Abstract
Background
Our previous work revealed significantly less acidosis in swine undergoing NOTES (Natural Orifice Translumenal Endoscopic Surgery) using endoscopic air insufflation than swine undergoing standard laparoscopy. We wanted to evaluate the differential effects of CO2 versus intraabdominal pressure as source for this finding. In addition, we investigated the endocrine stress response between swine undergoing NOTES peritoneoscopy with CO2 insufflation and animals undergoing standard diagnostic laparoscopy with CO2.
Materials and Methods
Twenty-eight (28) female 50 kg domestic pigs were randomly assigned to one of four groups using a permuted block randomization table: Group 1: NOTES using CO2 insufflation, Group 2: NOTES using air insufflation, Group 3: laparoscopy max pressure 12 mm Hg, Group 4: laparoscopy with max pressure 7 mm Hg. Invasive monitoring lines were placed. Pneumoperitoneum was established by the respective method and maintained for 90 minutes, visualizing liver, spleen and colon. Arterial blood gas was obtained at baseline and 4 additional time points. Serum TNF-α for POD (postoperative day) 1 and cumulative urine adrenaline for the procedure was determined by ELISA. ANOVA and t-test were used for statistical comparison. The study was IACUC (Institutional Animal Care and Use Committees) approved.
Results
All experiments were completed as outlined. Blood pH showed a significant difference between groups. Serum TNF-α revealed higher levels for NOTES CO2 on POD1 than standard laparoscopy (p= 0.03).
Conclusion
NOTES animals with CO2 insufflation initially experienced similar pH compared to standard laparoscopy but recovered to levels seen in low pressure laparoscopy and NOTES with air. NOTES with CO2 appears to elicit a stronger stress response in this study than standard or low pressure laparoscopy or NOTES with air.
Keywords: bowel, clinical papers/trials/research, CO2, pneumoperitoneum, endoscopy
INTRODUCTION
Our prior work compared the physiologic effects of NOTES (Natural Orifice Translumenal Endoscopic Surgery) using manual air insufflation as used for endoscopy and standard laparoscopy with preset routine CO2 insufflation. Pilot data from these experiments showed a significant drop in diastolic blood pressure associated with a significant decrease in heart rate for NOTES animals compared to animals undergoing standard laparoscopy, despite significantly lower intra-abdominal pressure in the NOTES group. As a consequence of the CO2 insufflation, the laparoscopic group experienced greater acidemia than the NOTES animals. Are the hemodynamic differences solely related to the difference in insufflation gas and insufflation pressure? Does a missing catecholamine surge or a significant parasympathetic stimulation in the NOTES animals contribute to these findings?
The aim of this study was to compare the cardiopulmonary results between animals undergoing NOTES peritoneoscopy with CO2 and animals undergoing diagnostic laparoscopy with CO2. We hypothesized that the difference in pulse pressure that was observed with air insufflation in NOTES would persist with CO2 insufflation compared to laparoscopy.
Our second aim was to compare the stress response between animals undergoing NOTES with animals undergoing laparoscopy. Our hypothesis here was that the animals with an abdominal incision (laparoscopy) would experience higher urine catecholamine levels and greater heart rate variability compared to NOTES animals.
METHODS
Animal preparation
Twenty-eight female 50 kg domestic pigs (Yorkshire swine) were acclimated five days prior to the planned procedure. If no condition rendered study participation infeasible, the swine were randomly assigned to one of four groups, using a permuted block randomization table (Figure 1). All pigs were given oral antibiotics (enrofloxacin) before the procedure and intramuscular (IM) injection of 600,000 units of a penicillin G benzathine + penicillin G procaine-based antibiotic at the start of the procedure. Perioperative procedures included: Intramuscular pre-medication (pig cocktail: 83.3 mg/ml ketamine and 16.7 mg/ml xylazine at a dose of 1 cc/8 kg or telazol 3-5 mg/kg), oral intubation, marginal ear vein venous access, and maintenance anesthesia with isoflurane.
Figure 1.
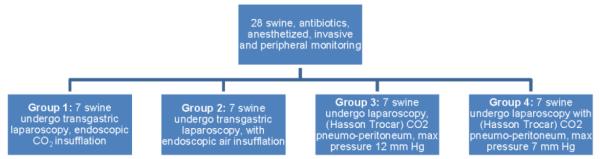
Schematic of study design
Monitoring and data collection
A urinary catheter was placed, calibrated at the pubic bone and light-protected for urine collection and intra-abdominal pressure measurements. A Veress needle without gas flow was placed for comparative intra-abdominal pressure measurements. A percutaneous arterial line was placed for blood draws and to follow cardiopulmonary parameters in 2.5 minutes (min) intervals. Electrocardiogram tracings were recorded at baseline and in 30 min intervals during and after the procedure for the duration of 10 min each to obtain a signal suitable for heart rate variability analysis. All animals had a peritoneal drain placed at the end of the procedure. Peritoneal fluid was obtained at postoperative day (POD) 1 and 2.
Group 1: NOTES with CO2
For animals undergoing NOTES with CO2 we used the following transgastric access procedure: the swine underwent endoscopy using a Cidex OSA treated endoscope through a sterile overtube placed into the oropharynx. The endoscope was connected through a pressure valve to a CO2 tank for gas inflow through the usual gas intake, replacing the room air. Adjustments to flow were manual.
After endoscopic irrigation, an adequate spot on the anterior gastric body was identified and a gastrotomy was created using the needle knife followed by a 15 mm dilating balloon over a wire to accommodate the passage of the endoscope. The endoscope was advanced into the peritoneal cavity; pneumoperitoneum was established and maintained for 90 minutes, visualizing liver, spleen and colon. Pressure was measured using the Veress needle and bladder catheter. Blood samples were obtained at the beginning of the procedure and at 10 min, 30 min, 60 min and 90 min from the start of the pneumoperitoneum. Complete blood count, arterial blood gas analysis and cytokines were obtained. Arterial blood pressure, heart rate, end-tidal CO2, O2 saturation and bladder pressure were monitored in 15-min intervals. Blood was frozen for additional laboratory analysis by blinded personnel. The pneumoperitoneum was aspirated, the endoscope withdrawn and the gastrotomy closed with jumbo clips. Urine from the light protected bladder catheter was collected for catecholamine measurements.
Group 2: NOTES with air control group
The same procedure as for group 1 was followed but the insufflation gas was standard air insufflation with manual control. All other procedure and data collection steps were the same as for group 1.
Group 3: Standard laparoscopy control group
The operative control group consisted of seven animals undergoing standard laparoscopy. After induction of anesthesia and antibiotic therapy, pneumoperitoneum was established using a periumbilical Hasson trocar connected to a CO2 insufflator with sterile tubing. Insufflation pressure was preset at 12 mm Hg. A Veress needle without flow was placed at a separate site to allow for monitoring identical to group 1. Cardiopulmonary and laboratory measurements were performed in the same timeframe with the same measurement parameters as for the experimental (NOTES) group.
Group 4: Low pressure laparoscopy control group
The same procedure as for group 3 was followed, only the insufflation pressure was preset at 7 mm Hg, which was the mean measured abdominal pressure in our prior NOTES experiments.
Laboratory values
Urine for analysis of catecholamines was collected into light protected urine collection bags (Bard) during surgery. After surgery the urine volume was measured, pH checked and aliquotted into labeled 1.5ml Microfuge tubes. The urine had 6M hydrochloric acid added (250ul per 50 ml urine) as a preservative prior to freezing at −20°C according to kit manufacturers instructions.
Once all urine samples were collected, the LDN (Labor Diagnostika Nord GmbH & Co ; Nordhorn, Germany) 2-Cat ELISA assay kit was used to assess urine concentrations of norepinephrine and epinephrine. Samples were thawed and prepared per kit instructions using 10ul sample aliquots. Samples, standards for standard curves and controls were run in duplicate. Incubation was performed for 60 min at 375 rpm for the extraction and enzymatic conversion steps. The assay was analyzed on a PowerWave X (Bio-Tek Instruments, Inc.) ELISA plate reader using KC 4 software.
Statistical methods
Repeated measures ANOVA models were used to estimate differences between the groups over time. Within the groups, changes from baseline to the end of the procedure were calculated. Assessment of ‘no change’ within each of the four treatment groups was made using a paired t-test. Testing for the same change from baseline to the end of procedure between the four treatment groups was assessed with ANOVA. When the overall p-value of this comparison was less than 0.05, a t-test was used for the six pairwise comparisons of treatment groups. To account for multiple comparisons with a Bonferroni correction, a p-value of 0.008 was considered significant. Power calculations were based on data arising from a previously published study.[1] A sample size of n=7 in each group was necessary to achieve a power of 80% to detect a mean difference in the diastolic blood pressure equal to that observed in the referenced study [mean change (sd) of +2.5 (13.2) in the laparoscopic group and −7 (6.4) in the NOTES group, autocorrelation=0.65]. The sample sizes were determined by adjusting the corresponding 2-sample t-test sample size determination for the relative efficiency of repeated measures by multiplying the calculated sample size by 1 minus the autocorrelation parameter. All t-test power calculations were accomplished with NCSS software (Version 2.5). All repeated measures models were fit using SAS Version 9.1 for Windows.
RESULTS
All experiments were completed as outlined. One animal died of respiratory compromise in recovery (group 2). There was no difference between groups in temperature, weight-gain or behavior for 14 days following the procedure. Necropsy was performed on POD 14.
Hemodynamics
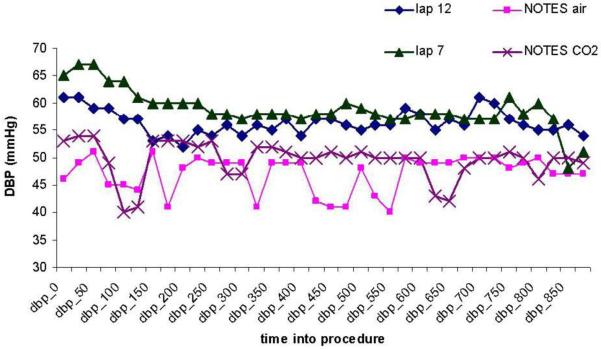
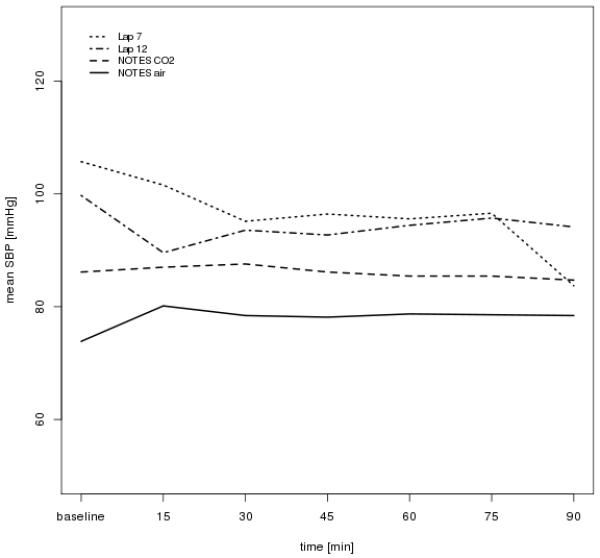
There was no significant difference in blood pressure between the four groups at baseline. The mean systolic blood pressure (SBP) ranged from 88 to 100 mmHg (p=0.30) and the mean diastolic blood pressure (DBP) ranged from 55 to 63 mmHg (p=0.40). All groups experienced a decrease in SBP over time, ranging from 5.5 mmHg (Lap/12mm) to 15.4 mmHg (Lap/7mm). However, there was no difference between the groups (p=0.62). The low pressure laparoscopy group showed a mean decrease of 15.4 mmHg (sd 14.8), which was significantly different from the ‘no change’ assumption, p=0.03. The other groups did not show this change. Similarly, the DBP decreased in all groups, ranging from 7.2 mmHg (NOTES/Air) to 10.5 mmHg (Lap/7mm). The rate of decrease was not significantly different between groups (p=0.90). Two of the groups exhibited a decrease in DBP significantly different from ‘no change’, in the NOTES/CO2 group (p=0.004) the mean decrease was 8.7 (sd 4.9) and in the Lap/7mm group (p=0.02) the mean decrease was 10.5 (sd 8.5). Repeated measures analysis showed a difference between groups across time in both DBP (p=0.012) and SBP (p=0.012). (Figures 2 and 3).
Figure 2.
Diastolic blood pressure (dbp) across time
Figure 3.
Systolic blood pressure (sbp) across time
The correlation of DBP with Veress needle pressure was estimated for each time point from baseline to 90 min, by 2.5 min increments. The highest correlation was found at 2.5 min after the procedure was begun, r=0.76. At all other time points the correlation ranged from 0.47 (at 5 min) to r=-0.21 (at 15 min). The mean Veress needle pressure across time in the NOTES animals (NOTES CO2 and NOTES air) was between 3.8 and 7.4.
The mean heart rate did not differ significantly between groups at baseline with means ranging from 90 bpm (sd 22) to 104 bpm (sd 18) (p=0.43), nor at 10 or 90 min after procedure start (p=0.23 and 0.27 respectively). The heart rate change ranged from an increase of 11 bpm (sd 14) from baseline to 90 min in the high pressure laparoscopy group (Lap/12mm) to a decrease of 18 bpm (sd 14) in the low pressure laparoscopy group (Lap/7mm). Without accounting for multiple comparisons among the four treatment groups, there was a significant difference in mean change in heart rate (p=0.02), with pairwise significant differences detected for NOTES/Air versus Lap/7mm (p=0.01) and for Lap/12mm versus Lap/7mm (p=0.002).
In the low pressure laparoscopy group (Lap/7mm), the heart rate dropped over the duration of the procedure by 18 bpm (sd 14.0), p=0.02. In the Lap/12mm group there was a mean increase of 11 bpm (sd 14.4), p=0.08, in the Notes/Air group a mean increase of 7 bpm (sd 17.4), p=0.33, and in the Notes/CO2 group a mean decrease of 4 bpm (sd 21.0), p=0.66.
Respiratory
No clinical events of respiratory distress were noted. O2 saturation was measured by the pulse oximeter was recorded between 99% and 100% for all animals throughout. End-tidal CO2 was not measured. Blood pH was not significantly different among the four groups at the beginning of the procedure [p=0.13, means ranged from 7.47(Lap/12mm) to 7.55 (Notes/Air)]. At 10 min, 30 min and 60 min into the procedure a significant difference among the four groups was noted (p=0.001, p=0.003 and 0.002). There was no significant difference at the end of the procedure, p=0.10 (Figure 4).
Figure 4.
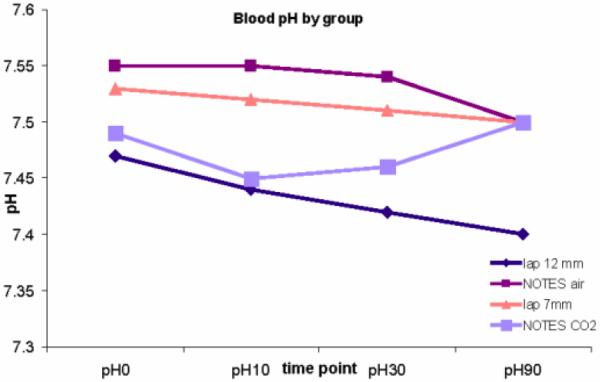
Serum pH by group over time
The change in pH was preceded by significant difference in pCO2 between the groups at 10 min and 30 min into the procedure (p=0.004, means ranging from 34.5 (4.2) to 42.7 (4.8), and p=0.036, means ranging from 35.0 (3.0) to 42.7 (3.8)). A higher pCO2 was seen in the lap 12 and NOTES CO2 groups compared to NOTES air and lap 7 (42.7 and 42.5 vs 34.5 and 37.9 at 10 min; 42.7 and 40.0 vs 35.0 and 37.4 at 30 min).
For the pO2 the group means were different at the different time points (Table 1). The change from baseline was not different between the groups (p=0.99).
Table 1.
Partial oxygen pressure (pO2) by group across time
| pO2 baseline (sd) | 10 min | 30 min | 60 min | 90 min (end of procedure) |
|
|---|---|---|---|---|---|
| NOTES CO2 Group 1 |
167 (32) | 128 | 154 | 150 | 164 |
| NOTES air Group 2 |
218 (57) | 213 (72) | 202 | 206 | 213 |
| Lap 12 Group 3 |
175 (+24) | 147 (33) | 141 | 150 | 138 |
| Lap 7 Group 4 |
204 (74) | 194 | 193 | 205 | 192 |
| p-value (ANOVA) |
0.041 | 0.017 | 0.024 | 0.022 | 0.073 |
Min = minutes
Lap = laparoscopy
Hematology
There was no statistically significant difference in serum white blood cell (WBC) count or hematocrit between the groups at baseline, POD 1, 2, 7 or 14, p>0.05. Platelet count was not significantly different among groups at baseline, POD 1, 2, 7 or 14, nor was peritoneal total WBC count, p>0.05. The proportion of neutrophils was significantly different among the four groups on POD 1, p=0.005, means ranging from 40 (sd 18.8) in the Lap/7mm group to 89 (sd 11.3) in the Notes/CO2 group. This was not seen on POD 2, p=0.19, means ranging from 45 to 72.
Cytokines
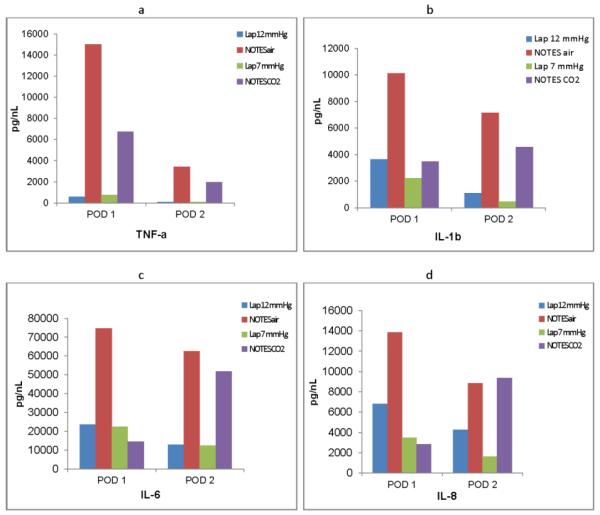
TNF-α, IL-1b, IL-6 and IL-8 in the serum, plasma and peritoneal fluid were evaluated at POD 1 and 2. For the serum ELISA for IL-8, only 25/270 (9%) specimen tested revealed a measurable reaction. Plasma was therefore used. Serum TNF-α revealed higher levels for NOTES CO2 on POD1 than standard laparoscopy (12 vs 6 , p= 0.03) (Figure 5a).
Figure 5.
Mean cytokine levels by group for postoperative day 1 (POD 1) and postoperative day 2 (POD 2). a) TNF-α levels, b) IL-1B levels, c) IL-6 levels, d) IL-8 levels. Lap 12=laparoscopy with 12 mmHg pressure, NOTES air=NOTES with air, Lap 7=laparoscopy with 7 mm pressure, NOTES CO2=NOTES with CO2
Group differences were seen on POD 1 in IL-1b peritoneal fluid (p=0.04) with NOTES air having the highest mean value of 10140pg/ml (4485) and Lap/7mm the lowest at 3097pg/ml (3210) (Figure 5b). There was no significant difference among the four groups on POD 2, p=0.13. There were no statistically significant differences between the groups for IL-6 on POD 1 or 2, p=0.08 and 0.07 respectively (Figure 5c). On POD 1 there was a significant difference for IL-8 among groups, p=0.005, with a range of mean value from 13840pg/ml in NOTES/Air to 2863 pg/ml in NOTES/CO2. On POD 2 the differences among groups was no longer statistically significant, p=0.09 (Figure 5d). As we experienced some missing values (difficulty obtaining drain fluid on different days) we did not consider a change from baseline to add significant value in our small study.
Catecholamines
There was a borderline significant difference in adrenaline among the four groups, p=0.0504, with means from 4.7 (sd 4.6) in the Lap/7mm group to 10.6 (sd 21.6) in the NOTES/CO2 group. There was no significance for nor-adrenaline, p=0.13 with means ranging from 3.3 (sd 1.4) in Lap/7mm to 7.9 (sd 5.3) in the NOTES/Air group (Figure 6).
Figure 6.
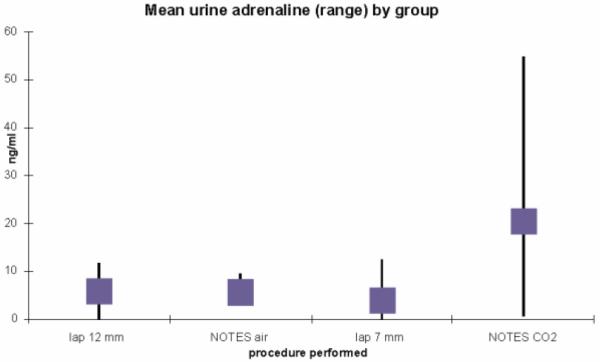
Mean urine adrenaline
DISCUSSION
Patients prefer surgical procedures that enable fast recovery, limit postoperative pain, are safe and have good cosmetic outcomes.[2, 3] When comparing minimally invasive surgical procedures with open resections, differences are often reportable through different morbidity and mortality. When developing new minimally invasive surgical (MIS) procedures, such as NOTES, smaller differences are expected and sometimes difficult to demonstrate.[4, 5] The proposed benefits of NOTES include decreased invasiveness by avoiding the abdominal wall as access to the peritoneal cavity[6-17] theoretically leading to less operative trauma with decreased inflammatory and catecholamine response. However, entry through the gastrointestinal tract has not been well studied in relation to the inflammatory response and invasiveness. Our previous work revealed significant hemodynamic differences between swine undergoing NOTES and swine undergoing standard laparoscopy.[1, 18, 19] Decreased preload, increased respiratory pressures, parasympathetic stimulation from intestinal distension and inflammatory response cascades all can influence hemodynamic stability during an operative procedure involving pneumoperitoneum and/or endoscopy. Traditionally, CO2 laparoscopy has been considered safer than air insufflation while conferring a negative hemodynamic impact compared to abdominal wall lifting devices. [5] As an endoscopic method, the NOTES concept was initiated using intra- and extralumenal air insufflation without gauge valve control, potentially causing uncontrolled intra-abdominal pressure and hemodynamic instability, including the potential for air emboli.[16] Increased interest exists in the utilization of CO2 as insufflation gas for both endoscopy and translumenal procedures with or without pressure control. CO2 and pressure control for endoscopy increase the complexity and cost of the instrumentation. We investigated if the hemodynamic differences noted in our prior experiments, which were conducted with air insufflation, persisted with CO2 utilization for transluminal procedures. Our experiments were structured to control for the differences in abdominal pressure seen in NOTES and laparoscopy. We were not able to include additional control groups such as a sham procedure or a laparotomy given the financial constraints of the study, thus we chose the scenarios that a clinician may have to decide between. These control groups were however included in a study by Trunzo et al [20] and the sham procedures revealed little inflammatory reaction in the first 48 hours and the open procedures showed a reaction multiple times higher than the laparoscopic or NOTES procedures.
The results from this study indicate that low pressure laparoscopy appears to have a smaller footprint on the porcine stress response than high pressure laparoscopy or NOTES peritoneoscopy using CO2. While there was no clinically detectable difference between the outcomes in any of the porcine groups, the hemodynamic, respiratory and the immunologic data from this study support this conclusion. The intra-abdominal pressure in the NOTES groups is usually lower than with standard laparoscopy and comparable to the low pressure laparoscopy, as we have reported in our prior study[19] and as it was here. The systemic vascular resistance and animal stress response certainly could be affected by higher intra-abdominal pressure; the laparoscopic 7 mm group may have been closest to the physiologic pressures and pH and thus ‘settled down’ after the initial trauma. It is possible that the volume of intraluminal and intraperitoneal CO2 in the NOTES CO2 group leads to some of the observed differences. Intestinal inflammatory response from the transluminal access could also be a contributor. Similarly, a laparoscopic approach with an intestinal injury or repair, might lead to an increase in the inflammatory response, which we did not control for in this setting. The measured hemodynamic and respiratory differences between the findings are not very large and may not be clinically significant in a healthy patient; when a borderline stable patient is concerned these findings should be kept in mind. In current clinical practice the findings of our study are applicable in situations such as transmural pancreatic necrosectomy, endoscopic PEG (percutaneous endoscopic gastrostomy) rescue, endoscopic treatment of large anastomotic leaks or fistulae with stents, clipping or suturing procedures.
CONCLUSION
Low pressure laparoscopy appeared to have the smallest impact when comparing the four different approaches. After initial equilibration, abdominal pressure/CO2 did not correlate well with hemodynamic changes. CO2 in NOTES animals had differential effect compared to air.
Acknowledgments
Funding: Research reported in this manuscript was funded through a NOSCAR grant (Natural Orifice Surgery Consortium for Assessment and Research) and in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK93553. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES Juliane Bingener - Surgeon Advisory Board, Titan Medical Inc.
Erica Loomis - Nothing to disclose
Marianne Huebner - Nothing to disclose
Christopher Gostout - Nothing to disclose
REFERENCES
- 1.Bingener J, Michalek J, Winston J, Van Sickle K, Haines V, Schwesinger W, Lawrence V. Randomized blinded trial comparing the cardiopulmonary effects of NOTES with standard laparoscopy in a porcine survival model. Surgical Endoscopy. 2008;22:1430–1434. doi: 10.1007/s00464-008-9898-8. [DOI] [PubMed] [Google Scholar]
- 2.Bingener J, Sloan JA, Ghosh K, McConico A, Mariani A. Qualitative and quantitative analysis of women’s perceptions of transvaginal surgery. Surgical Endoscopy. 2012;26:998–1004. doi: 10.1007/s00464-011-1984-7. [DOI] [PubMed] [Google Scholar]
- 3.Detsky A. What patients really want from health care. JAMA. 2011;306:2500–2501. doi: 10.1001/jama.2011.1819. [DOI] [PubMed] [Google Scholar]
- 4.Bickel A, Yahalom M, Roguin N, Frankel R, Breslava J, Ivry S, Eitan A. Power spectral analysis of heart rate variability during positive pressure pneumoperitoneum: the significance of increased cardiac sympathetic expression. Surgical Endoscopy. 2002;16:1341–1344. doi: 10.1007/s00464-001-9211-6. [DOI] [PubMed] [Google Scholar]
- 5.Galizia G, Prizo G, Lieto E, Castellano P, Pelosio L, Imperatore V, Ferrara A, Pignatelli C. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall-lifting cholecystectomy: A prospective randomized study. Surgical Endoscopy. 2001;15:477–483. doi: 10.1007/s004640000343. [DOI] [PubMed] [Google Scholar]
- 6.Hochberger J, Lamade W. Transgastric surgery in the abdomen: the dawn of a new era? Gastrointestinal Endoscopy. 2005;62:293–296. doi: 10.1016/j.gie.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Ponsky J. Gastroenterologists as surgeons: What they need to know. Gastrointestinal Endoscopy. 2005;61:454. doi: 10.1016/s0016-5107(04)02632-x. [DOI] [PubMed] [Google Scholar]
- 8.Kalloo A, Singh V, Jagannath S, Niiyama H, Hill S, Vaughn C, Magee C, Kantsevoy S. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointestinal Endoscopy. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 9.Jagannath S, Kantsevoy S, Vaughn C, Chung S, Cotton P, Gostout C, Hawes R, Pasricha P, Scorpio D, Magee C, Pipitone L, Kalloo A. Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointestinal Endoscopy. 2005;61:449–453. doi: 10.1016/s0016-5107(04)02828-7. [DOI] [PubMed] [Google Scholar]
- 10.Kantsevoy S, Jagannath S, Niiyama H, Chung S, Cotton P, Gostout C, Hawes R, Pasricha P, Magee C, Vaughn C, Barlow D, Shimonaka H, Kalloo A. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointestinal Endoscopy. 2005;62:287–292. doi: 10.1016/s0016-5107(05)01565-8. [DOI] [PubMed] [Google Scholar]
- 11.Park P, Bergstrom M, Ikeda K, Fritscher-Ravens A, Swain P. Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis. Gastrointestinal Endoscopy. 2005;61:601–606. doi: 10.1016/s0016-5107(04)02774-9. [DOI] [PubMed] [Google Scholar]
- 12.Swanstrom L, Kozarek R, Pasricha P, Gross S, Birkett D, Park P, Saadat V, Ewers R, Swain P. Development of a new access device for transgastric surgery. Journal of Gastronintestinal Surgery. 2005;9:1129–1137. doi: 10.1016/j.gassur.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Wagh MS, Merrifield BF, Thompson CC. Endoscopic transgastric abdominal exploration and organ resection: initial experience in a porcine model. Clinical Gastroenterology & Hepatology. 2005;3:892–896. doi: 10.1016/s1542-3565(05)00296-x. [DOI] [PubMed] [Google Scholar]
- 14.Wagh M, Merrifield B, Thompson C. Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointestinal Endoscopy. 2006;63:473–478. doi: 10.1016/j.gie.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Kantsevoy S, Hu B, Jagannath S, Vaughn C, Beitler D, Chung S, Cotton P, Gostout C, Hawes R, Pasricha P, Magee C, Pipitone L, Talamini M, Kalloo A. Peroral transgastric endoscopic splenectomy: is it possible? Surgical Endoscopy. 2006;20:522–525. doi: 10.1007/s00464-005-0263-x. [DOI] [PubMed] [Google Scholar]
- 16.Rattner D, Kalloo A. ASGE/SAGES Working group on natural orifice translumenal endoscopic surgery. Surgical Endoscopy. 2006;20:329–333. doi: 10.1007/s00464-005-3006-0. [DOI] [PubMed] [Google Scholar]
- 17.Pai RD, Fong DG, Bundga ME, Odze RD, Rattner DW, Thompson CC. Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model. Gastrointestinal Endoscopy. 2006;64:428–434. doi: 10.1016/j.gie.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Bingener J, Moran E, Gostout CJ, Buck L, Schwesinger W, Van Sickle K, Huebner M. Randomized study of natural orifice transluminal endoscopic surgery and endoscopy shows similar hemodynamic impact in a porcine model. Surg Endosc. 2011;25:1065–1069. doi: 10.1007/s00464-010-1317-2. [DOI] [PubMed] [Google Scholar]
- 19.Moran EA, Gostout CJ, McConico AL, Bingener J. Natural orifice translumenal endoscopic surgery used for perforated viscus repair is feasible using lower peritoneal pressures than laparoscopy in a porcine model. J Am Coll Surg. 2010;210:474–479. doi: 10.1016/j.jamcollsurg.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Trunzo JA, McGee MF, Cavazzola LT, Schomisch S, Nikfarjam M, Bailey J, Mishra T, Poulose BK, Lee Y-J, Ponsky JL, Marks JM. Peritoneal inflammatory response of natural orifice translumenal endoscopic surgery (NOTES) versus laparoscopy with carbon dioxide and air pneumoperitoneum. Surg Endosc. 2010;24:1727–1736. doi: 10.1007/s00464-009-0839-y. [DOI] [PubMed] [Google Scholar]