Abstract
Matrigel and similar commercial products are extracts of the Engelbreth-Holm–Swarm sarcoma that provide a basement-membrane-like attachment substrate or gel that is used to grow cells on or in, respectively. To ascertain further what proteins may be present in Matrigel, besides its major basement-membrane constituents, an analysis of the expressed liquid of gelled Matrigel was performed using proteome array technology. Among the growth factors/cytokines assayed, high positive detection was found for IGFBP1, IGFBP3, LIF, platelet factor 4, PlGF-2, and VEGF; moderate reactivity was found for cyr61, IGFBP2, IGFBP6, IL-1ra, and NOV; and low, but detectable, responses occurred for aFGF, IL-13, IL-23, M-CSF, and VEGF-B. Among the chemokines assayed, high positive detection was found for MIG and serpin E1; moderate reactivity was found for IP-10, MCP-1, and MCP-5, and low, but detectable, responses occurred for CXCL16, I-TAC, and MIP-1α. Among the other biologically active proteins assayed, high positive detection was found for adiponectin, C5a, endocan, lipocalin-2, sICAM-1, MMP-3, and TIMP-1; moderate reactivity was found for C-reactive protein, coagulation factor III, endoglin, endostatin/collagen XVIII, endothelin-1, ICAM-1, MMP-9, osteopontin, pentraxin-3, and RANTES; and low, but detectable, responses occurred for fetuin A, MMP-8, pentraxin-2, RBP4, resistin, and TIMP-4. The study found several growth factors, chemokines, and biologically active proteins not previously identified in Matrigel, and this may have significance to the interpretations of observed cellular responses when cells are grown on or in Matrigel.
Keywords: Cell culture, Extracellular matrix, Matrigel, Protein array
Introduction
Matrigel, or similar products sold as Cultrex or EHS matrix, is a basement-membrane-like matrix extracted from the Engelbreth-Holm–Swarm (EHS) mouse sarcoma (Kleinman and Martin 2005). It is primarily used for the in vitro culture of cells, either as an aid to cell attachment and growth or as a 3D biological gel in which cells are suspended and grown (Schuetz et al. 1988; Miyazaki et al. 2002; Kleinman and Martin 2005; Talbot et al. 2010; Nguyen-Ngoc and Ewald 2013). The EHS tumor is propagated in vivo and the extracellular matrix-like material extracted from it is mainly comprised of laminin (~60 %), collagen IV(~30 %), nidogen (~5 %), the heparan sulfate proteoglycan perlecan (~3 %), and entactin (~1 %) (Orkin et al. 1977; Kleinman et al. 1982, 1986). In addition, however, Matrigel has been found to contain various other biological components including MMP-2, MMP-9, urokinase [urokinase-type plasminogen activator (uPA)], tissue-type plasminogen activator, amylase, transferrin, and clusterin (Dirami et al. 1995; Gillette et al. 2003; Kleinman and Martin 2005).
Growth factors have also been identified in Matrigel. It was shown to contain transforming growth factor beta (TGFβ), EGF, IGF-1, FGF-2, PDGF, and nerve growth factor (Vukicevic et al. 1992; BD Biosciences Matrigel Product Data Sheet). Because of the various biological effects of these growth factors on a wide array of cell types, attempts were soon made to reduce their concentration, and “growth-factor reduced” Matrigel products are commercially available (Vukicevic et al. 1992; BD Biosciences). More recently, large scale proteomic analyses of Matrigel have been reported in an effort to qualitatively identify the less abundant proteins/peptides contained in it (Hansen et al. 2009; Hughes et al. 2010). These efforts identified the known extracellular matrix components that comprise the bulk of Matrigel and also over one-thousand other proteins. However, nearly without exception the other proteins identified were cellular proteins that are not secretory in nature, i.e., intracellular and membrane component proteins (Hansen et al. 2009; Hughes et al. 2010). What biologically active proteins/peptides that can be reproducibly found in Matrigel, lot-to-lot, is otherwise unreported or unknown.
In an attempt to broaden the knowledge of what biologically active proteins Matrigel contains, we have analyzed the liquid component of Matrigel (centrifrugally expressed from gelled Matrigel) using commercially available mouse specific proteome arrays that purport to define the expression of 106 separate proteins in a semi-quantitative way. The results show that Matrigel contains many more biologically active proteins than has previously been reported, and their potential influences on cells in culture, particularly, embryonic stem cells (ESC) and induced pluripotent stem cells (iPCS), is discussed.
Materials and methods
Matrigel basement-membrane-matrix liquid component preparation
Four separate lots of Matrigel basement membrane matrix (catalog no. 356234) were obtained from BD Biosciences (Bedford, MA, USA). After thawing on ice, two 900 µl aliquots of Matrigel were gelled at 37 °C in two 1.5 ml ultracentrifuge tubes (Beckman Coulter, Inc.; Danvers, MA, USA). The supernatants were collected from the compressed gel after centrifugation at 125,000×g for 30 min at 4 °C. The gel was then subjected to a second centrifugation at 125,000×g for 30 min at 4 °C, and supernatants were again collected and combined with those from the first centrifugation. From each 1.8 ml sample of Matrigel approximately 1.4 ml of total supernatant could be collected.
Proteome antibody array analysis
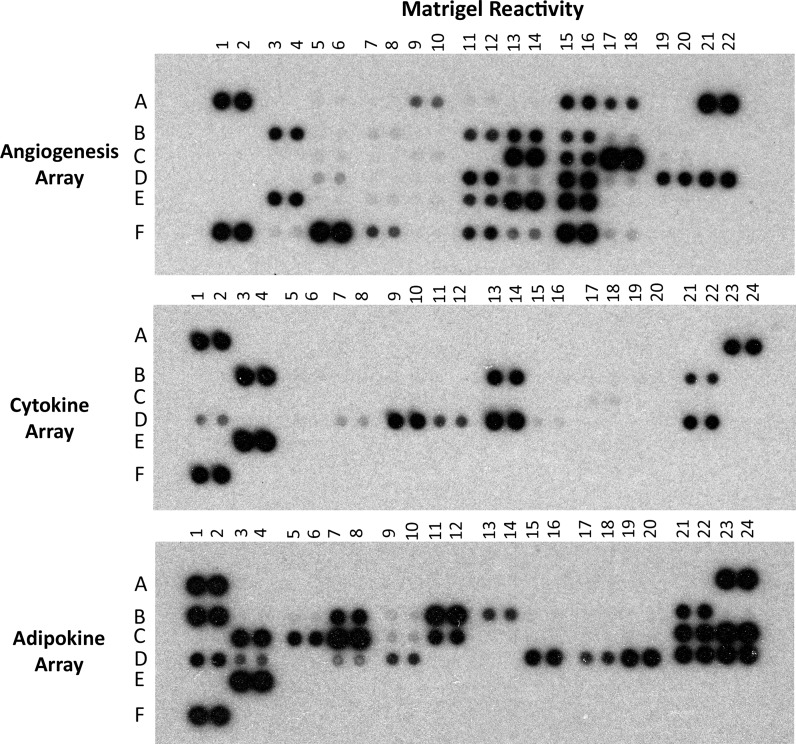
Semi-quantitative protein analysis of four independent lots of Matrigel supernatant, diluted 1:3, was performed according to the manufactures instructions on three protein antibody arrays that detect a total of 106 mouse growth factors, chemokines, extracellular matrix factors, and other biologically active proteins (R&D Systems, Inc., Minneapolis, MN, USA; Cat. No. ARY013, ARY006, and ARY015). The arrays’ capture antibodies (antibodies to the specified proteins), and positive and negative controls, are printed in duplicate (the results therefore being the average of two reactions) on nitrocellulose membranes and four membranes in total are included. The arrays’ chemiluminescent autoradiographs (Fig. 1) were measured by densitometry, corrected for background density, and expressed relative to each array’s positive controls (ImageQuant TL Software, GE Healthcare, Piscataway, NJ, USA).
Fig. 1.
Representative reactivity of the three proteome arrays after exposure to a 1:3 dilution of gelled-Matrigel liquid-extract. Positive controls are positioned in the corners of each array, and a single negative control is positioned in the lower right corner of each array (R&D Systems, Inc.)
Results
Semi-quantitative protein analysis of four separate lots of Matrigel was performed and representative array results are shown in Fig. 1. The densitometry values ± their standard deviation are listed in Table 1 by array designation (“angiogenesis”, “cytokine”, or “adipokine”) for a total of 106 mouse growth factors, chemokines or other biologically active proteins. The coordinates of each target protein are listed in the first column of Table 1, and their specific locations on the arrays are also available on-line from R&D Systems, Inc.
Table 1.
Protein array densitometry of Matrigel
| Array no. | Angiogenesis array | Mean | SD |
|---|---|---|---|
| A1, A2, A21, A22, F1, F2 | Positive control | 100.00 | 17.50 |
| F19, F20 | Negative control | 1.01 | 1.02 |
| A5, A6 | ADAMTS1 | 0.39 | 0.57 |
| A7, A8 | Amphiregulin | 0.12 | 0.54 |
| A9, A10 | Angiogenin | 6.51 | 2.06 |
| A11, A12 | Angiopoietin-1 | 0.80 | 0.43 |
| A13, A14 | Angiopoietin-3 | 0.30 | 0.51 |
| A15, A16 | Coagulation factor III | 36.60 | 5.60 |
| A17, A18 | CXCL16 | 18.31 | 7.42 |
| B3, B4 | Cyr61 | 22.18 | 8.34 |
| B5, B6 | DLL4 | 0.69 | 0.26 |
| B7, B8 | DPPIV | 1.76 | 1.07 |
| B9, B10 | EGFa | 0.41 | 0.46 |
| B11, B12 | Endoglin/CD105 | 22.07 | 3.44 |
| B13, B14 | Endostatin/Collagen XVIII | 38.36 | 6.22 |
| B15, B16 | Endothelin-1 | 29.04 | 6.73 |
| B17, B18 | FGF-1/aFGF | 5.45 | 2.28 |
| B19, B20 | FGF-2/bFGFa | 1.49 | 0.79 |
| C3, C4 | FGF-7/KGF | 0.65 | 0.49 |
| C5, C6 | Fractalkine/CX3CL1 | 1.11 | 0.39 |
| C7, C8 | GM-CSF | 0.72 | 0.83 |
| C9, C10 | HB-EGF | 1.65 | 0.35 |
| C11, C12 | HGF | 2.62 | 1.15 |
| C13, C14 | IGFBP-1 | 125.98 | 21.66 |
| C15, C16 | IGFBP-2 | 62.86 | 11.38 |
| C17, C18 | IGFBP-3 | 231.04 | 33.77 |
| C19, C20 | IL-1α | 2.92 | 1.16 |
| C21, C22 | IL-1β | 1.18 | 1.04 |
| D3, D4 | IL-10 | 1.03 | 0.26 |
| D5, D6 | IP-10/CXCL10 | 3.70 | 1.25 |
| D7, D8 | KC/CXCL1/GROα | 0.85 | 0.29 |
| D9, D10 | Leptin | 0.89 | 0.31 |
| D11, D12 | MCP-1/CCL2/JE | 44.18 | 17.68 |
| D13, D14 | MIP-1α/CCL3 | 12.02 | 4.06 |
| D15, D16 | MMP-3 (pro/mature form) | 100.87 | 35.72 |
| D17, D18 | MMP-8 (pro form) | 10.61 | 3.68 |
| D19, D20 | MMP-9 (pro/active form) | 36.99 | 6.05 |
| D21, D22 | NOV/CCN3 | 57.01 | 14.70 |
| E3, E4 | Osteopontin | 33.68 | 4.38 |
| E5, E6 | PD-ECGF | 1.65 | 0.44 |
| E7, E8 | PDGF-AA | 1.90 | 0.26 |
| E9, E10 | PDGF-AB/PDGF-BBa | 1.32 | 0.37 |
| E11, E12 | Pentraxin-3/TSG-14 | 23.18 | 6.39 |
| E13, E14 | Platelet factor-4/CXCL4 | 123.71 | 20.97 |
| E15, E16 | PlGF-2 | 112.56 | 30.26 |
| E17, E18 | Prolactin | 2.79 | 1.33 |
| E19, E20 | Proliferin | 1.85 | 1.26 |
| F3, F4 | SDF-1/CXCL12 | 3.34 | 0.92 |
| F5, F6 | Serpin E1/PAI-1 | 160.49 | 8.97 |
| F7, F8 | Serpin F1/PEDFb | 12.37 | 2.05 |
| F9, F10 | Thrombospondin-2 | 1.28 | 0.52 |
| F11, F12 | TIMP-1 | 27.11 | 4.28 |
| F13, F14 | TIMP-4 | 9.23 | 3.17 |
| F15, F16 | VEGF | 142.52 | 29.32 |
| F17, F18 | VEGF-B | 4.67 | 1.09 |
| Array no. | Cytokine array | Mean | SD |
|---|---|---|---|
| A1, A2, A23, A24, F1, F2 | Positive control | 100.01 | 17.32 |
| F23, F24 | PBS (negative control) | 1.39 | 1.24 |
| B1, B2 | BLC/CXCL13 | 2.05 | 0.97 |
| B3, B4 | C5a | 127.99 | 16.76 |
| B5, B6 | G-CSF | 1.75 | 0.52 |
| B7, B8 | GM-CSF | 0.56 | 0.40 |
| B9, B10 | I-309/CCL1 | 0.67 | 0.48 |
| B11, B12 | Eotaxin/CCL11 | 0.48 | 0.65 |
| B13, B14 | sICAM-1/CD54 | 96.60 | 19.23 |
| B15, B16 | IFN-γ | 1.43 | 0.20 |
| B17, B18 | IL-1α | 1.81 | 0.87 |
| B19, B20 | IL-1β | 1.96 | 1.43 |
| B21, B22 | IL-1ra | 43.89 | 23.18 |
| B23, B24 | IL-2 | 1.57 | 1.11 |
| C1, C2 | IL-3 | 1.52 | 0.95 |
| C3, C4 | IL-4 | 3.37 | 1.31 |
| C5, C6 | IL-5 | 0.55 | 0.12 |
| C7, C8 | IL-6 | 0.56 | 0.39 |
| C9, C10 | IL-7 | 2.12 | 0.75 |
| C11, C12 | IL-10 | 1.10 | 0.77 |
| C13, C14 | IL-13 | 5.60 | 2.85 |
| C15, C16 | IL-12 p70 | 1.21 | 0.65 |
| C17, C18 | IL-16 | 7.14 | 6.71 |
| C19, C20 | IL-17 | 1.56 | 1.25 |
| C21, C22 | IL-23 | 4.92 | 3.17 |
| C23, C24 | IL-27 | 1.52 | 1.50 |
| D1, D2 | IP-10/CXCL10 | 22.86 | 13.55 |
| D3, D4 | I-TAC/CXCL11 | 7.06 | 1.83 |
| D5, D6 | KC/CXCL1/GROα | 1.92 | 0.52 |
| D7, D8 | M-CSF/CSF-1 | 4.39 | 1.42 |
| D9, D10 | MCP-1/CCL2/JE | 95.71 | 18.61 |
| D11, D12 | MCP-5/CCL12 | 20.07 | 2.49 |
| D13, D14 | MIG/CXCL9 | 145.30 | 39.04 |
| D15, D16 | MIP-1α/CCL3 | 4.73 | 1.90 |
| D17, D18 | MIP-1β/CCL4 | 1.64 | 1.09 |
| D19, D20 | MIP-2/CXCL2 | 2.09 | 1.51 |
| D21, D22 | RANTES/CCL5 | 89.25 | 52.54 |
| D23, D24 | SDF-1/CXCL12 | 2.21 | 1.37 |
| E1, E2 | TARC/CCL17 | 3.98 | 1.59 |
| E3, E4 | TIMP-1 | 241.36 | 27.62 |
| E5, E6 | TNF-α | 2.34 | 0.54 |
| E7, E8 | TREM-1 | 0.92 | 0.72 |
| Array no. | Adipokine array | Mean | SD |
|---|---|---|---|
| A1, A2, A23, A24, F1, F2 | Positive control | 100.00 | 17.58 |
| F23, F24 | PBS (negative control) | 1.65 | 3.17 |
| B1, B2 | Adiponectin | 92.55 | 6.89 |
| B3, B4 | AgRP | 1.72 | 0.83 |
| B5, B6 | ANGPT-L3 | 1.89 | 0.61 |
| B7, B8 | C-reactive protein | 43.67 | 10.55 |
| B9, B10 | DPPIV | 2.88 | 1.67 |
| B11, B12 | Endocan/EMS-1 | 106.74 | 31.06 |
| B13, B14 | Fetuin A | 19.82 | 7.28 |
| B15, B16 | FGF acidic | 0.73 | 0.56 |
| B17, B18 | FGF-21 | 0.05 | 0.13 |
| B19, B20 | HGF | 0.30 | 0.20 |
| B21, B22 | ICAM-1 | 44.00 | 8.32 |
| B23, B24 | IGF-1a | 2.33 | 1.39 |
| C1, C2 | IGF-2 | 3.48 | 1.55 |
| C3, C4 | IGFBP-1 | 72.15 | 8.38 |
| C5, C6 | IGFBP-2 | 40.94 | 6.24 |
| C7, C8 | IGFBP-3 | 134.18 | 11.20 |
| C9, C10 | IGFBP-5 | 4.87 | 3.32 |
| C11, C12 | IGFBP-6 | 47.42 | 19.39 |
| C13, C14 | IL-6 | 1.18 | 0.92 |
| C15, C16 | IL-10 | 1.48 | 0.78 |
| C17, C18 | IL-11 | 0.76 | 0.48 |
| C19, C20 | Leptin | 1.95 | 1.00 |
| C21, C22 | LIF | 94.83 | 16.67 |
| C23, C24 | Lipocalin-2 | 142.33 | 8.73 |
| D1, D2 | MCP-1/CCL2/JE | 30.51 | 11.74 |
| D3, D4 | M-CSF//CSF-1 | 18.73 | 3.69 |
| D5, D6 | Oncostatin M | 1.72 | 0.80 |
| D7, D8 | Pentraxin-2 | 7.35 | 3.27 |
| D9, D10 | Pentraxin-3/TSG-14 | 16.33 | 10.58 |
| D11, D12 | Pref-1/DLK-1 | 1.13 | 0.66 |
| D13, D14 | RAGE | 0.63 | 0.45 |
| D15, D16 | RANTES/CCL5 | 46.70 | 22.04 |
| D17, D18 | RBP4 | 17.01 | 5.61 |
| D19, D20 | Resistin | 63.66 | 15.75 |
| D21, D22 | Serpin E1/PAI-1 | 91.93 | 13.82 |
| D23, D24 | TIMP-1 | 119.32 | 16.48 |
| E1, E2 | TNF-α | 2.66 | 0.94 |
| E3, E4 | VEGF | 124.05 | 10.27 |
aProteins previously reported in Matrigel as detected by immunoassay
bProteins previously reported in Matrigel as detected by mass spectroscopy
The strongest detection signals (densitometry value ≥ 50) among the growth factors/cytokines assayed were found for IGFBP-1, IGFBP-3, LIF, platelet factor 4, PlGF-2, and VEGF. A moderate positive response (densitometry values approximately between 20 and 49) was found for cyr61, IGFBP-2, IGFBP-6, IL-1ra, and NOV. Low, but detectable, responses occurred for aFGF, IL-13, IL-23, M-CSF, and VEGF-B (densitometry values approximately between 5 and 19). The chemkines assayed by the arrays showed high positive detection for MIG and serpin E1 while moderate reactivity was found for IP-10, MCP-1, and MCP-5, and low, but detectable, responses occurred for CXCL16, I-TAC, and MIP-1α. Among the other biologically active proteins assayed, high positive detection was found for adiponectin, C5a, endocan, lipocalin-2, sICAM-1, MMP-3, and TIMP-1 while moderate reactivity was found for C-reactive protein, coagulation factor III, endoglin, endostatin/collagen XVIII, endothelin-1, ICAM-1, MMP-9, osteopontin, pentraxin-3, and RANTES, and low, but detectable, responses occurred for fetuin A, MMP-8, pentraxin-2, RBP4, resistin, and TIMP-4.
Discussion
The proteome array results indicated the consistent presence of numerous secreted/soluble proteins present in four independent lots of commercially obtained EHS tumor extract, i.e., Matrigel (BD Biosciences). The results also highlight the apparent absence of several dozen other secreted/soluble proteins in Matrigel (Table 1). Despite recent proteomic analyses of Matrigel employing mass spectroscopy (Hansen et al. 2009; Hughes et al. 2010), the immunoassay analysis presented here identified many secreted/soluble proteins not previously identified in Matrigel, and did so in a semi-quantitative manner. Many of the newly identified proteins have various and well described effects on cell growth, differentiation, or maintenance in general. Because of the wide interest in stem cell biology and the frequent use of Matrigel in various in vitro stem cell assays, some discussion of the result in this context is exemplary and pertinent (Xu et al. 2001; Philp et al. 2005; Kleinman and Martin 2005; Ma et al. 2008; Uemura et al. 2010).
Under the category of growth factors/cytokines, the proteome arrays identified relatively high levels of IGFBP-1 (mean score of 72 and 125 units on separate arrays; relative to the arrays internal negative and positive controls), IGFBP-2 (41 and 63 units on separate arrays), IGFBP -3 (134 and 231 units on separate arrays), and IGFBP-6 (47 units), LIF (95 units), platelet factor-4 (124 units), and PlGF-2 (112 units). Insulin-like growth factor binding proteins sustain and mediate the action of IGF-1 and IGF-2, and IGF-1 signaling was found to be necessary for maintenance of human ESC (hESC; Wang et al. 2007). Also in a stem cell context, IGFBP-3, which had the highest response of the IGFBPs detected in Matrigel, is involved in various stem cell processes including vascular endothelial cell differentiation from hematopoietic endothelial precursor cells (Chang et al. 2007), inhibition of neural progenitor cells proliferation (Kalluri and Dempsey 2011), and modulation of liver regeneration from the hepatic stem cell compartment (Steiger-Luther et al. 2010). Besides the detection of IGFBP-1, -2, -3, and -6 with the proteome array, preliminary ELISA data also indicated that Matrigel contains >1 ng/ml IGFBP-4 (unpublished data). Thus, in using Matrigel, it should be understood that it will probably have effects on IGF-1/IGF-2 signal activation. Leukemia inhibitory factor is a key factor in maintaining the undifferentiated state of mouse ESC (mESC; Pease et al. 1990). It’s presence in Matrigel, therefore, could have significant effects on assessments of mESC growth and differentiation that should be taken into consideration when using Matrigel and mESC together (Greenlee et al. 2005; Zhou et al. 2010; Massumi et al. 2012). Platelet factor-4 (PF4) is a marker of megakaryocytes and has angiostatic effects (Strieter et al. 1995; Pick et al. 2013). Its relatively high levels in Matrigel might affect hematopoietic differentiation and vasculogenesis from ESC (Gerecht-Nir et al. 2003). Conversely, PlGF-2 is a positive factor for angiogenesis and endothelial cell proliferation via its binding to the VEGF receptor, and its presence in Matrigel would also be expected to influence Matrigel-based stem cell assays involving blood cell formation and vasculogenesis (Zhou et al. 2013). Finally, VEGF itself was detected as a high responder, and again, would mean that Matrigel could, in and of itself, affect ESC hematopoiesis and vasculogenesis, and hematopoietic stem cell differentiation, growth or survival (Nakayama et al. 1998; Gerber et al. 2002; Gerecht-Nir et al. 2003).
Chemokines that were indicated to be at high levels in Matrigel by the proteome array results were MIG (145 units) and serpin E1 (160 and 92 units in separate arrays), and some others, MCP-1 (96, 44, and 30 units in separate arrays) and MCP-5 (20 units), were detected at lower levels. These and other chemokines are being found to play important roles in stem cell biology. For example, it was recently shown that MCP-1 (a.k.a. CCL2) stimulated core ESC inducing factors Klf4, Nanog, Sox2, and Tbx3, and, that in conjunction with LIF, maintains pluripotency in mESC and mouse induced pluripotent stem cells (miPSC; Hasegawa et al. 2011). Other reports indicated chemokine participation in stem cell-mediated angiogenesis and cardiogenesis (Chamberlain et al. 2011; Tamura et al. 2011; Bronckaers et al. 2013; Lee et al. 2013).
High positive detection was found for adiponectin (92 units), C5a (127 units), endocan (106 units), lipocalin-2 (142 units), sICAM-1 (97 units), MMP-3 (101 units), and TIMP-1 (241, 119, and 27 units from three separate arrays) in Matrigel. The elevated levels of these functionally diverse proteins in Matrigel may be caused by the effects of the Matrigel-source-tumor on the host mouse’s physiology as it grows in the body. That is, these proteins, with the exception of adiponectin, are inflammation related and tissue integrity/remodeling related. Similarly, the other disparate proteins found in Matrigel at moderately high levels, i.e., C-reactive protein (44 units), coagulation factor III (37 units), endoglin (22 units), endostatin/collagen XVIII (38 units), endothelin-1 (29 units), ICAM-1 (44 units), IL-1ra (44 units), MMP-9 (37 units), osteopontin (34 units), pentraxin-3 (23 and 16 units in separate arrays), and RANTES (89 and 47 units in separate arrays) are also involved with inflammation and tissue integrity/remodeling. Be that as it may, some of these proteins can have profound effects on ESC maintenance, growth, and differentiation. For example, matrix remodeling by metalloproteinases (MMP) can support self-renewal of ESC, presumably by mobilizing pluripotency factors sequestered in the surrounding cell matrix (Przybyla et al. 2013). Another example is the potentiating role of MMP-3 in cardiac muscle differentiation in ESC embryoid bodies (Hong et al. 2010). Finally, it is interesting to note the recent report highlighting a connection between the activation of innate cellular inflammatory processes and its enhancement of nuclear reprogramming (Lee et al. 2012). Here, activation of toll-like receptors (TLR), particularly TLR3, led to epigenetic remodeling that render a cell’s chromatin more accessible to reprogramming factors and higher reprogramming efficiency. Although very speculative, some of the downstream inflammatory effector molecules linked to TLR activation, and that are found in Matrigel, such as C-reactive protein, endothelin-1, ICAM-1, IL-1ra, pentraxin-3, and RANTES might have a similar effect on nuclear reprogramming. What is sure, however, is that the presence of these inflammatory and cell–matrix remodeling proteins in Matrigel should be taken into account in biological assays using Matrigel because of their wide spread effects on a variety of cell types (Albini et al. 1987; Draper et al. 2004; Kleinman and Martin 2005; Lo et al. 2012).
Some previously reported growth factor components of Matrigel were not detected by the proteome arrays, i.e., FGF-2, IGF-1, PDGF, and EGF. This may reflect a factors low level in Matrigel, i.e., ~1 pg/ml for FGF-2 and ~3–12 pg/ml for PDGF (Vukicevic et al. 1992; BD Biosciences), and the limits of detection of the proteome array. However, proteome array sensitivity would not seem to explain the lack of detection for EGF and IGF-1 since these factors were previously reported to be in Matrigel in nanogram amounts; 3–4 ng/ml for EGF and 6–7 ng/ml (Vukicevic et al. 1992) or even 15 ng/ml for IGF-1 (BD Biosciences). We have previously noted some dissimilar results when comparing proteome array results to the results obtained from commercially available ELISA when measuring growth factors in conditioned cell culture medium (Talbot et al. 2012).
Other apparent anomalies may also be present in the results from the proteome arrays. Across the three arrays, some of the same proteins were targeted on different arrays. In comparing these instances, there were some pronounced differences in the resulting signal, e.g., TIMP-1, IGFBP-1 and -3, and RANTES (Table 1). The small differences between the arrays’ positive controls do not explain the wide differences found for these and a few other duplicated proteins on the arrays. This would suggest an inconsistency of the array proteome technology or that separate arrays are using different capture antibodies for the same protein target. Whether this variation is a quality control issue or illustrates the semi-quantitative nature of the proteome array data, this indicates that the data presented here need independent verification by alternative and more quantitative protein detection methods.
Acknowledgments
The authors thank Dr. Le Ann Blomberg for comments on the manuscript and Mr. Paul Graninger and Ms. Lori Schreier for their assistance in conducting the proteome array assays.
Abbreviations
- ADAMTS1
A disintegrin and metalloproteinase with thrombospondin motifs 1
- AgRP
Agouti-related protein; a.k.a. the protein product of the agouti-related transcript (ART)
- ANGPT-L3
Angiopoietin-like 3
- BLC
B lymphocyte chemoattractant; a.k.a. CXCL13; B-cell-attracting chemokine 1 (BCA-1)
- CRP
C-reactive protein
- C5a
Complement component 5a
- CCL
Chemokine (C-C motif) ligand - #
- CXCL
Chemokine (C-X-C motif) ligand- #
- Cyr61
Cysteine-rich protein 61, a.k.a. IGFBP-10
- DLL4
Delta-like ligand 4
- DPPIV
Dipeptidyl peptidase IV; a.k.a., cluster of differentiation 26 (CD26)
- EGF
Epidermal growth factor
- ESM-1
Endothelial cell-specific molecule-1; a.k.a. endocan
- FGF-1
Fibroblast growth factor-1; a.k.a. acidic FGF (aFGF)
- FGF-2
Fibroblast growth factor-2; a.k.a. basic FGF (bFGF)
- FGF-7
Fibroblast growth factor-7; a.k.a. keratinocyte growth factor (KGF)
- G-CSF
Granulocyte-colony stimulating factor
- GM-CSF
Granulocyte-macrophage-colony-stimulating factor
- HB-EGF
Heparin-binding EGF-like growth factor
- HGF
Hepatocyte growth factor
- I-309
a.k.a., CCL1 and T-cell activation-3 (TCA-3)
- ICAM-1
Intercellular adhesion molecule-1; a.k.a. CD54
- IFN-γ
Interferon-gamma
- IGF
Insulin-like growth factor-1 and -2
- IGFBP
Insulin-like growth factor binding-protein-1, -2, -3, -5, and -6
- IL
Interleukin-1α, -1β, -1ra, -2 thru -7, -10 thru -13, -16, -17, -23, and -27
- IP-10
Interferon-inducible protein-10; a.k.a. CXCL10 and cytokine responsive gene-2 (CRG-2)
- I-TAC
Interferon-inducible T-cell alpha chemoattractant; a.k.a. CXCL11
- KC
Keratinocyte-derived chemokine; a.k.a. CXCL1 and growth-related oncogene alpha (GROα)
- LIF
Leukemia inhibitory factor
- MCP-1
Monocyte chemotactic protein-1; a.k.a. CCL2 and junctional epithelium chemokine (JE)
- MCP-5
Monocyte chemotactic protein-5; a.k.a. CCL12
- M-CSF
Macrophage-colony stimulating factor; a.k.a. CSF-1
- MIG
Monokine induced by gamma-interferon; a.k.a. CXCL9
- MIP
Macrophage inflammatory protein-1α; a.k.a. CCL3; -1β/CCL4; -2/CXCL2
- MMP
Matrix metalloproteinase-3, -8, -9, and -14
- NOV
Nephroblastoma overexpressed gene; a.k.a. CCN3 and IGFBP9
- TSG-14
Tumor necrosis factor-stimulated gene-14; a.k.a. pentraxin-3
- Pref-1
Preadipocyte factor 1, a.k.a. DLK-1 (delta-like protein-1)
- RAGE
Receptor for advanced glycation endproducts
- RANTES
Regulated on activation, normal T-cell expressed and secreted; a.k.a. CCL5
- RBP4
Retinol-binding protein-4
- PAI-1
Plasminogen activator inhibitor-1, a.k.a. serpin E1
- PDGF
Platelet-derived growth factor A-chain homodimer (PDGF-AA), PDGF-BB and PDGF-AB
- PD-ECGF
Platelet-derived endothelial cell growth factor
- PlGF-2
Placenta growth factor 2
- Serpin E1
Serine protease inhibitor, clade E, member 1; a.k.a. plasminogen activator inhibitor type 1 (PAI-1)
- Serpin F1
Serine protease inhibitor, clade F, member 1; a.k.a. pigment epithelium-derived factor (PEDF)
- SPARC
Secreted protein acidic and rich in cysteine
- SDF-1
Stromal cell-derived factor-1; a.k.a. CXCL12
- TARC
Thymus and activation-regulated chemokine; a.k.a. CCL17
- TIMP
Tissue inhibitor of metalloproteinases-1 and -4
- TNF-α
Tumor necrosis factor-alpha
- TREM-1
Triggering receptor expressed on myeloid cells 1
- VEGF
Vascular endothelial growth factor
Footnotes
Mention of trade name, proprietary product or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture or imply its approval to the exclusion of other products or vendors that also may be suitable.
References
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain G, Smith H, Rainger GE, Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS One. 2011;6:e25663. doi: 10.1371/journal.pone.0025663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Calzi SL, Sullivan SM, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirami G, Papadopoulos V, Kleinman HK, Defreese DC, Musto NA, Dym M. Identification of transferrin and inhibin-like proteins in matrigel. In Vitro Cell Dev Biol Anim. 1995;31:404–411. doi: 10.1007/BF02634247. [DOI] [PubMed] [Google Scholar]
- Draper JS, Moore HD, Ruban LN, Gokhale PJ, Andrews PW. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820. doi: 10.1097/01.LAB.0000106502.41391.F0. [DOI] [PubMed] [Google Scholar]
- Gillette KM, Forbes K, Sehgal I. Detection of matrix metalloproteinases (MMP), tissue inhibitor of metalloproteinase-2, urokinase and plasminogen activator inhibitor-1 within matrigel and growth factor-reduced matrigel basement membrane. Tumori. 2003;89:421–425. doi: 10.1177/030089160308900415. [DOI] [PubMed] [Google Scholar]
- Greenlee AR, Kronenwetter-Koepel TA, Kaiser SJ, Liu K. Comparison of Matrigel and gelatin substrata for feeder-free culture of undifferentiated mouse embryonic stem cells for toxicity testing. Toxicol In Vitro. 2005;19:389–397. doi: 10.1016/j.tiv.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hansen KC, Kiemele L, Maller O, O’Brien J, Shankar A, Fornetti J, Schedin P. An in-solution ultrasonication-assisted digestion method for improved extracellular matrix proteome coverage. Mol Cell Proteomics. 2009;8:1648–1657. doi: 10.1074/mcp.M900039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Takahashi N, Forrest AR, Shin JW, Kinoshita Y, Suzuki H, Hayashizaki Y. CC chemokine ligand 2 and leukemia inhibitory factor cooperatively promote pluripotency in mouse induced pluripotent cells. Stem Cells. 2011;29:1196–1205. doi: 10.1002/stem.673. [DOI] [PubMed] [Google Scholar]
- Hong S, Kang JK, Park JJ, Ryu ES, Choi SS, Lee SH, Lee JH, Seo JS. Association of matrix metalloproteinase-3 with cardiogenic activity during Noggin-induced differentiation of mouse embryonic stem cells. Int J Cardiol. 2010;141:49–60. doi: 10.1016/j.ijcard.2008.11.156. [DOI] [PubMed] [Google Scholar]
- Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Dempsey RJ. IGFBP-3 inhibits the proliferation of neural progenitor cells. Neurochem Res. 2011;36:406–411. doi: 10.1007/s11064-010-0349-2. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparin sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kang JE, Lee JK, Bae JS, Jin HK. Bone-marrow-derived mesenchymal stem cells promote proliferation and neuronal differentiation of Niemann-Pick Type C mouse neural stem cells by upregulation and secretion of CCL2. Hum Gene Ther. 2013;24:655–669. doi: 10.1089/hum.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AT, Mori H, Mott J, Bissell MJ. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia. 2012;17:103–110. doi: 10.1007/s10911-012-9251-7. [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massumi M, Abasi M, Babaloo H, Terraf P, Safi M, Saeed M, Barzin J, Zandi M, Soleimani M. The effect of topography on differentiation fates of matrigel-coated mouse embryonic stem cells cultured on PLGA nanofibrous scaffolds. Tissue Eng Part A. 2012;18:609–620. doi: 10.1089/ten.tea.2011.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Imai M, Hirayama T, Saburi S, Tanaka M, Maruyama M, Matsuo C, Meguro H, Nishibashi K, Inoue F, Djiane J, Gertler A, Tachi S, Imakawa K, Tachi C. Establishment of feeder-independent cloned caprine trophoblast cell line which expresses placental lactogen and interferon tau. Placenta. 2002;23:613–630. doi: 10.1053/plac.2002.0846. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Fang I, Elliott G. Natural killer and B-lymphoid potential in CD34+ cells derived from embryonic stem cells differentiated in the presence of vascular endothelial growth factor. Blood. 1998;91:2283–2295. [PubMed] [Google Scholar]
- Nguyen-Ngoc KV, Ewald AJ. Mammary ductal elongation and myoepithelial migration are regulated by the composition of the extracellular matrix. J Microsc. 2013;251:212–223. doi: 10.1111/jmi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm RA. Murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease S, Braghetta P, Gearing D, Grail D, Williams RL. Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF) Dev Biol. 1990;141:344–352. doi: 10.1016/0012-1606(90)90390-5. [DOI] [PubMed] [Google Scholar]
- Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- Pick M, Azzola L, Osborne E, Stanley EG, Elefanty AG. Generation of megakaryocytic progenitors from human embryonic stem cells in a feeder- and serum-free medium. PLoS One. 2013;8:e55530. doi: 10.1371/journal.pone.0055530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla LM, Theunissen TW, Jaenisch R, Voldman J. Matrix remodeling maintains embryonic stem cell self-renewal by activating Stat3. Stem Cells. 2013;31:1097–1106. doi: 10.1002/stem.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Li D, Omiecinski CJ, Muller-Eberhard U, Kleinman HK, Elswick B, Guzelian PS. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988;134:309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Steiger-Luther NC, Darwiche H, Oh SH, Williams JM, Petersen BE (2010) Insulin-like growth factor binding protein-3 is required for the regulation of rat oval cell proliferation and differentiation in the 2AAF/PHX model. Hepat Med 2010:13-32 [DOI] [PMC free article] [PubMed]
- Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Talbot NC, Blomberg LA, Garrett WM, Caperna TJ. Feeder-independent continuous culture of the PICM-19 pig liver stem cell line. In Vitro Cell Dev Biol Anim. 2010;46:746–757. doi: 10.1007/s11626-010-9336-9. [DOI] [PubMed] [Google Scholar]
- Talbot NC, Sparks WO, Powell AM, Kahl S, Caperna TJ. Quantitative and semiquantitative immunoassay of growth factors and cytokines in the conditioned medium of STO and CF-1 mouse feeder cells. In Vitro Cell Dev Biol Anim. 2012;48:1–11. doi: 10.1007/s11626-011-9467-7. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Matsumura K, Sano M, Tabata H, Kimura K, Ieda M, Arai T, Ohno Y, Kanazawa H, Yuasa S, Kaneda R, Makino S, Nakajima K, Okano H, Fukuda K. Neural crest-derived stem cells migrate and differentiate into cardiomyocytes after myocardial infarction. Arterioscler Thromb Vasc Biol. 2011;31:582–589. doi: 10.1161/ATVBAHA.110.214726. [DOI] [PubMed] [Google Scholar]
- Uemura M, Refaat MM, Shinoyama M, Hayashi H, Hashimoto N, Takahashi J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-Q. [DOI] [PubMed] [Google Scholar]
- Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, Brimble SN, McLean A, Galeano MJ, Uhl EW, D’Amour KA, Chesnut JD, Rao MS, Blau CA, Robins AJ. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Lin Q, Liu Z, Wang H, Duan C, Wang Y, Hao T, Wu K, Wang C. Embryoid bodies formation and differentiation from mouse embryonic stem cells in collagen/Matrigel scaffolds. J Genet Genomics. 2010;37:451–460. doi: 10.1016/S1673-8527(09)60064-3. [DOI] [PubMed] [Google Scholar]
- Zhou X, Barsky LW, Adams GB. Placental growth factor expression is required for bone marrow endothelial cell support of primitive murine hematopoietic cells. PLoS One. 2013;8:e67861. doi: 10.1371/journal.pone.0067861. [DOI] [PMC free article] [PubMed] [Google Scholar]