Abstract
Current cell-based cartilage therapies relay on articular cartilage-derived autologous chondrocytes as a cell source, which possesses disadvantages, such as, donor site morbidity and dedifferentiation of chondrocytes during in vitro expansion. Due to these and other limitations, novel cell sources and production strategies are needed. Bone marrow-derived mesenchymal stromal cells (BM-MSCs) are a fascinating alternative, but they are not spontaneously capable of producing hyaline cartilage-like repair tissue in vivo. In vitro pre-differentiation of BM-MSCs could be used to produce chondrocytes for clinical applications. However, clinically compatible defined and xeno-free differentiation protocol is lacking. Hence, this study aimed to develop such chondrogenic differentiation medium for human BM-MSCs. We assessed the feasibility of the medium using three human BM-MSCs donors and validated the method by comparing BM-MSCs to three other cell types holding potential for articular cartilage repair. The effectiveness of the method was compared to conventional serum-free and commercially available chondrogenic differentiation media. The results show that the defined xeno-free differentiation medium is at least as efficient as conventionally used serum-free chondrogenic medium and performed significantly better on all cell types tested compared to the commercially available chondrogenic medium.
Keywords: Mesenchymal stromal cells, Xeno-free, Clinical grade, Chondrogenesis
Introduction
Articular cartilage is a highly specialized type of connective tissue that covers the ends of long bones facilitating transfer of load and providing a smooth, lubricated surface for joint movement. Due to its avascular and alymphatic structure, articular cartilage displays limited capacity for self-repair and defects left untreated often produce long-lasting musculoskeletal morbidity.
This study responds to a clinical need for novel cell-based therapies for articular cartilage defect repair such as autologous chondrocyte implantation (ACI). ACI utilizes chondrocytes isolated from the relatively non-weight bearing areas of the patient’s own hyaline cartilage tissue. However, the need for a substitutive cell source is apparent since there are many limitations related to this technique such as restrained quantity of biopsy material, donor site morbidity, dedifferentiation of chondrocytes during in vitro expansion, and mixed phenotype of the repair tissue (Dell’Accio et al. 2003; Horas et al. 2003; Matricali et al. 2010; Vasiliadis et al. 2010). Mesenchymal stromal cells (MSCs) possess great potential as an alternative solution as they are relatively easy to harvest, isolate, expand, and they are capable of chondrogenic differentiation (Dominici et al. 2006). In addition, previous studies have shown that human MSCs derived from bone marrow display better chondrogenic differentiation compared with other sources, e.g., adipose tissue (Afizah et al. 2007; Bernardo et al. 2007; Hennig et al. 2007).
Chondrogenic differentiation protocols for MSCs remained a challenge for decades, until two pioneering studies demonstrated successful in vitro chondrogenesis of cells isolated from postnatal mammalian bone marrow in 1998 (Johnstone et al. 1998; Mackay et al. 1998). Since then, many culture protocols that facilitate chondrogenic in vitro differentiation have been developed, but the challenges of producing nonhypertrophic stable articular chondrocytes are evident (De Bari et al. 2004; Pelttari et al. 2006; Grayson et al. 2010). Today it is obvious, nonetheless not fully understood, that in vitro chondrogenesis of MSCs requires particular culture conditions with correctly timed exposure to relevant growth factors, such as fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), and wingless-type (Wnt) proteins (Cleary et al. 2013).
However, despite all the current enthusiasm around MSCs, we are still in infancy regarding their use for cartilage repair. Few preclinical and clinical studies have been performed, and although MSCs have been reported to be as effective as autologous chondrocytes in cartilage repair (Nejadnik et al. 2010), no reliable spontaneous production of hyaline cartilage-like repair tissue upon transplantation into cartilage lesions has been accomplished (Ponticiello et al. 2000; Wakitani et al. 2002; 2004; Kuroda et al. 2007; Wakitani et al. 2007; Yan and Yu 2007; Chang et al. 2008; Mrugala et al. 2008; Wakitani et al. 2011). We reasoned that chondrogenic treatment of the cells prior to transplantation could be one possible solution to enhance their regenerative capacity in vivo (Zscharnack et al. 2010; Marquass et al. 2011). However, clinically compatible defined and xeno-free differentiation protocol is lacking.
Therefore, the aim of this study was to develop a defined xeno-free chondrogenic differentiation condition for MSCs that can be used for clinical-grade cell production. It is important to emphasize that our xeno-free method spans from MSC isolation and expansion and covers the whole differentiation process to acquire truly xeno-free MSC-derived chondrocytes for cartilage repair. Conventional cell culture conditions contain many animal-derived components mainly because they are easily available at low expense. However, use of animal-derived cell culture reagents increases the risk of zoonoses and host immune reactions (Horwitz et al. 2002; Spees et al. 2004; Heiskanen et al. 2007), namely antibody responses against animal-derived proteins or glycans. In addition, xeno-antigens have been shown to induce alterations in crucial stem cell-specific signaling systems that maintain self-renewal and pluripotency of MSCs (Nystedt et al. 2010). Thus, replacement of animal-derived cell culture reagents with defined xeno-free formulation would significantly enhance the safety and quality of cell-based products.
We tested the potency of our novel defined xeno-free chondrogenic differentiation medium with bone marrow-derived MSCs (BM-MSCs) from three donors and validated the method with three other cell types holding potential for articular cartilage repair. In addition to the potency tests for functional suitability, we compared the genotypic response of the cells from one donor between the defined xeno-free and the conventional chondrogenic media during a prolonged culture time. The effectiveness of the method was compared to a conventional serum-free and a commercially available chondrogenic differentiation media.
Materials and methods
Cell culture
MSCs were obtained from bone marrow aspirates of three healthy volunteer donors (DONOR 1–3), aged 18–33 years, after signed informed consent according to the Declaration of Helsinki. The protocol was approved by the Ethics committee of the Hospital District of Helsinki and Uusimaa (Finland). Bone marrow was aspirated under local anesthesia from the posterior iliac crest and collected in heparinized tubes. The isolation of BM-MSCs has been described in detail earlier (Mitkari et al. 2013). The xeno-free expansion of BM-MSCs was based either on 10 % platelet-rich plasma (Mitkari et al. 2013) or 0.5 % platelet-rich lysate with 2.5 % pooled human serum (Octapharma, Vantaa, Finland). The cells were used at second passage (P2).
Human adult chondrocytes (hACs) originated from two Swedish patients, aged 18–24, undergoing ACI. These cells were kindly provided by Professor Anders Lindahl (Sahlgrenska University Hospital, Gothenburg, Sweden). The exclusion criteria for the ACI treatment included inflammatory joint disease, and diffuse and advanced degeneration of the joint. Chondrocytes were maintained in media composed of DMEM/F-12 (GIBCO/Invitrogen, Carlsbad, CA, USA) supplemented with antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) (GIBCO/Invitrogen), 2 mM l-Glutamine (GIBCO/Invitrogen), 25 µg/ml Sodium l-ascorbate (Sigma-Aldrich, St. Louis, MO, USA), and with 10 % human serum (HS; Valley Biomedical, Winchester, VA, USA). For ACI the cells are expanded in vitro up to P2. However, as these cells were changed from cell transplant to research material, it is possible that they were expanded further. The maximum passage of the hACs in these experiments was P5. Human osteoarthritic knee-derived chondrocytes (OA chondrocytes) were obtained from a Finnish elderly patient undergoing knee replacement surgery as approved by the Ethics committee of the Hospital District of Helsinki and Uusimaa (Finland). Intact cartilage was harvested from distal femoral head and minced. The cartilage pieces were incubated overnight in digestion medium consisting of 0.5 mg/ml collagenase type 1A (Worthington/Sigma-Aldrich) dissolved in DMEM/F-12 (Gibco/Invitrogen) supplemented with antibiotics [100 U/ml penicillin, 100 mg/ml streptomycin, 2.5 µg/ml fungizone (Gibco/Invitrogen)], 2 mM l-Glutamine (Gibco/Invitrogen), 25 µg/ml sodium l-ascorbate (Sigma-Aldrich), and 10 % FBS (HyClone/Thermo Scientific, Logan, UT, USA). The digested cartilage was filtered through 70 μm nylon mesh (Prinsal, Tuusula, Finland), the chondrocytes were collected by centrifugation, and washed once with phosphate-buffered saline (PBS). The proliferation medium for OA chondrocytes was as stated above, apart from collagenase. The cells were used at P2.
Human embryonic stem cell-derived mesenchymal progenitors (hES-MPs; Cellartis AB, Gothenburg, Sweden) were also kindly provided by Professor Lindahl. These commercially available cells are a homogeneous population of progenitor cells with a high resemblance to adult human MSCs and without donor to donor variability in the cell population (Karlsson et al. 2009). The proliferation medium for hES-MPs was composed of DMEM High Glucose with GlutaMAX™ and without pyruvate (Gibco/Invitrogen), supplemented with antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin), 10 % FBS, and 4 ng/ml human recombinant basic FGF (hrbFGF; Invitrogen). The cells were used at P4.
Chondrogenic differentiation
For the pellet culture the cells were harvested using TrypLE™ Express (Gibco/Invitrogen). Each sample contained 3 × 105 cells in a 15 ml conical polypropylene tube with 400 µl of appropriate medium. The cells were centrifuged at 300 g for 4 min to form pellets. Duration of the pellet culture was 14, 28 or 84 days and the medium was changed twice a week. The defined xeno-free differentiation medium was compared to conventional serum-free differentiation medium, commercially available STEMPRO® Chondrogenesis Differentiation Kit (Gibco/Invitrogen) medium, and cell type specific proliferation media (described in previous section). The composition of each differentiation medium is described in detail in Table 1.
Table 1.
Composition of chondrogenic differentiation media
| Reagent | Concentration | Manufacturer |
|---|---|---|
| Defined xeno-free chondrogenic medium | ||
| DMEM-HG w/l-glutamine | Gibco/Invitrogen | |
| Penicillin G sodium | 100 U/ml | Gibco/Invitrogen |
| Streptomycin sulfate | 100 µg/ml | Gibco/Invitrogen |
| TGF-β1 (human, natural) | 10 ng/ml | R&D Systems |
| l-ascorbic acid sodium salt | 25 µg/ml | Sigma-Aldrich |
| l-proline | 40 µg/ml | Sigma-Aldrich |
| Oradexon® (dexamethasone) | 40 ng/ml | Schering-Plough/Organon |
| Insuman® Rapid (insulin, human, recombinant) | 10 µg/ml | Sanofi Aventis |
| Holo-Transferrin (human, natural) | 8 µg/ml | Sigma-Aldrich |
| Sodium selenite | 10 ng/ml | Sigma-Aldrich |
| Linoleic acid | 5.5 µg/ml | Sigma-Aldrich |
| Human serum albumin (natural) | 1.5 mg/ml | Sigma-Aldrich |
| Conventional serum-free chondrogenic medium | ||
| DMEM-HG w/l-glutamine | Gibco/Invitrogen | |
| Penicillin G sodium | 100 U/ml | Gibco/Invitrogen |
| Streptomycin sulfate | 100 µg/ml | Gibco/Invitrogen |
| TGF-β1 (human, natural) | 10 ng/ml | R&D Systems |
| L-ascorbic acid sodium salt | 25 µg/ml | Sigma-Aldrich |
| l-proline | 40 µg/ml | Sigma-Aldrich |
| Oradexon® (dexamethasone) | 40 ng/ml | Schering-Plough/Organon |
| ITS + ™ Premix | 1 % | BD Biosciences |
| STEMPRO® Chondrogenesis Differentiation Kit | ||
| STEMPRO® Differentiation Basal Medium | Gibco/Invitrogen | |
| Penicillin G sodium | 100 U/ml | Gibco/Invitrogen |
| Streptomycin sulfate | 100 µg/ml | Gibco/Invitrogen |
| STEMPRO® Chondrogenesis Supplement | 10 % | Gibco/Invitrogen |
For verification of the defined xeno-free medium, the number of BM-MSC pellets was 6–8 for glycosaminoglycan (GAG) detection and three for histochemical staining from all three donors (DONOR 1–3). The samples were collected at 14 and 28 days of culture. From DONOR 1, additional 6 samples were made for qRT-PCR and collected after 14, 28 and 84 days of culture.
For comparison of defined xeno-free differentiation medium, three parallel pellets were formed from BM-MSCs (DONOR 1), hACs (two donors), OA chondrocytes (single donor) and hES-MPs (commercial cell line) for each condition [cell type specific proliferation medium (without any added chondrogenic factors), defined xeno-free chondrogenic medium, conventional serum-free chondrogenic medium, and STEMPRO® Chondrogenesis Differentiation Kit medium]. For the comparison, DONOR 1 cells were selected as a representative of the three BM-MSC donors. The pellets were collected after 14 days of culture and subsequently processed to histological samples.
Histology
Histological samples were fixed with 10 % buffered formalin, dehydrated in ascending ethanol series and embedded in paraffin. Formalin fixed paraffin embedded (FFPE) samples were sectioned into 5 µm sections. Tissue sections on slides were deparaffinized and rehydrated before histological staining. For Safranin-O staining the slides were placed in filtered 0.5 % Safranin-O solution (0.1 M acetate buffer at pH 4.6) for 10 min and subsequently washed with running tap water for 10 min. For thionine staining the slides were placed in 0.04 % thionine solution (15 mM acetate buffer at pH 4.5) for 5 min and subsequently in 70 % ethanol for 10 s to clear the staining.
Immunohistochemistry
The FFPE sample sections were subjected to immunohistochemical detection of collagen type II and collagen type X. Deparaffinized and rehydrated sections were digested with 2 mg/ml hyaluronidase (Sigma-Aldrich) and 2 mg/ml pronase (Calbiochem/Merck, Darmstadt, Germany) in PBS for one hour at +37 °C. Sections were incubated with 2 % hydrogen peroxide (Sigma-Aldrich) for 10 min at room temperature (RT) to block endogenous peroxidase activity. To prevent non-specific staining the sections were treated with 10 % goat serum (DAKO, Glostrup, Denmark) for 20 min at RT. Primary antibodies were used at 2 µg/ml in PBS with 1 % bovine serum albumin (BSA; Bovogen, Keilor East, VIC, Australia) and 0.1 % Triton X-100 (Sigma-Aldrich). Sections were incubated with primary antibodies at +4 °C overnight. Antibodies against collagen type II (ab34712) and collagen type X (ab58632) were obtained from Abcam (Cambridge, U.K). HRP-conjugated goat-anti-rabbit/mouse secondary antibody (EnVision + Dual Link goat anti-rabbit/mouse, DAKO) was subsequently applied on the slides and incubated for 35 min at RT. AEC + substrate chromogen (DAKO) was used to visualize the antibody binding. Samples were incubated with the chromogen for 10 min at RT, and subsequently washed twice with water. For counterstain Mayer’s Hematoxylin was used for 20 s, followed by 10 min wash with running tap water. Negative controls lacked the treatment with the primary antibodies or were incubated with rabbit immunoglobulin fraction 2 µg/ml (DAKO).
Dimethylmethylene blue-based assay
The amount of sulfated GAGs in the pellet cultures was quantified using the dimethylmethylene blue-based (DMB) assay. Six to eight individual samples per donor were analyzed. The samples were digested in 0.5 mg/ml Proteinase K in PBS overnight at +60 °C. Chondroitin 4-sulfate (Sigma-Aldrich) was used to create the standard curve. After digestion the samples were pipetted in duplets into 96-well microplates, 1,9-dimethylmethylene blue (at pH 3.0) was added, and the absorbance was measured with a microplate reader at 525 nm. DNA content of the pellets was quantified for the normalization of the DMB assay. The Quant-iT™ PicoGreen dsDNA Kit (Invitrogen) was used according to the manufacturer’s instructions. Briefly, the samples were pipetted in duplets into 96-microplates. Lambda DNA in 10 mM Tris–HCl, 1 mM EDTA (pH 7.5) was used as a standard. Aqueous working solution of Quant-iT™ PicoGreen dsDNA reagent was added to each sample and incubated for 3 min at RT. The fluorescence was measured with a microplate reader at 480/520 nm.
Imaging
Histologically and immunohistochemically stained slides were analyzed with an Axioplan 2 microscope, and digital images were acquired using AxioCam MRc camera and MRGrap 1.0.0.4 software (all from Carl Zeiss MicroImaging GmbH, Jena, Germany).
RNA isolation
The samples were placed in pre-freezed Tissue Lyser II holder (Qiagen, Helsinki, Finland) together with Tungsten Carbide Beads (Qiagen) and the instrument was operated at 30 Hz for 2 min at a time, and the runs were repeated 2–4 times, until the samples were completely disrupted. Sample disruption was followed by treatment with 1 ml of QIAzol Lysis Reagent (Qiagen) and Tissue Lyser II was operated for another set of 2 min runs at 30 Hz to homogenize the samples. Subsequently, 200 μl of chloroform (Sigma-Aldrich) was added, the tubes were shaken vigorously for 15 s, and centrifuged at 12,000g at 4 °C for 15 min. The aqueous phase containing the nucleic acids was transferred into a new collection tube and one volume of 70 % ethanol was added. Further purification was performed with RNeasy Mini Kit (Qiagen) according to manufacturer’s protocol. During RNA purification on-column DNase digestion was performed with the RNase-Free DNAse Set (Qiagen) according to manufacturer’s instructions to eliminate genomic DNA contamination. Isolated and purified RNA was eluted in 30 μl of nuclease-free water (Amresco, Solon, OH, USA), the total RNA concentrations were measured using NanoDrop 2000 Micro-Volume UV–Vis Spectrophotometer (Thermo Fischer Scientific, Wilmington, DE, USA), and the samples were stored at −80 °C for no longer than three months.
Reverse transcription and quantitative real-time PCR analysis
Reverse transcription of total RNA to single-stranded cDNA was performed with RevertAid First Strand cDNA Synthesis Kit (Fermentas/Fischer Scientific, Vantaa, Finland) according to manufacturer’s protocols. Quantitative real-time PCR (qRT-PCR) amplification was performed using cDNA as a template. TaqMan® Gene Expression Master Mix and sequence-specific TaqMan® Gene Expression Assays (both from Applied Biosystems, Hämeenlinna, Finland) were used to amplify the targeted cDNA. Reactions were prepared and performed according to manufacturer’s protocol. The expression of SRY (sex determining region Y)-box 9(SOX9; Hs00165814_m1), collagen type II alpha 1(COL2A1;(Hs00264051_m1), aggrecan(ACAN; Hs00153936_m1), runt-related transcription factor 2(RUNX2; Hs00231692_m1), collagen type X alpha 1(COL10A1; Hs00166657_m1) and alkaline phosphatase (ALP), liver/bone/kidney (ALPL; Hs01029144_m1) were measured by qRT-PCR, with glyceraldehyde-3-phosphate-dehydrogenease (GAPDH; Hs99999905_m1) as reference gene. Relative quantification of marker gene expression was performed applying the efficiency-corrected ΔΔCt method.
Statistics
The data are expressed as mean ± standard deviation and 95 % confidence intervals, which were obtained by bias-corrected bootstrapping (5,000 replications). The comparison between groups at different time points was made by permutation test for two independent samples. The linearity across the time points was tested by permutate type analysis of variance with an appropriate contrast. Bonferroni correction was performed to adjust for multiple testing. All statistical results were considered significant for P values less than 0.05.
Results
The defined xeno-free differentiation medium produces extracellular proteoglycan-rich matrix comparable to that of the conventional serum-free medium
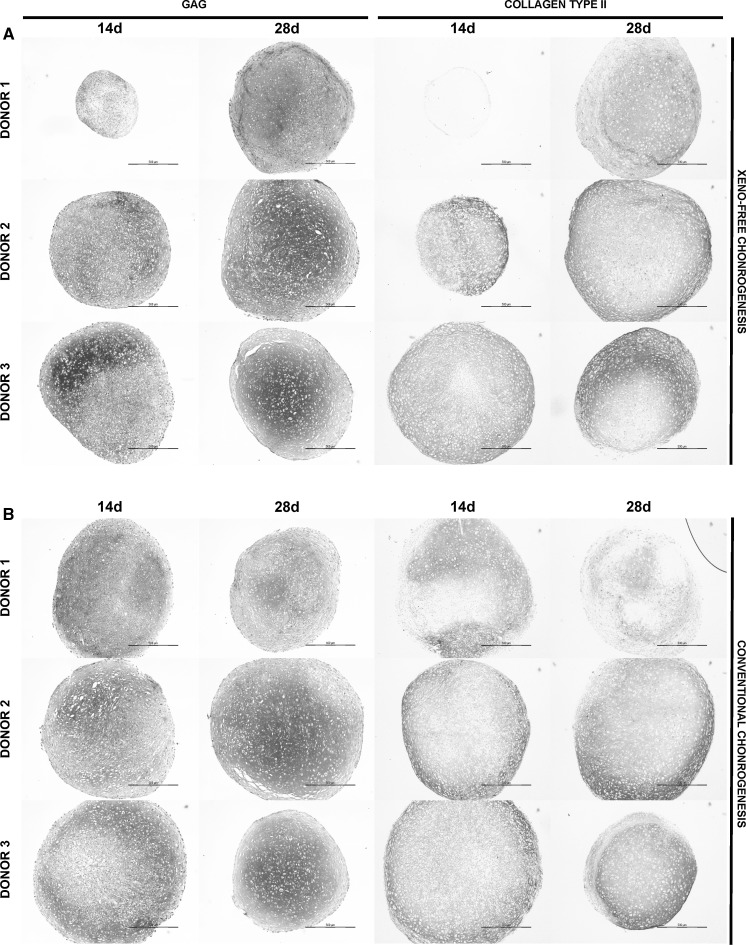
To verify the quality of the extracellular matrix (ECM) composition of the BM-MSC pellet cultures from the three donors (DONOR 1–3), we performed GAG detection by thionine and collagen type II by immunohistochemical staining. Although donor variation differentially influenced the ECM maturation of the pellets after 14 days of chondrogenic induction, no major differences in pellet size or GAG accumulation in the ECM between xeno-free and conventional conditions were observed by end of the 28-day culture (Fig. 1). Although there were variations between individual BM-MSC pellets both within and between the donors in xeno-free condition, the production of cartilaginous ECM, as indicated by collagen type II immunostaining, enhanced during the 28-day culture demonstrating the potency of the defined xeno-free differentiation medium (Fig. 1). The collagen type II rich matrix produced with the defined xeno-free medium after 28 days of culture was at least comparable to that produced by the conventional serum-free differentiation protocol. Overall, pellets produced from cells of the DONOR1 showed slightly poorer morphology compared to the pellets from donors 2 and 3. Nevertheless, these cells were able to produce more cartilaginous matrix in the xeno-free condition, indicating more profound differentiation capacity of the novel medium.
Fig. 1.
Defined xeno-free differentiation medium produces extracellular proteoglycan-rich matrix morphologically and structurally comparable to that of the conventional serum-free medium. Thionine staining of GAGs and immunohistochemical detection of collagen type II in the ECM of the BM-MSC pellets cultured in (a) the defined xeno-free or (b) conventional serum-free chondrogenic medium for 14 and 28 days. Exemplified light microscopic photographs of pellets (n = 3) from the three donors (DONOR 1–3). Scale bar: 500 µm
The total amount of DNA, GAG and GAG/DNA of the BM-MSC pellets from the three donors (DONOR 1–3) after 14- and 28-day chondrogenesis in defined xeno-free and conventional serum-free media are presented in Table 2. After adjustment for multiple testing, the differences between defined xeno-free and conventional serum-free media that remained statistically significant are also presented in Table 2. At day 14 of culture, the differentiation of DONOR 2 cells in the defined xeno-free and conventional serum-free media resulted in statistically different amounts of DNA in the pellets (P ≤ 0.05). At day 28 of culture, in addition to DONOR 2 cells the pellets of DONOR 1 cells showed statistically significant difference in the total DNA amount (P ≤ 0.01). The foremost statistically significant difference between the two differentiation conditions was observed in the amount of GAG and GAG/DNA ratio of the DONOR 1 pellets at day 14 (P ≤ 0.001).
Table 2.
DNA, GAG and GAG/DNA content of the BM-MSC pellets after 14 and 28 days of differentiation in defined xeno-free or conventional serum-free chondrogenic media
| 14-day chondrogenic differentiation | 28-day chondrogenic differentiation | |||||
|---|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | Donor 1 | Donor 2 | Donor 3 | |
| DNA (µg) | ||||||
| Xeno-free | 0.5 ± 0.3 | 1.5 ± 0.1 | 1.3 ± 0.4 | 0.6 ± 0.1 | 1.6 ± 0.1 | 0.5 ± 0.2 |
| Conventional | 0.9 ± 0.4 | 1.7 ± 0.1* | 1.9 ± 0.7 | 0.9 ± 0.2** | 1.8 ± 0.1** | 0.7 ± 0.1 |
| GAG (µg) | ||||||
| Xeno-free | 3.9 ± 1.3 | 43.7 ± 2.9 | 55.8 ± 4.1 | 51.4 ± 23.0 | 38.9 ± 10.1 | 42.0 ± 17.2 |
| Conventional | 50.4 ± 15.7*** | 48.1 ± 2.1 | 51.9 ± 3.8 | 52.7 ± 23.2 | 42.5 ± 15.4 | 38.4 ± 8.9 |
| GAG/DNA | ||||||
| Xeno-free | 7.3 ± 6.3 | 30.2 ± 4.7 | 48.3 ± 22.0 | 93.5 ± 57.0 | 25.1 ± 6.6 | 95.5 ± 65.7 |
| Conventional | 66.1 ± 39.3*** | 28.3 ± 2.2 | 30.4 ± 9.3 | 66.3 ± 40.7 | 24.3 ± 8.9 | 59.0 ± 16.1 |
Data are presented as average ± standard deviation of 6–8 pellets
Statistically significant difference between defined xeno-free and conventional serum-free conditions after adjustment for multiple comparisons
* P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001
Despite donor-dependent differences, the data provide important proof-of-principle evidence of the potency of the novel defined xeno-free chondrogenesis medium to be able to induce chondrogenic differentiation of BM-MSCs. The ECM of the cartilaginous pellets produced with the defined xeno-free protocol was morphologically and structurally at least comparable to the conventional serum-free medium.
Chondrogenic markers are upregulated under xeno-free conditions
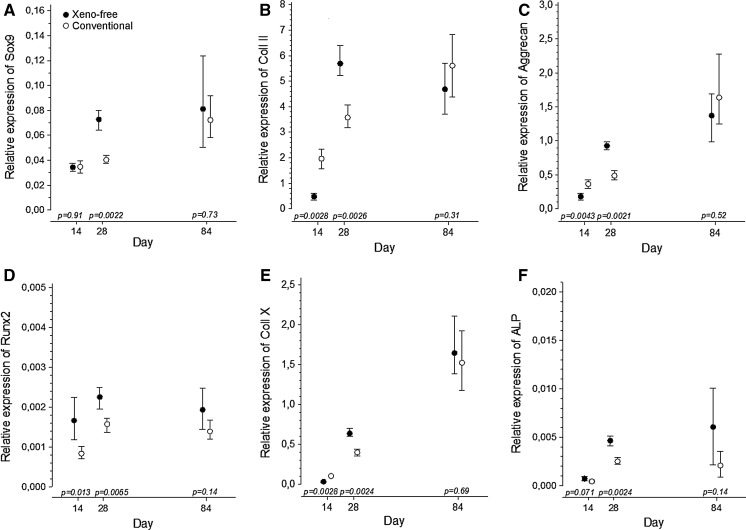
Transcript analysis of BM-MSCs from DONOR 1 showed that the chondrogenic genes, SOX9, COL2A1 and ACAN, were upregulated both in the defined xeno-free and conventional serum-free chondrogenic media during the 14 to 84 days of culture (P ≤ 0.01 for linearity). By the end of the 84-day culture, these genes were equally expressed (Fig. 2a–c). Interestingly, at 28 days of culture, the xeno-free samples showed significantly higher expression levels of these markers compared to the conventional serum-free condition, possibly indicating more vigorous chondrogenesis with the defined xeno-free medium.
Fig. 2.
Chondrogenic markers are upregulated under xeno-free conditions. Relative RNA expression of a SOX9, b COL2A1, c ACAN, d RUNX2, e COL10A1 and f ALP in the BM-MSC pellets cultured in defined xeno-free and conventional serum-free chondrogenic medium for 14, 28 and 84 days. The mRNA levels were normalized to glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). Data were expressed as the mean (dot) with 95 % confidence intervals (whiskers) of six biological replicates from one donor (DONOR 1)
The relative expression of Runx2 transcription factor, an indicator for osteogenic commitment, was significantly higher in xeno-free medium when compared to the conventional medium at days 14 and 28 of culture. At the end of the 84-day culture, the relative expression of RUNX2 reached comparable levels in xeno-free and conventional culture conditions (Fig. 2d). The trend in RUNX2 expression during the 84-day culture showed steady upregulation in conventional serum-free condition (P ≤ 0.05 for linearity) but remained statistically constant in the defined xeno-free culture (P ≥ 0.05 for linearity) during the 84-day chondrogenic induction. During the differentiation period, the collagen type X was clearly upregulated in both conditions (P ≤ 0.001 for linearity), and by the end of the 84-day culture, the expression levels of COL10A1 were comparable (Fig. 2e). The relative expression of ALP, an enzyme essential for calcification, was upregulated in the both conditions tested (P ≤ 0.01 for linearity in defined xeno-free medium; P ≤ 0.05 for linearity in conventional serum-free medium). ALP expression reached similar levels in xeno-free and conventional conditions after the 84-day culture period, albeit high variation between pellets in xeno-free condition was observed (Fig. 2f).
Taken together, these data suggest that at least in this individual donor (DONOR 1), the expression of chondrogenic and hypertrophic markers in defined xeno-free chondrogenic medium is at least comparable to that of the conventional serum-free differentiation method. In addition, the expression data of the chondrogenic genes indicates that the chondrogenic induction of the BM-MSCs might be more efficient in the xeno-free condition.
Chondrogenically differentiated BM-MSCs under defined xeno-free conditions produce collagen-rich extracellular matrix in a similar manner as chondrocytes
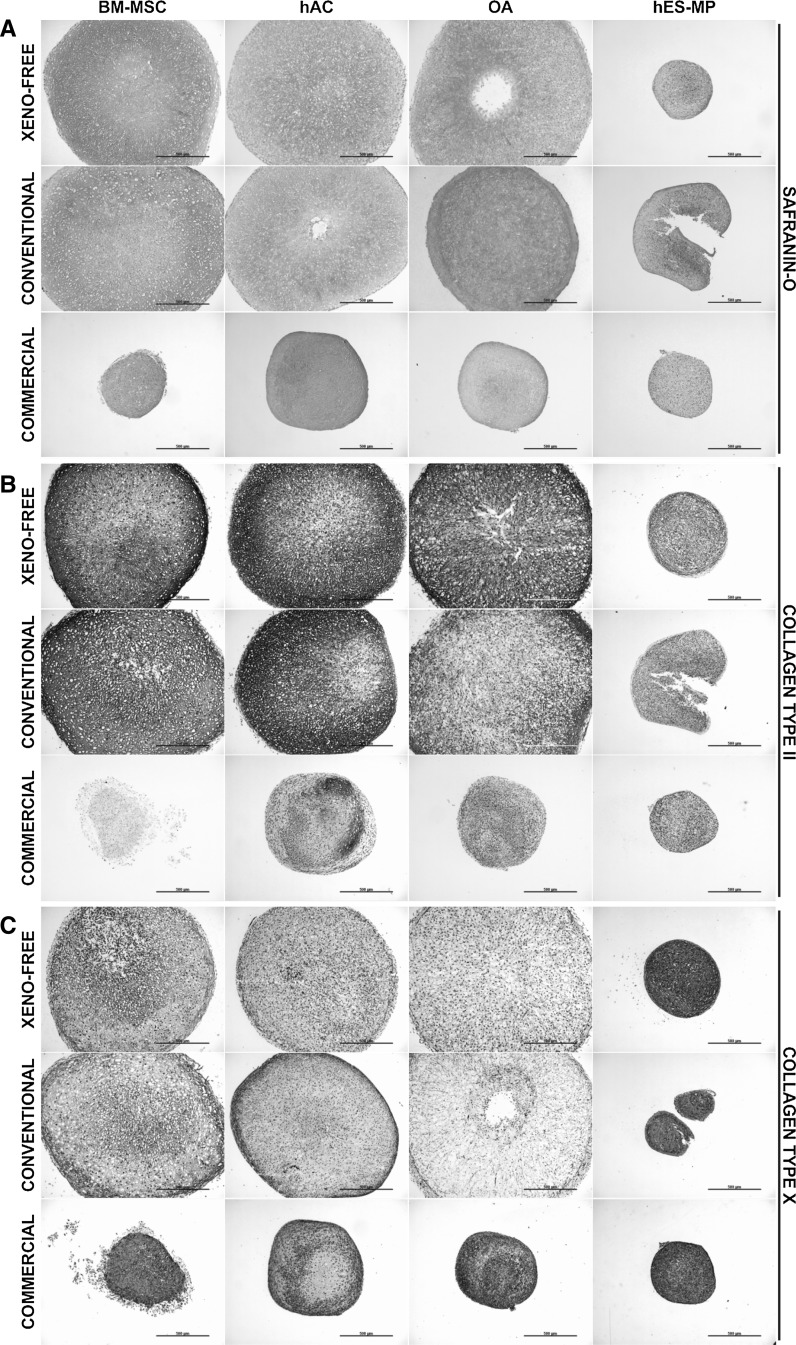
To assess the efficiency of chondrogenic differentiation we visualized the GAGs in the ECM by Safranin-O staining. After 14 days of differentiation pellets of BM-MSCs (DONOR 1), hACs, and OA chondrocytes showed equally efficient chondrogenesis both in defined xeno-free differentiation medium and in conventional serum-free chondrogenic medium (Fig. 3a). These pellets had considerably increased in size over the 14-day differentiation period and it was clear that growth resulted from ECM production. In contrast, hES-MP pellets failed to show any progress in differentiation in the defined xeno-free differentiation condition and only slight GAG deposition could be detected in the conventional serum-free chondrogenic medium (Fig. 3a). OA chondrocyte and hAC pellets cultured in commercially available chondrogenic medium showed minor growth in pellet size and some GAG production, while pellets formed with BM-MSCs or hES-MPs failed to differentiate (Fig. 3a). Moreover, all pellets cultured in the respective cell type specific proliferation medium had either remained the same or decreased in size, and no GAG production could be observed (data not shown).
Fig. 3.
BM-MSCs differentiate in defined xeno-free chondrogenic differentiation medium as efficiently as chondrocytes and produce ECM similar to that of chondrocyte pellets. Triplicate samples of BM-MSCs, hACs, OA chondrocytes, and hES-MPs were placed under defined xeno-free chondrogenic differentiation medium, conventional serum-free chondrogenic differentiation medium, and commercially available differentiation medium for 14 days as pellet cultures. Exemplified light microscopic photographs of pellets stained with Safranin-O (a), against collagen type II (b), and collagen type X (c). Scale bar: 500 µm
To compare the ECM composition of the pellet cultures we performed immunohistochemical staining for collagen type II and X. After 14 days of differentiation the pellets of BM-MSCs, hACs, and OA chondrocytes in defined xeno-free and conventional serum-free chondrogenic media stained strongly for collagen type II (Fig. 3b), but staining for collagen type X was clearly weaker (Fig. 3c). In these cell types chondrogenic differentiation with defined xeno-free or conventional serum-free differentiation media produced comparable pellet structure where collagen distribution was regular and even. In contrast, the use of commercially available differentiation medium led to weak collagen type II staining (Fig. 3b), but substantially stronger collagen type X staining (Fig. 3c). In addition, the collagen distribution was markedly irregular. The collagen production and pellet structure in cell type specific proliferation medium was comparable to that of the commercially available medium (data not shown). Deviant from the others, hES-MP pellets stained weakly for collagen type II under all conditions (Fig. 3b), but collagen type X staining was clearly stronger compared to other cell types (Fig. 3c). In these pellets distribution of collagens and the pellet structure were disturbed and irregular.
Altogether, these data indicate that BM-MSCs differentiate towards chondrocytic lineage both in defined xeno-free and conventional serum-free chondrogenic media as efficiently as chondrocytes (i.e., hACs and OA chondrocytes). In addition, under these conditions BM-MSC and chondrocyte pellets produce ECM similar in composition and structure.
Discussion
The potential of BM-MSCs for cartilage repair was demonstrated in a recent study that compared the clinical outcomes of patients with first generation ACI to patients treated with autologous BM-MSC transplants (Nejadnik et al. 2010). BM-MSCs resulted to be less time-consuming and less expensive compared to the ACI procedures, and without donor site morbidity. However, previous studies have also demonstrated that MSCs are not spontaneously capable of producing hyaline cartilage-like repair tissue in vivo (Wakitani et al. 2002; Kuroda et al. 2007; Centeno et al. 2008; Wakitani et al. 2011). Chondrogenic treatment of the cells prior to transplantation could be one possible solution to enhance their regenerative capacity in vivo (Zscharnack et al. 2010; Marquass et al. 2011). To date, no clinical results on the use of in vitro pre-differentiated BM-MSCs for cartilage repair have been reported, and there are currently no registered clinical trials utilizing such cells (clinicaltrials.gov; search words: cartilage and mesenchymal; search date August 2013). Moreover, a defined and xeno-free differentiation method for BM-MSC-derived chondrocytic cells, a prerequisite for clinical applications, has not been published thus far.
Today, serum-free in vitro chondrogenesis is a standard procedure and various studies around this area have been conducted over the years (Barry et al. 2001; Awad et al. 2003; Lee et al. 2009). However, serum-free and xeno-free concepts are quite distinct from each other. Customary medium supplements (which may be nominally serum-free, but usually contain undefined products extracted from animal or human serum) used for serum-free differentiation are not compatible with clinical grade cell production, and thus, those should be replaced with specific products of highest quality (Moore 2006; Sensebé 2008; Sensebé and Bourin 2009). Furthermore, from the regulatory perspective, consistence of the production methods and traceability of materials and reagents are of particular interest when producing MSCs for clinical applications (Sensebé et al. 2010). This study is the first one to directly challenge the conventional serum-free chondrogenic differentiation protocol with a defined xeno-free differentiation medium.
During this study we observed that the defined xeno-free chondrogenic differentiation medium produced pellets with proteoglycan-rich ECM resembling those grown in conventional serum-free chondrogenic differentiation medium, but performance of the commercially available differentiation medium was clearly weaker. These observations were further strengthened by immunohistochemical stainings that showed the production of collagen type II in the xeno-free and conventional serum-free media. In addition, our results demonstrated that BM-MSCs differentiated at least as efficiently as ex vivo chondrocytes, but performance of hES-MPs was significantly weaker. The results of the expression patterns of chondrogenic genes SOX9, COL2A1, and ACAN, indicated elevated potency for chondrogenic differentiation with the xeno-free method. This was verified in matrix production, where cells differentiated with the defined xeno-free chondrogenic medium were more capable of producing GAGs in their surroundings.
The large inter-donor variability is a common phenomenon, and a true clinical problem with BM-MSCs. Based on our results on biochemical analysis of proteoglycans, this problem was also evident in the present study. Moreover, as the photomicrographs and standard deviations of biochemical and expression analyses demonstrated, there was also a large intra-donor variation. For example, in the GAG analysis, statistically significant difference (P ≤ 0.001) of DONOR 1 pellets was detected between the two differentiation media at day 14; pellets grown in the defined xeno-free differentiation medium showed clearly weaker GAG deposition compared to pellets grown in the conventional serum-free differentiation medium. However, it was also observed that at day 28 the xeno-free chondrogenic pellets had superseded the conventional pellets. Differentially timed response to chondrogenic stimuli under these two conditions is most likely caused by the fact that BM-MSC cultures are heterogeneous mixtures of cells at various stages and this type of variation is a common phenomenon in MSC experiments (Phinney et al. 1999; Pelttari et al. 2006; Larson et al. 2008). Although the linear trend of the expression pattern of RUNX2 during the 84-day culture period showed statistically significant change in the conventional serum free medium (increase in relative expression during the culture time), these differences are probably biologically insignificant. Premature induction of hypertrophy is one of the major problems of in vitro chondrogenesis of MSCs; cells adopt a transient chondrocyte phenotype that is merely an intermediate state on the way towards bone formation (Dickhut et al. 2009; Farrell et al. 2009; Hellingman et al. 2010; Gelse et al. 2012). Even though collagen type X is normally expressed at low or moderate levels in mature articular cartilage (Rucklidge et al. 1996; Wachsmuth et al. 2006), its production, marking the transition towards hypertrophy, is usually not desirable in chondrogenesis experiments. From our results it could be observed that hypertrophic markers were expressed in similar manner under defined xeno-free and conventional serum-free differentiation conditions and collagen type X production of MSCs was similar to that of chondrocytes. Nevertheless, the scope of this study was not to investigate the process or prevention of hypertrophy in vitro, but to validate a method for chondrogenesis that can be translated to clinical practice. The true potency of the medium described herein must be tested in patients. However, in clinical practice, the in vitro induction of chondrogenesis of BM-MSCs and the construction of the tissue engineered chondrogenic/cartilaginous tissue for implantation, will most likely span only through few weeks. Based on our gene expression and ECM production results, the chondrogenic induction time of 14 days in the defined xeno-free medium seems to be a promising preinduction time for future clinical investigations.
Alongside the differentiation procedure, the effect of preceding cell manipulation on cell behavior through expansion culture should not be underestimated. Here, the inferior performance of the commercially available differentiation medium was most likely due to incompatibility between expansion and differentiation conditions, which might have had a negative effect on the differentiation potential of the cells. In addition, the commercial cell line, hES-MPs, showed signs of differentiation only under conventional serum-free differentiation medium, indicating that the respective cell type specific expansion medium favors this subsequent differentiation condition and is not compatible with the other differentiation methods. Overall inferior performance of hES–MPs is interesting, as the functionality of these cells is controversial. It may be that these cells do not represent true mesenchymal progenitors, regardless their morphology and surface markers, although their differentiation potential towards mesodermal lineage has been previously demonstrated (Karlsson et al. 2009). Alternatively, the differentiation potential of these cells may be, in fact, highly dependent on multiple factors (e.g., culture conditions, freeze–thaw cycles, and passaging) despite their embryonic origin. As previous studies have shown, xeno-free expansion culture possesses many advantages over serum supplemented culture in MSC production (Doucet et al. 2005; Lange et al. 2007; Bieback et al. 2009; Miwa et al. 2012; Chase et al. 2012). In addition, MSCs are very sensitive about their culture conditions and do not cope well with unstable or changing environment (Sotiropoulou et al. 2006). Our results demonstrated that expansion and subsequent differentiation of human BM-MSCs under xeno-free condition is a good alternative system to generate cartilage in vitro and, thanks to its intrinsic characteristics, safer to be used for clinical application in patients.
Conclusion
This study has shown that it is possible to develop a defined and xeno-free chondrogenic differentiation protocol for BM-MSCs that is at least as effective as conventionally used serum-free chondrogenic medium. This is an important finding because in general xeno-free and defined media are considered less effective than those containing animal/human sera or other animal-derived additives, and are therefore refrained as a true alternative for large-scale cell production. In addition, customary medium supplements are not proven safe for clinical use and thus, we need to seek for alternative reagents of higher quality to produce cell culture protocols competent for clinical use.
Acknowledgments
The authors wish to acknowledge the European Science Foundation’s Regenerative Medicine (REMEDIC) activity, the Maud Kuistila Memorial Foundation and the Emil Aaltonen Foundation for their financial support (V.M.). Viikki Doctoral Programme in Molecular Biosciences (VGSB) is acknowledged for financial support (M.S.). The hACs and hES-MPs were kindly provided by Professor Anders Lindahl (Sahlgrenska University Hospital, Gothenburg, Sweden). Hannu Kautiainen, BA, and Salme Järvenpää, MSc, are acknowledged for their valuable contribution for the statistical analysis (MedCare Ltd, Äänekoski, Finland). The authors thank Sirkka Hirschovits-Gerz for excellent technical assistance with BM-MSC culture (Finnish Red Cross Blood Service, Helsinki, Finland).
Footnotes
Maria Skog and Virpi Muhonen have contributed equally to this work.
References
- Afizah H, Yang Z, Hui JH, Quyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- Awad HA, Halvorsen YD, Gimble JM. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Emons JA, Karperien M, Nauta AJ, Willemze R, Roelofs H, Romeo S, Marchini A, Rappold GA, Vukicevic S, Locatelli F, Fibbe WE. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007;48:132–140. doi: 10.1080/03008200701228464. [DOI] [PubMed] [Google Scholar]
- Bieback K, Hecker A, Kocaömer A, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T, Ochiai N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26:18–26. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- Chase LG, Yang S, Zachar V, Yang Z, Lakshmipathy U, Bradford J, Boucher SE, Vemuri MC. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl Med. 2012;1:750–758. doi: 10.5966/sctm.2012-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MA, van Osch GJ, Brama PA, Hellingman CA, Narcisi R (2013) FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med. doi: 10.1002/term.1744 [Epub ahead of print] [DOI] [PubMed]
- De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–150. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- Dell’Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16–27. doi: 10.1016/S0014-4827(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Dickhut A, Pelttari K, Janicki P, Wagner W, Eckstein V, Egermann M, Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- Farrell E, van der Jagt OP, Koevoet W, Kops N, van Manen CJ, Hellingman CA, Jahr H, O’Brien FJ, Verhaar JA, Weinans H, van Osch GJ. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods. 2009;15:285–295. doi: 10.1089/ten.tec.2008.0297. [DOI] [PubMed] [Google Scholar]
- Gelse K, Ekici AB, Cipa F, Swoboda B, Carl HD, Olk A, Hennig FF, Klinger P. Molecular differentiation between osteophytic and articular cartilage–clues for a transient and permanent chondrocyte phenotype. Osteoarthr Cartil. 2012;20:162–171. doi: 10.1016/j.joca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthr Cartil. 2010;18:714–723. doi: 10.1016/j.joca.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U, Mikkola M, Olsson C, Miller-Podraza H, Blomqvist M, Olonen A, Salo H, Lehenkari P, Tuuri T, Otonkoski T, Natunen J, Saarinen J, Laine J. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25:197–202. doi: 10.1634/stemcells.2006-0444. [DOI] [PubMed] [Google Scholar]
- Hellingman CA, Koevoet W, Kops N, Farrell E, Jahr H, Liu W, Baatenburg de Jong RJ, Frenz DA, van Osch GJ. Fibroblast growth factor receptors in in vitro and in vivo chondrogenesis: relating tissue engineering using adult mesenchymal stem cells to embryonic development. Tissue Eng Part A. 2010;16:545–556. doi: 10.1089/ten.tea.2008.0551. [DOI] [PubMed] [Google Scholar]
- Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Emanuelsson K, Wessberg F, Kajic K, Axell MZ, Eriksson PS, Lindahl A, Hyllner J, Strehl R. Human embryonic stem cell-derived mesenchymal progenitors–potential in regenerative medicine. Stem Cell Res. 2009;3:39–50. doi: 10.1016/j.scr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohqushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthr Cartil. 2007;15:226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213:18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- Larson BL, Ylöstalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008;26:193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim JH, Jo CH, Seong SC, Lee JC, Lee MC. Effect of serum and growth factors on chondrogenic differentiation of synovium-derived stromal cells. Tissue Eng Part A. 2009;15:3401–3415. doi: 10.1089/ten.tea.2008.0466. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Marquass B, Schulz R, Hepp P, Zscharnack M, Aigner T, Schmidt S, Stein F, Richter R, Osterhoff G, Aust G, Josten C, Bader A. Matrix-associated implantation of pre-differentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39:1401–1412. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- Matricali GA, Dereymaeker GP, Luyten FP. Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Belg. 2010;76:669–674. [PubMed] [Google Scholar]
- Mitkari B, Kerkelä E, Nystedt J, Korhonen M, Mikkonen V, Huhtala T, Jolkkonen J. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol. 2013;239:158–162. doi: 10.1016/j.expneurol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Miwa H, Hashimoto Y, Tensho K, Wakitani S, Takagi M. Xeno-free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology. 2012;64:301–308. doi: 10.1007/s10616-011-9400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. The medium is the message. Nat Biotechnol. 2006;24:160–161. doi: 10.1038/nbt0206-160. [DOI] [PubMed] [Google Scholar]
- Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, Jorgensen C, Noël D. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67:288–295. doi: 10.1136/ard.2007.076620. [DOI] [PubMed] [Google Scholar]
- Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- Nystedt J, Anderson H, Hirvonen T, Impola U, Jaatinen T, Heiskanen A, Blonqvist M, Satomaa T, Natunen J, Saarinen J, Lehenkari P, Valmu L, Laine J. Human CMP-N-acetylneuraminic acid hydroxylase is a novel stem cell marker linked to stem cell-specific mechanisms. Stem Cells. 2010;28:258–267. doi: 10.1002/stem.250. [DOI] [PubMed] [Google Scholar]
- Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. doi: 10.1002/(SICI)1097-4644(19991201)75:3<424::AID-JCB8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246–255. doi: 10.1002/1097-4636(200011)52:2<246::AID-JBM2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Rucklidge G, Milne G, Robins S. Collagen type X: a component of normal human, pig, and rat articular cartilage. Biochem Biophys Res Commun. 1996;224:297–302. doi: 10.1006/bbrc.1996.1024. [DOI] [PubMed] [Google Scholar]
- Sensebé L. Clinical grade production of mesenchymal stem cells. Biomed Mater Eng. 2008;18:S3–S10. [PubMed] [Google Scholar]
- Sensebé L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- Sensebé L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal stem cells for clinical application. Vox Sang. 2010;98:93–107. doi: 10.1111/j.1423-0410.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943–949. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- Wachsmuth L, Söder S, Fan Z, Finger F, Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol Histopathol. 2006;21:477–485. doi: 10.14670/HH-21.477. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transpl. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, Kurosaka M, Yoshiya S, Hattori K, Ohgushi H. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B. Repair of chronic osteochondral defects using pre-differentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38:1857–1869. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]