Abstract
INTRODUCTION
This preliminary study determined if magnetic resonance imaging (MRI) markers of cervical muscle degeneration [elevated muscle fatty infiltration (MFI), cross-sectional area (CSA), and reduced relative muscle CSA (rmCSA)] could be modified with exercise in patients with chronic whiplash.
METHODS
Five women with chronic whiplash undertook 10 weeks of neck exercise. MRI measures of the cervical multifidus (posterior) and longus capitus/colli (anterior) muscles, neck muscle strength, and self-reported neck disability, were recorded at baseline and at the completion of the exercise program.
RESULTS
Overall significant increases in CSA and rmCSA were observed for both muscles but significant reductions in MFI were only evident in the cervical multifidus muscle. These changes coincided with increased muscle strength and reduced neck disability.
CONCLUSIONS
MRI markers of muscle morphology in individuals with chronic whiplash appear to be modifiable with exercise.
Keywords: whiplash, muscle degeneration, exercise, MRI, rehabilitation
INTRODUCTION
Persistent pain-related disability associated with chronic whiplash constitutes a sizeable financial and economic cost for society, and conservative management of these patients remains a challenge.1–3 One of the major challenges in the management of whiplash-associated disorders (WAD) has been the lack of identifiable patho-anatomical features of the condition that could potentially direct management. However, recent magnetic resonance imaging (MRI) studies have identified distinctive MRI markers of cervical muscle degeneration that appear unique to patients who are transitioning to persistent WAD.4,5
Using conventional T1-weighted MRI,5–7 muscle degeneration has been identified both in patients who transition to, and those already with, persistent moderate to severe symptoms associated with WAD. In particular, levels of cervical muscle fatty infiltration (MFI) have been found to be elevated (MFI index of ≥ 0.24) in those with poor functional recovery.5 Elevated MFI levels have been shown to develop between 1and 3 months following whiplash injury with minimal variability beyond 3 months.5 The significance of such findings is that elevated levels of MFI are not present in those who report full-recovery, healthy individuals, or in those with chronic idiopathic neck pain,4 suggesting traumatic factors play a role. In addition, larger cross sectional areas (CSA) of the deep posterior (cervical multifidus) and anterior (deep cervical flexors) muscles were observed in patients with WAD in comparison to healthy controls.7,8 Larger CSA recordings may represent muscle pseudohypertrophy in chronic WAD due to the higher fat content of the muscles expanding the musculofascial borders. This has been supported by further evaluation of the existing data,7,8 whereby a measure of relative muscle CSA (rmCSA) was developed to adjust for the influence of MFI on CSA in patients with WAD. In 93% of muscles examined, the rmCSA was found to be the same or significantly smaller in patients with WAD compared to asymptomatic controls.9
These morphological muscle changes in individuals with chronic WAD may signal a reduction in the contractile elements that could diminish their capacity to generate and/or sustain force.10,11 Reports of reduced strength12,13 and endurance14 in the neck muscles in this population supports this theory. While it is acknowledged that other factors may influence measurements of muscle performance (e.g. fear of movement12,15 and/or reduced activity),16 the coexistence of muscle degeneration and reduced muscle performance (strength/endurance) provide some insight into the potential biological issues in patients with WAD. The cervical vertebral column is heavily dependent on the active support of muscles for physical support.17 In particular, the deep sleeve of muscles that envelope the cervical spine is considered to play a vital role in segmental cervical motion18,19 particularly in controlling buckling and unwanted rotary intersegmental motion associated with contraction of large multi-segmental muscles during daily tasks.20 Interestingly, our previous work has indicated the greatest levels of MFI in chronic whiplash are evident in this deep sleeve of muscles both posterior (cervical multifidus)4,6,8 and anterior (longus capitis/colli)7 to the cervical column. Potentially positive benefits may be attained if these distinct morphological changes in these neck muscles could be reversed with targeted exercise.
There is evidence that exercise can change the morphology of healthy muscles,21 but there have been no investigations of morphological changes in the WAD population in response to exercise. The primary purpose of this pilot study was to gain some initial evidence of whether morphological changes in cervical muscles could be modified with exercise in patients with WAD. Based on our previous work we focused this pilot investigation on the cervical multifidii and longus capitis/colli muscles shown to be the most severely affected in this patient group.4,6–8 We hypothesized that exercise targeted to maximize muscle hypertrophy22,23 would reduce the characteristic signal intensity associated with higher levels of MFI and increase the rmCSA in these muscles in individuals with persistent WAD. Secondarily we hypothesized that changes in muscle morphology in response to exercise would be associated with increases in cervical muscle strength and reductions in neck pain-related disability.
METHODS
Experimental Design
A pilot study was designed to assess the effect of progressive resisted exercise on cervical muscle morphology as measured with MRI at 3 time intervals (pre-training, mid-training at 6 weeks, and post-training at 10 weeks) in 5 patients with persistent WAD.
Participants
Five women volunteers participated in the study with a mean age (SD) of 30.8 (6.2) years and a mean symptom duration of 21.3 (9.5) months. Participants were included if they reported a history of persistent neck pain and disability as a result of a motor vehicle collision consistent with a WAD Grade II (neck pain, reduced range of cervical spine motion, and point tenderness in the neck region on physical examination).24 Considering the heterogeneity of WAD, a screening MRI scan was performed on each participant to ensure they had the necessary MFI value of ≥0.24, as this has been reported to discriminate between chronic WAD and insidious onset neck pain.4
Potential subjects with WAD Grade II were not considered if they had any features precluding them from an MRI examination, had evidence of MFI values that were below a 0.24 cut-off, or had formally trained their neck or shoulder girdle muscles within the last 6 months. A specialist musculoskeletal physiotherapist (SOL) with advanced clinical skills in the assessment of patients with chronic WAD performed all clinical screening.
Ethical approval for the study was granted by the Institutional Medical Research Ethics Committee and was conducted in accordance with the Declaration of Helsinki. All participants received verbal and written information about the study and signed an informed consent form.
Measurements
MRI Measures of Muscle Morphology
Magnetic resonance images were obtained using a Siemens 1.5T Sonata MRI system (Erlangen, Germany) with a conventional T1-weighted spin-echo pulse sequence of 24 slices, 4 millimeters (mm) slice thickness, 200 mm field of view read, 448 millisecond (ms) repetition time, 14 ms echo time, 256*256 matrix size, in plane resolution of 0.9 mm x 0.9 mm, and 8.42 second (s) acquisition time. These image parameters were chosen to acquire images with reasonable tissue contrast of fat and muscle. Sagittal and axial slices of the cervical spine were obtained from the mid-point of the cerebellum through the segmental level of the first thoracic vertebra to ensure capture of the entire extensor and flexor musculature. 4,6–8 Similar to our previous protocols,6,7 measures of the cervical multifidus were recorded bilaterally at each vertebral level between C3 and C7 (5 slices), and bilateral measures of longus capitis/colli at the C0, C2, C5, and C7 (4 slices) vertebral levels with measurements for the left and right sides averaged. For the purposes of this study an average of the measurements (MFI, CSA, rmCSA) recorded at each slice were used for analysis. Scout images were utilized from the T2-weighted sagittal slices to ensure location and measurement accuracy. Careful attention was taken to position the subject on the table consistently at each MRI session. This, in tandem with the sagittal scout images and time-dependent co-registration of all images, ensured that each muscle/fat measure was taken from the same anatomical location for each MRI session. Measures were made by a rater experienced in these measures (JE) using MRIcro software.25 The rater was blind to which of the 3 time-points were being analyzed to minimize the potential for bias.
MFI Measurement
A measure of fat in muscle, which has been previously reported in detail,4,6,8,26 was created from the pixel intensity profile with MRIcro software. The MFI measure, based on the contrasting pixel intensities of fat and soft-aqueous skeletal muscle on T1-weighted imaging (bright and dark, respectively), permits a simple quantitative comparison of the relative amounts of intramuscular fat between individuals.4,6,8,26 The measure quantifies the average pixel intensity profiles of individually traced muscles as a ratio of that of an average pixel intensity profile from a standardized region of intermuscular fat at the C2 level, thus allowing comparisons between individuals. This measure, based on the contrasting pixel intensities of fat and muscle on T1-weighted imaging (bright and dark, respectively), allowed for the simple process of quantifying intramuscular fat relative to a recognized area of intermuscular fat. The MFI measures are highly reliable (intra- and inter-rater).27
CSA and rmCSA Measurement
CSA measurements utilized the identical imaging protocol as described for the MFI measurement. Analysis consisted of manually tracing user-defined regions of interest over each of the cervical multifidus and longus capitis/colli muscles bilaterally on each slice of the axial T1-weighted images. Histograms were created from the summated user-defined region of interest, displaying each particular image pixel intensity profile. The CSA of muscles were calculated by the number of pixels under each individual region of interest in the -x and -y axes (mm * mm = mm2) with MRIcro software, which has been shown to be a highly reliable (intra- and inter-rater) measure.8 The rmCSA was then calculated using the equation: rmCSA = CSA*(1-MFI), where the characteristic bright signal from fat on T1-weighted imaging was removed from the CSA measure.
Neck Disability Index (NDI)
The NDI contains 10 items: 7 related to activities of daily living, 2 related to pain, and 1 related to concentration.28 Each item is scored from 0 to 5, and the total score is expressed as a percentage (total possible score, 100%), with higher scores corresponding to greater disability. The NDI has shown responsiveness in the ability to detect change in chronic neck pain disorders.29
Cervical Muscle Strength
Dynamometry measurements of strength of the flexor and extensor neck muscles were recorded at the cervico-thoracic (CT) junction using an established dynamometry protocol30 to evaluate change in the force-producing capacity of the cervical muscles. Participants performed three maximal voluntary contraction (MVC) trials with a rest period of 1 minute between trials and 5 minutes rest between testing of the flexor and extensor muscle groups. During each trial, standardized verbal and visual feedback were provided. The peak torque value of the 3 trials was recorded as the MVC measurement for analysis.
Exercise
Participants undertook a supervised progressive resistance exercise program for 10 weeks in accordance with guidelines for muscle hypertrophy.22,23 Although the program was standardized across the 5 participants, there was the potential for modification if an individual, based on symptom response, required such modification, and this decision was left to the discretion of both the treating physiotherapist and the patient. A 10-week program was chosen to maximize the potential for observing any morphological changes in the exercised cervical spine muscles. The same experienced physiotherapist (LVW) supervised the entire program for all participants. Participants were permitted to continue any current medications and their usual level of other physical activity for the duration of the study.
Participants attended 2 supervised sessions a week (total of 20 sessions lasting 30–60 minutes per session) at the university physiotherapy clinic. Exercise at the clinic was performed with a dynamometer. This dynamometer, which has been described in detail elsewhere,30 permitted specific isometric and isotonic exercise to be targeted at the cranio-cervical (CC) (upper neck) and CT (lower neck) flexor and extensor muscles at a set intensity level (percentage of MVC). In this manner exercise was performed in 4 different directions (CC flexion, CC extension, CT flexion, CT extension) either in an isometric or isotonic mode. During isometric exercise the axes of the dynamometer were locked, and participants were provided continual visual feedback of their muscle torque effort to ensure accurate performance of exercise intensity. For isotonic exercise the axes of the device were unlocked to permit exercise to be performed through range against a resistance provided by a calibrated weight (providing a resistance corresponding to the allocated %MVC) that counteracts the resistance lever of the dynamometer.
The progression of the exercise program is shown in Table 1. All 4 exercises were performed in all exercises sessions (CC flexion, CC extension, CT flexion, CT extension) with all following the same progression (1–3 sets, 50–70%MVC) for the first 5 weeks. From weeks 6 to 10, CC flexion and extension exercise remained isometric and was progressed to 80% MVC, but CT flexion and extension exercise was performed in an isotonic mode (isometric ceased) and progressed from 60–80% MVC. Exercise was progressed from isometric to isotonic to expose the cervical musculature with a different functional stimulus. To account for gains in strength over the duration of the exercise program, the reference MVC measurements against which exercise intensity was calculated were repeated at weeks 4 and 8. In addition to the supervised training sessions, patients also performed home exercises on non-training days. These exercises mimicked the dynamometry exercises (i.e. CC and CT flexion/extension) but were only performed against the resistance of gravity to ensure consistent, but non-fatiguing stimulation of the targeted cervical muscles.
Table 1.
Exercise parameters over the duration of the 10-week program. Parameters were identical for cranio-cervical (CC) and cervico-thoracic (CT) exercises in the first 5 weeks, with the CT exercises performed in an isotonic contraction mode over the last 5 weeks. For all muscle groups the exercise load [e.g. 50% maximal voluntary contraction (MVC)] was based specifically on the MVC performance of that muscle group (e.g. CC Flexors), and this was re-evaluted at weeks 4 and 8 for each muscle group.
| Exercise Parameters (Sets x Repetitions at Load – Contraction Mode) | ||
|---|---|---|
| Week | CC Flexion and Extension | CT Flexion and Extension |
| 1 | 1 x 10 at 50%MVC - Isometric | |
| 2 | 2 x 10 at 50%MVC - Isometric | |
| 3 | 3 x 10 at 50%MVC - Isometric | |
| 4 | 3 x 10 at 60%MVC - Isometric | |
| 5 | 3 x 10 at 70%MVC - Isometric | |
| 6 | 3 x 10 at 80%MVC - Isometric | 3 x 10 at 60%MVC - Isotonic |
| 7 | 3 x 10 at 80%MVC - Isometric | 3 x 10 at 70%MVC - Isotonic |
| 8 | 3 x 10 at 80%MVC - Isometric | 3 x 10 at 80%MVC - Isotonic |
| 9 | 3 x 10 at 80%MVC - Isometric | 3 x 10 at 80%MVC - Isotonic |
| 10 | 3 x 10 at 80%MVC - Isometric | 3 x 10 at 80%MVC - Isotonic |
The supervising physiotherapist recorded attendance at the 20 exercise sessions at the university clinic. There was no attempt to monitor compliance in the home exercises, as supervised exercises were considered to be the key exercises to induce morphological changes in the neck muscles.
Procedure
Volunteers were screened for eligibility prior to undertaking baseline MRI, cervical muscle strength, and NDI, measurements. Participants then commenced their 10 week supervised progressive resistance exercise program. MRI measures of MFI and CSA/rmCSA were repeated at 6 weeks following the commencement of the program (mid-training morphological measures) and again at the completion of training (post-training measures). The cervical strength and NDI measures were also repeated following the completion of the exercise program at 10 weeks.
Statistical Analysis
Separate repeated measures analyses of variance were performed to evaluate the effect of time on each outcome measure (MFI, CSA, rmCSA, muscle strength). Effect sizes were calculated using partial eta squared (η2). Effect sizes of 0.1 – 0.3 were interpreted as a modest effect, 0.3–0.5 a moderate effect, and > 0.5 a strong effect.31 A priori pairwise comparisons with Bonferroni corrections were used to compare changes in outcome across each time point (baseline, 6 weeks, 10 weeks) For all analyses the significance level was P ≤ 0.05, and where applicable, P values adjusted for multiple comparisons are reported. All analyses were performed using IBM SPSS 21.0 statistical software (IBM, USA).
RESULTS
Each participant attended all 20 supervised sessions with the exception of 1 participant who attended 19 of 20 sessions.
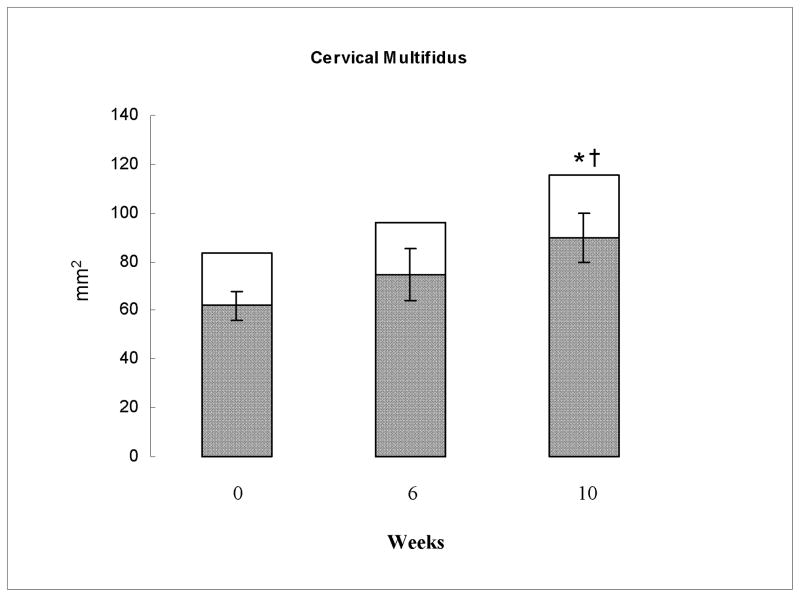
Significant reductions in MFI (P < 0.01, η2 = 0.9), and increases in CSA (P < 0.001, η2 = 0.91) and rmCSA (P < 0.001, η2 = 0.94), were observed in the cervical multifidus muscles in response to the exercise program. As shown in Table 2 and Figure 1, these significant changes were only evident at 10-weeks [MFI (P < 0.03), CSA (P < 0.01), rmCSA (P < 0.01)] and not at the 6 week time point [MFI (P = 0.14), CSA (P = 0.18), rmCSA (P > 0.05)].
Table 2.
Group means (SD) for the Magnetic Resonance Imaging (MRI) measures recorded at baseline, 6 weeks (mid-exercise period), and 10 weeks (completion of exercise period), following the commencement of the exercise program, for both the cervical multifidus and longus capitus/colli muscles. Muscle MRI measures include muscle fatty infiltration (MFI) recorded as a proportion, and cross sectional area (CSA) and relative muscle CSA (rmCSA) recorded in millimeters (mm)2. For comparison, normative MFI values across all anterior/posterior muscles have been reported previously as [mean (± SD) 0.16 ± (0.033)].7
| Muscle Variable | Measurement Point | Time Effect | Effect Size | ||
|---|---|---|---|---|---|
|
| |||||
| Baseline | 6 weeks | 10 weeks | P-Value | η2 | |
| Cervical Multifidus | |||||
| MFI | 0.26 ± 0.03 | 0.22 ± 0.03 | 0.23 ± 0.02 | < 0.01 | 0.9 |
| CSA | 83.68 ± 8.39 | 96 ± 15.72 | 115.74 ± 13.73 | < 0.001 | 0.91 |
| rmCSA | 61.99 ± 6.97 | 74.59 ± 11.97 | 89.65 ± 11.37 | < 0.001 | 0.94 |
| Longus Capitus/Colli | |||||
| MFI | 0.18 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.04 | 0.28 | 0.28 |
| CSA | 81.55 ± 12.35 | 94.68 ± 7.4 | 115.95 ± 14.45 | < 0.001 | 0.86 |
| rmCSA | 66.84 ± 10.79 | 79.06 ± 6.14 | 96.63 ± 12.87 | < 0.001 | 0.87 |
Figure 1.
Group mean total cross sectional area (CSA) (entire column) and relative muscle CSA (rmCSA) (shaded portion of column) of the cervical multifidus muscle at 0, 6, and 10 weeks of exercise recorded in millimeters (mm)2. 95% confidence interval error bars are shown for the rmCSA. * significant difference in CSA to baseline. † significant difference in rmCSA to baseline.
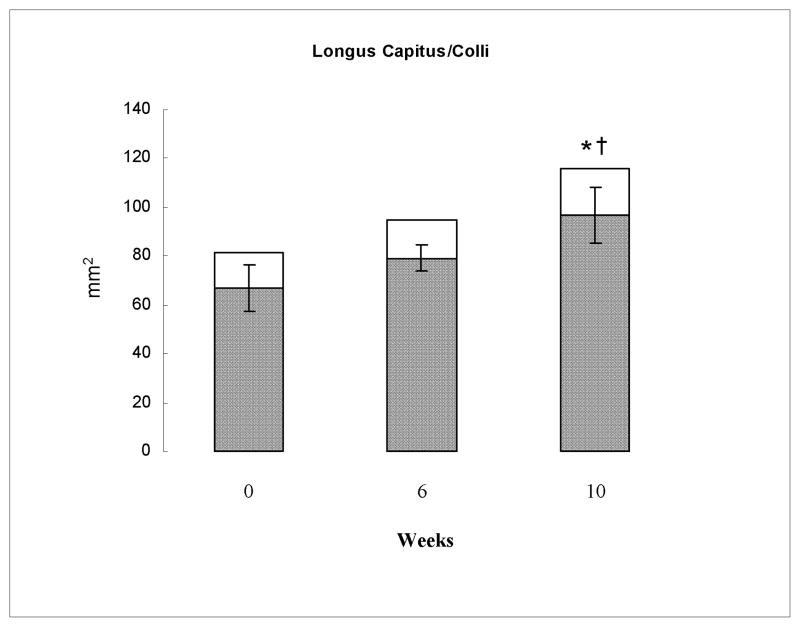
Overall significant increases in CSA (P < 0.001, η2 = 0.86) and rmCSA (P < 0.001, η2 = 0.87) were observed for the longus capitis/colli muscle but not for the MFI (P = 0.28, η2 = 0.28) measure in response to the exercise program. These significant effects were only evident at 10-weeks [CSA (P < 0.01), rmCSA (P < 0.01)] and not at 6-weeks [CSA (P = 0.26), rmCSA (P = 0.18)] as shown in Table 2 and Figure 2.
Figure 2.
Group mean total cross sectional area (CSA) (entire column) and relative muscle CSA (rmCSA) (shaded portion of column) of the longus capitus/colli muscle at 0, 6, and 10 weeks of exercise recorded in millimeters (mm)2. 95% confidence interval error bars are shown for the rmCSA. * significant difference in CSA to baseline. † significant difference in rmCSA to baseline.
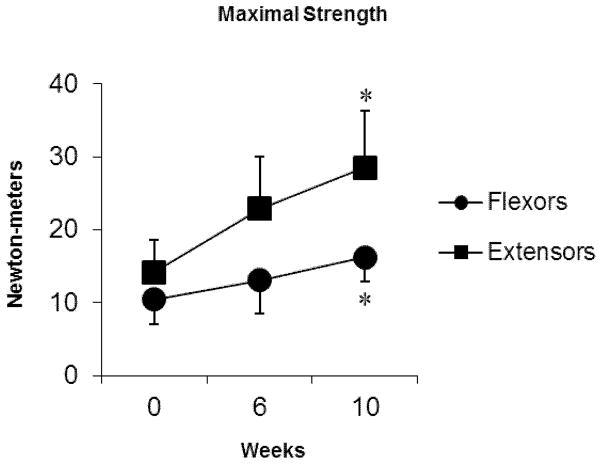
In tandem with the morphological changes in these muscles were significant increases in both extensor [10 weeks (P = 0.006)] and flexor [10 weeks (P = 0.04)] muscle strength (Figure 3) and significant reductions in self-reported levels of neck disability [median NDI scores/100 points; baseline = 38 %, post-exercise (10 weeks) = 20 %, P = 0.04].
Figure 3.
Group mean maximal strength (error bars, SD) of the cervical flexor and extensor muscles at 0, 6, and 10 weeks of exercise. * significant difference in strength to baseline.
DISCUSSION
The hypotheses of this study were upheld, providing preliminary evidence that morphological changes (CSA and MFI) in the neck muscles of patients with WAD can be altered with exercise. Significant reductions in MFI coinciding with increases in CSA and rmCSA were evident in cervical multifidus muscle, especially following completion of the 10-week exercise program. However, results were not consistent across both muscle groups examined. Despite significant increases in CSA and rmCSA of the longus capitis/colli after 10 weeks of training, reductions in MFI for this muscle group were not observed. This may reflect the lower levels of MFI in the longus capitis/colli muscle at baseline compared to that recorded for the cervical multifidus muscle. The lower level of MFI in longus capitis/colli compared to cervical multifidus muscle is consistent with our previously reported studies in patients with chronic WAD.4,6,7 The discrepant findings of MFI in these different muscle groups is not well understood at this stage, though they could reflect factors related to differences in fiber types, 18,32 denervation, inflammatory response to trauma, and/or disuse-induced atrophy.33–37 The changes in MFI and rmCSA are not linear, and this may be due to common disturbances in the local magnetic field (B0 inhomogeneity and/or magnetic susceptibility of adjacent tissues e.g. muscle and bone), which can influence signal intensities in MRI.38,39 Alternatively, it may be due to differences in muscle morphology such as fiber typing or internal structure complexity. In T1-weighted images, intermuscular fat is clearly identified, but smaller proportions of homogeneous intramuscular fat cannot be defined accurately40 and may even be invisible.41 Irrespective, these findings, particularly those for the cervical multifidus, indicate modification of the relative proportion of muscle constituents (fat vs. muscle components) towards that previously reported in healthy individuals.9 Future mechanistic work, where controlled experimental lesions to peripheral tissues in an animal model would help resolve questions related to the differential expression of muscle atrophy and associated fat infiltration.
The reductions in MFI and increases in rmCSA observed in the cervical multifidus muscle in response to exercise in this study suggest that the 2 measures may be related, however the exact nature of this inverse relationship is largely unknown. It would be reasonable to suggest that the observed increase in rmCSA is probably due to training parameters that aimed specifically to produce muscle hypertrophy.22,23 However, the mechanisms underlying the observed reductions in MFI are not as clear. Potentially, the visible MR signal of muscle fat on conventional T1-weighted sequences may be reduced artificially (i.e. ‘squashed’) to accommodate the muscle fiber hypertrophy. However, the coinciding increase in total CSA of the muscles in response to exercise (Table 2, Figures 1 and 2) suggests that the fat content of the muscle may not need to be ‘squashed’ within a confined fascial space. While increased rmCSA may have reduced the relative proportion of fat signal (ie. reduced MFI), the actual CSA of fat may have remained similar, accommodated by the concurrent increase in total CSA. Alternatively, the reduction in MFI may signal some other process of fat metabolism in skeletal muscle following exercise.42 At this stage these propositions are merely speculative and represent an area of mechanistic study in our laboratories with both animal43 and human models.
The capacity to modify these changes in the muscles of these patients is of potential value. Muscles account for approximately 80% of the physical support of the cervical vertebral column.17 In particular, our previous studies have shown the most severe muscle changes in chronic whiplash occur in the deep cervical muscle layers.4,6–8 These muscles envelope and physically support the cervical column.18–20,44 They also have direct insertions onto pain-sensitive cervical structures such as the capsules of cervical facet joints45,46 which have been implicated as a likely source of persistent symptoms in whiplash.46–48 We contend that potentially reversing the muscle changes, or at least restoring more normal proportions of fat and contractile tissue in these cervical muscles may be of potential benefit for improving functional rates of recovery. While the observed muscle changes in this study coincided with increased strength (Figure 3) and reduced neck disability, the small sample size precludes any robust inferences to be drawn between reversal of muscle changes and clinical outcome. However, it is interesting to note that the increase in extensor muscle strength (101% increase) is more substantial than that attained by the flexor muscles (55% increase). While this coincides with the more consistent morphological changes observed in the extensor muscles compared to the flexors, it might also suggest greater relative weakness of the extensors at baseline; the extensor:flexor strength ratio increased from 1.35 at baseline to 1.76 at 10 weeks, which is more consistent with that previously reported in healthy women (1.84)30 using the same dynamometer. Potentially the large changes in strength could also reflect improved neural drive to the muscles21 that may at least in part be due to a reduced fear of movement due to training. However, changes in muscle strength also seemed to be associated with the physical changes in the muscles (rmCSA, CSA), since neither strength nor physical changes improved significantly at 6 weeks, but both concurrently improved significantly at 10 weeks. Clarification of this relationship is an area for further study.
This study has several limitations; the largest is the small sample size (n = 5), limited to women participants only. Due to the costs of MRI, it was designed as a pilot study. The large effect sizes give increased confidence for the effect of exercise on these unique muscle changes in individuals with WAD. Thus these findings provide justification for a larger investigation of the mechanisms for effect of exercise on morphological changes in this specific patient group and its relevance to clinical outcome. There are also some limitations related to the MRI measurements utilized in this study. In the main, MRI has advanced, and there is a call for using known and clinically available techniques in the quantification of tissue fat [e.g. 3-dimensional chemical shift water-fat imaging (Dixon) and Proton-Density Fat Fraction].41 While there is evidence that T1-weighted MRI is a robust measure of muscle fat when compared to gold-standard histology,49 emerging evidence demonstrates water-fat imaging may provide an accurate and more time-efficient measure.43,50
It is also acknowledged that 2-dimensional CSA measurements on MRI are inherently limited, as 2-dimensional images are more sensitive to the evolving radio frequency slice profiles when compared to 3-dimensional images. Accordingly, the reported measures of muscle size in this study and others8,51,52 may have partial volumes. Going forward, a 3-dimensional volume with image registration may produce a more accurate metric for measuring muscle volume changes over time. In this instance, however, we propose that the 2-dimensional registered measure of muscle size provides a relevant and easily accessible radiological marker of the viable contractile elements of the muscle. The diagnostic and prognostic value of serial muscular imaging with MRI in patients with persistent whiplash has yet to be determined.
In conclusion, this study provides preliminary evidence that the distinctive MRI markers of muscle degeneration known to be a unique feature of patients with persistent WAD can be modified with exercise and justifies further larger scale investigation.
Acknowledgments
Shaun O’Leary was supported by an NHMRC of Australia Research Training Fellowship, and a Health Practitioner Research Fellowship (Queensland Health (Royal Brisbane and Women’s Hospital) and University of Queensland (CCRE Spinal Pain, Injury and Health)). James Elliott is supported by NIH KL2 - KL2 RR025740 – NUCATS – Northwestern University.
ABBREVIATIONS
- MRI
magnetic resonance imaging
- MFI
muscle fatty infiltration
- CSA
cross-sectional area
- rmCSA
relative muscle CSA
- WAD
whiplash-associated disorder
- SD
standard deviation
- mm
millimetres
- ms
millisecond
- s
seconds
- NDI
neck disability index
- CT
cervico-thoracic
- MVC
maximal voluntary contraction
- η2
partial eta squared
- CC
cranio-cervical
Footnotes
Elliott has an investment/ownership interest in a medical consulting start-up, Pain ID, LLC
References
- 1.Jull G, Kenardy J, Hendrikz J, Cohen M, Sterling M. Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments. Pain. 2013;154(9):1798–1806. doi: 10.1016/j.pain.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 2.Lamb SE, Gates S, Williams MA, Williamson EM, Mt-Isa S, Withers EJ, Castelnuovo E, Smith J, Ashby D, Cooke MW, Petrou S, Underwood MR. Emergency department treatments and physiotherapy for acute whiplash: a pragmatic, two-step, randomised controlled trial. Lancet. 2013;381(9866):546–556. doi: 10.1016/S0140-6736(12)61304-X. [DOI] [PubMed] [Google Scholar]
- 3.Michaleff ZA, Maher CG, Lin CW, Rebbeck T, Jull G, Latimer J, Connelly L, Sterling M. Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60457-8. [DOI] [PubMed] [Google Scholar]
- 4.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol. 2008;63(6):681–687. doi: 10.1016/j.crad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Elliott J, Pedler A, Kenardy J, Galloway G, Jull G, Sterling M. The temporal development of Fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS One. 2011;6(6):e21194. doi: 10.1371/journal.pone.0021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine. 2006;31(22):E847–855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 7.Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976) 2010;35(9):948–954. doi: 10.1097/BRS.0b013e3181bb0e55. [DOI] [PubMed] [Google Scholar]
- 8.Elliott J, Jull G, Noteboom JT, Galloway G. MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD) Man Ther. 2008;13(3):258–265. doi: 10.1016/j.math.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O’Leary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine (Phila Pa 1976) 2014;39(1):39–47. doi: 10.1097/BRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 10.Cannon J, Kay D, Tarpenning K, Marino F. Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct Imaging. 2007;27:91–100. doi: 10.1111/j.1475-097X.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol. 588(Pt 18):3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson I, Reichert A, De Serres SJ, Dumas JP, Cote JN. Maximal voluntary isometric neck strength deficits in adults with whiplash-associated disorders and association with pain and fear of movement. J Orthop Sports Phys Ther. 2009;39(3):179–187. doi: 10.2519/jospt.2009.2950. [DOI] [PubMed] [Google Scholar]
- 13.Prushansky T, Gepstein R, Gordon C, Dvir Z. Cervical muscles weakness in chronic whiplash patients. Clin Biomech (Bristol, Avon) 2005;20(8):794–798. doi: 10.1016/j.clinbiomech.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Kumbhare DA, Balsor B, Parkinson WL, Harding Bsckin P, Bedard M, Papaioannou A, Adachi JD. Measurement of cervical flexor endurance following whiplash. Disabil Rehabil. 2005;27(14):801–807. doi: 10.1080/09638280400020615. [DOI] [PubMed] [Google Scholar]
- 15.Carroll LJ, Hogg-Johnson S, van der Velde G, Haldeman S, Holm LW, Carragee EJ, Hurwitz EL, Cote P, Nordin M, Peloso PM, Guzman J, Cassidy JD. Course and prognostic factors for neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S75–82. doi: 10.1097/BRS.0b013e31816445be. [DOI] [PubMed] [Google Scholar]
- 16.Manini T, Clark B, Nalls M, Goodpaster B, Ploutz-Snyder L, Harris T. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 17.Panjabi MM, Cholewicki J, Nibu K, Grauer J, Babat LB, Dvorak J. Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech. 1998;13(1):11–17. doi: 10.1016/s0268-0033(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 18.Boyd-Clark LC, Briggs CA, Galea MP. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine. 2002;27(7):694–701. doi: 10.1097/00007632-200204010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Mayoux Benhamou M, Revel M, Vallee C, Roudier R, Barbet J, Bargy F. Longus colli has a postural function on cervical curvature. Surg Radiol Anat. 1994;16:367–371. doi: 10.1007/BF01627655. [DOI] [PubMed] [Google Scholar]
- 20.Winters JM, Peles JD. Neck muscle activity and 3-D head kinematics during quasi-static and dynamic tracking movements. In: Winters JM, Woo SLY, editors. Multiple Muscle Systems: Biomechanics and Movement Organisation. New York: Springer-Verlag; 1990. pp. 461–480. [Google Scholar]
- 21.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness. A review of the acute programme variables. Sports Med. 2005;35(10):841–851. doi: 10.2165/00007256-200535100-00002. [DOI] [PubMed] [Google Scholar]
- 23.Wernbom M, Augustsson J, Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37(3):225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20(8 Suppl):1S–73S. [PubMed] [Google Scholar]
- 25.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 26.Elliott JM, Jull GA, Noteboom JT, Durbridge GL, Gibbon WW. Magnetic resonance imaging study of cross-sectional area of the cervical extensor musculature in an asymptomatic cohort. Clin Anat. 2007;20(1):35–40. doi: 10.1002/ca.20252. [DOI] [PubMed] [Google Scholar]
- 27.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006;31(22):E847–855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 28.Vernon H, Mior S. The neck disability index: A study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 29.Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the Neck Disability Index and patient specific functional scale in patients with cervical radiculopathy. Spine. 2006;31(5):598–602. doi: 10.1097/01.brs.0000201241.90914.22. [DOI] [PubMed] [Google Scholar]
- 30.Van Wyk L, Jull G, Vicenzino B, Greaves M, O’Leary S. A comparison of craniocervical and cervicothoracic muscle strength in healthy individuals. J Appl Biomech. 2010;26(4):400–406. doi: 10.1123/jab.26.4.400. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 32.Boyd-Clark LCBC, Galea MP. Comparative Histochemical Composition of Muscle Fibers in a Pre- and a Postvertebral Muscle of the Cervical Spine. J Anat. 2001;199(pt. 6):709–716. doi: 10.1046/j.1469-7580.2001.19960709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulor JP, Cambon B, Vigneron P, Reyne Y, Nougues J, Casteilla L, Bacou F. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998;439(1–2):89–92. doi: 10.1016/s0014-5793(98)01216-2. [DOI] [PubMed] [Google Scholar]
- 34.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31(25):2926–2933. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 35.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Borisov AB, Dedkov EI, Carlson BM. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec. 2001;264(2):203–218. doi: 10.1002/ar.1155. [DOI] [PubMed] [Google Scholar]
- 37.Lefaucheur JP, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. J Neuroimmunol. 1996;70(1):37–44. doi: 10.1016/s0165-5728(96)00099-9. [DOI] [PubMed] [Google Scholar]
- 38.McMahon KL, Cowin G, Galloway G. Magnetic resonance imaging: the underlying principles. J Orthop Sports Phys Ther. 2011;41(11):806–819. doi: 10.2519/jospt.2011.3576. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Mao W, Qiu M, Smith M, Constable T. Factors influencing flip angle mapping in MRI: RF pulse shape, slice-select gradients, off-resonance excitation, and B0 inhomogeneities. Magn Reson Med. 2006;56:463–468. doi: 10.1002/mrm.20947. [DOI] [PubMed] [Google Scholar]
- 40.Schick F, Machann J, Brechtel K, Strempfer A, Klumpp B, Stein D, Jacob S. MRI of muscular fat. Magentic Resonance in Medicine. 2002;47:720–727. doi: 10.1002/mrm.10107. [DOI] [PubMed] [Google Scholar]
- 41.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeukendrup AE. Regulation of fat metabolism in skeletal muscle. Ann N Y Acad Sci. 2002;967:217–235. doi: 10.1111/j.1749-6632.2002.tb04278.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith AC, Parrish TB, Abbott R, Hoggarth MA, Mendoza K, Chen YF, Elliott JM. Muscle-fat magnetic resonance imaging: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. 2014 doi: 10.1002/mus.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyd-Clark LC, Briggs CA, Galea MP. Comparative histochemical composition of muscle fibres in a pre- and a postvertebral muscle of the cervical spine. J Anat. 2001;199(Pt 6):709–716. doi: 10.1046/j.1469-7580.2001.19960709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelstein BA, McLendon RE, Barbir A, Myers BS. An anatomical investigation of the human cervical facet capsule, quantifying muscle insertion area. J Anat. 2001;198(Pt 4):455–461. doi: 10.1046/j.1469-7580.2001.19840455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine (Phila Pa 1976) 2000;25(10):1238–1246. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 47.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21(15):1737–1744. doi: 10.1097/00007632-199608010-00005. discussion 1744–1735. [DOI] [PubMed] [Google Scholar]
- 48.Panjabi MM, Cholewicki J, Nibu K, Grauer JN, Babat LB, Dvorak J. Mechanism of whiplash injury. Clin Biomech (Bristol, Avon) 1998;13(4–5):239–249. doi: 10.1016/s0268-0033(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhi-Jun H, Wen-Bin X, Shuai C, Zhi-Jie Z, Feng-Dong Z, Xiao-Jing Y, Ji-Ying W, Li-Li H, Feng J, Guo-Xiang F, Dan-Ju W, Shun-Wu F, Xiang-Qian F. Accuracy of magnetic resonance imaging signal intensity ratio measurements in the evaluation of multifidus muscle injury and atrophy relative to that of histological examinations. Spine (Phila Pa 1976) 2014;39(10):E623–629. doi: 10.1097/BRS.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 50.Elliott JM, Walton DM, Rademaker A, Parrish TB. Quantification of cervical spine muscle fat: a comparison between T1-weighted and multi-echo gradient echo imaging using a variable projection algorithm (VARPRO) BMC Med Imaging. 2013;13:30. doi: 10.1186/1471-2342-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto M, Ichihara D, Okada E, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Takahata T. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers - 10year follow-up MR study. Injury. 2012;43(6):912–916. doi: 10.1016/j.injury.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Ulbrich EJ, Aeberhard R, Wetli S, Busato A, Boesch C, Zimmermann H, Hodler J, Anderson SE, Sturzenegger M. Cervical muscle area measurements in whiplash patients: Acute, 3, and 6 months of follow-up. J Magn Reson Imaging. 2012;36(6):1413–1420. doi: 10.1002/jmri.23769. [DOI] [PubMed] [Google Scholar]