Abstract
The multipotent and immunosuppressive capacities of mesenchymal stem cells (MSCs) attract several scientists worldwide towards translational research focusing on treatment of diseases including liver failure. Though MSC’s have been isolated from different sources, researchers do not concur on the best source for expansion and clinical translation. In this study, we have compared the isolation, proliferation and expansion of MSCs from umbilical cord blood (UCB), Wharton’s Jelly (WJ), bone marrow (BM) and adipose tissue (AT). MSCs were isolated by density gradient separation from UCB, BM and AT and by both enzymatic and explant method for WJ. The MSCs are characterized by their ability to adhere to plastic, expression of positive (CD105, CD73, CD90, CD29, CD44) and negative (CD45, CD14, CD34) markers by flow cytometry and also by their in vitro adipogenic, osteogenic and chondrogenic differentiation. This comprehensive study clearly shows that WJ is better than UCB both in terms of rapidity, yield and ease of procedure. AT and BM are autologous sources for MSC’s but the specimen collection involves cumbersome and painful procedures and an invasive approach. However being autologous, they are safe and probable candidates for therapeutic future applications.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-014-9718-z) contains supplementary material, which is available to authorized users.
Keywords: Mesenchymal stem cells, Stem cell therapy, Explant method, Oct-4
Introduction
Mesenchymal stem cells have generated interest in a wide variety of biomedical disciplines including clinical therapy applications. They can be sourced from various tissues like blood, bone marrow (BM), trabecular bone, adipose tissue (AT), dermis, synovium, skeletal muscle, and pericytes (Tuan et al. 2003). Using Umbilical cord, MSCs have been isolated from cord blood, umbilical vein, sub endothelium and Wharton’s jelly (WJ) as well as from three relatively indistinct regions of the umbilical cord matrix: the perivascular zone, the intervascular zone, and subamnion (Karahuseyinoglu et al. 2007). Many earlier reports show that these cells have therapeutic potential, possibly as a substitute cell for BM-derived mesenchymal stem cells for cellular therapy.
Scientific reports are varied in their protocols for isolation, expansion and characterization. Hence, mesenchymal and tissue stem cell committee of the International Society for Cellular Therapy (ISCT) has proposed minimal criteria to define human MSCs. MSCs must be plastic adherent, express CD105, CD73 and CD90 and lack expression of CD45, CD34, and CD14 OR CD19 and HLA-DR surface molecules, as well as be capable of differentiation to osteogenic, adipogenic and chondrogenic lineages (Dominici et al. 2006). The ability to form colony forming units (CFU) which demonstrates self renewal property is a characteristic feature of stem cells (La Rocca et al. 2009). In this study, the MSCs derived from WJ, umbilical cord blood (UCB), AT and BM developed colonies as well as fulfilled the minimum criteria of ISCT.
Bone marrow, a frequently used source for MSCs, engages a painful collection procedure. Umbilical cord matrix (WJ) is found to be a good source of MSCs compared with UCB and BM with regard to isolation and expansion potential (Mustapha et al. 2010). AT was also found to be a good autologous source of MSCs (Zuk et al. 2002).
In this work, four independent sources have been compared to study the efficiency of MSC isolation and proliferation. We have also investigated the effectiveness of isolation of MSC’s from WJ by the simple explant method compared to an enzymatic method. The characterization has also been pursued following the criteria laid down by the International Society of Cellular Therapy in each of these cases. A comprehensive study of this magnitude covering sources, methods of isolation as well as comparison of the characteristics by various techniques has not been attempted before and therefore this work is a new and fresh look at these aspects.
Materials and methods
Collection, isolation and culture of MSCs
Samples were collected and processed after obtaining informed consent and with clearances from Stem cell ethical committee and Review Board of the Government Stanley Hospital.
Umbilical cord blood
Umbilical cord blood was collected from 35 mothers undergoing cesarean section at RSRM hospital, an obstetrics section of the Government Stanley Medical College and Hospital (Chennai, India). The cord blood was collected into Vacutainers K2 EDTA (BD Biosciences, Gurgaon, Haryana, India). Mononuclear cells were separated using Ficoll hypaque (Sigma, St. Louis, MO, USA) gradient method and cultured using DMEM—low glucose (Sigma) with 20 % FBS (Sigma, F2442) at seeding density of 9 × 104 cells/cm2. Medium was changed every 48–72 h. On reaching 70 % confluency, the cells were passaged in the split ratio of 1:2 (Boyum 1968; Needham 1987).
Cord matrix/Wharton jelly
The umbilical cord, devoid of blood (35 samples), was cut and placed into Phosphate Buffered Saline (PBS) (Sigma, USA, D5652) with antibiotics (300 U of penicillin and 300 µg of streptomycin) and processed within 1–3 h by the two methods mentioned below.
Explant method
Dices of umbilical cord, 0.5 mm thick were cultured in DMEM-low glucose with 20 % fetal bovine serum (FBS) (Sigma) at 37 °C, 5 % CO2, and left undisturbed for 7 days to allow for migration of cells from explants. On day 7, explants were removed. Medium was changed thereafter every 48–72 h. On reaching 70 % confluency, the cells were passaged in the split ratio of 1:2 (Freshney et al. 2007).
Enzymatic method
Dices of umbilical cord, 0.5 cm thick were transferred to 50 ml tubes with serum free DMEM (Sigma) and centrifuged at 2,000 rpm for 10 min. The supernatant was discarded and the pellet was immersed in 0.1 % collagenase (Gibco Cat No. 17104019) and kept overnight. Double volume of PBS (Sigma, D5652) was added and centrifuged at 2,000 rpm for 10 min. The supernatant was discarded and the pellet was treated with 2.5 % trypsin (Sigma) at 37 °C for 30 min. FBS was added to neutralize the excess trypsin. After a wash in culture medium the cells were resuspended and seeded.
Bone marrow
Twenty milliliter of BM was collected from seven patients at the Institute of Surgical Gastroenterology, Government Stanley Hospital (Chennai, India) into containers containing anti-coagulant citrate dextrose (ACD) (Sigma, C3821) in a ratio of 1:5. Mononuclear cells were separated using the Ficoll hypaque (Sigma) gradient method. The cells were seeded at a density of 15 × 104/cm2 in alpha-MEM (Sigma), 10 % FBS, 2 mM l-glutamine (Sigma), 300/mL Penicillin, 300 μg/mL Streptomycin, 5 μM Hydrocortisone (Sigma), 100 μM 2-mercaptoethanol (Sigma, M7522) and incubated at 37 °C and 5 % CO2 for 3 days and later the medium was refreshed every 48 h till confluence was reached. On >70 % confluence the cells were trypsinised (0.05 % trypsin–0.2 % EDTA) and subsequently passaged by splitting them into two or three flasks (Freshney et al. 2007).
Adipose tissue
Two AT samples from the abdominal region were obtained during elective abdominal surgeries. AT was finely minced with the help of a scalpel (SC and Omental) and digested using Collagenase type IV (Gibco Cat No. 17104019) for 10 min at room temperature. The dissociated tissue was centrifuged for 5 min at 500 g at 37 °C. The supernatant was decanted and the pellet (stromal vascular fraction—SVF) resuspended with MesenproRS medium (Invitrogen, Carlsbad, CA, USA, Cat No. 12746012) and seeded at a density of 14 × 104 cells/cm2. The efficiency of isolation was calculated by the time to reach confluency and proliferation rate by BrdU assay.
Characterization
Flow cytometry analysis
The cells were washed with PBS containing 2 % FBS. FITC conjugated anti human CD45, and anti CD14 and PE conjugated anti human CD 34, CD29, CD73, CD90 and PerCP-Cy 5.5 conjugated anti human CD105 antibodies were used for staining the cells. All antibodies were purchased from BD Biosciences. Analysis was done on Flow cytometer (BD FACS ARIA II) using FACS DIVA software (Version 6.1.2). Propidium iodide (PI) stains dead cells alone. Hence the cells unstained by PI during flowcytometric analysis represent the viable cell population (Hao et al. 1998; Mckenzie et al. 2007).
BrdU cell proliferation assay
BrdU assay was performed to determine the proliferation of cells by following the BrdU cell proliferation kit protocol (Cell Signaling Technology, Danvers, MA, USA, Cat No: 6813). Briefly, the cells were incubated for 24 h in culture medium incorporating BrdU solution, fixed, and stained with detection antibody (anti-BrdU antibody) for 1 h and HRP-conjugated anti-mouse IgG antibody (both antibodies were included in the kit from Cell Signaling Technology) added, washed after 30 min and then 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added for 30 min. Absorbance was read at 450 nm after stop solution.
Immunocytochemistry
The cultured cells were fixed with 4 % paraformaldehyde (Sigma, 6148), and permeabilised using Triton-×-100 (Sigma, 93443). Immunohistochemical staining was done using smooth muscle actin (SMA) and vimentin antibodies (BioGenex, Hyderabad, India). Vimentin and SMA are present in mesenchymal stem cells (Mafi et al. 2011). Immunostaining was performed according to the manufacturer’s protocol (Biogenex—super sensitive polymer—HRP-IHC detection system).
Immunofluorescence
The cells were washed with PBS, fixed with 4 % paraformaldehyde and permeabilized with 0.25 % Triton-×-100. After blocking with 1 % BSA, the cells were incubated with Anti-Vimentin antibody overnight at 4 °C, followed by FITC-conjugated secondary antibody incubation for 1 h. After PBS wash, the slides were mounted using glycerol and viewed under microscope (Karaoz et al. 2009).
Colony forming unit (CFU)
The cells were seeded in duplicate at a density of 1 × 105 in 100 mm petri dishes containing DMEM/10 % FBS. The cells were incubated in 5 % CO2 at 37 °C and the medium was changed after 7 days and subsequently every 3 days. After 14 days of culture, the colonies were stained with 0.05 % crystal violet and counted. Clusters of more than 50 cells were considered as colonies.
RT-PCR
The RNA was isolated from MSCs at 90 % confluency using TRI reagent (Sigma, Cat No. T9424) according to the manufacturer’s protocol in a PCR apparatus (Eppendorf, Hamburg, Germany: Mastercycler Gradient 5331). The cDNA conversion was carried out using the High-Capacity cDNA Reverse Transcription Kit (AB Applied Biosystems, Bangalore, India, Cat No. 4368814) and amplified using PCR Master Mix (Thermo Scientific, Mumbai, India, Cat No. K0171) with primers of the following sequences:
Oct4: Forward 5′-GAGAATTTGTTCCTGCAGTGC-3′ and reverse 5′-GTTCCCAATTCCTTAGTG-3′;
Sox2: Forward 5′-GGCAGCTACAGCATGATGCAGGAGC-3′ and reverse 5′-CTGGTCACATGGAGTTGTACTGCAGG-3′;
Nanog: Forward 5′-ACCTATGCCTGTGATTTGTGG-3′ and reverse 5′-AAGAGTAGAGGCTGGGGTAGG-3′.
GAPDH: Forward 5’-ATGTTCGTCATGGGTGTGAA-3’ and reverse 5’-GTCTTCTGGGTGGCAGTGAT-3’.
The PCR reaction protocol for Oct-4 and Nanog was 94 °C for 3 min, 94 °C for 30 s, 62 °C for 40 s, 72 for 45 s and 72 °C for 10 min for 35 cycles. For SOX-2, the PCR reaction protocol was 94 °C for 3 min, 94 °C for 45 s, 65 °C for 45 s, 72 for 1 min and 72 °C for 5 min for 35 cycles. One percent agarose gel was run and the bands were quantified by gel documentation (UVP Bioimaging System, Upland, CA, USA, Vision works LS image acquisition and Analysis software).
Osteogenic, adipogenic and chondrogenic differentiation
The cultured cells were differentiated into osteogenic, adipogenic and chondrogenic lineage by culturing in osteogenic medium [DMEM supplemented with 10−8 M dexamethasone (Sigma, D4902), 10 mM β glycerophosphate (Sigma, G9422), and 50 µg/ml ascorbic acid], Adipogenic medium [DMEM supplemented with 10 mM 3 isobutyl-1-methylxanthine (Sigma, 17018), 0.1 mM indomethacin (Sigma, 17378), 10 µg/ml insulin (Sigma, I6634), 10−6 dexamethasone] and Chondrogenic medium (Stempro, Invitrogen) and confirmed by staining with Alizarin red (Sigma, A5533), Oil red O (Sigma, O0625) and Alcian blue (Himedia Laboratories, Mumbai, India: Cat no RM471-1g staining, respectively).
Mean values have been used for comparisons/analysis.
Statistics: The chi Square test was carried out to determine the statistical significance (in Figs. 1 and 4).
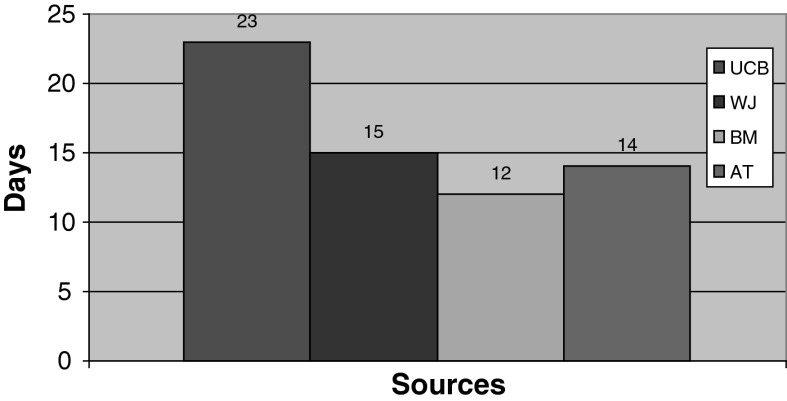
Fig. 1.
Bone marrox (BM) MSCs reached 70 % confluency in a shorter period (12 days) when compared to WJ (15 days), AT (14 days) and UCB (23 days) derived MSCs
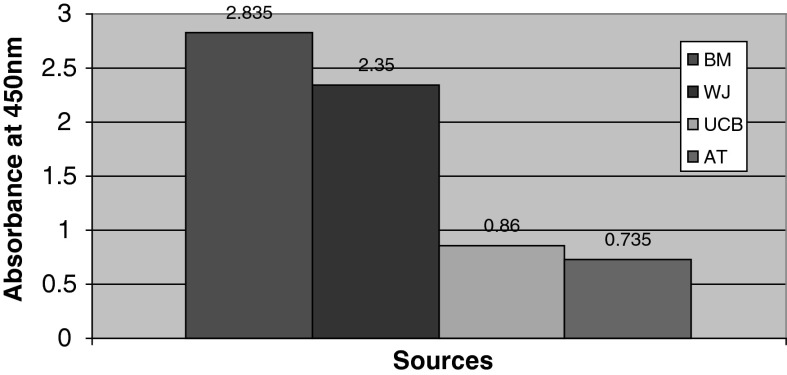
Fig. 4.
The proliferative potential of MSCs was analyzed using BrdU cell proliferation assay. The bone marrow MSCs have high proliferative rate (2.835) when compared with WJ (2.35), UCB (0.86) and AT (0.735) derived MSCs
Results
Time to reach confluency
Mesenchymal stem cells have been separated from BM, WJ, UCB and AT by their adherence capacity. BM MSCs reached confluency in 12 days whereas MSCs from WJ, UCB and AT reached confluency in 15, 23 and 14 days, respectively (Fig. 1).
Differentiation potential of MSCs
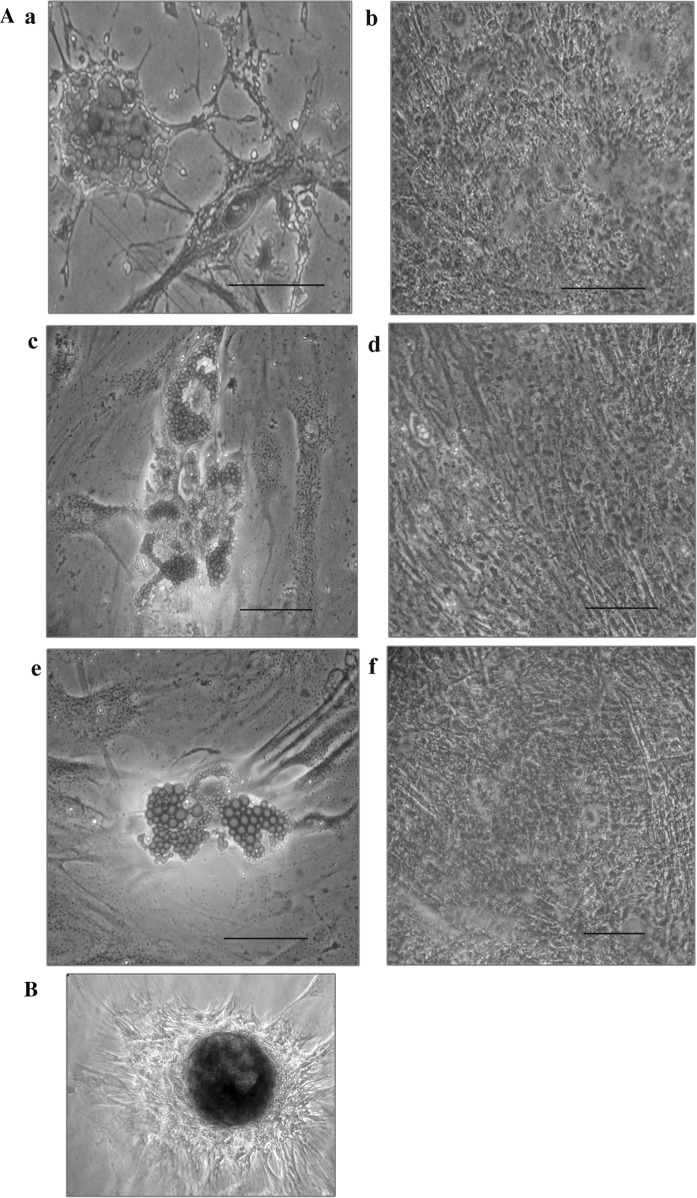
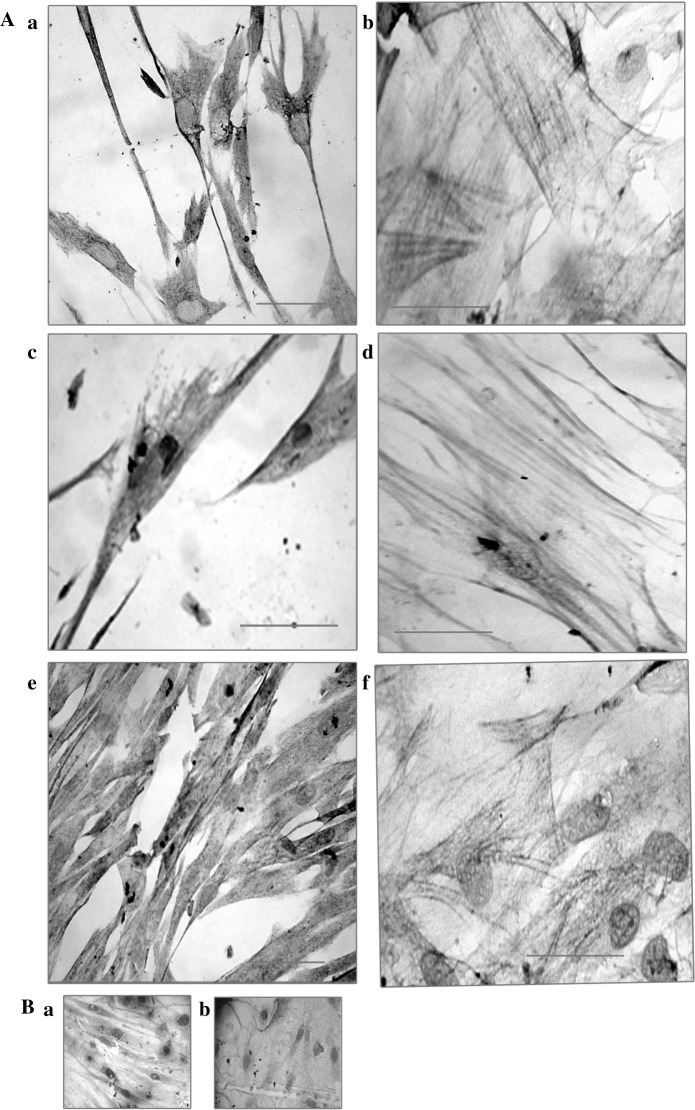
The differentiation potential of MSCs was identified by their osteogenic, adipogenic and chondrogenic differentiation and demonstrated by positive staining with Alizarin red, Oil O red and Alcian blue staining, respectively (Fig. 3).
Fig. 3.
MSCs were characterized by their differentiation potential to adipogenic, osteogenic and chondrogenic lineages by Oil red O, Alizarin red and Alcian blue staining, respectively. (A) a Adipocytes positive for Oil red-O staining—BM cells. b Osteocytes positive for alizarin staining—BM cells. c. Adipocytes positive for Oil red-O staining—WJ cells. d Osteocytes positive for alizarin staining—WJ cells. e Adipocytes positive for Oil red-O staining—UCB cells. f Osteocytes positive for alizarin staining—UCB cells. B Representative image of Alcian blue staining of Chondrocytes derived from WJ MSCs
Flow cytometry
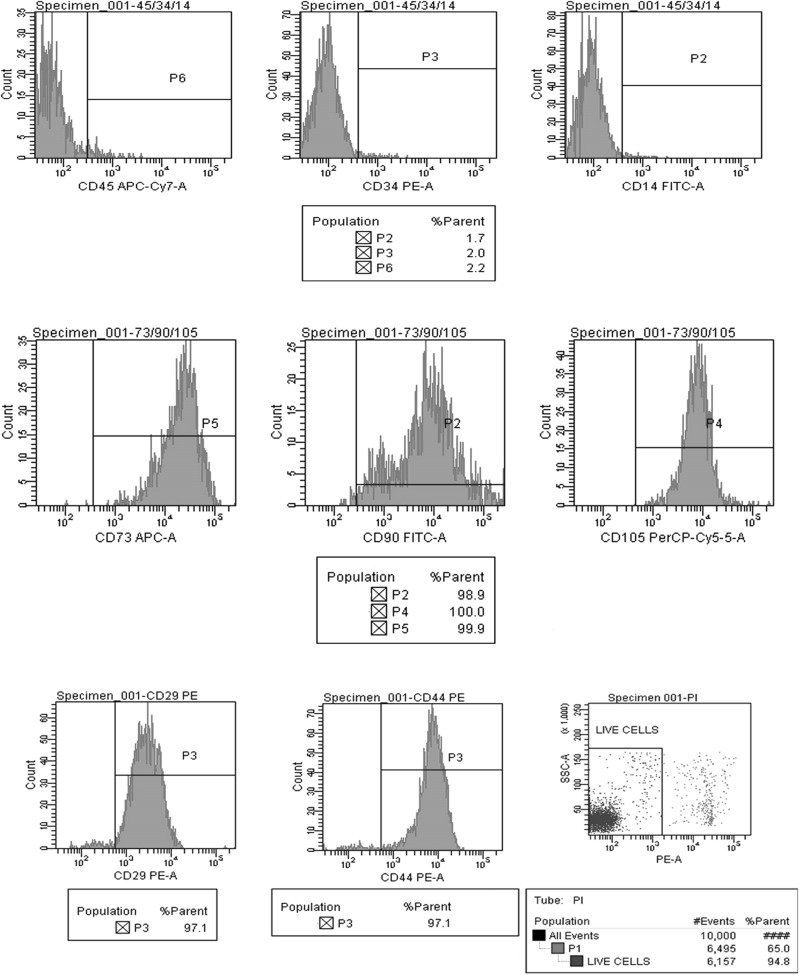
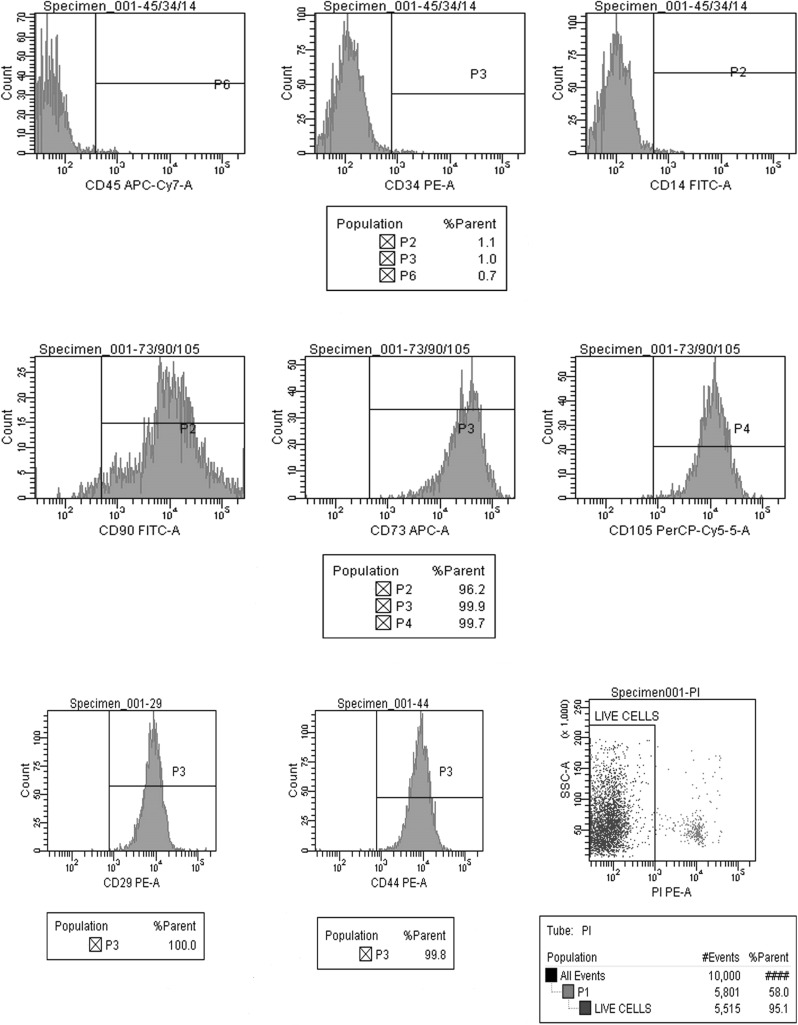
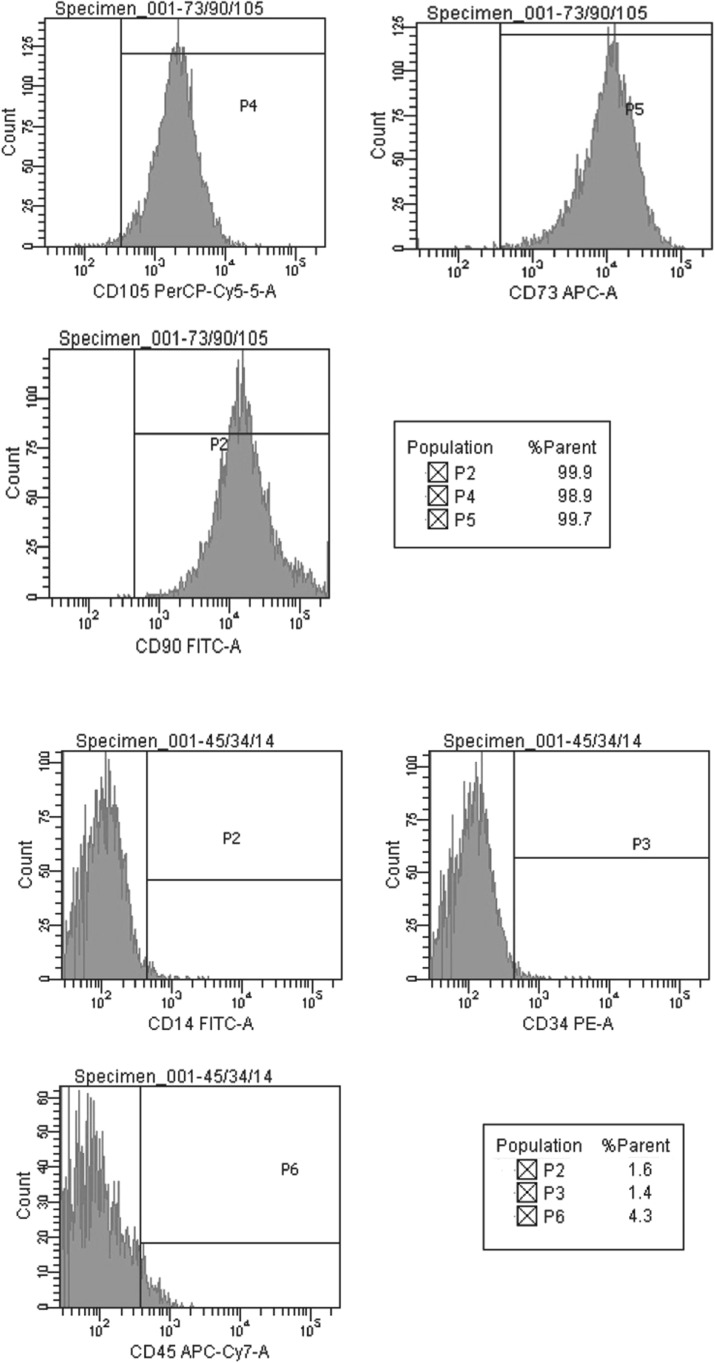
We have analyzed the expression pattern of positive (CD29, CD44, CD90, CD105, and CD73) and negative (CD45, CD14 and CD34) markers of MSCs derived from BM, WJ, AT and UCB. Positive expression of CD 29, CD90, CD 105, and CD73, was observed in MSCs derived from BM, WJ, AT and UCB. CD45, CD14 and CD34 markers were negative in all the four groups, thereby fulfilling the minimum criteria proposed by ISCT. However, the percentage of live cells as identified by negative staining with PI, differed between the sources (Figs. 6, 7, 8, 9).
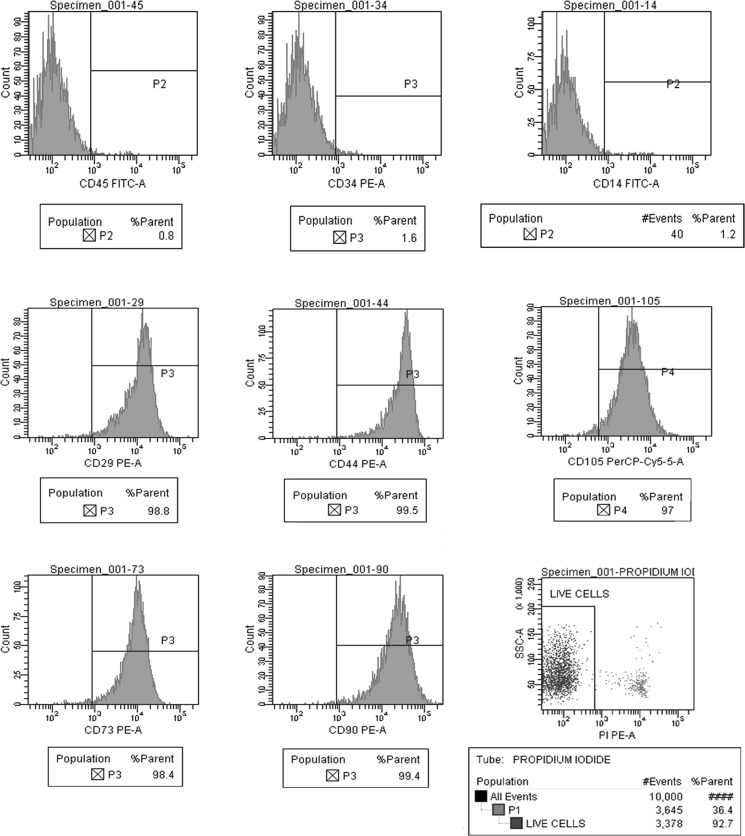
Fig. 6.
Positive expression of mesenchymal stem cell markers (CD105, CD73, CD90, CD44, and CD29) and negative expression of hematopoietic markers (CD45, CD34 and CD14) in UCB derived cells at passage 2 and cell viability by propidium iodide
Fig. 7.
Positive expression of mesenchymal stem cell markers (CD105, CD73, CD90, CD44, and CD29) and negative expression of hematopoietic markers (CD45, CD34 and CD14) in WJ derived cells at passage 2 and cell viability by propidium iodide
Fig. 8.
Positive expression of mesenchymal stem cell markers (CD105, CD73, CD90, CD44, and CD29) and negative expression of hematopoietic markers (CD45, CD34 and CD14) in BM derived cells at passage 2 and cell viability by propidium iodide
Fig. 9.
Positive expression of mesenchymal stem cell markers (CD105, CD 73 and CD90) and negative expression of hematopoietic markers (CD45, CD34 and CD14) in adipose tissue derived cells at passage 2
BrdU cell proliferation assay and CFU
The proliferative capacity of the isolated MSCs was calculated using BrdU assay. The mean value for BM was higher when compared with the other three sources (Fig. 4). The MSCs derived from all the four sources have shown colony forming potential—a characteristic feature of stem cells (Supplement Figure 1).
Immunocytochemistry and Immunofluorescence
Vimentin and SMA are expressed by few cell types including MSCs. In this study, MSCs derived from BM, WJ, UCB and AT were positive for Vimentin and SMA by Immunocytochemistry and Immunofluorescence (Figs. 5, 10).
Fig. 5.
MSCs were characterized by the expression of the markers vimentin and SMA using Immunocytochemistry. A Positive staining of vimentin and SMA was observed in BM (a, b), WJ (c, d) and UCB (e, f) derived MSCs respectively. B Negative controls without antibody (a) and Buccal cells (b). Bar 50 μm
Fig. 10.
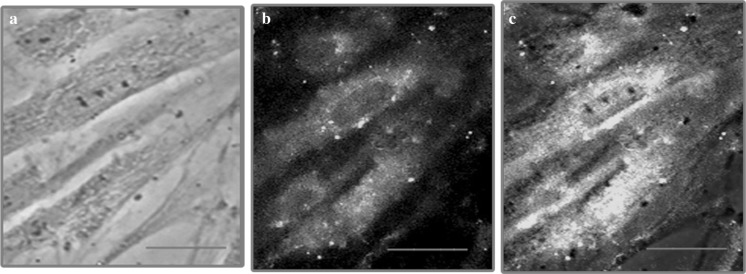
Immunofluorescence analysis of vimentin expression in bone marrow mesenchymal stem cell was done to further characterize MSCs. a Phase contrast image of mesenchymal stem cells. b Expression of vimentin in bone marrow MSCs and c overlay
RT-PCR analysis
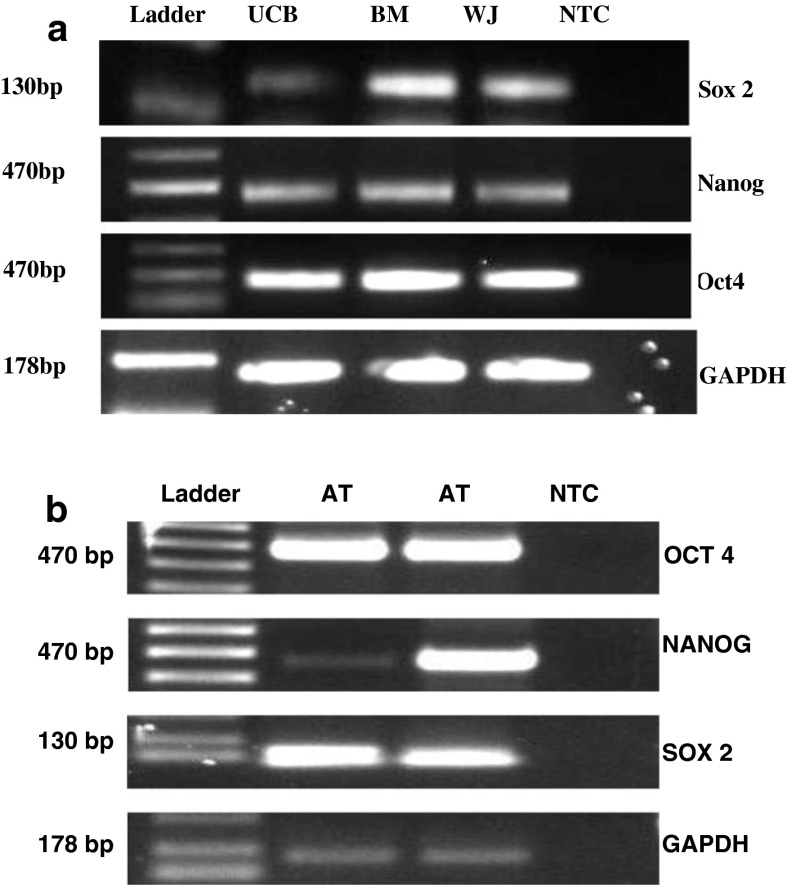
RT-PCR was performed to characterize MSCs based on their expression of pluripotency markers. MSCs from all sources in this study expressed Oct-4, Nanog and Sox-2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control (Fig. 11). The expression levels of all the three markers was less in UCB compared with BM and WJ. Increased expression of Oct-4 and Sox-2 was observed in AT derived MSCs.
Fig. 11.
RT-PCR was performed to characterize MSCs based on their pluripotent potential. a Expression of Oct-4, Nanog and Sox-2 was observed in BM, WJ and UCB MSCs. However, the level of expression of all the three markers was less in UCB than in BM and WJ. b Increased expression of Oct-4 and Sox-2 was observed in AT derived MSCs. Whereas, aberrant expression of Nanog was observed in AT derived MSCs. GAPDH was used as an internal control. NTC No template control
Discussion
Friedenstein et al. (1970) found that BM stromal cells (that were later identified as mesenchymal stem cells), had the potential to form colonies (Mafi et al. 2011). MSCs have been isolated from different adult derived tissues like peripheral blood, AT, lung, heart, synovium, skeletal muscle, periosteum, dermis and dental pulp, as well as fetal/neonatal tissues like amniotic fluid, amniotic membrane, chorion membrane, chorion villi, deciduas, placenta, cord blood, WJ and umbilical cord (Malgieri et al. 2010; Miao et al. 2006; Romanov et al. 2003; Wang et al. 2004; Hass et al. 2011; Zomorodian and Eslaminejad 2012).
Bone marrow and AT are autologous sources for MSCs, of which BM is a frequently used source. The use of the BM directly for seeding yields higher numbers of adhered cells on plastic dishes and reduced loss of MSCs compared to density gradient separation methods (Mareschi et al. 2012). However use of the whole BM aspirate may yield heterogeneous mixture of cells, such as hematopoietic cells at different differentiation/commitment stages, endothelial cells and endothelial progenitor cells in addition to MSCs. The use of BM mononuclear cells for seeding upon separation by density gradient centrifugation is bound to result in a more homogeneous MSC population. In the last decade AT has been increasingly used by many researchers as a source of autologous MSCs (Pikuła et al. 2013). Allogenic MSCs sourced from various tissues were being used by researchers in recent clinical trials (Patel and Genovese 2011).
Autologous cell sources would be the ideal choice but in situations where this is not possible, when the patient is too ill to provide his own tissues or if the time is not enough to allow expansion, then an allogenic source (where the expanded cells are often already available) would be an option. In the present study, we have compared the efficiency of isolation, proliferation and expansion of MSCs derived from BM, UCB, AT and WJ.
A near 100 % efficiency was observed in the isolation of MSCs from BM, AT and WJ and 40 % with UCB. This is concordant with the report of Rebelatto et al. (2008).
On initial seeding of sample, spindling indicates the initiation of proliferation. For BM, WJ, AT and UCB derived MSCs, spindling/proliferation occurred at different time points—day 8 for UCB, day 7 for WJ and AT and day 3 for BM (Table 1). Yield is calculated by analyzing the number of cells obtained during the initial passages 1 and 2. Though UCB as a source for MSC has the advantage of having a non-invasive collection procedure (Ali and Mull 2012), in the present study, the yield of MSCs from UCB was lower than from BM and AT. This was also the finding of Kern et al. (2006).
Table 1.
Time taken for the initial appearance of Spindle Cells and Passage
| UCB (days) | WJ (days) | BM (days) | Adipose tissue (days) | |
|---|---|---|---|---|
| Start of spindle cells | 8 | 7 | 3 | 7 |
| Passage 1 | 23 | 15 | 12 | 18 |
| Passage 2 | 28 | 20 | 17 | 23 |
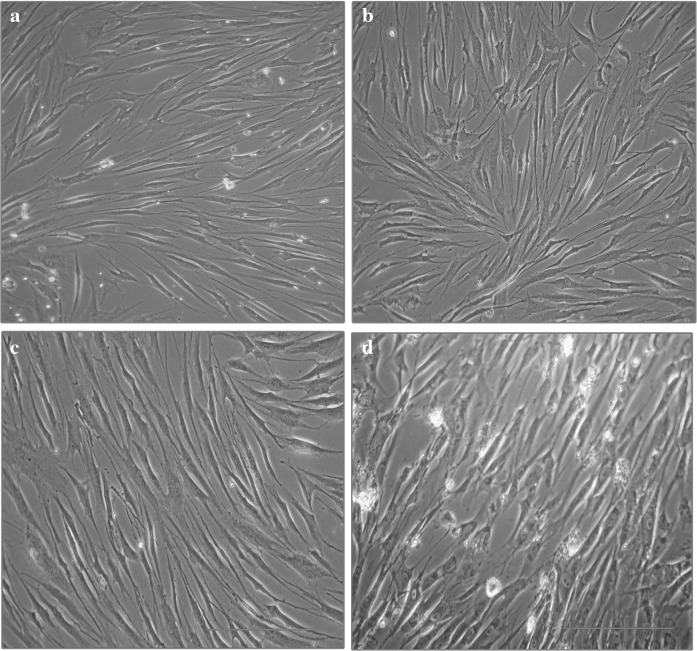
As mentioned by Shetty et al. (2010), in the present study also, the time to reach confluency of MSCs from all the three sources was comparable—lower in BM MSCs compared to WJ MSCs, AT MSCs and UCB MSCs (Fig. 1). Besides, the BM MSCs reached 70 % confluency in a shorter period compared to AT, WJ and UCB MSCs (Figs. 2, 3). The proliferative capacities of MSCs by BrdU assay also showed that BM derived MSCs were more proliferative compared with WJ and UCB (Fig. 4).
Fig. 2.
Images of BM (a), UCB (b), AT (c) and WJ (d) derived MSCs at day 12. Scale bar 50 μm
The unstained cells on trypan blue staining (Neubauer’s chamber) and PI staining (flow cytometry) represent the viable population. Viable MSCs were higher with BM derived MSCs indicating that this is a good source to get a higher yield of MSCs compared to the other three sources. However, WJ is a freely available source, engaging a noninvasive collection procedure with the added advantage of having a higher yield of MSCs compared to BM, AT and UCB (data not shown).
Researchers have used various methods for the isolation of MSCs from WJ. Some studies used enzymes including collagenase/hyaluronidase/trypsin (CHT), collagenase/trypsin (CT) and trypsin (Trp), while other researchers opted for the removal of arteries and veins for explant method (Koliakos et al. 2011; Salehinejad et al. 2012). In this study we directly used dices of Umbilical Cord without removing the blood vessels. This simpler procedure was compared with an enzymatic method using trypsin. The efficiency of both isolation procedures was compared. Spindling was observed on day 9 on using the enzymatic method and day 7 for the explant method (Supplement table 1). The yield of MSCs was also higher with the explant method. The umbilical cord is an easy to obtain, large volume source (approximately 50–60 cm/cord), making its use a cost effective source for MSC expansion.
The ability to generate clones from a single cell is a formal demonstration of the self renewal ability, a characteristic of stem cells (La rocca et al. 2009). We observed CFUs during MSC derivation from all four sources (Supplement Figure 1). These colonies had round as well as fusiform/spindle shaped cells.
The minimum criteria proposed by ISCT were fulfilled by MSCs derived in his study. MSCs typically are plastic adherent. MSC’s differentiation to osteogenic, adipogenic and chondrogenic lineages was also demonstrated. In addition, Flow cytometric analysis for typical markers defining MSCs as proposed by Mesenchymal and Tissue cell committee of the ISCT (La rocca et al. 2009; Dominici et al. 2006) was also done. Positive expression of CD29, CD44, CD90, CD105 and CD73, was observed in MSCs derived from all sources while CD45, CD14 and CD34 markers were negative. However, differences were observed in the percentage of expression among the four sources as depicted in Figs. 6, 7, 8 and 9. The expression of Vimentin and SMA by the MSC’s from all the four sources was demonstrated by immunocytochemistry (Fig. 5) and immunofluorescence (Fig. 9).
Riekstina et al. (2009), Yoon et al. (2013), Riekstina et al. (2009) and Habich et al. (2006) have demonstrated the pluripotency markers in MSCs from BM, WJ, AT and UCB, respectively. The same was observed in the present study. The expression of Oct-4, Nanog and Sox-2 was higher in BM MSCs when compared with WJ and UCB (Fig. 10).
Several researchers have focussed on the use of MSCs as a therapeutic option for treating liver diseases. Autologous transplantation of BM stem cells for the therapy of chronic liver diseases has been attempted for a few critically ill patients (Pai et al. 2012). Mohamadnejad et al. (2007) showed that autologous MSC transplantation is feasible and safe for treating decompensated liver disease following cirrhosis. Improvement of liver function was also observed after injection of autologous MSC in liver cirrhosis patients during phase I–II clinical trial (Kharaziha et al. 2009). BM derived Haematopoetic Stem cells also have been shown to be free of side effects and providing 60 % success rate during phase I trial involving patients with liver insufficiency (Gordon et al. 2006).
Hence these four sources were used to study the feasibility and efficiency of isolation and expansion of MSC’s as they may have implications in the future for clinical translation options, including liver diseases (Fig. 11).
Conclusion
Mesenchymal stem cells were isolated, expanded, characterized and analysed from BM, WJ, UCB and AT and they fulfilled the criteria as laid down by ISCT. BM and AT derived MSCs have a good proliferative capacity, as well as the advantage of being autologous sources. However they involve invasive and cumbersome collection processes, often from an already ill patient. On the other hand, the Umbilical Cord, a wasted tissue after birth, is an easily available large volume source and engages a noninvasive, easy and painless collection process and the umbilical cord matrix—WJ has a fairly good proliferative potential, ranking next to BM. The explant method for MSC isolation is simple, and contributes to making the UC a cost effective source for derivation of allogenic MSCs. Future clinical research involving use of Umbilical Cord derived MSCs as a therapeutic option is worth pursuing.
Electronic supplementary material
Acknowledgments
This study is the preliminary part of the project entitled “Hepatic progenitor cells: isolation from various sources, characterization, expansion and transplantation” funded by Indian Council of Medical Research, New Delhi.
References
- Ali H, Mull FA. Defining umbilical cord blood mesenchymal stem cells. Stem Cell Discov. 2012;2:15–23. doi: 10.4236/scd.2012.21003. [DOI] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;21:77–89. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. doi: 10.1186/1423-0127-18-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slapercortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Gordon MY, Levicar N, Pai M. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- Habich A, Jurga M, Markiewicz I, Lukomska B, Bany-Laszewicz U, Domanska-Janik K. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp Hematol. 2006;34:914–925. doi: 10.1016/j.exphem.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hao QL, Smogorzewska EM, Barsky LW, Crooks GM. In vitro identification of single CD34+ CD 38− cells with both lymphoid and myeloid potential. Blood. 1998;91:4145–4151. [PubMed] [Google Scholar]
- Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney IR, Stacey GN, Auerbach JM. Culture of human stem cells. USA: Wiley; 2007. [Google Scholar]
- Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukan A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- Karaoz E, Aksoy A, Ayhan S, Sarıboya AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132:533–546. doi: 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR, Soleimani M. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I–II clinical trial. Eur J Gastroenterol Hepatol. 2009;10:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- Koliakos I, Tsagias N, Karagiannis V. Mesenchymal cells isolation from Wharton’s jelly, in perspective to clinical applications. J Biol Res-Thessalon. 2011;16:194–201. [Google Scholar]
- La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefsno A, Giannuzzi P, Marassa L, Cappello F, Zummo G, Farina G. Isolation and characterization of Oct-44+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267–282. doi: 10.1007/s00418-008-0519-3. [DOI] [PubMed] [Google Scholar]
- Mafi P, Hindocha S, Mafi R, Griffin M, Khan WS. Adult mesenchymal stem cells and cell surface characterization—a systematic review of the literature. Open Orthop J. 2011;5:253–260. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, Castiglia S, Risso A, Ferrero I, Tarella C, Fagioli F. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012;10:920581. doi: 10.1155/2012/920581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie JL, Takenaka K, Gan OI, Doedons M, Dick JE. Low rhodamine 123 retention identifies long-term human hematopoietic stem cells with in the Line CD34+ CD 38− population. Blood. 2007;109:543–545. doi: 10.1182/blood-2006-06-030270. [DOI] [PubMed] [Google Scholar]
- Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Quian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- Mustapha Z, Alexandra B, Biserka R, Claire J, Michel GM, André G, Chantal L, Yves B. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010;34:693–701. doi: 10.1042/CBI20090414. [DOI] [PubMed] [Google Scholar]
- Needham PL. Separation of human blood using “mono-poly resolving medium”. J Immunol Methods. 1987;99:283. doi: 10.1016/0022-1759(87)90139-6. [DOI] [PubMed] [Google Scholar]
- Pai M, Spalding D, Xi F, Habib F. Autologous bone marrow stem cells in the treatment of chronic liver disease. Int J Hepatol. 2012;2012:307165. doi: 10.1155/2012/307165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AN, Genovese J. Potential clinical applications of adult human mesenchymal stem cell (Prochymal®) therapy. Stem Cells Cloning: Adv Appl. 2011;4:61–72. doi: 10.2147/SCCAA.S11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuła M, Trzonkowska NM, Wardowska A, Renkielska A, Trzonkowski P. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther. 2013;13:1357–1370. doi: 10.1517/14712598.2013.823153. [DOI] [PubMed] [Google Scholar]
- Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–1376. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev Biol Anim. 2012;48:75–83. doi: 10.1007/s11626-011-9480-x. [DOI] [PubMed] [Google Scholar]
- Shetty P, Cooper K, Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci. 2010;4:14–24. doi: 10.4103/0973-6247.59386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soland MA, Bego MG, Colletti E, Porada CD, Zanjani ED, Stephen SJ, Graça AP. Modulation of human mesenchymal stem cell immunogenicity through forced expression of human cytomegalovirus US proteins. PLoS One. 2012;7:e36163. doi: 10.1371/journal.pone.0036163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Chang JY, Kim BJ, Jeon HW. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int. 2013;2013:428726. doi: 10.1155/2013/428726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomorodian E, Eslaminejad MB. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012;2012:1–9. doi: 10.1155/2012/980353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.