Abstract
Approximately one quarter of all strokes in humans occur in white matter, and the progressive nature of white matter lesions often results in severe physical and mental disability. Unlike cortical grey matter stroke, the pathology of white matter stroke revolves around disrupted connectivity and injured axons and glial cells, rather than neuronal cell bodies. Consequently, the mechanisms behind ischemic damage to white matter elements, the regenerative responses of glial cells and their signaling pathways, all differ significantly from those in grey matter. Development of effective therapies for white matter stroke would require an enhanced understanding of the complex cellular and molecular interactions within the white matter, leading to the identification of new therapeutic targets. This review will address the unique properties of the axon-glia unit during white matter stroke, describe the challenging process of promoting effective white matter repair, and discuss recently-identified signaling pathways which may hold potential targets for repair in this disease.
Keywords: White Matter, Stroke, Ischemia, Repair, Oligodendrocytes, Myelin
1. Introduction
Small vessel infarcts affecting brain white matter are an important clinical problem, accounting for up to 25% of all strokes (Arboix and Marti-Vilalta, 2009; Roger et al., 2012; Schneider et al., 2004). This percentage may grow in upcoming years due to the increasing prevalence of risk factors associated with small vessel disease, such as type II diabetes and metabolic syndrome (Bokura et al., 2008; Del Bene et al., 2013; Gouw et al., 2008). Many promising neuroprotective therapies for stroke failed the transition from animal studies to clinical trials, and a major reason for these failures may be the almost exclusive focus of preclinical studies on the neuroprotection of cerebral gray matter, with little attention to white matter tracts (Gladstone et al., 2002). A probable contributing factor is the predominant use of rodents in pre-clinical studies, whose white matter comprises only ~14% of total brain volume. Since white matter makes up to 50% of the volume in human brains, it is likely that the data from rodent studies misrepresents the relevance of white matter in human brain pathology (Matute, 2011; Zhang and Sejnowski, 2000). Although ischemic injuries in gray and white matter share some common characteristics, there are unique properties of stroke in white matter that are derived from the white matter elements: the axons, the oligodendrocytes that enwrap them in myelin, and fibrous astrocytes which interact with the former two. These, alongside microglia, progenitor cells and vasculature, form an intricate environment and a delicate homeostasis that is highly vulnerable to ischemic damage (Hamner et al., 2011; Matute et al., 2001; Matute, 2011; Pantoni et al., 1996; Stirling and Stys, 2010). Development of effective therapeutic strategies and identification of new targets for the treatment of white matter stroke (WMS) would require an enhanced understanding of the complex cellular and molecular architecture of white matter components. This article will review key mechanisms underlying the white matter response to ischemic WMS with focus on the axon-glia functional unit during stroke recovery.
2. The unique structure and function of brain white matter
The white matter is comprised primarily of axons and glial cells, and is devoid of neuronal cell bodies or their dendrites. Bundles of axons are topographically organized in white matter so that axons originating from specific regions form projections which occupy distinct parts of the white matter (Filley, 2010; Schmahmann et al., 2008). These tracts of axons enable rapid communication between non-adjacent brain regions as well as between peripheral and central areas.
The majority of white matter axons are enwrapped by oligodendrocytes which form segments of myelin sheaths around the axons. Myelin segments facilitate fast saltatory propagation of action potentials, and segregate the axonal membrane into defined regions: the node of Ranvier, where clusters of Na+ channels “propel” the action potential along the axon (Huxley and Stampfli, 1949; Waxman and Swadlow, 1977), paranode, K+ channel-rich juxtaparanode, and internode (Rios et al., 2003; Susuki and Rasband, 2008).
The lack of neuronal cell bodies and dendrites means there are no “classical” synapses in the white matter, but recent work demonstrates the existence of “axo-myelinic” synapses which involve vesicular transmitter release from axons, acting on receptors on the inner surface of the myelin sheath (Stys, 2011). Neurotransmitters released from unmyelinated white matter axons can also act on surrounding glia (Alix and Domingues, 2011). These types of signals are at the base of a bidirectional neuron-glia communication involving the secretion of endosome-derived vesicles by oligodendrocytes and their subsequent internalization by neurons through endocytosis (Fruhbeis et al., 2013). Whether this occurs in white matter is still unknown. Oligodendrocytes are also suggested to contribute to long-term axonal integrity by delivering products of aerobic glycolysis which are rapidly metabolized in axons (Funfschilling et al., 2012).
Additional important players in white matter homeostatic maintenance are fibrous astrocytes. The long processes of these cells run along axons and connect to blood vessels (Oberheim et al., 2009). White matter astrocytes are also an important source of energy, supplying axons with lactate converted from deposits of glycogen (Brown et al., 2003; Ransom and Fern, 1997). Astrocytic endfeet on brain capillaries are an important part of the blood–brain barrier (Abbott, 2005; Alvarez et al., 2013), and they also participate in regulating local microcirculation (Attwell et al., 2010; Gordon et al., 2008). In addition, astrocytic processes form contact with myelinated axons at the nodes of Ranvier, where they participate in “siphoning” of K+ ions that accumulate following action potential generation (Kamasawa et al., 2005; Rash, 2010).
White matter microglia play an important role in neurodegeneration and inflammation. They are activated by cytokines, neurotransmitters and modulators, and can also synthesize and release many cytokines, chemokines, reactive oxygen radicals and neurotrophins which can be either injurious or beneficial to the surrounding axons and oligodendrocytes (Raivich and Banati, 2004).
The white matter components and the complex interactions among them create an optimal environment for fast transmission of signals along tracts of axons. However, the low blood flow and little collateral blood supply in deep white matter compared to gray matter make this intricate milieu highly susceptible to ischemic injuries (Iadecola et al., 2009; O’Sullivan et al., 2002), which disrupt white matter function with oftentimes devastating consequences.
3. White matter pathology in humans and WMS models
White matter “lacunar infarcts” in humans range in size up to 15 mm and often result in severe physical and mental disability including vascular dementia (Dufouil et al., 2009; Goldberg and Ransom, 2003), with an elevated mid-long-term risk of recurrence (Arboix and Marti-Vilalta, 2009; Norrving, 2008). Smaller white matter “micro-infarcts” with mean diameters between 0.2 and 1 mm are thought to have a similar ischemic origin and are even more common, appearing in a third of cognitively normal elderly patients (Smith et al., 2012). Infarcts in white matter are characterized by an irregular lesion bordered by reactive astrocytes and microglia/macrophages in older lesions, while acute lesions feature a necrotic encephalomalacic core (Bailey et al., 2012). The axons adjacent to white matter stroke show altered nodal structure and molecular disorganization that indicates a peri-infarct region of partial damage that radiates out from the infarct (Hinman et al., 2015)
In animals, transient focal ischemia achieved via injection of a vasoconstricting drug into white matter is thus far the closest model for human white matter lacunar infarcts. These injections produce focal white matter injury, with wide-scale axonal and oligodendrocyte damage (Hughes et al., 2003; Sozmen et al., 2009). In the striatum, these models were also shown to produce lesions with a peri-infarct penumbra region displaying axon pathology (Frost et al., 2006; Lecrux et al., 2008). In subcortical white matter, similar focal lesions evolve over time and features oligodendrocyte and axonal loss at the core, and a penumbra region displaying demyelination, inflammation and axonal degeneration which are in part age-dependent (Rosenzweig and Carmichael, 2013; Sozmen et al., 2009).
The sensitivity of different white matter elements to ischemia has been demonstrated in numerous models of cerebral artery occlusion. Swelling of white matter glia has been observed as early as 30 minutes after middle cerebral artery occlusion, followed by loss of oligodendrocytes (Irving et al., 1997; Pantoni et al., 1996), and axonal degeneration within 120 minutes of ischemia (Dewar and Dawson, 1997; Valeriani et al., 2000). Short transient ischemia was sufficient to cause widespread striatal white matter injury with extensive glial and axon pathology (Kubo et al., 2009).
The progressive damage to oligodendrocytes, myelin and axons observed in white matter stroke models and in human white matter stroke may be attributed to glutamate-mediated excitotoxicity and downstream molecular pathways. Glutamate, released from astrocytes during ischemia due to reversal of Na+-dependent glutamate transporters, acts on ionotropic AMPA/kainate and NMDA receptors (Baltan et al., 2008; Micu et al., 2006; Tekkok et al., 2007). Prolonged activation of glutamate receptors in oligodendrocytes causes influx of Ca2+ and its accumulation within mitochondria, which prompts the production of radical oxygen species and activation of caspase-mediated cell death (Matute et al., 2006; Sanchez-Gomez et al., 2003). Oligodendrocytes are particularly sensitive to oxidative stress due to their low supplies of the cellular antioxidant glutathione, which is further depleted by excessive glutamate (Back et al., 1998; Thorburne and Juurlink, 1996).
A second source for the rise in cytosolic Ca2+ in oligodendrocytes is activation of metabotropic P2Y receptors and ionotropic P2X7 receptors by adenosine triphosphate (ATP) (James and Butt, 2001; Matute et al., 2007). ATP released from oligodendrocytes through pannexin hemichannels was shown to activate P2X7 receptors that cause oligodendrocyte death, myelin damage and axon dysfunction (Domercq et al., 2010). High levels of cytosolic Ca2+ were also shown to activate Ca2+-dependent enzymes such as calpains and phospholipases, resulting in white matter degradation (Stys, 2004; Vosler et al., 2008). In addition to oligodendrocytes, P2X7 receptors can be found in white matter microglia, and their activation mediates inflammation that could further contribute to ischemic damage (Xiang and Burnstock, 2005).
The effects of ischemia on oligodendrocytes are directly relevant to axonal function. Documented changes in the ultrastructure of myelinated axons following ischemia and Ca2+ activation include splitting, retraction of myelin at paranodes and separation of the myelin lamellae, suggestive of loss of axoglial contact (Fu et al., 2009; Mclver et al., 2010). Other changes documented in mice as early as 3 days after mild cerebral hypoperfusion include a progressive reduction of paranodal neurofascin signal and a loss of septate-like junctions, increase in nodal length and changes in the spatial distribution of myelin-associated glycoproteins (Reimer et al., 2011).
Such changes in the axon-glia structure disrupt the spatial segregation of Na+ and K+ channels in the node and juxtaparanode areas. This segregation is critical to proper axonal conduction of action potentials and its perturbation has been suggested to contribute to many pathological conditions that involve failure of axonal function (Arroyo et al., 2002; Hinman et al., 2006; Rasband, 2011; Salzer et al., 2008). Studies in models of multiple sclerosis demonstrate that disrupted or less efficient axonal conductance may manifest as severely as a complete loss of axonal function, due in most to the spatial and temporal summation requirements that are the hallmark of neuronal signaling; for a target neuron to reach threshold, several action potentials (from one or more axons) must arrive to its synaptic terminals within a narrow time window. Thus, even a small delay in conduction may equal a complete arrest of the signal downstream (Waxman et al., 1995). Many suggested repair strategies for white matter pathology, therefore, focus on restoring axonal function through oligodendrocytes and myelin repair.
4. Activity-dependent remyelination and oligodendrocyte response to white matter ischemia
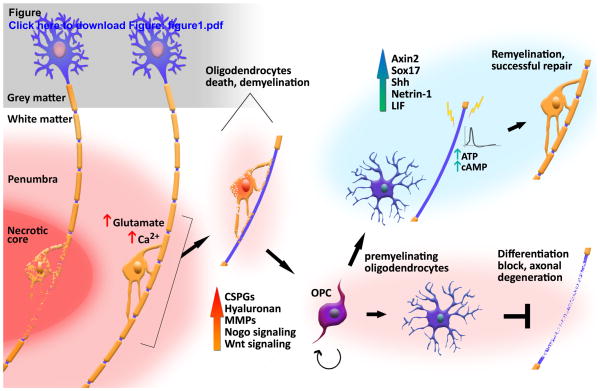
While myelination is, at its core, a developmental process, the adult mammalian central nervous system (CNS) retains at least some capability of remyelination and regeneration following injury (Duncan et al., 2009; Franklin and Ffrench-Constant, 2008). In the case of white matter ischemia, demyelination and axonal degradation occur quickly in the ischemic core, yet gradual restoration of oligodendrocytes and remyelination have been observed in the peri-infarct area (Gregersen et al., 2001; Tanaka et al., 2003). An important feature of this process is oligodendrogenesis, which occurs following ischemia. This proliferative response of oligodendrocyte progenitor cells (OPC), peaks at 5–7 days after stroke induction and declines 14–28 days later (Iwai et al., 2010; Sozmen et al., 2009). However, this robust regenerative response appears to encounter a roadblock of OPC differentiation failure, which ultimately limits recovery after demyelination (Syed et al., 2008). A deeper understanding of this regenerative failure is crucial, since remyelination is key in preventing axonal degeneration (Irvine and Blakemore, 2008). Figure 1 offers a schematic overview of potentially successful repair vs. regenerative failure.
Figure 1. Damage and repair following white matter stroke.
Complete loss of axons and glia in the necrotic ischemic core (left). Glutamate-mediated excitotoxicity and rising Ca2+ levels promote oligodendrocytes death and demyelination in the peri-infarct area. A regenerative response initiates with OPC proliferation, but inhibitory signals lead to a differentiation and maturation block. Vulnerable demyelinated axons may suffer secondary degeneration (bottom right). Alternatively, neuronal activity, trophic factors and positive regulators may contribute to oligodendrocytes maturation and successful remyelination (top right).
It is hypothesized that during regeneration there is recapitulation of many developmental processes. In the case of myelination, neuronal activity plays a crucial role during development. Suppressing neural activity during development reduces OPC proliferation and disrupts myelination (Barres and Raff, 1993; Demerens et al., 1996), while inducing neuronal activity via electrical stimulation promotes proliferation, survival and differentiation of oligodendrocytes, as well as axon myelination (Gary et al., 2012; Gibson et al., 2014; Ishibashi et al., 2006). In injured spinal cord, electrical activity was similarly demonstrated to promote OPC differentiation and remyelination, as well as improve locomotor recovery (Zhang et al., 2014).
The underlying mechanisms for this neuron-glia regulation are complex, and several known pathways and genes are discussed in the next section. A summarized view of these and other pathways is offered in Table 1. Some direct signals that have been identified include neurotransmitters such as glutamate and acetylcholine which are released from depolarized neurons and act on oligodendrocytes and OPC to modulate proliferation, migration, and differentiation (De Angelis et al., 2012; Gudz et al., 2006; Wake et al., 2011; Zonouzi et al., 2011).
Table 1.
Regulators of white matter damage and OPC regeneration, and their documented effects.
| Pathway/protein | Mechanism/activity | Outcome/effect | Reference |
|---|---|---|---|
| Activity-dependent signals | |||
| ATP | A1 adenosine receptor on oligodendrocytes | ↑ OPC differentiation, maturation and migration |
Othman et al., 2003 Stevens et al., 2002 |
| cAMP | MEK-ERK pathway (?) | ↑ T remyelination ↑ OPC maturation |
Malone et al., 2013 Sun et al., 2012 |
|
| |||
| ECM-related proteins | |||
| CSPGs | Inhibitory GAG chains | ↓ OPC differentiation, maturation and migration |
Lau et al., 2012 Sherman and Back, 2008 |
| hyaluronan | Digested by Hyaluronidase CD44, TLR2 signaling | ↓ OPC differentiation, maturation ↑ progressive demyelination |
Back et al., 2005 Sloane et al., 2010 |
| MMPs | Degradation of MBP, MAG and ECM proteins | ↑ T demyelination | Walker and Rosenberg, 2010 |
|
| |||
| Wnt signaling | |||
| Wnt/β-catenin | Nuclear β-catenin/tcf/LEF complex | ↓ OPC differentiation, maturation |
Fancy et al., 2009 Feigenson et al., 2009 |
| Axin2 | β-catenin degradation | ↑ remyelination | Fancy et al., 2011 |
| APC | β-catenin regulation cytoskeletal regulation | ↑ OPC proliferation, differentiation | Lang et al., 2013 |
| HIF | Autocrine Wnt7a/7b signaling | ↓ developmental myelination ↑ White matter angiogenesis |
Yuen et al., 2014 |
| Sox17 | β-catenin degradation | ↑ oligodendrocyte differentiation | Ming et al., 2013 |
|
| |||
| Nogo related proteins | |||
| NogoA/NgR | RhoA(?), Rho kinase ROCK (?) | ↓ remyelination, OPC recruitment |
Pourabdolhossein et al., 2014 Niederost et al., 2002 |
| LINGO-1 | self-association, homophilic intercellular interactions | ↓ OPC differentiation, maturation ↓ remyelination |
Mi et al., 2009 Jepson et al., 2012 |
|
| |||
| Additional regulators | |||
| Shh | Induces Olig1 and Olig2 expression | ↑ oligodendrogenesis ↑ OPC maturation, differentiation |
Harsan et al., 2008 Ferent et al., 2013 |
| Netrin-1 | Signaling through Dcc, RhoA inhibition | ↑ OPC proliferation, differentiation ↑ remyelination |
Rajasekharan et al., 2009 He et al., 2013 |
| LIF | Akt signaling, antioxidation | ↓ white matter injury, infarct volume | Rowe et al., 2014 |
Activity-dependent release of ATP enhances OPC differentiation and myelination (Ishibashi et al., 2006; Stevens et al., 2002). Oligodendrocytes express each of the different adenosine receptor subtypes (Othman et al., 2003), and treatment of OPC in culture with adenosine promotes their maturation and migration through a A1 adenosine receptor-mediated pathway (Othman et al., 2003; Stevens et al., 2002).
Cyclic adenosine monophosphate (cAMP) may be another activity-dependent regulator of myelination. Electrical stimulation increases neuronal cAMP in vivo (Udina et al., 2008), and depolarized neurons affect myelin protein processing in oligodendrocytes in a cAMP-dependent manner (Trajkovic et al., 2006). In addition, elevating cAMP levels enhanced remyelination in a cuprizone demyelination model (Sun et al., 2012). Recently, cAMP was demonstrated to act as an activity-dependent pathway in neurons to promote myelination (Malone et al., 2013).
5. Molecular pathways involved in ischemic white matter damage and repair
Ischemia is associated with alterations in multiple biological processes and molecular pathways, and many proteins greatly affect repair processes in white matter. This section will address some of the recently identified molecular systems and their potential function in white matter regeneration. At present, the majority of findings come from commonly-used demyelination models rather than models of WMS, but it is likely that signals which play a role in these models are, to some extent, relevant to WMS pathology.
5.1 Extracellular Matrix related proteins
The extracellular matrix affects proliferation, survival, migration and differentiation of oligodendrocytes, as well as process extension and myelination (Sherman and Back, 2008). CNS damage leads to accumulation of several extracellular matrix components, particularly Chondroitin Sulfate Proteoglycans (CSPGs) and hyaluronan, in and around the injured area (Back et al., 2005; Galtrey and Fawcett, 2007). In white matter, high levels of CSPGs like aggrecan, neurocan and versican, as well as high molecular weight hyaluronan and one of its receptors, CD44, are found in active multiple sclerosis demyelinating lesions and neonatal white matter injury (Back et al., 2005; Buser et al., 2012; Sobel and Ahmed, 2001). In models of traumatic spinal cord injury and lysolecithin-induced demyelination, accumulation of CSPGs inhibits OPC migration and their maturation into myelinating oligodendrocytes, while clearance of CSPGs was correlated with remyelination (Karimi-Abdolrezaee et al., 2012; Lau et al., 2012; Siebert et al., 2011).
High molecular weight hyaluronan blocks OPC differentiation and maturation both in vitro and in vivo (Back et al., 2005; Dean et al., 2011; Marret et al., 1994; Sloane et al., 2010). It is digested to bioactive forms by the hyaluronidase PH20 which is highly expressed by OPC and reactive astrocytes in demyelinated lesions. Overexpression of PH20 inhibits oligodendrocyte differentiation in vitro and hyaluronan fragments generated by PH20 block remyelination in vivo (Preston et al., 2013). Similarly, overexpression of CD44 in myelinating oligodendrocytes induces progressive demyelination (Tuohy et al., 2004). In addition to CD44, hyaluronan inhibits OPC differentiation by acting through Toll-like receptor 2 (Sloane et al., 2010).
Dysregulation of matrix metalloproteinases (MMPs), a family of zinc endopeptidases which degrade extracellular matrix proteins, was also linked to damage and repair after stroke and other neurological diseases (Alvarez-Sabin et al., 2004; Anthony et al., 1997; Horstmann et al., 2003). MMPs, and especially MMP-2 and MMP-9, are known to break down myelin basic protein and myelin-associated glycoprotein (MAG), two major components of the myelin sheath (Chandler et al., 1995; Gijbels et al., 1993). Transient global ischemia increases activity of MMP-2 enzyme in reactive astrocytes in corpus callosum, with significant suppression of delayed myelin degradation apparent after treatment with the MMP inhibitor BB-94 (Walker and Rosenberg, 2010).
5.2 Wnt signaling
The Wnt/β-catenin pathway is involved in many developmental processes, one of which is oligodendrogenesis (Ortega et al., 2013). Canonical Wnt signaling through the intranuclear mediator Tcf, which is upregulated in OPC during white matter pathology, has been linked to impaired myelin repair after injury (Fancy et al., 2009). The binding of Wnt ligands to OPC promotes the stabilization of a β-catenin/Tcf/LEF complex and activation of downstream genes which, during instances of high activity, inhibit the differentiation and maturation of OPC into myelinating oligodendrocytes, ultimately leading to impaired myelination (Fancy et al., 2009; Fancy et al., 2014; Feigenson et al., 2009; Ye et al., 2009).
In spinal cord, Wnt and bone morphogenic protein (BMP) signaling pathways have overlapping temporal activity and a similar effect of inhibiting oligodendrocytes differentiation (Feigenson et al., 2011). Blocking BMP signaling prevents Wnt-mediated inhibition of oligodendrocyte differentiation, but not vice versa, suggesting that BMP signals act downstream from Wnt (Feigenson et al., 2011). BMP signaling is active in oligodendroglia and astrocytes within the demyelinated corpus callosum, and infusion of the BMP antagonist Noggin into the brains of mice during demyelination promoted mature oligodendrocyte regeneration and remyelination (Sabo et al., 2011).
Axin2 is a transcriptional target of active Wnt signaling that also serves to autoregulate and inhibit Wnt signaling by promoting β-catenin degradation (Jho et al., 2002; Lustig et al., 2002). Axin2 expression is upregulated in OPC in neonatal white matter injury and active multiple sclerosis lesions, and its deletion in mice delays OPC maturation and impairs remyelination following spinal cord demyelination injury (Fancy et al., 2011). Administration of an Axin2 stabilizer was found to be sufficient to promote myelination in cerebellar slice cultures after acute hypoxia and in demyelinated spinal cord (Fancy et al., 2011).
Adenomatous Polyposis Coli (APC) is another regulator of Wnt/β-catenin signaling (Miller et al., 2009; Nathke, 2006). APC is transiently expressed in oligodendrocytes during development and demyelination- induced regeneration. It was found to enhance proliferation and differentiation of OPCs in a cell- autonomous manner (Lang et al., 2013). Its deletion in oligodendroglial lineage cells revealed that APC regulates oligodendrocyte differentiation through β-catenin-independent, as well as β-catenin-dependent, mechanisms, with the β-catenin-independent mechanism involving cytoskeletal regulation (Lang et al., 2013).
Recent work has demonstrated a role for Hypoxia-inducible factors (HIFs) in Wnt7a/7b-mediated OPC regulation (Yuen et al., 2014). These factors act as transcriptional mediators of the cellular response to hypoxia (Majmundar et al., 2010; Semenza and Prabhakar, 2012). OPC HIF1/2α activity, triggered by hypoxia, inhibits myelination via autocrine Wnt7a/7b signaling, which also has a novel paracrine role in promoting Wnt-dependent vessel growth into developing postnatal white matter tracts. Loss of OPC-encoded HIF1/2α function inhibits angiogenesis in developing white matter, resulting in catastrophic loss of corpus callosum axons (Yuen et al., 2014). This possible interaction between OPC differentiation and angiogenesis is a promising research link in stroke, given the natural occurrence of angiogenesis in stroke tissue reorganization. It is possible that the HIF-Wnt7a/7b pathway is activated by decreased oxygen levels that are associated with ischemic white matter injury, and that it plays a role in modulating OPC and blood vessels in infarcted areas, but this has yet to be studied.
The SRY-box 17 (Sox17) transcription factor is a regulator of oligodendrocyte differentiation (Sohn et al., 2006), which was found to affect Wnt signaling at multiple levels, including Sox17-mediated proteasomal degradation of β-catenin (Chen et al., 2013; Chew et al., 2011). Enhanced Sox17 expression in oligodendrocytes was detected in active remyelinating lesions (Moll et al., 2013), and transgenic overexpression of Sox17 was shown to promotes oligodendrocyte differentiation and attenuate lysolecithin-induced demyelination (Ming et al., 2013).
5.3 Nogo related proteins
Myelin-associated inhibitory factors such as NogoA and MAG are among the most well-known factors that inhibit regeneration in the CNS (McKerracher et al., 1994; Schwab, 2010). These factors bind to the Nogo receptor 1 and 2 (NgR1, NgR2) which are expressed by neurons and glial cells (Huang et al., 2012; Hunt et al., 2002). NgR1 is well known to inhibit regeneration processes in multiple pathological conditions, but most studies have focused on its effects on axonal sprouting and regeneration (Harvey et al., 2009; Wahl et al., 2014; Yu et al., 2008). Much less is known about the effect of myelin inhibitory factors on remyelination and oligodendrocyte regeneration. This information is important in light of growing evidence showing that blocking NgR and its ligands improves the outcomes in demyelination models (Karnezis et al., 2004; Petratos et al., 2012; Yang et al., 2010). Recent reports have indicated that NogoA plays a direct role in regulating myelination in vitro and in an in vivo model of focal demyelination (Chong et al., 2012). Furthermore, blocking NgR1 significantly enhanced the remyelination process, recruitment of proliferating OPC to the lesion site, and functional recovery after demyelination in the optic nerve (Pourabdolhossein et al., 2014). The mechanism for NgR1-mediated inhibition of neurite outgrowth involves activation of RhoA and its downstream effector Rho kinase ROCK (Niederost et al., 2002), however, whether this mechanism also plays a role in the regulation of OPC and remyelination is still unknown.
LINGO-1 is a single transmembrane protein specifically expressed in CNS neurons and oligodendrocytes. It is an essential signaling co-receptor within the NgR1 complex, and has no known direct ligands (Mi et al., 2008). Reduced function of LINGO-1 in OPC promotes differentiation and maturation in these cells, while overexpression of LINGO-1 inhibited these processes. Concordantly, increased myelination is present in LINGO-1 knockout animals (Lee et al., 2007; Mi et al., 2005; Zhao et al., 2007). An antagonist of LINGO-1 promoted remyelination and OPC differentiation in vivo after experimental demyelination (Mi et al., 2009). The mechanism for LINGO-1-mediated negative regulation of oligodendrocytes differentiation appears to involve self-association of the receptor in trans, as well as homophilic intercellular interactions (Jepson et al., 2012).
5.4 Positive regulators of repair and trophic factors
Several new positive regulators of white matter repair have emerged in recent years. The morphogen Sonic Hedgehog (Shh) controls the generation of oligodendrocytes during development and regulates their production in the adult white matter by inducing expression of the transcription factors Olig1 and Olig2 (Traiffort et al., 2010). Administration of Shh increases the number of OPC and premyelinating oligodendrocytes in the cerebral cortex and corpus callosum (Loulier et al., 2006), and an increase of Shh expression induces oligodendrogenesis and promotes recovery after chronic demyelination (Harsan et al., 2008). In a model of focal lysolecithin demyelination in mouse corpus callosum, Shh transcripts were upregulated in oligodendrocytes within the lesion but not in normal white matter, suggesting a broad reactivation of the Shh pathway. Adenovirus-mediated transfer of Shh into the lesioned brain attenuated the lesion extent and increased OPC maturation and differentiation, while blocking of Shh decreased OPC proliferation and differentiation, and prevented repair (Ferent et al., 2013).
Netrin-1 plays a role in axon guidance during development and contributes to white matter formation by influencing OPC proliferation, differentiation, and migration (Bradford et al., 2009; Tsai et al., 2006). The mechanism underlying the effect of Netrin-1 on OPC and oligodendrocytes involves signaling through the receptor Dcc, expressed by both cell types, and downstream inhibition of RhoA (Rajasekharan et al., 2009). Under pathological conditions, Netrin-1 inhibits inflammation and apoptosis, as well as promotes repair after ischemic stroke by increasing angiogenesis (Lu et al., 2012; Rosenberger et al., 2009; Sun et al., 2011). Overexpression of Netrin-1 increases proliferation and differentiation of OPC into mature oligodendrocytes, and promotes white matter remyelination and neurobehavioral outcomes after focal cerebral ischemia in mice (He et al., 2013).
Leukemia inhibitory factor (LIF) is a cytokine that exerts pleiotropic effects on cell survival (Metcalf, 2003). Intracerebral injections of LIF attenuate injury when administered after focal ischemia (Suzuki et al., 2005). LIF also promotes myelination by oligoderndrocytes when released by astrocytes in response to activity-dependent rise in ATP (Ishibashi et al., 2006). More recently, LIF effectively reduced infarct volume, reduced white matter injury and improved functional outcomes when administered to rats following permanent middle cerebral artery occlusion. The underlying mechanism for LIF-mediated white matter protection appears to involve activation of the Akt signaling pathway and antioxidation via inhibition of lactate dehydrogenase release from oligodendrocytes, reduction of superoxide dismutase activity and induction of peroxiredoxin 4 (Rowe et al., 2014).
6. Conclusion
Although white matter damage is an important part of many neurological disorders, and in particular white matter stroke, mechanisms of white matter damage and repair are relatively understudied compared to those in gray matter. The key to improving recovery, restoring function, and reducing long-term disability after white matter stroke lies in a better understanding of white matter biology and the changes that occur in different elements of the white matter following an ischemic insult. Signals transmitted between injured axons and glia, ionic imbalance, and downstream regulation of several genes and signaling pathways ultimately contribute to the complex pathology of white matter stroke. Animal studies have attempted to promote white matter repair and recovery in different models of white matter pathology, by identifying and manipulating specific factors, genes or signals involved in damage and repair processes. Considerable attention has been directed to preserving and restoring axonal integrity and conductance through remyelination, by targeting OPC and oligodendrocytes and promoting their differentiation. While some successes have been recorded, it is likely that an effective therapy for white matter stroke in humans would require a more comprehensive approach involving multiple targets.
Highlights.
Unique properties of stroke in white matter are derived from the main white matter components: axons and glia
A limited regenerative response takes place in the peri-infarct area following white matter stroke
Neuronal activity, trophic factors and positive regulators contribute to repair and remyelination
Acknowledgments
Supported by National Institutes of Health (NIH) RO1 NS071481 and American Heart Association UCLA Bugher Center 14BFSC17760005.
Abbreviations
- WMS
White matter stroke
- OPC
Oligodendrocyte progenitor cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix JJ, Domingues AM. White matter synapses: form, function, and dysfunction. Neurology. 2011;76:397–404. doi: 10.1212/WNL.0b013e3182088273. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–22. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–58. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–15. [PubMed] [Google Scholar]
- Arboix A, Marti-Vilalta JL. Lacunar stroke. Expert Rev Neurother. 2009;9:179–96. doi: 10.1586/14737175.9.2.179. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Grinspan J, Lambert S, Levinson SR, Brophy PJ, Peles E, Scherer SS. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci. 2002;22:1726–37. doi: 10.1523/JNEUROSCI.22-05-01726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Bailey EL, Smith C, Sudlow CL, Wardlaw JM. Pathology of lacunar ischemic stroke in humans--a systematic review. Brain Pathol. 2012;22:583–91. doi: 10.1111/j.1750-3639.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28:1479–89. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–60. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–9. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- Bradford D, Cole SJ, Cooper HM. Netrin-1: diversity in development. Int J Biochem Cell Biol. 2009;41:487–93. doi: 10.1016/j.biocel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. J Physiol. 2003;549:501–12. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–6. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- Chen HL, Chew LJ, Packer RJ, Gallo V. Modulation of the Wnt/beta-catenin pathway in human oligodendroglioma cells by Sox17 regulates proliferation and differentiation. Cancer Lett. 2013;335:361–71. doi: 10.1016/j.canlet.2013.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew LJ, Shen W, Ming X, Senatorov VV, Jr, Chen HL, Cheng Y, Hong E, Knoblach S, Gallo V. SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. J Neurosci. 2011;31:13921–35. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA. 2012;109:1299–304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol. 2012;72:713–28. doi: 10.1002/dneu.20976. [DOI] [PubMed] [Google Scholar]
- Dean JM, Riddle A, Maire J, Hansen KD, Preston M, Barnes AP, Sherman LS, Back SA. An organotypic slice culture model of chronic white matter injury with maturation arrest of oligodendrocyte progenitors. Mol Neurodegener. 2011;6:46. doi: 10.1186/1750-1326-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene A, Makin SD, Doubal FN, Inzitari D, Wardlaw JM. Variation in risk factors for recent small subcortical infarcts with infarct size, shape, and location. Stroke. 2013;44:3000–6. doi: 10.1161/STROKEAHA.113.002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar D, Dawson DA. Changes of cytoskeletal protein immunostaining in myelinated fibre tracts after focal cerebral ischaemia in the rat. Acta Neuropathol. 1997;93:71–7. doi: 10.1007/s004010050584. [DOI] [PubMed] [Google Scholar]
- Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–40. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Godin O, Chalmers J, Coskun O, MacMahon S, Tzourio-Mazoyer N, Bousser MG, Anderson C, Mazoyer B, Tzourio C, Investigators PMS. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke. 2009;40:2219–21. doi: 10.1161/STROKEAHA.108.540633. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci USA. 2009;106:6832–6. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–85. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–16. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Baranzini SE, Silbereis JC, Shiow LR, Yuen TJ, Huang EJ, Lomvardas S, Rowitch DH. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci. 2014;17:506–12. doi: 10.1038/nn.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–65. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw IE, Grinspan JB. Canonical Wnt signalling requires the BMP pathway to inhibit oligodendrocyte maturation. ASN Neuro. 2011;3:e00061. doi: 10.1042/AN20110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent J, Zimmer C, Durbec P, Ruat M, Traiffort E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J Neurosci. 2013;33:1759–72. doi: 10.1523/JNEUROSCI.3334-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM. White matter: organization and functional relevance. Neuropsychol Rev. 2010;20:158–73. doi: 10.1007/s11065-010-9127-9. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res. 2006;169:206–11. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Sun W, Shi Y, Shi R, Cheng JX. Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One. 2009;4:e6705. doi: 10.1371/journal.pone.0006705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gary DS, Malone M, Capestany P, Houdayer T, McDonald JW. Electrical stimulation promotes the survival of oligodendrocytes in mixed cortical cultures. J Neurosci Res. 2012;90:72–83. doi: 10.1002/jnr.22717. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36:432–40. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–36. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Ransom BR. New light on white matter. Stroke. 2003;34:330–2. doi: 10.1161/01.str.0000054048.22626.b9. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, van der Flier WM, Pantoni L, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Fazekas F, Scheltens P, Barkhof F. On the etiology of incident brain lacunes: longitudinal observations from the LADIS study. Stroke. 2008;39:3083–5. doi: 10.1161/STROKEAHA.108.521807. [DOI] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–92. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–66. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MA, Moller T, Ransom BR. Anaerobic function of CNS white matter declines with age. J Cereb Blood Flow Metab. 2011;31:996–1002. doi: 10.1038/jcbfm.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsan LA, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, Guignard B, Parizel N, Grucker D, Boehm N, Miller RH, Ghandour MS. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J Neurosci. 2008;28:14189–201. doi: 10.1523/JNEUROSCI.4453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PA, Lee DH, Qian F, Weinreb PH, Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–95. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li Y, Lu H, Zhang Z, Wang Y, Yang GY. Netrin-1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2013;33:1921–7. doi: 10.1038/jcbfm.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of Ranvier. J Comp Neurol. 2006;495:351–62. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman JD, Lee MD, Tung S, Vinters HV, Carmichael ST. Molecular disorganization of axons adjacent to human lacunar infarcts. Brain. 2015;138:736–45. doi: 10.1093/brain/awu398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003;34:2165–70. doi: 10.1161/01.STR.0000088062.86084.F2. [DOI] [PubMed] [Google Scholar]
- Huang JY, Wang YX, Gu WL, Fu SL, Li Y, Huang LD, Zhao Z, Hang Q, Zhu HQ, Lu PH. Expression and function of myelin-associated proteins and their common receptor NgR on oligodendrocyte progenitor cells. Brain Res. 2012;1437:1–15. doi: 10.1016/j.brainres.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Anthony DC, Ruddin M, Botham MS, Rankine EL, Sablone M, Baumann D, Mir AK, Perry VH. Focal lesions in the rat central nervous system induced by endothelin-1. J Neuropathol Exp Neurol. 2003;62:1276–86. doi: 10.1093/jnen/62.12.1276. [DOI] [PubMed] [Google Scholar]
- Hunt D, Mason MR, Campbell G, Coffin R, Anderson PN. Nogo receptor mRNA expression in intact and regenerating CNS neurons. Mol Cell Neurosci. 2002;20:537–52. doi: 10.1006/mcne.2002.1153. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Stampfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949;108:315–39. [PubMed] [Google Scholar]
- Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–4. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–77. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Irving EA, Yatsushiro K, McCulloch J, Dewar D. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: involvement of free radicals. J Cereb Blood Flow Metab. 1997;17:612–22. doi: 10.1097/00004647-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–7. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G, Butt AM. P2X and P2Y purinoreceptors mediate ATP-evoked calcium signalling in optic nerve glia in situ. Cell Calcium. 2001;30:251–9. doi: 10.1054/ceca.2001.0232. [DOI] [PubMed] [Google Scholar]
- Jepson S, Vought B, Gross CH, Gan L, Austen D, Frantz JD, Zwahlen J, Lowe D, Markland W, Krauss R. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J Biol Chem. 2012;287:22184–95. doi: 10.1074/jbc.M112.366179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Schut D, Wang J, Fehlings MG. Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS One. 2012;7:e37589. doi: 10.1371/journal.pone.0037589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T, Mandemakers W, McQualter JL, Zheng B, Ho PP, Jordan KA, Murray BM, Barres B, Tessier-Lavigne M, Bernard CC. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–44. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Kubo K, Nakao S, Jomura S, Sakamoto S, Miyamoto E, Xu Y, Tomimoto H, Inada T, Shingu K. Edaravone, a free radical scavenger, mitigates both gray and white matter damages after global cerebral ischemia in rats. Brain Res. 2009;1279:139–46. doi: 10.1016/j.brainres.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Maeda Y, Bannerman P, Xu J, Horiuchi M, Pleasure D, Guo F. Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci. 2013;33:3113–30. doi: 10.1523/JNEUROSCI.3467-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Doring A, Sloka S, Stirling DP, Rivest S, Yong VW. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–32. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- Lecrux C, McCabe C, Weir CJ, Gallagher L, Mullin J, Touzani O, Muir KW, Lees KR, Macrae IM. Effects of magnesium treatment in a model of internal capsule lesion in spontaneously hypertensive rats. Stroke. 2008;39:448–54. doi: 10.1161/STROKEAHA.107.492934. [DOI] [PubMed] [Google Scholar]
- Lee X, Yang Z, Shao Z, Rosenberg SS, Levesque M, Pepinsky RB, Qiu M, Miller RH, Chan JR, Mi S. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J Neurosci. 2007;27:220–5. doi: 10.1523/JNEUROSCI.4175-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulier K, Ruat M, Traiffort E. Increase of proliferating oligodendroglial progenitors in the adult mouse brain upon Sonic hedgehog delivery in the lateral ventricle. J Neurochem. 2006;98:530–42. doi: 10.1111/j.1471-4159.2006.03896.x. [DOI] [PubMed] [Google Scholar]
- Lu H, Wang Y, He X, Yuan F, Lin X, Xie B, Tang G, Huang J, Tang Y, Jin K, Chen S, Yang GY. Netrin-1 hyperexpression in mouse brain promotes angiogenesis and long-term neurological recovery after transient focal ischemia. Stroke. 2012;43:838–43. doi: 10.1161/STROKEAHA.111.635235. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M, Gary D, Yang IH, Miglioretti A, Houdayer T, Thakor N, McDonald J. Neuronal activity promotes myelination via a cAMP pathway. Glia. 2013;61:843–54. doi: 10.1002/glia.22476. [DOI] [PubMed] [Google Scholar]
- Marret S, Delpech B, Delpech A, Asou H, Girard N, Courel MN, Chauzy C, Maingonnat C, Fessard C. Expression and effects of hyaluronan and of the hyaluronan-binding protein hyaluronectin in newborn rat brain glial cell cultures. J Neurochem. 1994;62:1285–95. doi: 10.1046/j.1471-4159.1994.62041285.x. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–30. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–24. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, Arranz AM, Ravid R, Rodriguez-Antiguedad A, Sanchez-Gomez M, Domercq M. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:9525–33. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219:53–64. doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, Goldberg MP. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience. 2010;169:1364–75. doi: 10.1016/j.neuroscience.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–11. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The unsolved enigmas of leukemia inhibitory factor. Stem Cells. 2003;21:5–14. doi: 10.1634/stemcells.21-1-5. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–51. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40:1971–8. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chedotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–15. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Miller BW, Lau G, Grouios C, Mollica E, Barrios-Rodiles M, Liu Y, Datti A, Morris Q, Wrana JL, Attisano L. Application of an integrated physical and functional screening approach to identify inhibitors of the Wnt pathway. Mol Syst Biol. 2009;5:315. doi: 10.1038/msb.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Chew LJ, Gallo V. Transgenic overexpression of Sox17 promotes oligodendrocyte development and attenuates demyelination. J Neurosci. 2013;33:12528–42. doi: 10.1523/JNEUROSCI.0536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll NM, Hong E, Fauveau M, Naruse M, Kerninon C, Tepavcevic V, Klopstein A, Seilhean D, Chew LJ, Gallo V, Nait Oumesmar B. SOX17 is expressed in regenerating oligodendrocytes in experimental models of demyelination and in multiple sclerosis. Glia. 2013;61:1659–72. doi: 10.1002/glia.22547. [DOI] [PubMed] [Google Scholar]
- Nathke I. Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC. Nat Rev Cancer. 2006;6:967–74. doi: 10.1038/nrc2010. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–76. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrving B. Lacunar infarcts: no black holes in the brain are benign. Pract Neurol. 2008;8:222–8. doi: 10.1136/jnnp.2008.153601. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–6. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, Gascon S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15:602–13. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Othman T, Yan H, Rivkees SA. Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia. 2003;44:166–72. doi: 10.1002/glia.10281. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–6. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- Petratos S, Ozturk E, Azari MF, Kenny R, Lee JY, Magee KA, Harvey AR, McDonald C, Taghian K, Moussa L, Mun Aui P, Siatskas C, Litwak S, Fehlings MG, Strittmatter SM, Bernard CC. Limiting multiple sclerosis related axonopathy by blocking Nogo receptor and CRMP-2 phosphorylation. Brain. 2012;135:1794–818. doi: 10.1093/brain/aws100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabdolhossein F, Mozafari S, Morvan-Dubois G, Mirnajafi-Zadeh J, Lopez-Juarez A, Pierre-Simons J, Demeneix BA, Javan M. Nogo receptor inhibition enhances functional recovery following lysolecithin-induced demyelination in mouse optic chiasm. PLoS One. 2014;9:e106378. doi: 10.1371/journal.pone.0106378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–80. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–81. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–26. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Fern R. Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation? Glia. 1997;21:134–41. [PubMed] [Google Scholar]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Semin Cell Dev Biol. 2011;22:178–84. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience. 2010;168:982–1008. doi: 10.1016/j.neuroscience.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer MM, McQueen J, Searcy L, Scullion G, Zonta B, Desmazieres A, Holland PR, Smith J, Gliddon C, Wood ER, Herzyk P, Brophy PJ, McCulloch J, Horsburgh K. Rapid disruption of axon-glial integrity in response to mild cerebral hypoperfusion. J Neurosci. 2011;31:18185–94. doi: 10.1523/JNEUROSCI.4936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–11. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44:2579–86. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DD, Collier LA, Seifert HA, Chapman CB, Leonardo CC, Willing AE, Pennypacker KR. Leukemia inhibitor factor promotes functional recovery and oligodendrocyte survival in rat models of focal ischemia. Eur J Neurosci. 2014;40:3111–9. doi: 10.1111/ejn.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo JK, Aumann TD, Merlo D, Kilpatrick TJ, Cate HS. Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci. 2011;31:4504–10. doi: 10.1523/JNEUROSCI.5859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–40. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23:9519–28. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–6. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR. The role of hypoxia-inducible factors in oxygen sensing by the carotid body. Adv Exp Med Biol. 2012;758:1–5. doi: 10.1007/978-94-007-4584-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol. 2011;231:19–29. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–60. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–82. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- Sohn J, Natale J, Chew LJ, Belachew S, Cheng Y, Aguirre A, Lytle J, Nait-Oumesmar B, Kerninon C, Kanai-Azuma M, Kanai Y, Gallo V. Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. J Neurosci. 2006;26:9722–35. doi: 10.1523/JNEUROSCI.1716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009;180:261–72. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol Med. 2010;16:160–70. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Stys PK. The axo-myelinic synapse. Trends Neurosci. 2011;34:393–400. doi: 10.1016/j.tins.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sun H, Le T, Chang TT, Habib A, Wu S, Shen F, Young WL, Su H, Liu J. AAV-mediated netrin-1 overexpression increases peri-infarct blood vessel density and improves motor function recovery after experimental stroke. Neurobiol Dis. 2011;44:73–83. doi: 10.1016/j.nbd.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Liu Y, Liu B, Xiao Z, Zhang L. Rolipram promotes remyelination possibly via MEK-ERK signal pathway in cuprizone-induced demyelination mouse. Exp Neurol. 2012;237:304–11. doi: 10.1016/j.expneurol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–23. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, Suzuki N. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:685–93. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- Syed YA, Baer AS, Lubec G, Hoeger H, Widhalm G, Kotter MR. Inhibition of oligodendrocyte precursor cell differentiation by myelin-associated proteins. Neurosurg Focus. 2008;24:E5. doi: 10.3171/FOC/2008/24/3-4/E4. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–9. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Ye Z, Ransom BR. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metab. 2007;27:1540–52. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–22. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Angot E, Ruat M. Sonic Hedgehog signaling in the mammalian brain. J Neurochem. 2010;113:576–90. doi: 10.1111/j.1471-4159.2010.06642.x. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Dhaunchak AS, Goncalves JT, Wenzel D, Schneider A, Bunt G, Nave KA, Simons M. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J Cell Biol. 2006;172:937–48. doi: 10.1083/jcb.200509022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Macklin WB, Miller RH. Netrin-1 is required for the normal development of spinal cord oligodendrocytes. J Neurosci. 2006;26:1913–22. doi: 10.1523/JNEUROSCI.3571-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy TM, Wallingford N, Liu Y, Chan FH, Rizvi T, Xing R, Bebo B, Rao MS, Sherman LS. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia. 2004;47:335–45. doi: 10.1002/glia.20042. [DOI] [PubMed] [Google Scholar]
- Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238–47. doi: 10.1016/j.expneurol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Valeriani V, Dewar D, McCulloch J. Quantitative assessment of ischemic pathology in axons, oligodendrocytes, and neurons: attenuation of damage after transient ischemia. J Cereb Blood Flow Metab. 2000;20:765–71. doi: 10.1097/00004647-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–5. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–51. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EJ, Rosenberg GA. Divergent role for MMP-2 in myelin breakdown and oligodendrocyte death following transient global ischemia. J Neurosci Res. 2010;88:764–73. doi: 10.1002/jnr.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Swadlow HA. The conduction properties of axons in central white matter. Prog Neurobiol. 1977;8:297–324. doi: 10.1016/0301-0082(77)90009-0. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Pathophysiology of demyelinated axons. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon: Structure, function, and pathophysiology. Oxford University Press; New York: 1995. pp. 438–461. [Google Scholar]
- Xiang Z, Burnstock G. Expression of P2X receptors on rat microglial cells during early development. Glia. 2005;52:119–26. doi: 10.1002/glia.20227. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu Y, Wei P, Peng H, Winger R, Hussain RZ, Ben LH, Cravens PD, Gocke AR, Puttaparthi K, Racke MK, McTigue DM, Lovett-Racke AE. Silencing Nogo-A promotes functional recovery in demyelinating disease. Ann Neurol. 2010;67:498–507. doi: 10.1002/ana.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–38. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Huang L, Zou J, Yu Z, Wang Y, Wang X, Xu L, Liu X, Xu XM, Lu PH. Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats. Neurobiol Dis. 2008;32:535–42. doi: 10.1016/j.nbd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–96. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang G, Rong W, Wang A, Wu C, Huo X. Oscillating field stimulation promotes spinal cord remyelination by inducing differentiation of oligodendrocyte precursor cells after spinal cord injury. Biomed Mater Eng. 2014;24:3629–36. doi: 10.3233/BME-141190. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A. 2000;97:5621–6. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Jin WL, Ju G. An in vitro study on the involvement of LINGO-1 and Rho GTPases in Nogo-A regulated differentiation of oligodendrocyte precursor cells. Mol Cell Neurosci. 2007;36:260–9. doi: 10.1016/j.mcn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Zonouzi M, Renzi M, Farrant M, Cull-Candy SG. Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat Neurosci. 2011;14:1430–8. doi: 10.1038/nn.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]