55 min, 42 % A; 56–65 min, 90 % A; and 66–80 min, back to 21 % A before the next injection. Data were collected with the Hitachi D-7000 HPLC System Manager software (Ko et al. 1989).
Experimental animals
Three-month old Sprague–Dawley female rats (100–120 g) were purchased from the Animal House Colony (Giza, Egypt) and were maintained on standard lab diet (protein 160.4; fat 36.3 and fiber 41 g/kg). Animals were housed in a room free from any source of chemical contamination, artificially illuminated and thermally controlled, at the Animal House Lab. (National Research Centre, Dokki, Cairo, Egypt). All animals have received human care in compliance with the guidelines of the Animal Care and Use Committee of the National Research Center (Dokki, Cairo, Egypt).
Experimental design
Eighty animals were divided into eight groups (ten rats/group), housed in filter-top polycarbonate cages and were maintained on their respective diet for 12 weeks as follows: group 1, normal control animals; group 2, rats fed on basal diet and treated orally with P. ginseng extract (PGE, 20 mg/kg bw); group 3, rats fed on basal diet and treated orally with AFB1 (80 µg/kg bw) in corn oil; group 4, rats treated orally with FB1 (100 µg/kg bw) in corn oil; group 5, rats treated with a mixture of AFB1 and FB1 made with the respective same dose; group 6, rats treated with AFB1 plus PGE; group 7, rats treated with FB1 plus PGE and group 8, rats treated with a mixture of AFB1 and FB1 plus PGE. The animals were observed daily for any signs of toxicity. At the end of the treatment period (i.e. day 84) all animals were fasted for 12 h, then were killed by cervical dislocation and samples of kidney and liver were collected for analytical and genetics procedures.
Markers of oxidative status in liver and kidney
Lipid peroxidation (LP) was ascertained by the formation of MDA using thiobarbituric acid as one of the main products of lipid peroxidation as described by Yoshioka et al. (1979). Glutathione (GSH) content was determined according to Ellman (1959) and the product was measured spectrophotometrically at 412 nm using the extinction coefficient of 13.7/mM × cm.
Determination of gene expression in liver
RNA extraction
Liver tissue cells were ground in liquid nitrogen and total RNA was extracted from all experimental animals. The extraction of total RNA was performed using TRIzol reagent according to the manufacturer’s procedures. The concentration and purity of RNA was measured at 260/280 nm using ultraviolet spectrophotometer (ratios fell between 1.75 and 1.9, indicating very pure RNA in all cases). Equal amounts of RNA isolated from individual rats of each group were prepared for the semi-quantitative RT-PCR (Marone et al. 2000).
Semi-quantitative reverse transcription and PCR reaction
The first-strand cDNA was prepared from the 5 μg of total RNA using Fermentas kits. The used RT program was: 60 min at 42 °C (cDNA synthesis); 5 min at 94 °C (denaturation). Afterwards the reaction tubes containing RT preparations were ash-cooled in an ice chamber until used for DNA amplification through polymerase chain reaction (PCR) (Brun et al. 2006). The first-strand cDNA from different rat samples was used as the template for amplification by the PCR with the following pairs of specific primers (from 5′ to 3′): Cu–Zn SOD forward: GCAGAAGGCAAGCGGTGAAC, Cu–Zn SOD reverse: TAGCAGGACAGCAGATGAGT, GPx forward: CTCTCCGCGGTGGCACAGT, GPx reverse: CCACCACCGGGTCGGACATAC, and CAT forward: GCAGATACCTGTGAACTGTC, CAT reverse: GTAGAATGTCCGCACCTGAG (Harvey et al. 1995). β-Actin forward: CGTGACATTAAGGAGAAGCTGTGC, β-Actin reverse: CTCAGGAGGAGCAATGATCTTGAT a house-keeping gene, was used for normalizing mRNA levels of the target genes (Robert et al. 2002). The PCR cycling parameters were one cycle of 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 60 °C (Cu–Zn SOD and GPx genes, respectively) for 30 s, 70 °C for 40 s, and a final cycle at 72 °C for 5 min (CAT, β-actin, respectively). The PCR products were electrophoresed onto an ethidium bromide stained 2.0 % agarose gel. The ethidium bromide-stained gel bands were scanned and the signal intensities were quantified by the computerized Gel-Pro program image analyzer (Version 3.1 for Windows3).
DNA fragmentation assays for apoptosis protocol
DNA fragmentation in liver and kidney tissues was determined according to the method described by Perandones et al. (1993). In brief, 10–20 mg of liver or kidney tissues were ground in 400 μl hypotonic lysis buffer (10 mM Tris base, 1 mM EDTA and 0.2 % Triton X-100), centrifuged at 3,000×g for 15 min at 4 °C and the supernatant containing small DNA fragments was separated. One-half of the volume was used for gel electrophoresis and the other half together with the pellet containing large pieces of DNA were used for quantification of fragmented DNA by the Diphenyl amine. The samples were treated with equal volumes of absolute isopropyl alcohol and 0.5 M NaCl to precipitate the DNA, stored at −20 °C overnight and centrifuged at 2,000×g for 15 min.
Statistical analysis
All data were statistically analyzed using the General Linear Model Procedure of the Statistical Analysis System (SAS Institute Inc 1982). The significance of the differences among treatment groups was determined by Waller-Duncan k-ratio (Waller and Duncan 1969). All statements of significance were based on probability of P ≤ 0.05.
Results
Chemical composition of PGE
The HPLC analysis of PGE revealed that the concentrations of ginsenosides in mg/g were 0.54 Rg1, 3.16 Rg2, 4.04 Rg3, 0.88 Rh1, 0.11 Rh2, 3.72 Rb1, 1.71 Rb2, 0.95 Re, 1.02 Rf, 1.89 Rc, and 1.32 Rd with total ginsensoides 19.3 mg/g.
Lipid peroxidation (MDA) content
The effect of PGE treatment on the level of lipid peroxidation measured in terms of MDA in liver and kidney tissues of normal control or mycotoxins (AFB1 and/or FB1)-treated rats are shown in Table 1. These results indicated that treatment with PGE alone resulted in a significant decrease in MDA in liver; however, it did not significantly affect MDA in kidney. Treatment with AFB1 and/or FB1 resulted in a significant increase in MDA level in liver and kidney tissues. This increase recorded the highest level in the group treated with the two mycotoxins followed by the group treated with FB1 then those treated with AFB1. It is worthy to mention that the decrease of MDA production in liver and kidney by PGE was very efficient when rats were exposed to AFB1 or FB1 individually. However, the decrease was less pronounced when both toxins were present together (Table 1).
Table 1.
Effects of PGE on lipid peroxidation and glutathione content in kidney and liver of rats treated with AFB1 and/or FB1
| Treatment | MDA (µmol/g tissue) | GSH (µmol/g tissue) | ||
|---|---|---|---|---|
| Liver | Kidney | Liver | Kidney | |
| Control | 45.26 ± 2.17e | 30.78 ± 0.79d | 4.52 ± 0.22b | 4.03 ± 0.13b |
| PGE | 32.89 ± 1.57c | 28.97 ± 0.98d | 5.66 ± 0.25a | 5.04 ± 0.19a |
| AFB1 | 81.28 ± 3.29b | 53.23 ± 1.91a | 2.86 ± 0.12d | 3.18 ± 0.13c |
| FB1 | 90.43 ± 2.74a | 58.26 ± 1.54a | 2.06 ± 0.05d,e | 2.65 ± 0.20d |
| AFB1 + FB1 | 92.50 ± 3.16a | 58.54 ± 3.2a | 1.80 ± 0.07e | 2.28 ± 0.15d |
| AFB1 + PGE | 49.58 ± 3.07e | 36.0 ± 2.79c | 3.79 ± 0.22c | 3.41 ± 0.18c |
| FB1 + PGE | 56.62 ± 2.52c | 38.15 ± 1.77c | 4.32 ± 0.2b | 3.95 ± 0.09b |
| AFB1 + FB1 + PGE | 66.74 ± 3.39c | 44.16 ± 1.57b | 3.59 ± 0.19c | 3.07 ± 0.03c |
Data are means ± SEM, within each column, means superscript with different letters are significantly different (P ≤ 0.05) compared to the control
Glutathione (GSH) level
The results presented in Table 1 indicated that treatment with PGE alone resulted in a significant increase in GSH content in liver and kidney compared with normal control values. Treatment with AFB1 and/or FB1 resulted in a significant decrease in GSH in liver and kidney tissue. This decrease in GSH in liver and kidney tissues was more pronounced in the group having received AFB1 plus FB1 followed by the group having received FB1 alone than in those having received AFB1 alone. Treatment with PGE succeeded to induce a significant improvement in GSH in liver but did not induce a significant effect in the kidney tissues. This improvement was more pronounced in the group treated with FB1 followed by that treated with AFB1 then the group having received AFB1 plus FB1.
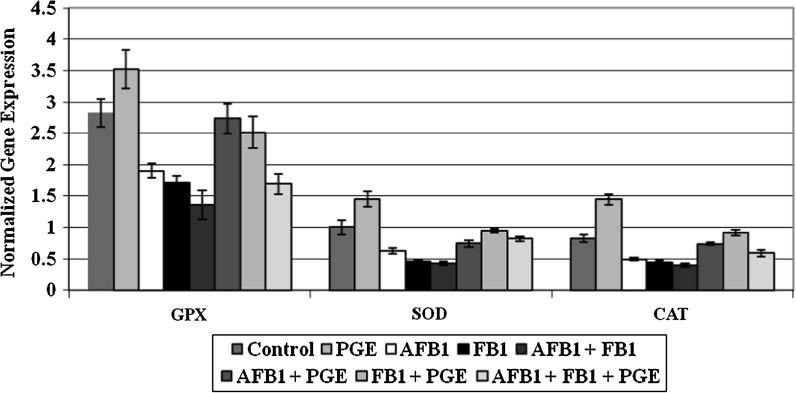
Evaluation of gene expression
In the current study, the bands produced from amplifying cDNA of GPx, SOD1, CAT and the house keeping gene β-Actin as a control were analyzed and the results were expressed as the ratio between maximum optical density (max OD) for each band of the target amplification product and the corresponding max OD of β-actin. The data of the expression of GPx, SOD1 and CAT mRNA in livers of the different treatment groups are summarized in Fig 1. These results showed that there was a highly significant decrease in the gene expression of these enzymes in the groups treated with AFB1 and/or FB1 compared to the other groups. Treatment with PGE alone, however, resulted in a significant increase in the expression of GPx, SOD1 and CAT mRNA. Treatment with PGE in rats having received AFB1 and/or FB1 significantly up-regulated the expression of GPx, SOD1 and CAT mRNA although the gene expressions of these enzymes were still different from the control group.
Fig. 1.
Effects of Panax ginseng extract (PGE) on catalase (CAT), Super Oxide Dismutase (SOD) and glutathione peroxidase (GPx) mRNA expression in liver of rats treated with AFB1 and/or FB1 compared to the control group
DNA fragmentation
The current results indicated that treatment with AFB1 and/or FB1 caused marked increase in DNA fragmentation percentage in liver and kidney compared to the untreated control. The percentage of DNA fragmentation was more pronounced in rats treated with the combined treatments (AFB1 and FB1) compared to the single treatment with either AFB1 or FB1 (Table 2). Moreover, the liver was more sensitive to damage than the kidney. Rats treated with PGE were comparable to the control regarding DNA fragmentation. However, those treated with PGE and the mycotoxins showed a decrease in DNA fragmentation only in liver (Table 2).
Table 2.
Effects of PGE on DNA fragmentation percentage in liver and kidney of rats treated with AFB1 and/or FB1
| Treatments | DNA fragmentation % in liver | Changes | DNA fragmentation % in kidney | Changes |
|---|---|---|---|---|
| Control | 5.0 | − | 3.38 | − |
| PGE | 4.4 | −0.6 | 3.08 | −0.30 |
| AFB1 | 36.4 | +31.4 | 17.4 | +14.02 |
| FB1 | 16.5 | +11.5 | 12.48 | +9.1 |
| AFB1 + FB1 | 44.78 | +39.78 | 22.2 | +18.82 |
| AFB1 + PGE | 14.46 | +9.46 | 11.3 | +7.92 |
| FB1 + PGE | 11.32 | +6.32 | 12.5 | +9.12 |
| AFB1 + FB1 + PGE | 22.52 | +17.52 | 14.5 | +11.12 |
Discussion
The current study revealed that PGE is composed of ginsenosides (ginseng saponins) including Rg1, Rg2, Rg3, Rh1, Rh2, R, Rb2, Re, Rf, Rc and Rd. These results were similar to those reported previously (Li and Liu 2008; Liu et al. 2002a) These ginsenosides appear to be responsible for most of the activities of ginseng including vasorelaxation, antioxidation, antiinflammation and anticancer properties (Lü et al. 2009). The antioxidant activity of ginsenosides was confirmed by Zhong and Jiang (1997) who found that the ginsenosides Rb1, Rc, Re, Rg1, Rg2, and Rh1 counteracted the action of free radicals induced by xanthine. Moreover, Wang et al. (2007) demonstrated that the endothelial dysfunction induced by homocysteine and HIV protease inhibitors was effectively blocked by Rb1 and other ginsenosides suggesting that Rb1 and other ginsenosides fully block reactive oxygen species production.
The natural co-occurrence of AFB1 and FB1, especially in corn, is a worldwide problem and has been associated with a high incidence of human hepatocellular carcinoma (CAST 2003). Moreover, it is also likely that the oxidative stress exerted individually by AFB1 and FB1 may be enhanced by co-exposure to both mycotoxins (Abdel-Wahhab et al. 2010, 2012). The toxicology of AFB1 involves its biotransformation through cytochrome P450 to the highly reactive AFB1-exo-8,9-epoxide, which forms adducts with DNA (Guengerich et al. 1998). Besides, AFB1 is able to induce ROS generation (Matur et al. 2011; Adedara et al. 2010; Abdel-Wahhab et al. 2012), possibly requiring the activation of cytochrome P450. In addition, FB1 can produce oxidative stress and/or apoptosis, depending on the species and the cell types (Stockmann-Juvala and Savolainen 2008). It is widely accepted that the cytotoxic effect of AFB1 and/or FB1 on normal differentiated cells is due to the production of ROS at high levels (Towner et al. 2003; Hassan et al. 2010, 2012). Oxidative damage induced by ROS cause tissue damage by a variety of mechanisms including DNA damage, lipid peroxidation, protein oxidation and depletion of thiols.
In the current study, experimental mycotoxicoses in rats were developed in order to characterize the genetic alterations induced by subchronic treatment of known levels of AFB1 and FB1, individually or as a mixture, which could mimic those found in nature. Moreover, the possible protective role of PGE against the oxidative stress and DNA damage in rats was evaluated. The selected doses of AFB1, FB1 and PGE were based on our previous work (Abdel-Wahhab et al. 2010). No animal mortality was observed in any of the treatment groups except only two rats died in the group treated with AF1 plus FB1. However, the animals within the other treatment groups appeared healthy and active. The results revealed that the levels of LP as an oxidative stress marker induced in liver and kidney was significantly increased while the levels of antioxidant GSH were significantly decreased in AFB1 and/or FB1-treated groups compared to their levels in the normal controls. These results are in agreement with those reported previously (Verma and Mathuria 2009; Abdel-Wahhab et al. 2010; Hassan et al. 2010, 2012; Mary et al. 2012) who reported that the increased LP level with the concomitant decrease in antioxidant enzyme activities were the most pronounced markers for AFB1 and/or FB1 toxicity and carcinogenicity. Lipid peroxidation of polyunsaturated fatty acids which is an important outcome of oxidative stress is one of the main manifestations of oxidative damage and has been found to play an important role in cellular damage, necrosis and apoptosis (Kulanthaivel et al. 2012). Several reports indicated that aflatoxin administration resulted in excessive LP (Abdel-Wahhab et al. 2006; Abdel-Aziem et al. 2011) with concomitant decrease in reduced glutathione (Abdel-Wahhab et al. 2010), increased protein oxidation and DNA damage (Gross-Steinmeyer and Eaton 2012; Hassan et al. 2012) in rat liver and kidney. It is well documented that AFB1 is activated mainly by the cytochrome P450 group of enzymes to form the reactive intermediates AFB1-8,9-epoxide (Gallagher et al. 1996), consequently, it induce the formation of ROS (Preetha et al. 2006) and lipid peroxidation (Shen et al. 1995). Furthermore, FB1 inhibits the ceramide synthetase activity and disrupts the sphingolipid metabolism (Pinelli et al. 1999; Abdel-Wahhab et al. 2004, 2010) and increases concentrations of arachidonic acid metabolites, including prostaglandins in bronchial epithelial cells (Poux et al. 2000).
In addition, FB1 produces oxidative stress and/or apoptosis (Hassan et al. 2010), depending on the species and the cell types (Stockmann-Juvala and Savolainen 2008). The mechanisms used by FB1 to induce ROS have begun to be evaluated in recent years (Domijan and Abramov 2011), but have not been clearly understood yet. However, administration of FB1 to rats enhanced LP which is presumably a result of free radical-mediated toxicity and carcinogenicity (Abdel-Wahhab et al. 2004; El-Nekeety et al. 2007; Stockmann-Juvala et al. 2004).
The present data revealed marked depletion in GSH content as well as the gene expression of the antioxidant enzymes, GPx, SOD and CAT in the liver tissues of mycotoxins-treated rats. GSH, a potent inhibitor of genotoxicity, plays an important role as an endogenous antioxidant system that is found at particularly high concentration in the liver and is known to have a key function in the protective process (Sinclair et al. 1990). Furthermore, the depletion of GSH content and the decrease in antioxidant enzyme activities was reported previously (Hassan et al. 2010, 2012; Noaman et al. 2008) which was associated with an increase in LP (Abdel-Aziem et al. 2011). The decrease in GSH, SOD and CAT mRNA expression reported herein is in accordance with previous reports which indicated the detection of low liver GST activity in SEC-bearing mice (Kwiecien et al. 2006) and in lung cancer-bearing animals (Selvendiran et al. 2005). The reduction in the mRNA of SOD and GPx in mycotoxins-treated rats could be either due to the oxidation of transcription factors or due to the decrease in the half lives of mRNAs (Alam et al. 1999). Thus, the decrease in GSH enzyme led to an indirect increase in oxidative DNA damage indicating that GSH plays a role in the suppression of oxygen free radical formation and NO generation in liver and kidney (Verma and Mathuria 2009). Consequently, under this pathological condition, the active process of cellular self-destruction, DNA fragmentation and apoptosis, might occur (Hassan et al. 2012).
The increased DNA fragmentation reported in the current study was consistent with the previous reports which indicated that AFB1 induce DNA fragmentation (Abdel-Wahhab et al. 1998; Hassan et al. 2012). The mutagenicity of AFs especially AFB1 arising from the toxin molecules which might form covalent-adducts which cause a disturbance of DNA replication (Bonnett and Taylor 1989). The formation of AFB1-DNA adducts is regarded as a critical step in the initiation of AFB1-induced hepatocarcinogenesis (Preston and Williams 2005; Abdel-Wahhab et al. 2006; Pfohl-Leszkowicz 2008), p53 gene mutation (Habib et al. 2006) and indicates that oxidative stress is an apoptosis inducer (Meki et al. 2004). The proteolytic activation of DNA ladder formation is a key step in the apoptotic cascade (Maruyama et al. 2001). However, for FB1, Wang and Groopman (1999) reported that they are the only mycotoxins with carcinogenic potency that are not direct DNA-damaging agents and that they increase carcinogenesis by increasing the number of DNA replication (Dragan et al. 2001). Furthermore, it was suggested that the oxidative damage induced by FB1 might indirectly lead to mutagenicity and genotoxicity (Hassan et al. 2010). This observation is the possible explanation for the damage induced in hepatic DNA after exposure to FB1. The interaction of AFB1 and FB1 in the induction of DNA damage and its correlation with biomarkers of cellular oxidative status revealed a clear synergism of these tested mycotoxins to induce genetic damage. These results supported those reported by Mary et al. (2012) who demonstrated that AFB1 and FB1, alone or as a mixture, affect the oxidative status in spleen mononuclear cells, by increasing ROS levels and biomolecular oxidative damage. The mixture of both mycotoxins induced the greatest oxidative stress due to its stronger pro-oxidant action, resulting from the interaction of AFB1 and FB1.
Previous studies revealed that PGE contains many classes of compounds, including ginsenosides, essential oil, peptidoglycans, polysaccharides, nitrogen-containing compounds, fatty acids and phenolic compounds (Lee et al. 2010). Currently, more than 30 different ginsenosides from PGE have been isolated and characterized, and these ginsenosides are known to have different pharmacologic effects. Moreover, new ginsenosides were also isolated from red ginseng, which are not usually found in raw ginseng (Park 1996; Kim et al. 2000). It also contains phenolic compounds including salicylic acid, caffeic acid and maltol which have antioxidant activity (Park 1996). In addition, the ginseng root contains amino acids, vitamins A, B1, B2, B12, C, and E and niacin as well as inorganic elements such as sodium, potassium, calcium, magnesium, phosphorus, iodine, iron, zinc, copper, manganese, and selenium (Choi et al. 2009). These components are important to enhance the antioxidant capacity of the body including the enzymatic antioxidants such as SOD, GPx, CAT and GSH and the nonenzymatic antioxidants such as vitamins C and E (Ramesh et al. 2012).
In the present study, PGE upregulated GSH and gene expression of antioxidant enzymes as well as suppressed LP and DNA fragmentation percentage in AFB1 and/or FB1-treated rats. This may suggest that PGE exerts antioxidant and anti-apoptotic effects. The potential protective mechanisms of PGE to induce both activity and expression of the antioxidant enzymes was assessed using semi-quantitative RT-PCR. The current results suggested that the protective effect PGE against mycotoxicosis resulted from the increased antioxidant system in the cell (Ramesh et al. 2012). Moreover, it is well documented that the antioxidant enzymes (SOD, GSH and CAT) are considered the first line of the antioxidant defense system against ROS generated during oxidative stress (Ray and Husain 2002). Generally, these results indicated that PGE have protective effects against liver injury induced by the two mycotoxins and it plays a role in increasing the antioxidant status as well as lowering the oxidative damage of nucleic acids in the body (Abdel-Wahhab et al. 2010; Mannaa et al. 2006). Accordingly, an aqueous extract of ginseng has been reported to restore the expression of these antioxidant enzymes in H2O2-injured primary cultures of rat astrocytes (Naval et al. 2007). In the same respect, Li et al. (2010) reported that ginseng saponins induced a protective role against alcohol-induced hepatic injury in mice by up-regulating the expression of the antioxidant enzymes. Furthermore, the decrease in DNA fragmentation in PGE-treated rats suggested that the protective effect of PGE during mycotoxicosis resulted from the reduction of apoptosis via the increase in the expression of antioxidants enzymes. These results also are consistent with previous observations of anti-apoptotic effects of ginseng which suggested that ginsenosides inhibit cardiomyocyte apoptosis by inhibiting expression of the pro-apoptotic FAS gene in rats (Liu et al. 2002a). Moreover, they exert protective effects against the progression of oxidative stress-induced DNA damage (Choi et al. 2003), protect against the disturbances in sphingolipid metabolism (Pinelli et al. 1999) and decrease arachidonic acid metabolites (Poux et al. 2000).
Conclusion
The results of the present study demonstrated that AFB1 and FB1, alone or in combination affected the oxidative status in rats, by increasing ROS generation as indicated by the elevation of MDA and the reduction of GSH levels in liver and kidney. These mycotoxins also down-regulated mRNA gene expression of antioxidant enzymes in liver and increased DNA fragmentation. The mixture of the two mycotoxins induced the greatest oxidative stress due to its stronger pro-oxidant action, resulting of the interaction of AFB1 and FB1. Besides, this study provides relevant information about the mechanisms by which the mycotoxins induced cytotoxic effects: ROS may have a dual role, by acting as toxic bio-products that alter the cellular function and viability, and also as key participants in the cellular regulation and signaling. PGE induced a potential protective effect against the cytotoxicity of these mycotoxins. This effect was due to its ability to prevent lipid peroxidation by enhancing enzyme and nonenzyme antioxidant defense systems and suppressing the oxidative stress in rats.
Acknowledgments
This work was supported by the National Research Centre, Dokki, Cairo, Egypt Project # S90402.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdel-Aziem SH, Hassan AM, Abdel-Wahhab MA. Dietary supplementation with whey protein and ginseng extract counteract the oxidative stress and DNA damage in rats fed aflatoxins-contaminated diet. Mutat Res. 2011;723:65–71. doi: 10.1016/j.mrgentox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Ahmed HH. Protective effects of Korean Panaxginseng against chromium VI toxicity and free radical generation in rats. J Ginseng Res. 2004;28:11–17. doi: 10.5142/JGR.2004.28.1.011. [DOI] [Google Scholar]
- Abdel-Wahhab MA, Nada SA, Farag IM, Abbas NF, Amra HA. Potential protective effect of HSCAS and bentonite against dietary aflatoxicosis in rat: with special reference to chromosomal aberrations. Nat Toxins. 1998;6:211–218. doi: 10.1002/(SICI)1522-7189(199809/10)6:5<211::AID-NT31>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Hassan AM, Amer HA, Naguib KM. Prevention of fumonisin-induced maternal and developmental toxicity in rats by certain plant extracts. J Appl Toxicol. 2004;24:469–474. doi: 10.1002/jat.1000. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Ahmed HH, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol. 2006;26:229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Hassan NS, El-Kady AA, Mohamed YA, El-Nekeety AA, Mohamed SR, Sharaf HA, Mannaa FA. Red ginseng protects against aflatoxin B1 and fumonisin-induced hepatic pre-cancerous lesions in rats. Food Chem Toxicol. 2010;48:733–742. doi: 10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, Ibrahim AA, El-Nekeety AA, Hassan NS, Mohamed AA. Panax ginseng C.A. Meyer extract counteracts the oxidative stress in rats fed multi-mycotoxins-contaminated diet. Comun Sci. 2012;3:143–153. [Google Scholar]
- Adedara IA, Owumi SE, Uwaifo AO, Farombi EO. Aflatoxin B1 and ethanol co-exposure induces hepatic oxidative damage in mice. Toxicol Ind Health. 2010;26:717–724. doi: 10.1177/0748233710377772. [DOI] [PubMed] [Google Scholar]
- Alam K, Nagi MN, Badary OA, Al-Shabanah OA, Al-Rikabi AC, Al-Bekairi AM. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacol Res. 1999;40:159–163. doi: 10.1006/phrs.1999.0472. [DOI] [PubMed] [Google Scholar]
- Bonnett M, Taylor ER. The structure of the aflatoxin B1-DNA adduct at N7 of guanine. Theoretical intercalation and covalent adduct models. J Biomol Struct Dyn. 1989;7:127–149. doi: 10.1080/07391102.1989.10507756. [DOI] [PubMed] [Google Scholar]
- Brun ME, Gasca S, Girard C, Bouton K, De Massy B, De Sario A. Characterization and expression analysis during embryo development of the mouse ortholog of MLL3. Gene. 2006;371:25–33. doi: 10.1016/j.gene.2005.11.013. [DOI] [PubMed] [Google Scholar]
- CAST (2003) Mycotoxins: risks in plant, animal, and human systems. In: Richard JL, Payne GA (Eds), Council for Agricultural Science and Technology Task Force report no. 139, Ames, IA, USA. ISBN: 1-887383-22-0
- Choi HJ, Han HS, Park JH, Son JH, Bae JH, Seung TS, Choi C. Antioxidantive, phospholipase A2 inhibiting, and anticancer effect of polyphenol rich fractions from Panaxginseng C.A. Meyer. J Korean Soc Agric Chem Biotechnol. 2003;46:251–256. [Google Scholar]
- Choi MK, Kang MH, Kim MH. The analysis of copper, selenium, and molybdenum contents in frequently consumed foods and an estimation of their daily intake in Korean adults. Biol Trace Elem Res. 2009;128:104–117. doi: 10.1007/s12011-008-8260-2. [DOI] [PubMed] [Google Scholar]
- Chu FS, Li GY. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl Environ Microbiol. 1994;60:847–852. doi: 10.1128/aem.60.3.847-852.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domijan AM, Abramov AY. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis-implication to mechanism of cell toxicity. Int J Biochem Cell Biol. 2011;43:897–904. doi: 10.1016/j.biocel.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Dragan YP, Bidlack WR, Cohen SM, Goldsworthy TL, Hard GC, Howard PC, Riley RT, Voss KA. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: fumonisin B1 as an example. Toxicol Sci. 2001;61:6–17. doi: 10.1093/toxsci/61.1.6. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- EFSA Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 2007;446:1–127. [Google Scholar]
- Ellman GL. Tissue sulfahydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon. 2007;50:256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- FAO . Food nutrition and agriculture (food for the future), FAO. Rome: FAO; 1991. [Google Scholar]
- Gallagher EP, Kunze KL, Stapleton PL, Eaton DL. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- Galvano F, Campisi A, Russo A, Galvano G, Palumbo M, Renis M, Barcellona ML, Perez-Polo ML, Vanella JRA. DNA damage in astrocytes exposed to fumonisin B1. Neurochem Res. 2002;27:345–351. doi: 10.1023/A:1014971515377. [DOI] [PubMed] [Google Scholar]
- Galvano F, Russo A, Cardile V, Galvano G, Vanella A, Renis M. DNA damage in human fibroblasts exposed to fumonisin B1. Food Chem Toxicol. 2002;40:25–31. doi: 10.1016/S0278-6915(01)00083-7. [DOI] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Eaton DL. Dietary modulation of the biotransformation and genotoxicity of aflatoxin B1. Toxicology. 2012;299:69–79. doi: 10.1016/j.tox.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Johnson WW, Shimada T, Ueng YF, Yamazaki H, Langouet S. Activation and detoxication of aflatoxin B1. Mutat Res. 1998;402:121–128. doi: 10.1016/S0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- Habib SL, Said B, Awad AT, Mostafa MH, Shank RC. Novel adenine adducts, N7-guanine-AFB1 adducts, and p53 mutationsin patients with schistosomiasis and aflatoxin exposure. Cancer Detect Prev. 2006;30:491–498. doi: 10.1016/j.cdp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Harvey MB, Arcellana-Panlilio MY, Zhang X, Schultz GA, Watson AJ. Expression of genes encoding antioxidant enzymes in preimplantation mouse and cow embryos and primary bovine oviduct cultures employed for embryo coculture. Biol Reprod. 1995;53:532–540. doi: 10.1095/biolreprod53.3.532. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Hassan NS, Mohamed SR, El-Nekeety AA, Abdel-Wahhab MA. Aquilegia vulgaris L. extract counteracts oxidative stress and cytotoxicity of fumonisin in rats. Toxicon. 2010;56:8–18. doi: 10.1016/j.toxicon.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Abdel-Aziem SH, Abdel-Wahhab MA. Modulation of DNA damage and alteration of gene expression during aflatoxicosis via dietary supplementation of Spirulina (Arthrospira) and whey protein concentrate. Ecotoxicol Environ Saf. 2012;79:294–300. doi: 10.1016/j.ecoenv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Huang YC, Chen CT, Chen SC, Lai PH, Liang HC, Chang Y, Yu LC, Sung HW. A natural compound (ginsenoside Re) isolated from Panax ginseng as a novel angiogenic agent for tissue regeneration. Pharm Res. 2005;22:636–646. doi: 10.1007/s11095-005-2500-3. [DOI] [PubMed] [Google Scholar]
- IARC . IARC Monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC; 1993. Aflatoxins; pp. 243–395. [PMC free article] [PubMed] [Google Scholar]
- IARC Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Monogr Eval Carcino Risks Hum. 2002;82:21. [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Vea M, González-Peñas E, Lizarraga E, López de Cerain A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in barley from a northern region of Spain. Food Chem. 2012;132:35–42. doi: 10.1016/j.foodchem.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Jestoi M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—a review. Crit Rev Food Sci Nutr. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- Jung CH, Seog HM, Choi IW, Choi HD, Cho HY. Effects of wild ginseng (Panaxginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98:245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Khalil WKB, Hassan AM, Ahmed KA, Park MH, Kim Y, Park HH, Abdel-Wahhab MA. Protective effects of Panax ginseng extract standardized with ginsenoside Rg3 against EDTA-induced toxicity in male rats. Arch Toxicol. 2008;82:183–195. doi: 10.1007/s00204-007-0237-y. [DOI] [PubMed] [Google Scholar]
- Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- Klaric MS, Pepeljnjak S, Domijan AM, Petrik J. Lipid peroxidation and glutathione levels in porcine kidney PK15 cells after individual and combined treatment with fumonisin B1, beauvericin and ochratoxin A. Basic Clin Pharmacol Toxicol. 2007;100:157–164. doi: 10.1111/j.1742-7843.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Ko SR, Choi KJ, Kim SC, Kim MW. Contents of crude saponin and ginsenosides in white ginsengs. Koren J Pharmacol. 1989;20:170–174. [Google Scholar]
- Kulanthaivel L, Srinivasan P, Shanmugam V, Periyasamy BM. Therapeutic efficacy of kaempferol against AFB1 induced experimental hepatocarcinogenesis with reference to lipid peroxidation, antioxidants and biotransformation enzymes. Biomed Prev Nutr. 2012;2:252–259. doi: 10.1016/j.bionut.2012.04.002. [DOI] [Google Scholar]
- Kwiecień I, Michalska M, Włodek L. The selective effect of cystathionine on doxorubicin hepatotoxicity in tumor-bearing mice. Eur J Pharmacol. 2006;550:39–46. doi: 10.1016/j.ejphar.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim YK, Park N, Kim CS, Lee CY, Park SU. Chemical constituents and biological activities of the berry of Panax ginseng. J Med Plants Res. 2010;5:349–353. [Google Scholar]
- Li GX, Liu ZQ. The protective effects of ginsenosides on human erythrocytes against hemin induced hemolysis. Food Chem Toxicol. 2008;46:886–892. doi: 10.1016/j.fct.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Li YG, Ji DF, Zhong S, Shi LG, Hu GY, Chen S. Saponins from Panaxjaponicas protect against alcohol-induced hepatic injury in mice by up-regulating the expression of GPX3, SOD1 and SOD3. Alcohol Alcohol. 2010;45:320–331. doi: 10.1093/alcalc/agq034. [DOI] [PubMed] [Google Scholar]
- Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin induced diabetic rats. Mol Cell Biochem. 2003;243:147–152. doi: 10.1023/A:1021620414979. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Z, Liu X. Effect of ginsenoside Re on cardiomyocyte apoptosis and expression of Bcl-2/Bax gene after ischemia and reperfusion in rats. J Huazhong Univ Sci Technol. 2002;22:305–309. doi: 10.1007/BF02896771. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Luo XY, Sun YX, Chen YP, Wang ZC. Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochim Biophys Acta. 2002;1572:58–66. doi: 10.1016/S0304-4165(02)00281-7. [DOI] [PubMed] [Google Scholar]
- Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaa F, Abdel-Wahhab MA, Ahmed HH, Park MH. Protective role of Panax ginseng extract standardized with ginsenoside Rg3 against acrylamide induced neurotoxicity in rats. J Appl Toxicol. 2006;26:198–206. doi: 10.1002/jat.1128. [DOI] [PubMed] [Google Scholar]
- Marasas WFO. Fumonisins: history, world-wide occurrence and impact. Adv Exp Med Biol. 1996;392:1–17. doi: 10.1007/978-1-4899-1379-1_1. [DOI] [PubMed] [Google Scholar]
- Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online. 2000;3:19–25. doi: 10.1251/bpo20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama W, Youdim MB, Naoi M. Antiapoptotic properties of rasagiline, N-propargylamine-1(R)-aminoindan, and its optical (S)-isomer, TV1022. Ann NY Acad Sci. 2001;939:320–329. doi: 10.1111/j.1749-6632.2001.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Mary VS, Theumer MG, Arias SL, Rubinstein HR. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 2012;302:299–307. doi: 10.1016/j.tox.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Matur E, Ergul E, Akyazi I, Eraslan E, Inal G, Bilgic S, Demircan H. Effects of Saccharomycescerevisiae extract on haematological parameters, immune function and the antioxidant defence system in breeder hens fed aflatoxin contaminated diets. Br Poult Sci. 2011;52:541–550. doi: 10.1080/00071668.2011.617726. [DOI] [PubMed] [Google Scholar]
- Meki AM, Esmail EF, Hussein AA, Hassanein HM. Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: effect of melatonin. Toxicon. 2004;43:93–100. doi: 10.1016/j.toxicon.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Naval MV, Gomez-Serranillos MP, Carretero ME, Villar AM. Neuroprotective effect of a ginseng (Panaxginseng) root extract on astrocytes primary culture. J Ethnopharmacol. 2007;112:262–270. doi: 10.1016/j.jep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Noaman E, Badr El-Din NK, Bibars MA, Abou Mossallam AA, Ghoneum M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008;268:348–359. doi: 10.1016/j.canlet.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Park JD. Recent studies on the chemical constituents of Korean ginseng (Panaxginseng C.A. Meyer) J Ginseng Sci. 1996;20:389–415. [Google Scholar]
- Perandones CE, Illera VA, Peckham D, Stunzl LL, Ashman RF. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993;151:3521–3529. [PubMed] [Google Scholar]
- Pfohl-Leszkowicz A. Formation, persistence and significance of DNA adduct formation in relation to some pollutants from a board perspective. Adv Toxicol. 2008;2:183–240. doi: 10.1016/S1872-0854(07)02007-3. [DOI] [Google Scholar]
- Pfohl-Leszkowicz A, Petkova-Bocharova T, Cherozemsky IN, Castegnaro M. Balkan endemic nephropathy and associated urinary tract tumors: a review on etiological causes and the potential role of mycotoxins. Food Addit Contam. 2002;19:282–302. doi: 10.1080/02652030110079815. [DOI] [PubMed] [Google Scholar]
- Pinelli E, Poux N, Garren L, Pipy B, Castegnaro M, Miller DJ, Pfohl-Leszkowicz A. Activation of mitogen-activated protein kinase by fumonisin B1 stimulates cPLA2 phosphorylation, the arachidonic acid cascade and cAMP production. Carcinogenesis. 1999;20:1683–1688. doi: 10.1093/carcin/20.9.1683. [DOI] [PubMed] [Google Scholar]
- Poux N, Pinelli E, Castegnaro M, Miller DJ, Pfohl-Leszkowicz A. Effects of fumonisin B1 on cell signal transduction pathways: main role of MAPKs. In: de Koe WJ, Samson RA, Van Egmond HP, Gilbert J, Sabino M, editors. Mycotoxins and phycotoxins in perspective at the turn of the millenium. The Netherland: WJ Koe; 2000. pp. 251–257. [Google Scholar]
- Preetha SP, Kanniappan M, Selvakumar E, Nagaraj M, Varalakshmi P. Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp Biochem Physiol C Comp Pharmacol. 2006;143:333–339. doi: 10.1016/j.cbpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Williams GM. DNA-reactive carcinogens: mode of action and human cancer hazard. Crit Rev Toxicol. 2005;35:673–683. doi: 10.1080/10408440591007278. [DOI] [PubMed] [Google Scholar]
- Ramesh T, Kim S, Hwang SY, Sohn SH, Yoo SK, Kim SK. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:6. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- Robert C, McGraw S, Massicotte L, Pravetoni M, Gandolfi F, Sirard MA. Quantification of housekeeping transcript levels during the development of bovine preimplantation embryos. Biol Reprod. 2002;67:1465–1472. doi: 10.1095/biolreprod.102.006320. [DOI] [PubMed] [Google Scholar]
- Sahu SC, Eppley RM, Page SW, Gray GC, Barton CN, O’Donnell MW. Peroxidation of membrane lipids and oxidative DNA damage by fumonisin B1 in isolated rat liver nuclei. Cancer Lett. 1998;125:117–121. doi: 10.1016/S0304-3835(97)00521-1. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS user’s guide: statistics. Cary, NC, USA: SAS Institute; 1982. [Google Scholar]
- Selvendiran K, Thirunavukkarasu C, Singh JP, Padmavathi R, Sakthisekaran D. Chemopreventive effect of piperine on mitochondrial TCA cycle and phase-I and glutathione-metabolizing enzymes in benzo(α)pyrene induced lung carcinogenesis in Swiss albino mice. Mol Cell Biochem. 2005;271:101–106. doi: 10.1007/s11010-005-5615-2. [DOI] [PubMed] [Google Scholar]
- Shen HM, Ong CN, Shi CY. Involvement of reactive oxygen species in aflatoxin B1-induced cell injury in cultured rat hepatocytes. Toxicology. 1995;99:115–123. doi: 10.1016/0300-483X(94)03008-P. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Barnett AH, Luine J. Free radical and auto-oxidant system in health and disease. Br Hosp Med. 1990;43:334–344. [PubMed] [Google Scholar]
- Stockmann-Juvala H, Savolainen K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum Exp Toxicol. 2008;27:799–809. doi: 10.1177/0960327108099525. [DOI] [PubMed] [Google Scholar]
- Stockmann-Juvala H, Mikkola J, Naarala J, Loikkanen J, Elovaara E, Savolainen K. Fumonisin B1-induced toxicity and oxidative damage in U-118MG glioblastoma cells. Toxicology. 2004;202:173–183. doi: 10.1016/j.tox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Theumer MG, Canepa MC, Lopez AG, Mary VS, Dambolena JS, Rubinstein HR. Subchronic mycotoxicoses in Wistar rats: assessment of the in vivo and in vitro genotoxicity induced by fumonisins and aflatoxin B1, and oxidative stress biomarkers status. Toxicology. 2010;268:104–110. doi: 10.1016/j.tox.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Thiel PG, Marasas WFO, Sydenham EW, Shephard GS, Gelderblom WCA. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia. 1992;117:3–9. doi: 10.1007/BF00497272. [DOI] [PubMed] [Google Scholar]
- Towner RA, Qian SY, Kadiiska MB, Mason RP. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic Biol Med. 2003;35:1330–1340. doi: 10.1016/j.freeradbiomed.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Verma RJ, Mathuria N. Effect of curcumin on aflatoxin-induced biochemical changes in testis of mice. Fertil Steril. 2009;91:597–601. doi: 10.1016/j.fertnstert.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Waller RA, Duncan DB. A Bayes rule for the symmetric multiple comparison problems. J Am Stat Assoc. 1969;64:1484–1503. [Google Scholar]
- Wang JS, Groopman JD. DNA damage by mycotoxins. Mutat Res. 1999;424:167–181. doi: 10.1016/S0027-5107(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- WHO . Forty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva: WHO Technical Report Series. WHO; 1998. [Google Scholar]
- Yokozawa T, Liu ZW. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren Fail. 2000;22:115–127. doi: 10.1081/JDI-100100858. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. doi: 10.1016/0002-9378(79)90708-7. [DOI] [PubMed] [Google Scholar]
- Zhong G, Jiang Y. Calcium channel blockage and anti-free-radical actions of ginsenosides. Chin Med J. 1997;110:28–29. [PubMed] [Google Scholar]