Abstract
Cytogenetic effects of Anilofos which was widely used in agriculture, was evaluated in Allium cepa root meristematic cells. In the Allium root growth inhibition test EC50 value was determined 50 ppm and 1/2× EC50 (25 ppm), EC50 (50 ppm) and 2 × EC50 (100 ppm) concentrations of Anilofos were applied to onion roots. A negative and positive control were used in the experiment in parallel. According to results mitotic index decreased with increasing the Anilofos concentrations in all application groups and each exposure time, while disturbed anaphase–telophase, choromosome laggard(s), stickiness and anaphase bridge(s) were observed. In anaphase–telophase cells, c-metaphase, disturbed nucleus and binuclear cells were observed in other anomalies. The results were also analyzed statistically by using Dunnett t test (2-tailed) and all concentrations of Anilofos were found significant.
Keywords: Allium test, Anilofos, Chromosome aberrations, Mitotic index
Introduction
Organophosphorus pesticides (OPs) are one of the most important groups of pesticides which have been widely used in agriculture, industry and hygiene (Bello-Ramírez et al. 2000; Ballesteros and Parrado 2004; Wu et al. 2007). OPs, such as herbicides and insecticides, increased economic profits significantly, however, the abusive usage of these pesticides during decades caused serious hazards on environmental and human health (Mann et al. 2009; Burridge et al. 2010). In addition, when OPs pesticides enter the environment and reach high concentrations (Fleischli et al. 2004), it can accumulate and can affect non-target species.
They act by inhibiting the hydrolysis reaction performed by acetylcholinesterase, an enzyme that is essential for central nervous system function in insects and humans. This inhibition leads to accumulation of the neurotransmitter acetylcholine, causing interruption of nervous impulses in the synapses (Eyer 2003). Exposure to even small amounts of an OPs compound can be fatal; death is generally caused by respiratory failure (Jokanović 2009).
Anilofos is also an OP which has an important role to control weeds and marsh grasses in rice. Hazarika and Sarkar (2001) reported that Anilofos inhibited cholinesterase enzyme activities of plasma, erytrocyte, blood, liver, and brain. This pesticide led to a reduction of total protein of plasma and liver. Results showed moderate toxic potential of Anilofos in mammals.
To analyze the effects of different substances, higher plants (Vicia faba, Tradescantia paludosa, Pisum sativum, Hordeum vulgare, Crepis capillaris and Allium cepa, etc.) have proven to be useful when used as bioindicators (Kluge and Podlesak 1985; Ma et al. 1995; Amer et al. 1999; Sang and Li 2004; Majer et al. 2005; Mišik et al. 2006; Gadeva and Dimitrov 2008; Enan 2009; Özkara et al. 2011). Among them, Allium test is one of the well-known and reliable test systems to determine the toxicity in the laboratories (Fiskesjo 1985; Saxena et al. 2005; Konuk et al. 2007; Liman et al. 2010). Onions are easy to store and to handle, and also macroscopic and microscopic parameters can be observed easily. Moreover this system correlates well with the data obtained from eukaryotic and prokaryotic systems (Fiskesjo 1988).
It is highlighted that the species A. cepa is widely employed for the determination of cytotoxic, genotoxic and mutagenic effects of various substances and also environmental samples. The mitotic index (MI), could be used as a reliable parameter for evaluating the cytotoxicity of various agents (Leme and Marin-Morales 2009). Thus, in this study, we aimed to evaluate the cytotoxic effect of Anilofos, an organophosphate pesticide (OP), by using the A. cepa test.
Materials and methods
Test organism/growth conditions
Equal-sized bulbs (25–30 mm in diameter, without any treatment) of a commercial variety of A. cepa L. (2n = 16) were used in the Allium test. The onions were kept cool and dry until the experiment. Just before use, the outer scales of the bulbs were carefully removed and the brownish bottom plates were scraped away without destroying the root primordia. The roots were protected from direct sunlight in order to minimize fluctuation of the rate of cell division (Evans et al. 1957). The experiment was carried out under laboratory conditions.
Chemicals
The test substance Anilofos was obtained from Fluka (Interlab A.S. Istanbul, Turkey) (CAS No. 64249-01-0) and dissolved in dimethyl sulphoxide (1 % DMSO, purity 99 %). All other chemicals were purchased from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany). Stock solutions of the test materials were prepared in distilled water or dimethyl sulphoxide (DMSO) and stored at room temperature in the dark. The structural formula of Anilofos is shown in Fig. 1.
Fig. 1.
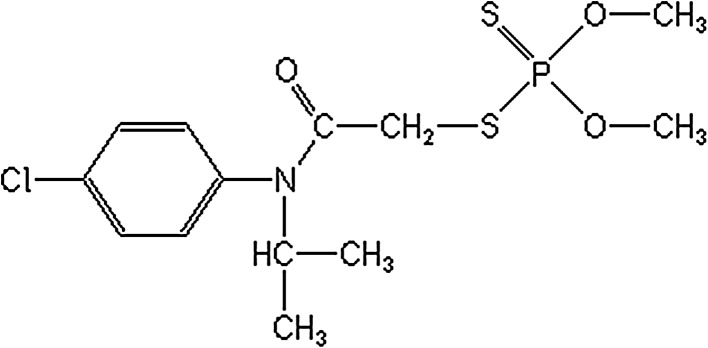
The chemical structure of Anilofos
Determination of EC50
Clean and healthy onion bulbs were placed onto test tubes filled with distilled water and allowed to produce roots in the dark at room temperature for 24 h. After this time, the best growing five bulbs were chosen for the experiment and treated with different concentrations of Anilofos (6.25, 12.5, 25, 50 and 100 ppm) at room temperature (~21 ± 4 °C) for 96 h. In the experiment, the test concentrations were refreshed at every 24 h. A set of bulbs was also treated with 1 % DMSO as negative control and methyl methane sulfonate (MMS) as positive control. On the 5th day, the length of the whole root bundle from control and experimental sets (lengths of ten roots from each bulb) were measured. Taking root lengths of control groups as 100 % lengths of application groups were plotted against test concentrations and the point showing 50 % growth was defined as EC50 concentration.
Cytogenetic Assay
A. cepa bulbs were placed in test tubes and rooted in distilled water for 24 h. The five bulbs which have approximately same root length were transferred to the control and application solutions. 1/2× EC50, EC50 and 2 × EC50 were used to determinate the application doses. Roots were treated with three different concentrations of Anilofos for 24, 48 and 72 h under the usual laboratory conditions. At the end of the exposure period, roots were cut and immediately treated in a chilled Carnoy’s fixative (ethanol: acetic acid = 3:1) for 24 h and kept at 4 °C overnight. The roots were transferred to 70 % alcohol and kept in refrigerator until use.
Staining and macroscopic parameters
After hydrolysis during 7 min in 1 N HCl at 60 °C, root tips were washed with distilled water three times. They were stained by means of the Feulgen reaction and the apical 2 mm were squashed in a drop of 45 % acetic acid. One slide was prepared per bulb. The MI was calculated in cytogenetic analysis for each concentration and exposure time. For MI, the different stages of mitosis were counted in at least 5,000 cells (1,000 cells/slide) per concentration, and expressed as a percentage.
Statistical analyzes
The data of root length, MI and mitotic phases were analyzed statistically using SPSS, ver. 18.0. Dunnett t test (two tailed) was performed in order to evaluate the significant differences between each treatment group and control. The level of statistical significance was in all cases p ≤ 0.05. Three independent experiments were carried out and each data point represents the arithmetic mean ± SD of at least.
Results
Effects on Allium root growth and mitotic index
Table 1 shows the results of Allium root growth inhibition test. Allium root growth found the effective concentration (EC50) value which retards 50 % root growth with approximately 50 ppm. The analysis of the results showed that the root growth decreased with increasing concentrations (6.25, 12.5, 25, 50 and 100 ppm) of Anilofos. The average length of roots was 4.42 ± 0.23 cm after 96 h of growth in the control. Dose–response curves obtained between the concentrations of Anilofos and the effective concentration (EC50) value was determined as 50 ppm and the root length after 96 h in EC50 was 2.73 ± 0.14 cm. After determination of EC50 value, 2 × EC50 (100 ppm), EC50 (50 ppm) and 1/2× EC50 (25 ppm) and control group were used at 24, 48 and 72 h treatment periods in the experiment.
Table 1.
Allium root growth inhibition test
| Test substance | Concentrations (ppm) | Mean of root length ± SD |
|---|---|---|
| Negative control | – | 4.42 ± 0.23 |
| Positive control | – | 1.04 ± 0.15* |
| Anilofos | 6.25 | 4.06 ± 0.14* |
| 12.5 | 3.89 ± 0.17* | |
| 25 | 2.86 ± 0.20* | |
| 50 | 2.73 ± 0.14* | |
| 100 | 2.29 ± 0.13* |
* Significantly different from negative control (p < 0.05 Dunnet t test, 2-sided) SD: Standard deviation
The effect of Anilofos on the MI (%) and mitotic phases of A. cepa root meristem cells were summarizes in Table 2. According to results MI significantly decreased in different concentrations of Anilofos at each exposure time compared to control. There were significant differences between Anilofos concentrations and the control.
Table 2.
The effects of Anilofos on MI and mitotic phases in the root cells of A. cepa
| Concentration (ppm) | Treatment time | Counted cell number | Mitotic index ± SD | Mitotic phases (%) ± SD | |||
|---|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | ||||
| Negative control | 24 h | 5,042 | 81.33 ± 10.63 | 78.27 ± 10.47 | 1.03 ± 0.18 | 1.02 ± 0.37 | 0.83 ± 0.38 |
| Positive control | 4,425 | 39.16 ± 4.45* | 36.99 ± 5.46* | 1.26 ± 0.17 | 0.59 ± 0.34 | 0.52 ± 0.28 | |
| 25 | 5,084 | 34.44 ± 3.72* | 31.04 ± 3.36* | 1.14 ± 0.39 | 0.87 ± 0.15 | 1.40 ± 0.43 | |
| 50 | 5,078 | 30.07 ± 8.92* | 26.93 ± 8.54* | 0.88 ± 0.27 | 0.92 ± 0.23 | 1.32 ± 0.26 | |
| 100 | 5,088 | 28.31 ± 1.61* | 24.75 ± 2.22* | 0.65 ± 0.45 | 1.75 ± 0.73 | 1.16 ± 0.32 | |
| Negative control | 48 h | 5,100 | 72.22 ± 1.55 | 71.83 ± 2.48 | 0.73 ± 0.37 | 0.26 ± 0.33 | 0.31 ± 0.24 |
| Positive control | 5,052 | 35.96 ± 2.11* | 32.85 ± 2.02* | 1.36 ± 0.38* | 0.66 ± 0.39* | 0.76 ± 0.42* | |
| 25 | 5,197 | 25.11 ± 2.50* | 23.85 ± 2.18* | 0.51 ± 0.21 | 0.85 ± 0.32 | 0.55 ± 0.22 | |
| 50 | 5,060 | 24.76 ± 1.73* | 22.19 ± 1.94* | 1.04 ± 0.47 | 0.91 ± 0.28 | 0.64 ± 0.35 | |
| 100 | 5,100 | 19.20 ± 4.55* | 17.75 ± 4.22* | 0.43 ± 0.15 | 0.71 ± 0.51 | 0.49 ± 0.46 | |
| Negative control | 72 h | 5,185 | 60.88 ± 2.18 | 58.96 ± 2.63 | 0.58 ± 0.32 | 0.42 ± 0.85 | 0.98 ± 0.65 |
| Positive control | 5,198 | 31.96 ± 4.12* | 28.86 ± 3.68* | 1.04 ± 0.47 | 0.89 ± 0.26 | 0.92 ± 0.45 | |
| 25 | 5,056 | 21.22 ± 5.44* | 18.68 ± 5.38* | 0.73 ± 0.37 | 1.03 ± 0.25 | 0.75 ± 0.15 | |
| 50 | 5,148 | 20.66 ± 3.68* | 18.79 ± 3.25* | 0.63 ± 0.34 | 1.00 ± 0.48 | 0.51 ± 0.33 | |
| 100 | 5,142 | 18.78 ± 4.98* | 17.53 ± 4.41* | 0.46 ± 0.32 | 0.65 ± 0.30 | 0.35 ± 0.23* | |
* Significantly different from negative control (p < 0.05 Dunnet t test. 2-sided)
SD standard deviation
The highest MI values were obtained from 24 h applications of 25 ppm with a score of 34.44 ± 3.72. The percentage of MI was low at 100 ppm at 72 h with a score of 18.78 ± 4.98 compared to other concentrations. In addition all MI results were found statistically significant for all concentrations of Anilofos and at each exposure time.
In this study, all concentrations of Anilofos caused changes percentage of the distribution of mitotic phases in comparison to the control (Table 2). The percentage values of particular mitotic phases of control in the 24 h experiment were for prophase 78.27 ± 10.47, for metaphase 1.03 ± 0.18, for anaphase 1.02 ± 0.37, for telophase 0.83 ± 0.38. For the 48 h experiment, the values were 71.83 ± 2.48, 0.73 ± 0.37, 0.26 ± 0.33, 0.31 ± 0.24 and for 72 h 58.96 ± 2.63, 0.58 ± 0.32, 0.42 ± 0.85, 0.98 ± 0.65, respectively. This pesticide decreased the percentages of the prophase stages significantly for all concentrations in 24, 48, 72 h. Anilofos caused differentiation in the stage of metaphase, anaphase and telophase when compared to control but these results were not found statistically significant.
Effects on chromosome aberrations
Allium cepa anaphase–telophase chromosome aberration test is shown in Table 3. The total mean percentages of anaphase-telophase aberrations (disturbed anaphase-telophase, choromosome laggard(s), stickiness, anaphase bridge) according to total cells with chromosome abberations were calculate in control groups as 40.9 ± 15.3, 37.6 ± 4.1 and 43.7 ± 3.7 for 24, 48, 72 h, respectively. The total mean percentages of other anomalies (c-metaphase, disturbed nucleus, polyploidy, binuclear cell) were also calculate in the experiment. Chromosome laggards were the most frequently observed chromosomal aberrations, although polyploidy was not observed in all application groups. The total percentage of chromosomal aberrations increased with an increase in the Anilofos concentrations and exposure time. According to results, the effect of Anilofos concentrations on chromosome aberration was statistically significant in all application groups as compared to the control. While the lowest anomalies were observed 0.4 % at the 50 ppm at 72 h, the highest one were observed 62.2 % at the 50 ppm at 48 h.
Table 3.
Percentage of chromosome aberrations of Anilofos at different times and concentrations obtained for the A. cepa anaphase–telophase test
| Concentration (ppm) | Counted cell numbers | Anaphase–teleophase anomalies (%) | Other anomalies (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disturbed anaphase–teleophase | Chromosome laggards | Stickiness | Anaphase bridge | Total Anomalies (% ± S.D)* | Counted cell numbers | C-metaphase | Disturbed nucleus | Polyploidy | Binuclear cell | Total anomalies (% ± SD)* | ||
| Control-24 | 344 | 12.1 | 16.4 | 6.3 | 5.2 | 40.9 ± 15.3 | 5,366 | 0.4 | 0.2 | – | 0.1 | 0.7 ± 0.4 |
| MMS (10 μg/ml) | 322 | 7.4 | 50.2 | 5.6 | 2 | 64.4 ± 7.4* | 5,219 | 0.1 | 0.1 | – | 0.1 | 0.2 ± 0.2* |
| 25 | 275 | 3.6 | 49.4 | 1 | 5 | 55 ± 9.4 | 5,084 | 0.8 | – | – | – | 0.2 ± 0.1* |
| 50 | 292 | 4.6 | 49.8 | 3.2 | 0.8 | 58.4 ± 7.2 | 5,078 | 0.1 | – | – | – | 0.1 ± 0.1* |
| 100 | 321 | 4.4 | 55.2 | 3.8 | 0.8 | 64.2 ± 11.1* | 5,088 | 0.2 | – | – | – | 0.1 ± 0.1* |
| Control-48 | 188 | 6.6 | 24.6 | 4 | 2.4 | 37.6 ± 4.1* | 5,217 | 0.1 | 0.2 | – | 0.2 | 0.4 ± 0.2 |
| MMS (10 μg/ml) | 370 | 7.6 | 53.4 | 7.1 | 6.1 | 74.1 ± 6* | 5,143 | – | – | – | – | 0.1 ± 0.1* |
| 25 | 315 | 3.8 | 55.2 | 2.8 | 1.2 | 63 ± 4* | 5,197 | 0.2 | – | – | – | 0.1 ± 0.1* |
| 50 | 355 | 4.1 | 62.2 | 3.7 | 1 | 71 ± 4.1* | 5,060 | 0.1 | – | – | – | 0.1 ± 0.1* |
| 100 | 387 | 6.5 | 66 | 4.6 | 0.2 | 77.4 ± 16.9* | 5,100 | 0.6 | – | – | – | 0.1 ± 0.1* |
| Control-72 | 218 | 6.5 | 26.2 | 8.9 | 2.2 | 43.7 ± 3.7 | 5,217 | 0.2 | 0.8 | – | 0.1 | 0.4 ± 0.5 |
| MMS (10 μg/ml) | 451 | 12 | 61.7 | 8 | 8.9 | 90.3 ± 11.9* | 5,083 | 0.1 | – | – | 0.1 | 0.1 ± 0.1* |
| 25 | 283 | 6.2 | 46.6 | 3 | 0.8 | 56.6 ± 6.7* | 5,056 | – | – | – | – | 0.1 ± 0.1* |
| 50 | 281 | 5.6 | 46.2 | 2.6 | 0.4 | 56.2 ± 6.6* | 5,148 | 0.4 | – | – | – | 0.1 ± 0.1* |
| 100 | 284 | 4 | 49.8 | 2.2 | 0.8 | 56.8 ± 2.2* | 5,142 | 0.4 | – | – | – | 0.1 ± 0.2* |
* Significantly different from negative control (p < 0.05 Dunnet t test. 2-sided) SD: Standard deviation
Discussion
Plants are used for determination of environmental pollutants such as pesticides because they are indirect recipients of agrotoxic, so they are important materials for genotoxic test and environmental monitoring of places affected by pollutants. Higher plants such as A. cepa, V. faba and T. paludosa have large monocentric chromosomes in reduced numbers and are accepted as suitable test material for the study of environmental mutagenesis (Rank and Nielsen 1998; Grover and Kaur 1999; Patra and Sharma 2002; Sharma and Vig 2012). Allium cepa assay is one of the best and extensively used system for the determination of cytotoxic and genotoxic impacts of pollutants, due to its sensitivity (Barberio et al. 2011). Also, A. cepa showed a good correlation with the results from other established test systems using eukaryotic as well prokaryotic cells (Fiskesjö 1985; Yıldız et al. 2009).
In Allium root growth test, Anilofos was found cytotoxic causing an inhibition in root growth of A. cepa. This inhibition was generally related to cell elongation during the differentiation (Fusconi et al. 2006), apical meristematic activity (Webster and MacLeod 1996), and the inhibition of protein synthesis (Seth et al. 2007). EC50 value was shown to be a useful parameter for choosing the test concentrations for the genotoxicity tests (Chauhan et al. 1999; Seth et al. 2007). In this study the effective concentration (EC50) was determinated 50 ppm and 2 × EC50 (100 ppm), EC50 (50 ppm) and 1/2× EC50 (25 ppm) and control group were used in the experiment.
The mitotic index is a reliable parameter which allows to estimate the frequency of cellular division (Marcano et al. 2004; Fernandes et al. 2007). Inhibition of mitotic activities is generally used for screening of cytotoxic agents (Linnainmaa et al. 1978). MIs higher than negative control may be derived from the result of the induction of cell division while MIs lower than negative control may be derived from the growth and development of exposed organisms having been affected by test compounds (Hoshina 2002).
The concentration-dependent inhibition of MI described the cytotoxic potential of Anilofos in A. cepa. In the present study, Anilofos was shown to have a cytotoxic effect due to decrease of MI. Similar effects on MI were reported by many earlier studies in the A. cepa test (Marcano et al. 2004; Yıldız and Arıkan 2008; Pandey 2008; Srivastava and Mishra 2009; Fındıklı and Türkoğlu 2010; Ozen et al. 2011; Andrioli et al. 2012).
Significant reduction of MI may be due to the mitodepressive action of substances therefore the chemicals used interfere in the normal cell cycle resulting in decrease in number of dividing cells (Sharma and Vig 2012). In addition it may be due to inhibition of DNA synthesis or blocking of G1, suppressing DNA synthesis or effect of test compound at G2 phase of the cell cycle (Sudhakar et al. 2001; El-Ghamery et al. 2000; Majewska et al. 2003). So, we can state the decline of MI in our study can be related to these reasons.
In addition, the cytotoxicity of Anilofos can be explained by its chemical structure. Anilofos is an important thiono organophosphate herbicide which is used as AchE inhibitor (Hazarika and Sarkar 2001). It is reported that OPs are worth nearly 40 % of the global market and that they are anticipated to maintain dominance for some time in the future (Singh and Walker 2006). This substance is readily absorbed through the mucosal membrane of the digestive tract by the blood and other body tissues and can spread through the respiratory system. Thiono OPs have no direct inhibitory effects on AChE and thioesters of these compounds (P=S) gain inhibitory properties after conversion to the oxo (P=O) form. Therefore, the chemicals which are containing thioester bonds must turn to oxo forms for becoming active (Marrs 1993; Maroni et al. 2000). Anilofos contains a P=S bond; therefore, in our opinion, this compound may be cytotoxic due to its chemical structure and biological activity in the cell. There are several supporting data about OPs in previous studies parallel to our study (Arañez and Rubio 1993; Lamsal et al. 2010; Türkoğlu 2012).
There are a few studies on the potential genotoxic activity of Anilofos in the literature. Hazarika and Sarkar (2001) investigated the toxicity of Anilofos in rats and they showed that this substance has moderate toxicity in mammals. In another study, Aggarwal et al. (2007) investigated the embryo-fetal toxicity of Anilofos in groundwater containing arsenic. According to this research, Anilofos led to very important changes in the embryo-fetal development both alone and when combined with arsenic.
In this study Anilofos caused changes in the frequencies of the different mitotic phases. Their frequencies depended on the duration of treatment and concentration of the chemical applied. Anilofos decreased the percentages of the prophase stages significantly at all concentrations at all exposure time and caused differentiation in the stage of metaphase, anaphase and telophase when compared to control but these results were not found statistically significant. When pesticides once penetrate the cell and accumulate in the cell, they may be highly toxic (Antunes-Madeira and Madeira 1979). The phases of mitotic division may be affected the accumulation of the pesticides in the cell. The results of this study conformed with many earlier studies (Pandey 2008; Liman et al. 2010; Yıldız and Arıkan 2008; Srivastava and Mishra 2009; Andrioli et al. 2012).
The total mean percentages of anaphase-telophase aberrations and the total mean percentages of other anomalies were determined in this study. Four main types of chromosome aberrations were observed in anaphase-telophase aberrations: disturbed anaphase-telophase, choromosome laggard(s), stickiness, anaphase bridge(s). Other aberrations (c-metaphase, disturbed nucleus, polyploidy, binuclear cell) were also evaluated in the experiment. The total percentage of chromosomal aberrations increased with an increase in the Anilofos concentrations and exposure time. Chromosome laggards were the most frequently observed chromosomal aberrations, although polyploidy was not observed in all application groups.
Choromosome laggards are the result of disturbances in the spindle fiber formation caused by the different chemicals (Kuriyama and Sakai 1974; Haliem 1990).
Stickiness is considered to be a chromatid type aberration (Badr 1986). Darlington and Mc-Leish (1951) proposed that stickiness might be due to degradation or depolymerization of chromosomal DNA. Besides, many researchers reported that its occurrences during the study could be caused of sub-chromatid linkage between chromosomes (Chauhan et al. 1986; Ajay and Sarbhoy 1988; Kovalchuk et al. 1998; Liman et al. 2010). The bridges involving one or more chromosomes were the most important and frequent type in addition to sticky chromosomes. These bridges cause structural chromosome mutations. Anaphase bridges could happen during the translocation of the unequal chromatid exchange, due to dicentric chromosome presence, or due to the breakage and fusion of chromosomes and chromatids. (El-Ghamery et al. 2000; Luo et al. 2004). In addition, bridges may arise due to chromosome breaks, stickiness and breakage and reunion of the broken ends (Badr et al. 1992). Other aberrations, such as c-mitosis, binuclear cell and disturbed nucleus, were also observed in the experiment. Binuclear cells are accepted as the inhibition of cytokinesis in any control point of the cell cycle (Ateeq et al. 2002). C-mitosis, might occur due to disturbed microtubules (Fiskesjo 1988; Odeigah et al. 1997) or might be the result of disturbances in the spindle fiber formation (Haliem 1990).
In conclusion, Anilofos was found to be cytotoxic and genotoxic due to decrease of MI and induction of chromosome aberrations in Allium test. Plant test systems are practical techniques due to their sensitivity and easyness to apply to detect the genotoxicity of environmental pollutants. These test systems also point to the importance of understanding the main characteristics of the toxicity of pollutants.
References
- Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, Darzi A. Proving the effectiveness of virtual reality simulation for laparoscopic surgical training. Ann Surg. 2007;246:771–779. doi: 10.1097/SLA.0b013e3180f61b09. [DOI] [PubMed] [Google Scholar]
- Ajay KL, Sarbhoy RK. Cytogenetic studies on the effect of some chlorinated pesticides. Cytologia. 1988;53:427–436. doi: 10.1508/cytologia.53.427. [DOI] [Google Scholar]
- Amer SM, Mohammed FI, Ashry ZM. Cytogenetic effects of the fungicide benomyl on Vicia faba and Pisum sativum. Bull Nat Res Cent Egypt. 1999;24:481–494. [Google Scholar]
- Andrioli NB, Soloneski S, Larramendy ML, Mudry MD. Cytogenetic and microtubule array effects of the zineb-containing commercial fungicide formulation Azzurro(®) on meristematic root cells of Allium cepa L. Mutat Res. 2012;742:48–53. doi: 10.1016/j.mrgentox.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Antunes-Madeira MC, Madeira VMC (1979) Interaction of insecticides with lipid membranes. Biocihim Biophys Acta 550:384–392 [DOI] [PubMed]
- Arañez A, Rubio R. Genotoxicity of two organophosphate insecticides based on allium test. Sci Diliman. 1993;5:2. [Google Scholar]
- Ateeq B, Farah MA, Ali MN, Ahmad W. Clastogenicity of pentachlorophenol, 2, 4D and butachlor evaluated by Allium root tip test. Muta-t Res. 2002;514:105–113. doi: 10.1016/S1383-5718(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Badr A. Effect of the s-triazine herbicide terbutryn on mitosis chromosomes and nucleic acids in root tips of Vicia faba. Cytologia. 1986;51:571–578. doi: 10.1508/cytologia.51.571. [DOI] [Google Scholar]
- Badr A, Ghareeb A, Eldin HM (1992) Cytotoxicity of some pesticides in mitotic cells of V. faba roots. Egyptian J Appl Sci 7:457–468
- Ballesteros E, Parrado MJ. Continuous solid-phase extraction and gas chromatographic determination of organophosphorus pesticides in natural and drinking waters. J Chromatogr A. 2004;1029:267–273. doi: 10.1016/j.chroma.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Barberio A, Voltolini JC, Mello MLS. Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology. 2011;20:927–935. doi: 10.1007/s10646-011-0602-8. [DOI] [PubMed] [Google Scholar]
- Bello-Ramírez AM, Carreón-Garabito BY, Nava-Ocampo AA. A theoretical oxidation of organophosphorus pesticides. Toxicology. 2000;149:63–68. doi: 10.1016/S0300-483X(00)00222-5. [DOI] [PubMed] [Google Scholar]
- Burridge L, Weis JS, Cabello F, Pizarro J, Bostick K. Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture. 2010;306:7–23. doi: 10.1016/j.aquaculture.2010.05.020. [DOI] [Google Scholar]
- Chauhan LKS, Dikshith TSS, Sundararaman V. Effect of deltamethrin on plant cells. Cytological effects on the root meristem cells of Allium cepa. Mutat Res. 1986;171:25–30. doi: 10.1016/0165-1218(86)90005-4. [DOI] [Google Scholar]
- Chauhan LKS, Saxena PN, Gupta SK. Cytogenetic effects of cypermethrin and fenvalerate on the root meristem cells of Allium cepa. Environ Exp Bot. 1999;42:181–189. doi: 10.1016/S0098-8472(99)00033-7. [DOI] [Google Scholar]
- Darlington CD, Mc-Leish L. Action of maleic hydrazide on the cell. Nature. 1951;167:407–408. doi: 10.1038/167407a0. [DOI] [PubMed] [Google Scholar]
- El-Ghamery AA, El-Nahas AI, Mansour MM. The action of atrazine herbicide as an inhibitor of cell division on chromosomes and nucleic acids content in root meristems of Allium cepa and Vicia faba. Cytologia. 2000;65:277–287. doi: 10.1508/cytologia.65.277. [DOI] [Google Scholar]
- Enan MR. Genotoxicity of the herbicide 2, 4-dichlorophenoxyacetic acid (2, 4-D): higher plants as monitoring systems. Am Eurasian J Sustain Agric AEJSA. 2009;3:452–459. [Google Scholar]
- Evans HJ, Meary GJ, Tomkinson SN (1957) The use of colchicine as an indicator of mitotic rate in broad bean root meristem. J Genet 55:487–502
- Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–190. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol. 2007;88:252–259. doi: 10.1016/j.pestbp.2006.12.003. [DOI] [Google Scholar]
- Fındıklı Z, Türkoğlu Ş. The effects of glyphos and DDVP on mitotic division and chromosomes in Allium cepa L. J Sci Cumhuriyet Univ. 2010;31:49–62. [Google Scholar]
- Fiskesjo G. The Allium test as standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Fiskesjo G. The Allium test-an alternative in environmental studies: the relative toxicity of metal ions. Mutat Res. 1988;197:243–260. doi: 10.1016/0027-5107(88)90096-6. [DOI] [PubMed] [Google Scholar]
- Fleischli MA, Franson JC, Thomas NJ, Finley DL, Riley W. Avian mortality events in the United States caused by anticholinesterase pesticides: a retrospective summary of National Wildlife Health Center records from 1980 to 2000 Arch. Environ Contam Toxicol. 2004;46:542–550. doi: 10.1007/s00244-003-3065-y. [DOI] [PubMed] [Google Scholar]
- Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, Berta G. Effect of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv, Frisson seedlings. Environ Exp Bot. 2006;58:253–260. doi: 10.1016/j.envexpbot.2005.09.008. [DOI] [Google Scholar]
- Gadeva P, Dimitrov B. Genotoxic effects of the pesticides Rubigan, Omite and Rovral in root-meristem cells of Crepis capillaris L. Genet Toxicol Environ Mutagen. 2008;652:191–197. doi: 10.1016/j.mrgentox.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Grover IS, Kaur S. Genotoxicity of waste water samples from sewage and industrial effluent detected by the Allium cepa root anaphase aberration and micronucleus assay. Mutat Res. 1999;426:183–188. doi: 10.1016/S0027-5107(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Haliem AS. Cytological effect of the herbicide sencorer on mitosis of A. cepa. Egypt J Botany. 1990;33:93–104. [Google Scholar]
- Hazarika A, Sarkar SN. Effect of isoproturon pretreatment on the biochemical toxicodynamics of anilofos in male rats. Toxicol. 2001;165:87–95. doi: 10.1016/S0300-483X(01)00411-5. [DOI] [PubMed] [Google Scholar]
- Hoshina MM (2002) Evaluation of a possible contamination of the waters of the Claro River municipality of Rio Claro, part of the Corumbatai River basin, with the mutagenicity tests using Allium cepa. State University of Sao Paulo, Rio Claro, SP (in Portuguese)
- Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107–115. doi: 10.1016/j.toxlet.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Kluge R, Podlesak W. Plant critical levels for the evaluation of boron toxicity in spring barley (Hordeum vulgare L.) Plant Soil. 1985;83:381–388. doi: 10.1007/BF02184450. [DOI] [Google Scholar]
- Konuk M, Liman R, Cigerci IH. Determination of genotoxic effect of boron on Allium cepa root meristematic cells. Pak J Bot. 2007;39:73–79. [Google Scholar]
- Kovalchuk O, Arkhipov I, Telyuk A, Hohn P, Kovalchuk L. The Allium cepa chromosome aberration test reliably measures genotoxicity of soils of inhabited areas in the Ukraine contaminated by the Chernobyl accident. Mutat Res. 1998;415:47–57. doi: 10.1016/S1383-5718(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Sakai H. Role of Tubulin-Sh groups in polymerization to microtubules. Functional-Sh groups in tubulin for polymerization. J Biochem. 1974;76:651–654. doi: 10.1093/oxfordjournals.jbchem.a130609. [DOI] [PubMed] [Google Scholar]
- Lamsal K, Ghimire BK, Sharma P, Ghimiray AK, Kim SW, Yu CY, Chung IM, Lee YS, Kim JS, Shakya SR. Genotoxicity evaluation of the insecticide ethion in root of Allium cepa L. Afric J Biotech. 2010;9:4204–4210. [Google Scholar]
- Leme DM, Marin-Morales MA. Allium cepa test in environmental monitoring: a review on its application. Mutat Res. 2009;682:71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Liman R, Akyıl D, Eren Y, Konuk M. Testing of the mutagenicity and genotoxicity of metolcarb by using both Ames/Salmonella and Allium test. Chemosphere. 2010;80:1056–1061. doi: 10.1016/j.chemosphere.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Linnainmaa K, Meretoja T, Sorsa M, Vainto H. Cytogenetic effects of styrene and styrene oxide. Mutat Res. 1978;58:277–286. doi: 10.1016/0165-1218(78)90020-4. [DOI] [PubMed] [Google Scholar]
- Luo LZ, Werner KM, Gollin SM, Saunders WS (2004) Cigarette smoke induces anaphase bridges and genomic imbalances in normal cells. Mutat Res 554:375–385 [DOI] [PubMed]
- Ma TH, Xu Z, Xu C, McConnell H, Rabago EV, Arreola GA, Zhang H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat Res. 1995;334:185–195. doi: 10.1016/0165-1161(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Majer BJ, Grummt T, Uhl M, Knasmüller S. Use of plant bioassays for the detection of genotoxins in the aquatic environment. Acta Hydrochim Hydrobiol. 2005;33:45–55. doi: 10.1002/aheh.200300557. [DOI] [Google Scholar]
- Majewska AE, Wolska E, Sliwinska M, Furmanowa N, Urbanska A, Pietrosiuk A, Zobel A, Kuran M. Antimitotic effect, G2/M accumulation, chromosomal and ultrastructure changes in meristematic cells of Allium cepa L. root tips treated with the extract from Rhadiola rosea roots. Caryology. 2003;56:337–351. doi: 10.1080/00087114.2003.10589343. [DOI] [Google Scholar]
- Mann RM, Hyne RV, Choung CB, Wilson SP. Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Poll. 2009;157:2903–2927. doi: 10.1016/j.envpol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Marcano L, Carruyo I, Del Campo A, Montiel X. Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa L. Environ Res. 2004;94:221–226. doi: 10.1016/S0013-9351(03)00121-X. [DOI] [PubMed] [Google Scholar]
- Maroni M, Colosio C, Ferioli A, Fait A. Organophosphorus pesticides. Toxicol. 2000;143:9–37. doi: 10.1016/S0300-483X(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Marrs TC. Organophosphate poisoning. Pharmacol Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-M. [DOI] [PubMed] [Google Scholar]
- Mišik M, Solenska M, Mišcieta K, Mišikova K, Knasmuller S. In situ monitoring of clastogenicity of ambient air in Bratislava, Slovakia using the Tradescantia micronucleus assay and pollen abortion assays. Genet Toxicol Environ Mutagen. 2006;605:1–6. doi: 10.1016/j.mrgentox.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Odeigah PGC, Nurudeen O, Amund OO. Genotoxicity of oil field wastewater in Nigeria. Hereditas. 1997;126:161–167. doi: 10.1111/j.1601-5223.1997.00161.x. [DOI] [Google Scholar]
- Ozen E, Çiçek F, Gür B, Aydın N, Akıncı B, Topal M, Keser M, Çavuşoğlu K. The effects of paraquat on some cytotoxic and biochemical parameters in Allium cepa. J Sci Fırat Univ. 2011;23:117–124. [Google Scholar]
- Özkara A, Akyıl D, Erdoğmuş SF, Konuk M. Evaluation of germination, root growth and cytological effects of wastewater of sugar factory (Afyonkarahisar) using Hordeum vulgare bioassays. Environ Monit Assess. 2011;183:517–524. doi: 10.1007/s10661-011-1936-7. [DOI] [PubMed] [Google Scholar]
- Pandey RM. Cytotoxic effects of pesticides in somatic cells of Vicia faba L. Cytol Genet. 2008;42:373–377. doi: 10.3103/S0095452708060030. [DOI] [PubMed] [Google Scholar]
- Patra M, Sharma A. Relative efficacy of Allium cepa and Allium sativum in anaphase-telophase test screening metal genotoxicity. Biologia. 2002;57:409–414. [Google Scholar]
- Rank J, Nielsen MH. Genotoxicity testing of waste water sludge using the Allium cepa anaphase-telophase chromosome aberration assay. Mutat Res. 1998;418:113–119. doi: 10.1016/S1383-5718(98)00118-1. [DOI] [PubMed] [Google Scholar]
- Sang N, Li G. Genotoxicity of municipal landfill leachate on root tips of Vicia faba. Mutat Res. 2004;560:159–165. doi: 10.1016/j.mrgentox.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Saxena PN, Chauhan LKS, Gupta SK. Cytogenetic effects of commercial formulation of cypermethrin in root meristem cells of Allium sativum: spectroscopic basis of chromosome damage. Toxicology. 2005;216:244–252. doi: 10.1016/j.tox.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Seth CS, Chaturvedi PK, Misra V. Toxic effect of arsenate and cadmium alone and in combination on Giant Duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ Toxicol. 2007;22:539–549. doi: 10.1002/tox.20292. [DOI] [PubMed] [Google Scholar]
- Sharma S, Vig AP. Genotoxicity of atrazine, avenoxan, diuron and quizalofop-P-ethyl herbicides using the Allium cepa root chromosomal aberration assay. Terrest Aquat Environ Toxicol. 2012;6:90–95. [Google Scholar]
- Singh B, Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev. 2006;30:428–471. doi: 10.1111/j.1574-6976.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- Srivastava K, Mishra KK. Cytogenetic effects of commercially formulated atrazine on the somatic cells of Allium cepa and Vicia faba. Pestic Biochem Physiol. 2009;93:8–12. doi: 10.1016/j.pestbp.2008.08.001. [DOI] [Google Scholar]
- Sudhakar R, Gowda N, Venu G. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 2001;66:235–239. doi: 10.1508/cytologia.66.235. [DOI] [Google Scholar]
- Türkoğlu Ş. Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pesticide Biochem Physiol. 2012;103:224–230. doi: 10.1016/j.pestbp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Webster PL, MacLeod RD. The root apical meristem and its margin. In: Waishel Y, Eshel A, Kafkafi U, editors. Plant roots. The hidden half. 2. New York: Marcel Dekker; 1996. pp. 51–76. [Google Scholar]
- Wu J, Lin L, Luan T, Gilbert YSC, Lan C. Effects of organophosphorus pesticides and their ozonationbyproducts on gap junctional intercellular communication in rat liver cell line. Food Chem Toxicol. 2007;45:2057–2063. doi: 10.1016/j.fct.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Yıldız M, Arıkan ES. Genotoxicity testing of quizalofop-P-ethyl herbicide using the Allium cepa anaphase-telophase chromosome aberration assay. Caryologia. 2008;61:45–52. doi: 10.1080/00087114.2008.10589608. [DOI] [Google Scholar]
- Yıldız M, Cigerci IH, Konuk M, Fidan AF, Terzi H. Determination of genotoxic effects of copper sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere. 2009;75:934. doi: 10.1016/j.chemosphere.2009.01.023. [DOI] [PubMed] [Google Scholar]


