Abstract
Introduction
Ischemia-reperfusion injury (I-R) in skeletal muscle requires timely treatment.
Methods
Rodent models of I-R injury were used to test the efficacy of recombinant human MG53 (rhMG53) protein for protecting skeletal muscle.
Results
In a mouse I-R injury model, we found that mg53−/− mice are more susceptible to I-R injury. rhMG53 applied intravenously to the wild type mice protected I-R injured muscle, as demonstrated by reduced CK release and Evans blue staining. Histochemical studies confirmed beneficial effects of rhMG53. Interestingly, rhMG53 did not protect against I-R injury in rat skeletal muscle. This was likely due to the fact that the plasma level of endogenous MG53 protein is high in rats.
Discussion
Our data suggest that rhMG53 may be a potential therapy for protection against muscle trauma. A mouse model appears to be a better choice than a rat model for evaluating potential treatments for protecting skeletal muscle.
Keywords: muscle injury, TRIM72, tourniquet, creatine kinase, edema
Introduction
Extremity trauma is a major form of injury in civilian life and in combat1. It often involves skeletal muscle ischemia-reperfusion injury (I-R) due to vascular damage 2 or development of extremity compartment syndrome 3, potentially leading to permanent loss of limb function, amputation, or even death4. I-R is a complex injury that results in vascular, neural, and muscular damage5. In myofibers, free radicals generated during I-R promote membrane damage6, inciting a cascade of events that can lead to necrosis and apoptosis4. As such, increasing the antioxidant capacity of skeletal muscle prior to I-R injury is effective at mitigating tissue damage7. However, with trauma it may not be feasible to combat initial free radical production, raising the need to identify effective therapies directed at enhancing membrane repair8.
Rats and mice have been used for many traumatic injury-related studies. Different responses to injuries between these 2 species have been observed in various models, including spinal cord injury9,10, lung injury11, liver injury,12 and wound healing (observations from our laboratories). In general, rats are more resistant to injury and often recover quicker from injuries than do mice12,10. It is possible that rats might possess more efficient injury-repair machinery, but the exact mechanism(s) are largely unknown. Recently, Cai et al.13 identified Mitsugumin 53 (MG53), a muscle-specific TRIM family protein, as an essential molecule in regulating cell membrane repair 13,14. We further demonstrated MG53 knock-out mice develop progressive muscle weakness and defective muscle repair after exercise or injury15. To test the potential therapeutic application for MG53 in treatment of acute injury, we administrated recombinant human MG53 (rhMG53) to injured C2C12 myotubes or isolated myofibers in vitro and observed enhanced cell membrane repair16. Moreover, rhMG53 delivery to mdx and wild type mice improved the capacity to repair membrane damage caused by eccentric contractions or cardiotoxin17,16. Based on these observations, we hypothesized that delivery of rhMG53 would ameliorate skeletal muscle damage secondary to I-R injury. Using our standard rat tourniquet model we tested whether rhMG53 administration attenuated I-R in rats. Contrary to our expectations, histopathological measurements revealed similar muscle injury with or without the administration of rhMG53 in the rat model18. Interestingly, as part of the same study we found rhMG53 did improve C2C12 myotube viability upon H2O2 exposure in vitro18, which was consistent with other reports that indicated a therapeutic effect of MG53 in cardiac I-R19,20,16 and other forms of muscle injury in mice21,16.
In this study, we provide evidence that administration of rhMG53 provides significant protection against I-R injury to skeletal muscle in the mouse model. Our data suggest that endogenous MG53 plays an important role in protecting skeletal muscle from traumatic insults, and that rhMG53 may be a potential therapeutic reagent for protection against skeletal muscle I-R.
Methods
Mouse Studies
Animal protocols involving mice were approved by the Ohio State University Animal Care and Use Committee. Mice (C57BL/6J, weight 25 grams, purchased from Jackson Lab) were anesthetized with isoflurane (2% isoflurane, oxygen flow rate at 1 liter per minute) throughout the ischemic period. Body temperature was maintained using a water perfused heating pad (HP3119, Hallowell, EMC)(Gaymar Heat Therapy Pump, #TP650) during the entire procedure. Ischemia was induced in the left hind limb using a tension-controlled tourniquet around the upper thigh. The tourniquet was composed of 2-0 silk, 3.0 metric suture (Ethicon, LLC); suture tension was controlled by a calibrated spring (400 g). Following 45 minutes of ischemia, the tourniquet was released for 24-hour reperfusion. In the first mouse experiment, I-R was induced in mg53−/− mice or littermate wild type controls. Plasma samples were collected at indicated time points for creatine kinase (CK) measurement. In a second series of experiments, the protective effect of rhMG53 administration was evaluated in adult male C57BL/6J mice. rhMG53 was prepared by dissolving lyophilized rhMG53 (provided by TRIM-edicine, Inc.) in sterile saline (2 mg/ml). It was administered via tail vein injection (6 mg/kg body weight) 5 min prior to tourniquet application and 5 min prior to release of ischemia. Sham animals received only sterile saline. Twenty-four hours after reperfusion, mice were euthanized, and muscles were harvested. Gastrocnemius (Gas) and tibialis anterior (TA) muscles were weighed before and after drying at ~50°C for 7 days, and the wet:dry ratio served as an index of edema. TA muscle sections were stained with H&E for pathological examination. Muscle fiber cell membrane integrity was determined histologically in TA by microscopic visualization of Evans blue dye (Sigma, E-2129) [EBD; 1% w/v; applied intraperitoneally (i.p.) 16 hours before injury] inclusion within damaged cells. EBD positive muscle fibers were counted, and the percentage was calculated by an individual blinded to the treatments.
Rat Studies
Animal protocols involving rats were approved by the US Army Institute of Surgical Research Animal Care and Use Committee. This study adhered to National Institutes of Health guidelines for the care and use of laboratory animals (DHHS Publication, NIH, 86 to 23). Adult male Sprague-Dawley rats were administered either lyophilized rhMG53 dissolved in sterile saline or saline alone via tail vein injection (6 mg/kg body weight) 5 min prior to tourniquet application and 5 min prior to release. Both groups underwent 3 hour of pneumatic tourniquet induced I-R as previously described in detail 8. Two days after injury, rats were euthanized and muscles were harvested. Muscle fiber cell membrane integrity was determined histologically in TA muscles following the same procedure described above. GAS muscle were weighed before and after drying at ~50°C for 7 days, and the wet:dry ratio served as an index of edema.
Plasma levels of endogenous MG53 in mice, rats, and humans
For quantification of MG53 in plasma, plasma samples were collected from wild type C57BL/6J mice, Sprague Dawley rats (Harlan Laboratories, Inc. IN), and healthy, consented, human donors (samples purchased from Bioreclamation, LLC.) and analyzed by Western blot. The densitometry of protein bands was quantified by Image J software (NIH). Ponceau S (Sigma, P7170) staining was used for loading control.
Western blot analysis
To analyze expression levels of MG53, dysferlin, and caveolin-3 in skeletal muscles derived from mouse and rat, TA muscles were dissected from adult male C57BL/6J mice and adult male Sprague-Dawley rats. Proteins were extracted from the muscles by RIPA buffer supplied with protease inhibitor cocktail (Roche, 11697498001) and phosphatase inhibitor cocktail (Pierce, 78428). The proteins were separated by SDS-PAGE and transferred to PVDF membrane. The membranes were probed with antibodies against MG53 (1:3000, generated by our laboratory), dysferlin (1:1000, Novocastra Laboratories), and caveolin-3 (1:2000, BD Transduction Laboratories). We used 2 monoclonal antibodies derived from mouse and rabbit, both of which recognize MG53 in mouse, rat, and human tissues. Characterization of these antibodies has been reported in our previous publications13,15,16,25. Horseradish peroxidase conjugated secondary antibodies were incubated with the membrane. Peroxidase activity was determined with ECL kits (Pierce, 32106). The ECL signals were captured by HyBlot CL autoradiography film (Denville, Inc).
Statistics
Statistical differences between groups were determined using independent t-tests for continuous data and Mann–Whitney U tests for interval data. P-values <0.05 were used as criteria for statistical significance.
Results
mg53−/− mice are more susceptible to I-R injury in skeletal muscle
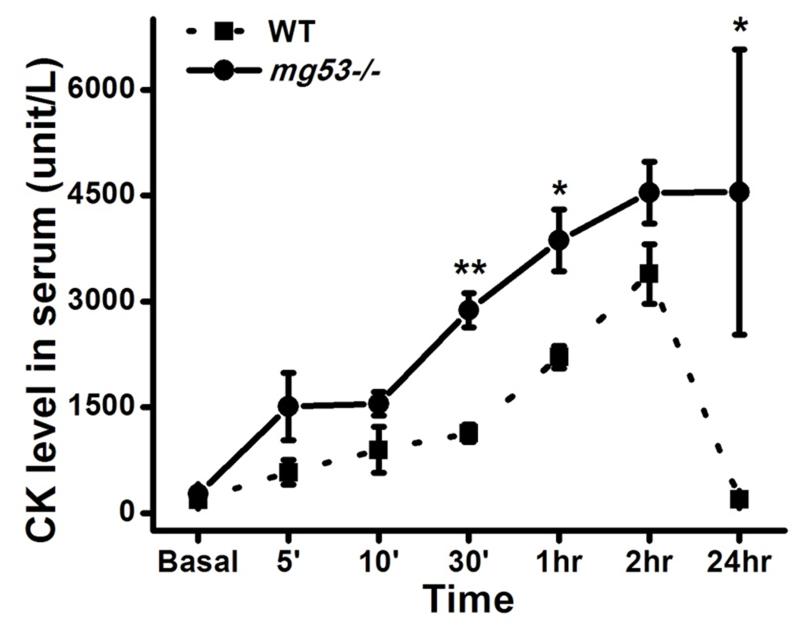
To test the role of MG53 in protection against I-R injury in skeletal muscle, we performed comparative studies with I-R injury in muscles from mg53−/− mice and wild type littermate controls. As shown in Fig. 1, circulating creatine kinase (CK) in mg53−/− mice following I-R injury was significantly higher at multiple time points as compared with wild type littermates, indicating that MG53 plays a critical role in protecting against I-R injury to muscle.
Figure 1. mg53−/− muscle is susceptible to ischemia-reperfusion (I/R) injury.
Creatine kinase levels in serum were measured at indicated time points. mg53−/− mice exhibited a different pattern of serum CK changes as compared to wild type control (n=6 pairs). The serum CK elevation was quicker and remained at high levels for a longer time in mg53−/− mice. Data are presented as mean ± SEM. *: P<0.05.
rhMG53 protects I-R induced muscle injury in mice
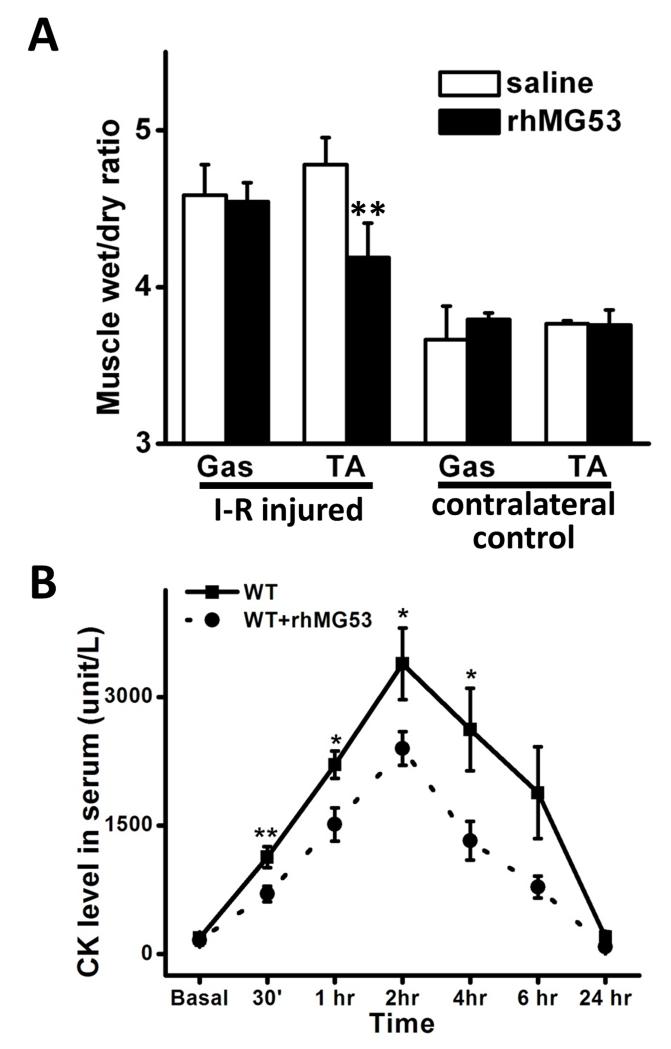
To examine if exogenous rhMG53 protein can protect against I-R injury in skeletal muscle, we delivered rhMG53 by tail vein injection to wild type mice. As shown in Fig. 2A, rhMG53 treatment reduced muscle edema significantly, as indicated by the measurement of wet:dry ratio, in I-R injured mouse skeletal muscle. The wet/dry ratio was reduced from 4.78±0.17 in the saline treatment group to 4.19±0.22 in the rhMG52 treated group (P<0.01). Furthermore, release of CK was also suppressed significantly by rhMG53 treatment, indicating improved membrane repair in rhMG53 treated animals (Fig. 2B).
Figure 2. rhMG53 prevents I-R induced skeletal muscle injury in mice.
(A) rhMG53 treatment (n=5) significantly reduced the wet:dry ratio of I-R injured muscle compared to saline treatment (n=4). **: P<0.01. (B) Serum CK concentration was measured at indicated time points (n=8 pairs). rhMG53 treated mice had lower CK in the blood than saline treated mice. Data are presented as mean ± SEM. *: P<0.05; **: P<0.01.
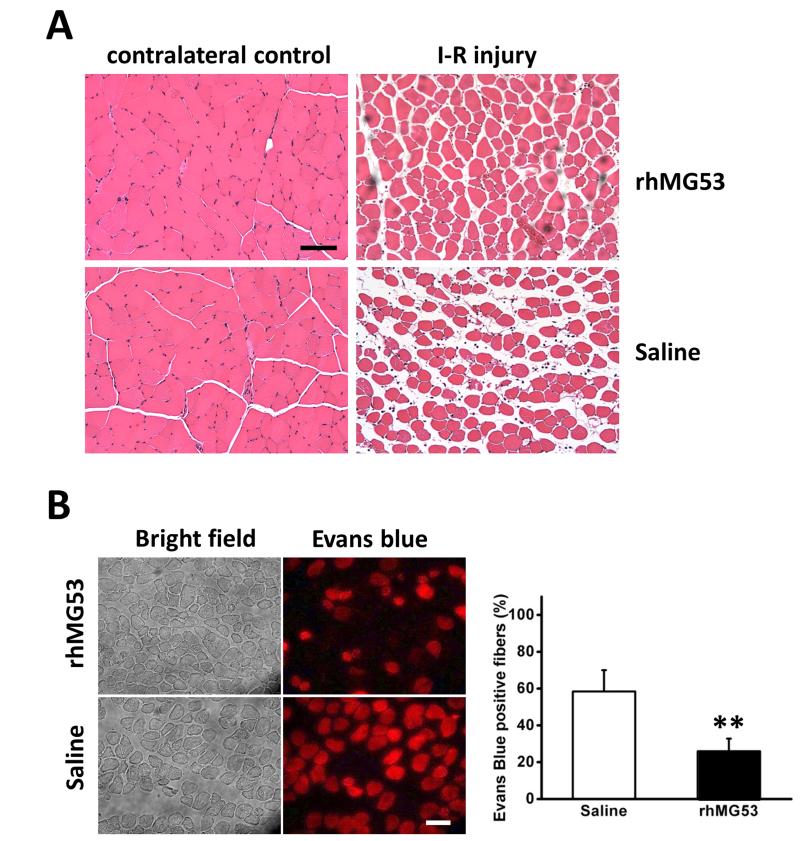
rhMG53 improves pathology of skeletal muscle associated with I-R injury
Similar to observations in Fig. 2A, H&E staining of the TA in the rhMG53 treated group also showed less swelling than saline controls (Fig. 3A). More necrotic fibers were evident by light-pink staining fibers in saline control muscle than in MG53 treated muscle (Fig. 3A). Evans blue dye (EBD) is commonly used to measure muscle membrane integrity 22. This membrane-impermeable fluorescent dye stains only muscle fibers with membrane damage. As shown in Fig. 3B, rhMG53 treatment significantly reduced EBD positive muscle fibers (26.0±6.9% in rhMG53 treatment group vs 58.4±11.6% in saline control group, P<0.01), indicating the membrane injury induced by I-R injury can be protected and/or repaired by rhMG53 delivery.
Figure 3. rhMG53 improves muscle structure associated with I-R injury.
(A) H&E staining of control (left) and injured (right) muscles. More necrotic fibers were seen by light pink staining in saline control muscle than that in MG53 treated muscle. n=3 pairs. (B). Fluorescent images of Evans blue positive fibers (right) showed less injury in rhMG53 treated muscle than control. Percentage of total damaged fibers is summarized in the lower panel. The data are mean ± SEM **: P<0.01. n=4 pairs. Scale bars, 50 μm.
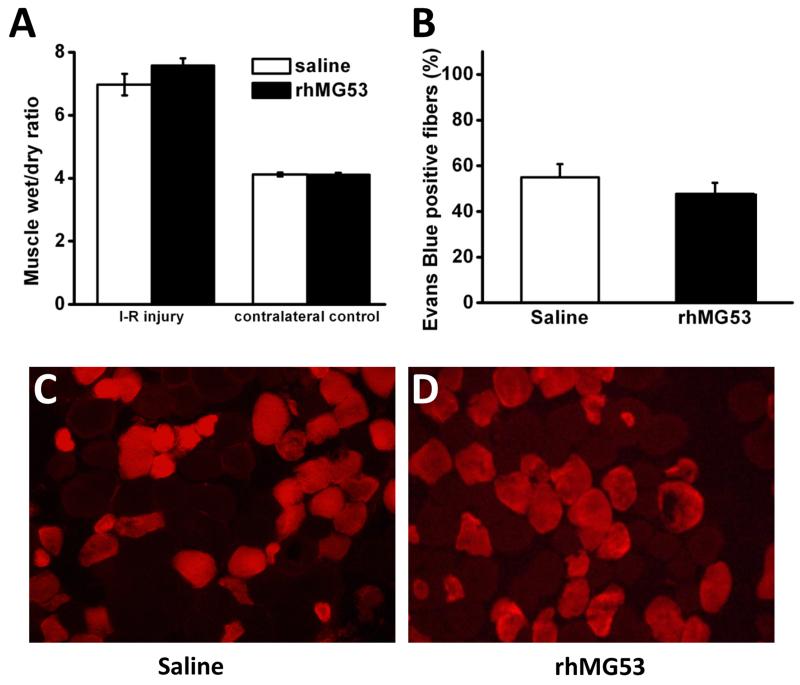
For comparative purposes, we repeated the I-R muscle injury studies using our established rat model18. We noted consistently that rat muscle was more resistant to I-R induced injury, as significantly longer ischemic times were required to cause detectable muscle injury. We found that the 45 min ischemia time we used in the mouse model did not cause measurable muscle injury in the rat model (not shown). We found it necessary to use 3 hours of tourniquet ischemia in order to achieve muscle injury in the rat model comparable to that in the mouse model. Under this condition, the percentage of EBD positive muscle fibers was 55.0±5.8% (Fig. 4B) in the rat with 3 hours of ischemia, and 58.4±11.6% in the mouse with 45 minutes of ischemia (Fig. 3B). Interestingly, administration of rhMG53 did not produce significant changes in the degree of muscle injury in the rat model; the percentage of EBD positive muscle fibers was not statistically different between saline and rhMG53 treated rats (55.0±5.8% vs 47.7 ± 4.9%, respectively, P = 0.46) (Fig. 4B). Edema, as indicated by muscle wet:dry ratio was also similar between groups (6.97±0.34 and 7.57±0.23 for saline and rhMG53 treated, respectively, P = 0.26) (Fig. 4A). These data were consistent with our previous report that showed only a marginal effect of rhMG53 in the I-R induced injury to the rat muscle 18.
Figure 4. rhMG53 exhibits minimal beneficial effects in I-R injury to rat skeletal muscle.
(A) Wet/dry weight in injured GAS muscle nearly doubles compared to uninjured muscle, which is unaffected by rhMG53 treatment. (B) Quantification of the number of muscle fibers that stain positive for EBD confirms the lack of effect of rhMG53 treatment in rats. (C-D) Representative fluorescent images of muscle cross-sections from (C) Control (PBS) and (D) rhMG53 treated rats clearly show similar numbers of EBD positively stained fibers (red) between treatments.
Plasma derived from rat contains higher level of endogenous MG53
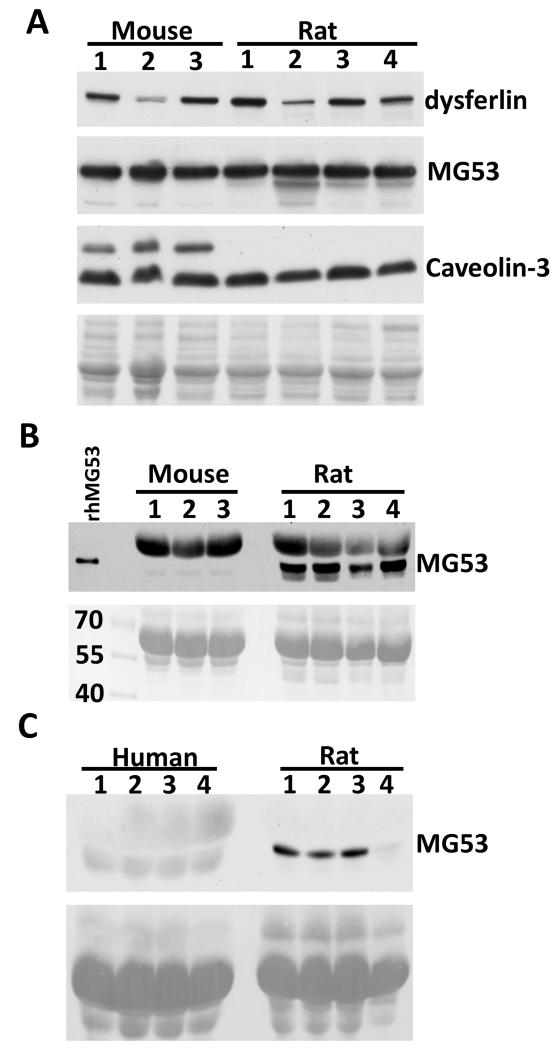
Such drastic differences between the responses of I-R injury and treatment of rhMG53 in the mouse and rat models raise an intriguing question that intrinsic muscle membrane repair mechanisms may be different between the 2 species. We performed a series of biochemical studies and found that several proteins that participate in repair of membrane injury, e.g. dysferlin, caveolin-3, and MG53, show similar expression levels in mouse and rat muscle (Fig. 5A). Thus, it is unlikely that difference in the intracellular membrane repair mechanism can account for the different response of I-R induced muscle injury in the 2 species. Interestingly, we found that endogenous circulating MG53 in rats is significantly higher than in mice (Fig. 5B). This finding might explain why the rats are less sensitive to skeletal muscle I-R injury. Furthermore, it might also explain why administration of rhMG53 had minimal effect on I-R injury in rat muscle.
Figure 5. Plasma samples derived from rat contain high level of endogenous MG53.
(A) Skeletal muscle samples from mouse and rat showed similar level of endogenous dysferlin, MG53, and caveolin-3. Ponceau S staining was used to show equal loading. Plasma samples from mice and rats (B) or rats and humans (C) were subjected to Western blot analysis. Representative Western blot figure shows that plasma derived from rat contains higher endogenous MG53 than plasma from mouse and human, indicating that mouse might be a better animal model to study MG53-mediated membrane repair. rhMG53 was used as positive control. The results were confirmed by 2 different MG53 antibodies generated from different animal species.
We also conducted comparative measurements with determination of MG53 in the human plasma. As shown in Fig. 5C, the level of MG53 in human plasma is similar to that in mouse. Thus, mouse skeletal I-R injury model might be a better preclinical model for studying MG53-mediated membrane repair.
Discussion
Extremity trauma is a large portion of both military 1 and civilian injuries 23, the majority of which include muscle trauma 24, often involving vascular injury and/or acute compartment syndrome culminating in I-R injury 25-27. Therapeutic agents that could reduce the magnitude of I-R and/or extend ischemic time would be beneficial. Here we showed the therapeutic benefit of rhMG53 for treatment of skeletal muscle I-R in mice. This represents an exciting and important finding.
In vitro studies and studies using MG53 knockout mice have provided compelling evidence that MG53 is an essential component required for repairing membrane damage in cardiac 13,19,20 and skeletal muscle28,15,29. Furthermore, delivery of exogenous rhMG53 has been shown to ameliorate the impact of eccentric contraction in dystrophic mice and cardiotoxin injury in skeletal muscle in normal mice 16 and acute lung injury30. We extend these observations by demonstrating in skeletal muscle that endogenous circulating MG53 contributes to protection against I-R injury, and treatment with rhMG53 ameliorates I-R induced skeletal muscle injury.
MG53 is a muscle-specific TRIM family protein (TRIM72) involved in vesicle trafficking and fusion with the plasma membrane during normal cellular physiology28 and during the emergency response of plasma membrane repair13,20,19. Through binding with phosphatidylserine, MG53 associates with intracellular vesicles underneath the sarcolemma of striated muscle. In muscle cells, MG53 interacts with dysferlin and caveolin-3 to form a dynamic vesicular complex 13. In the event of membrane disruption, MG53 is oxidized at a specific cystine residue in response to entry of the extracellular milieu into the cell, and disulfide-bond formation between MG53 molecules provides the nucleation mechanism for vesicle translocation to the membrane injury site.
We previously reported that rhMG53 did not show a significant protective effect against I-R skeletal muscle injury in rats 18. All published studies showing a benefit of rhMG53 administration have involved mice, suggesting that species differences may explain these disparate findings. No difference between rats and mice was found in endogenous MG53 levels in skeletal muscle. While most research has focused on MG53 levels resident in the tissue of study, endogenous MG53 is also present in circulating blood16. We found a more than 10-fold higher level of endogenous MG53 in rat plasma compared to mice. Based on this, we postulate that in rats baseline levels of endogenous MG53 are adequate to provide a protective effect without the need for exogenous rhMG53. This suggests that therapeutic levels of rhMG53 in mice (and possibly humans) may be much lower than those used in this and other studies and certainly warrants future study. Future studies with molecular or genetic manipulation to deplete MG53 in rats will provide a further test for the role of circulating MG53 in protection against muscle injuries. It was also important to determine what the native MG53 levels were in human blood, as high concentrations (as seen in rats) would suggest it would be of limited therapeutic value. To this end, Western blot analysis revealed that the level of plasma MG53 protein is much lower in humans than in rats, validating the pursuit of rhMG53 as a potential treatment in humans.
There are limitations to this study. We administered rhMG53 5 min prior to ischemia and again 5 minutes prior to reperfusion. While this was effective in demonstrating the beneficial effect of rhMG53, pretreatments are impractical in the context of trauma. Clearly, future experiments supporting the efficacy of post-injury treatment with rhMG53 will be required. Pretreatment is not a limitation for reconstructive surgery involving muscle flaps, functional muscle transfers, or even composite tissue allograft transplant surgery. Here pretreatment is indeed an option. In these cases rhMG53 may be of great benefit in extending surgical time. In addition, a recent study by Cea et al reported that altered expression of connexin hemichannels can enhance the permeability of sarcolemma to small molecules, such as Evans blue31. More studies will be needed to explore if the expression levels for connexins in skeletal muscle are different between rats and mice, and if the treatment with rhMG53 can alter the expression of connexins. This may account for some of the differences observed with Evans blue uptake in mouse vs rat muscle following I-R injuries.
Patient safety is a serious concern when considering any drug for clinical use. As already discussed, MG53 is present in circulating blood at low levels in both mouse and human. Toxicity tests in mice have reported no observable toxic effects with long-term administration of MG53 as measured by a wide range of indicators 16. Another advantage for the application of rhMG53 as a therapeutic agent is that there are established protocols for purification and scale-up production of the rhMG53 protein. Purified rhMG53 protein is stable at room temperature as a lyophilized powder, allowing for re-suspension in saline solution and delivery via different injection routes (e.g. intravenous, intramuscular, or subcutaneous methods).
Overall, this study provides additional support for the use of MG53 for protection and repair of tissue injury. Additionally, the beneficial therapeutic effect of rhMG53 in ameliorating I-R in skeletal muscle provides support for its potential as a treatment for I-R related to muscle trauma, including extremity compartment syndrome. Additional studies will be required to determine the effectiveness of rhMG53 as a post-injury treatment.
Acknowledgements
This work was funded by the U.S. Army Medical Research and Medical Command (grant: F_025_2010_USAISR) awarded to TJW, National Institutes of Health (grants: AR061385 and HL069000) awarded to JM, and American Heart Association (grant: 12SDG12070174) awarded to HZ. All components of this study including the decision where to publish were of the sole discretion of the authors. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense (AR 360-5) or the United States Government. TJW and JLR are employees of the U.S. government and this work was prepared as part of their official duties.
Abbreviations
- EBD
Evans blue dye
- H&E
Hematoxylin and eosin
- i.m.
intramuscular injection
- i.v.
intravenous injection
- I-R
Ischemia-reperfusion
- MG53
Mitsugumin 53
- rhMG53
recombinant human Mitsugumin 53
- TA
Tibialis anterior muscle
- TRIM
Tripartite motif
Footnotes
Competing interests:
JM founded TRIM-edicine, Inc, a university spin-off biotechnology company that is developing rhMG53 as therapeutic reagent for regenerative medicine. TT is an employee for TRIM-edicine, Inc.
Authors’ contributions:
HZ, JH, JR, KP, TT, YZ, LL and CZ performed the experiments; HZ, JL, ZL, JM and TW conceived and designed the study; HZ, JR, KP, JM and TW analysed the research; HZ, JM and TW wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Owens BD, Kragh JF, Jr., Macaitis J, Svoboda SJ, Wenke JC. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. Journal of orthopaedic trauma. 2007;21(4):254–257. doi: 10.1097/BOT.0b013e31802f78fb. [DOI] [PubMed] [Google Scholar]
- 2.White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in iraq and afghanistan. Ann Surg. 2011;253(6):1184–1189. doi: 10.1097/SLA.0b013e31820752e3. [DOI] [PubMed] [Google Scholar]
- 3.Ritenour AE, Dorlac WC, Fang R, Woods T, Jenkins DH, Flaherty SF, Wade CE, Holcomb JB. Complications after fasciotomy revision and delayed compartment release in combat patients. The Journal of trauma. 2008;64(2 Suppl):S153–161. doi: 10.1097/TA.0b013e3181607750. discussion S161-152. [DOI] [PubMed] [Google Scholar]
- 4.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10(6):620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 5.Criswell TL, Corona BT, Ward CL, Miller M, Patel M, Wang Z, Christ GJ, Soker S. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol. 2012;180(2):787–797. doi: 10.1016/j.ajpath.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Heppenstall RB, Scott R, Sapega A, Park YS, Chance B. A comparative study of the tolerance of skeletal muscle to ischemia. Tourniquet application compared with acute compartment syndrome. J Bone Joint Surg Am. 1986;68(6):820–828. [PubMed] [Google Scholar]
- 7.Bolcal C, Yildirim V, Doganci S, Sargin M, Aydin A, Eken A, Ozal E, Kuralay E, Demirkilic U, Tatar H. Protective effects of antioxidant medications on limb ischemia reperfusion injury. J Surg Res. 2007;139(2):274–279. doi: 10.1016/j.jss.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Walters TJ, Mase VJ, Jr., Roe JL, Dubick MA, Christy RJ. Poloxamer-188 reduces muscular edema after tourniquet-induced ischemia-reperfusion injury in rats. The Journal of trauma. 2011;70(5):1192–1197. doi: 10.1097/TA.0b013e318217879a. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes KR, Fricke ST, Faden AI. Neuropathological differences between rats and mice after spinal cord injury. Journal of magnetic resonance imaging: JMRI. 2010;32(4):836–846. doi: 10.1002/jmri.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. The Journal of comparative neurology. 2003;462(2):223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 11.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. American journal of physiology Lung cellular and molecular physiology. 2004;286(2):L231–246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 12.McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicology and applied pharmacology. 2012;264(3):387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11(1):56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders AK, Turnbull L, Whitchurch CB, North KN, Cooper ST. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33(12):5085–5094. doi: 10.1523/JNEUROSCI.3560-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284(23):15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4(139):139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisleder N, Lin P, Zhao X, Orange M, Zhu H, Ma J. Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp. 2011;52 doi: 10.3791/2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corona BT, Garg K, Roe JL, Zhu H, Park KH, Ma J, Walters TJ. The effect of recombinant human MG53 protein on tourniquet-induced ischemia reperfusion injury in rat muscle. Muscle & nerve. 2014 doi: 10.1002/mus.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107(1):76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 20.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121(23):2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, Cao CM, Xiao RP. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91(1):108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. American journal of physiology Cell physiology. 2004;286(2):C230–238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleet DA, Moffett DB. Framing the problem: injuries and public health. Fam Community Health. 2009;32(2):88–97. doi: 10.1097/01.FCH.0000347985.67681.9d. [DOI] [PubMed] [Google Scholar]
- 24.Owens BD, Kragh JF, Jr., Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. The Journal of trauma. 2008;64(2):295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 25.Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119–124. doi: 10.1177/1531003511401422. [DOI] [PubMed] [Google Scholar]
- 26.Gifford SM, Eliason JL, Clouse WD, Spencer JR, Burkhardt GE, Propper BW, Dixon PS, Zarzabal LA, Gelfond JA, Rasmussen TE. Early versus delayed restoration of flow with temporary vascular shunt reduces circulating markers of injury in a porcine model. The Journal of trauma. 2009;67(2):259–265. doi: 10.1097/TA.0b013e3181a5e99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox CJ, Gillespie DL, Cox ED, Mehta SG, Kragh JF, Jr., Salinas J, Holcomb JB. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. The Journal of trauma. 2008;64(2 Suppl):S99–106. doi: 10.1097/TA.0b013e3181608c4a. discussion S106-107. [DOI] [PubMed] [Google Scholar]
- 28.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 2009;284(5):3314–3322. doi: 10.1074/jbc.M808866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Communicative & integrative biology. 2009;2(3):225–226. doi: 10.4161/cib.2.3.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R, Wang Z, Yao Y, Li Y, Whitson BA, Duann P, Li H, Zhou X, Zhu H, Takeshima H, Hunter JC, McLeod RL, Weisleder N, Zeng C, Ma J. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nature communications. 2014;5:4387. doi: 10.1038/ncomms5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C, Willecke K, Latorre R, Saez JC. De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):16229–16234. doi: 10.1073/pnas.1312331110. [DOI] [PMC free article] [PubMed] [Google Scholar]