Abstract
Objective
Pain remains a critical medical challenge. Current treatments target nociception without addressing affective symptoms. Medically intractable pain is sometimes treated with cingulotomy or deep brain stimulation to increase tolerance of pain-related distress. Transcranial direct current stimulation (tDCS) may noninvasively modulate cortical areas related to sensation and pain representations. The present study aimed to test the hypothesis that cathodal (“inhibitory”) stimulation targeting left dorsal anterior cingulate cortex (dACC) would increase tolerance to distress from acute painful stimuli versus anodal stimulation.
Methods
Forty healthy volunteers received both anodal and cathodal stimulation. During stimulation, we measured pain distress tolerance with three tasks: pressure algometer, cold pressor, and breath holding. We measured pain intensity with a visual-analog scale before and after each task.
Results
Mixed ANOVA revealed that mean cold pressor tolerance tended to be higher with cathodal versus anodal stimulation (p = 0.055) for participants self-completing the task. Pressure algometer (p = 0.81) and breath holding tolerance (p = 0.19) did not significantly differ. The pressure algometer exhibited a statistically significant order effect irrespective of stimulation polarity (all p < 0.008). Pain intensity ratings increased acutely after cold pressor and pressure algometer tasks (both p < 0.01), but not after breath holding (p = 0.099). Cold pressor pain ratings tended to rise less after cathodal versus anodal tDCS (p = 0.072).
Conclusions
Although our primary results were nonsignificant, there is a preliminary suggestion that cathodal tDCS targeting left dACC may increase pain distress tolerance to cold pressor. Pressure algometer results are consistent with task-related sensitization. Future studies are needed to refine this novel approach for pain neuromodulation.
Keywords: neuromodulation, transcranial direct current stimulation, tDCS, pain, distress tolerance, noninvasive
Introduction
The costs of treating chronic pain may exceed those of treating coronary artery disease, cancer, and AIDS--combined (1). Existing treatments primarily target nociception, often via pharmaceutical agents such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and membrane stabilizers. These treatments suffer from poor or temporary efficacy as well as serious side effects including drug dependence and gastrointestinal damage. Chronic opiate use may, in fact, reduce pain tolerance--a phenomenon known as opioid-induced hyperalgesia. This phenomenon has been suggested by two recent prospective studies in chronic pain patients (2,3); however, other studies have shown conflicting results (4) in this growing area of research. Psychiatric sequelae of chronic pain include a maladaptive “vicious cycle” of avoidance (5) that may lead to depression and anxiety, both of which can be debilitating even in the absence of a pain syndrome. Non-pharmacological treatments, such as cognitive behavior therapy, have been developed to address these sequelae (6), but are under-utilized and difficult for the majority of pain patients to access.
There is growing evidence that the transition to chronic pain involves changes to cortical regions (7,8) including anterior cingulate cortex (9). This structure has one of the highest densities of opioid receptors in the brain, and these receptors have been implicated in chronic pain regulation (10). For half a century, medically intractable pain has at times been treated with cingulotomy, the ablation of the anterior cingulate cortex (11–14), with recipients reporting increased tolerability of pain even though intensity did not necessarily decrease. A recent open-label case series targeting the same region with deep brain stimulation showed reduction in the affective component of chronic pain (15), making the region an attractive target for neuromodulation.
Transcranial direct current stimulation (tDCS) is a noninvasive neuromodulation technique that alters cortical excitability via subthreshold modulation of neuronal resting membrane potentials by a constant weak electrical current (16). Based on studies of motor cortex stimulation, anodal tDCS generally increases the excitability of underlying cortex, while cathodal stimulation generally decreases excitability (16,17). The technique is being widely explored for a variety of neuropsychiatric applications and has been used to target cortical areas associated with sensation (18) and higher-order representations of pain (19). It is well-tolerated and generally considered safe in experimental settings; common side effects, such as tingling or itching sensations, skin redness, headache, or moderate fatigue, are generally transient and benign (16,17).
The present study used tDCS targeting left dorsal anterior cingulate cortex (dACC), a component of brain circuits mediating learning in response to emotionally charged experiences, to attempt to increase pain-related emotional distress tolerance in healthy participants. We hypothesized that non-invasive stimulation would selectively modulate the affective component of pain. Distress tolerance was measured during three tasks: the cold pressor, the pressure algometer, and breath holding. We expected that cathodal tDCS targeting the left dACC would increase tolerance to distress from acute painful stimuli, relative to anodal stimulation. Simultaneously, we expected no change in nociception as assessed by a visual analog pain rating scale, demonstrating selective modulation of the affective component of pain.
Methods
Participants
We recruited 40 healthy participants who met the following inclusion criteria: at least 18 years of age, naive to tDCS, fluent in English, and right-handed (the latter to eliminate potential confounds in task performance due to handedness). The study protocol was approved by the Butler Hospital Institutional Review Board. All participants provided written informed consent and were tested at the hospital, in a dedicated, quiet, and climate-controlled testing room. We employed convenience sampling via paper and online advertisements and accepted participants of any racial or ethnic group and of either sex. Participants were compensated for their time.
Potential participants were screened via a semi-structured interview using the Structured Clinical Interview for DSM-IV-TR (SCID) Research Version(20). We excluded individuals with current Axis I psychiatric disorders or current pain. We also excluded individuals with conditions that could possibly increase tDCS-associated risks, such as use of psychotropic medications, seizure disorder, a previous history of skull trauma or intracranial surgery, presence of metal in the cranial cavity, pacemakers or other implanted medical hardware, or pregnancy. For individuals successfully screened into the study, we requested no caffeine or nicotine use within three hours of starting the experiment to minimize any confounding effects of these substances on cognitive performance.
Study Setting
To maximize subject recruitment and retention, all testing occurred on a single day. The study used a within-subjects design, consisting of two 20-minute blocks separated by a 90-minute rest interval (Figure 1). Three pain distress tolerance tasks were administered during active tDCS. The rest interval was included because tDCS-associated effects can persist up to 90 minutes after stimulation ends (21). All testing was performed by a trained member of the research staff under the supervision of the investigators. The same individual conducted both testing blocks. When available, a second trained member of the research staff was present to assist with tDCS setup and data recording. For each participant, stimulation targeted left dACC. One electrode was placed at FC1 on the 10–20 electroencephalography (EEG) system, as used in prior studies targeting the left (pre) supplementary motor area, a structure overlying the left dACC (22–24). A reference electrode was placed over the contralateral mastoid process. Prior to stimulation, impedance of the electrode-to-scalp interface was checked with the stimulator to ensure that it remained at or below a predetermined safety cutoff level. Each participant received anodal stimulation targeting left dACC one block and cathodal stimulation over left dACC the other block; the order of stimulation polarity was randomized and counterbalanced.
Figure 1.
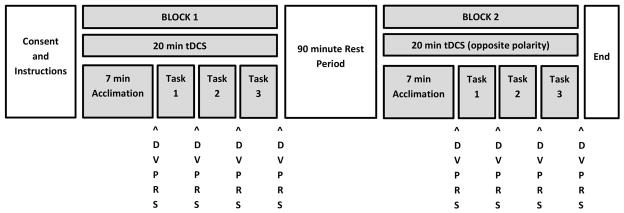
Diagram of experimental protocol. The two testing blocks were separated by a 90-minute rest interval. The polarity of tDCS received during Block 1 was randomized and counterbalanced; the opposite polarity of tDCS was delivered during Block 2. Within each testing block, there was a seven-minute acclimation period to tDCS (see text) followed by the three experimental tasks: cold pressor, pressure algometer, and breath holding. The order in which the tasks were presented was randomized and counterbalanced. The “DVPRS” labels indicate when DVPRS pain ratings were obtained over the course of the experiment.
Participants were blinded to stimulation polarity; experimenters were not. For all participants, tDCS stimulation amplitude was ramped up to 2 mA over 30 seconds and then maintained. Current was lowered only in the event of poor tolerability. Active stimulation was delivered in both testing blocks, as has often been used in studies of cognitive effects of tDCS. This within-participants design was chosen for two reasons: the potential difficulty of maintaining blinding with tDCS at a 2 mA stimulation amplitude (25) and to examine polarity-dependent neuromodulation targeting dACC with tDCS.
tDCS
During each testing block (Figure 1), tDCS was applied to the scalp over the target using a battery-powered Soterix 1X1 tDCS device (Figure 2; Soterix Medical Inc., New York, NY, USA). This single-channel device has one stimulating and one reference electrode. The device delivers a maximum of 2 mA of direct current stimulation for no more than 20 minutes. Stimulation was applied with carbon-rubber electrodes enclosed in disposable 3 × 5 cm sponge pockets. The sponges were saturated in normal saline (0.9% NaCl) and affixed to the head with a rubber headband adjusted to fit snugly but without discomfort. To enhance the connection, standard conductive gel (Microlyte, Coulbourn Instruments, Whitehall, PA, USA) was applied between the scalp and the sponge pocket. We started tDCS seven minutes before the first experimental task for participant acclimation; this was two to three minutes longer than similar studies demonstrating tDCS-associated effects on cognitive task performance (26,27).
Figure 2.
Photograph of the battery-powered tDCS device with leads, electrodes, sponges (in bag), and elastic headband.
Tasks
Pain intensity was rated with the validated and self-rated Defense and Veterans Pain Rating Scale (DVPRS), an 11-point numerical visual analog scale developed by the Office of the Army Surgeon General Pain Management Task Force (28). A score of 0 indicates no pain and 10 indicates the most severe pain. Baseline ratings were obtained during each testing block immediately after the seven-minute tDCS acclimation period but before administering the first pain distress tolerance task. Additional DVPRS ratings were obtained immediately after each of the three pain distress tolerance tasks described below. For these tasks, threshold was defined as the time elapsed (in seconds) from task onset to when the participant first reported pain, and tolerance was defined as time elapsed from task onset to when the participant ended the task (29,30).
The pain distress tolerance tasks were administered during tDCS, with order of task presentation being randomized and counterbalanced. If the preprogrammed 20 minutes of tDCS elapsed before all tasks in a testing block concluded, those tasks were completed as if the tDCS were still active (i.e. electrodes remained on the participant’s head until conclusion of all tasks in a testing block). The cold pressor task (31) involved immersing the participant’s dominant (right) hand and arm in a container of ice water. Our apparatus was comprised of an insulated cooler, inside of which was placed an open-topped wire mesh cylinder 16 cm diameter by 30 cm tall. The area outside of the mesh was filled with crushed ice. The cooler was then filled with water. Temperature was closely monitored inside the mesh cylinder until it equilibrated to a temperature of 33 ± 1° F. The researcher measured (1) time elapsed until the participant reported that the sensation was painful (defined as pain threshold) and (2) total time elapsed until the participant reported that their distress was no longer bearable (defined as pain tolerance). The participant was free to remove his or her hand at any time. The task had a five-minute safety limit after which time the participant was asked to remove his or her hand from the ice water bath and a pain tolerance time of 300 s was recorded. The pressure algometer apparatus was used to apply a fixed amount of non-harmful pressure to the index finger of the non-dominant (left) hand between the proximal and distal interphalangeal joints, following a previously described experimental design (32). This custom apparatus is constructed from a 37 cm long hinge. The final 5 cm are bent at a 90 degree angle to create a blunted tip approximately 1.5 mm by 5.0 mm that rests on the finger. A stack of washers bolted to the hinge produces a downward force of 7.6 N at the finger, causing a sensation akin to a dull butter knife that intensifies with time but does not cause tissue damage; it is equally effective with the dominant and non-dominant hand (30). The researcher similarly measured time elapsed until (1) the sensation became painful and (2) the sensation became so uncomfortable that the participant wanted it off of the finger (tolerance). This task also had a five-minute safety limit. The same apparatus was used to test all participants. In the breath holding task (33,34), participants were asked to take a deep breath and hold it as long as possible.
Data Analysis
For pain intensity ratings, the difference between the post-task DVPRS and baseline DVPRS ratings was calculated for each pain distress tolerance task for each participant. This difference was used for statistical analyses.
For the cold pressor and pressure algometer tasks, endurance was calculated as the difference between threshold and tolerance (29,30). It is a measure of how much longer a participant can continue a task after considering it painful. There was no measure of endurance for the breath holding task because participants were not asked to report pain during breath holding.
Statistical analysis included mixed ANOVA and t-tests, common for the psychological tasks used (29–32,35–38), as well as Shapiro-Wilk tests of dataset normality. These analyses were performed with R 3.0.0 (http://www.r-project.org/) using the open-source RKWard 0.6.1 graphical user interface and the rk.anova 0.01-17 plug-in (http://rkward.sourceforge.net). Analyses were confirmed with SPSS 21.0 (IBM, New York, NY, USA). Stimulation polarity was a within-subjects factor and stimulation order (cathodal-first vs. anodal-first) was a between-subjects factor. The significance level was set at the usual p < 0.05, two-tailed. For the cold pressor task specifically, an additional sub-analysis was performed excluding subjects who achieved the five-minute safety limit for both testing blocks.
Results
Demographically, participants had a mean age of 28.3 years (standard deviation 9.6, range 18 to 59), median age of 25 years. Seventeen participants (42.5%) were female.
Eight of 40 participants reported sensations of itching, tingling, or burning under the tDCS electrodes; one participant reported mild dizziness. In four of these, stimulation amplitude had to be reduced (from 2 mA to 1.1 – 1.8 mA) for some or all trials for tolerability (three had amplitude reduced during one testing block and one had it reduced for both blocks). In a fifth participant, adjusting the electrodes was sufficient. These sensations were consistent with those commonly reported for tDCS (16,17). For 12 of 240 tasks (5.0%) administered during the study, the 20 minutes of tDCS passed before task conclusion.
Threshold
Mixed ANOVAs also revealed no significant main or interaction effects of stimulation on cold pressor or pressure algometer threshold (all F(1,38) < 2.6, all p > 0.1).
Tolerance
A mixed ANOVA (Figure 3) revealed that mean cold pressor tolerance was higher with cathodal (97.8 seconds) versus anodal (82.2 seconds) stimulation, but the difference was not significant (F(1,38) = 3.6, p = 0.064). However, it was noted that five participants “maxed out” the cold pressor task by achieving the five-minute safety limit for both testing blocks. When these participants were excluded from the analysis, the significance improved to p = 0.055 (F(1,33) = 3.9, cathodal mean 68.6 seconds, anodal mean 51.1 seconds). Although published studies using such psychological tasks typically employ parametric statistics such as ANOVA that assume dataset normality (29–32,35–38), for rigor we performed the Shapiro-Wilk normality test, which suggested rejecting the null hypothesis that our data were normally distributed. We therefore re-analyzed the main effect of polarity on mean cold pressor tolerance for all participants with the non-parametric, paired, two-tailed, exact Wilcoxon signed-rank test, and obtained comparable results (WS = 416, p = 0.099).
Figure 3.
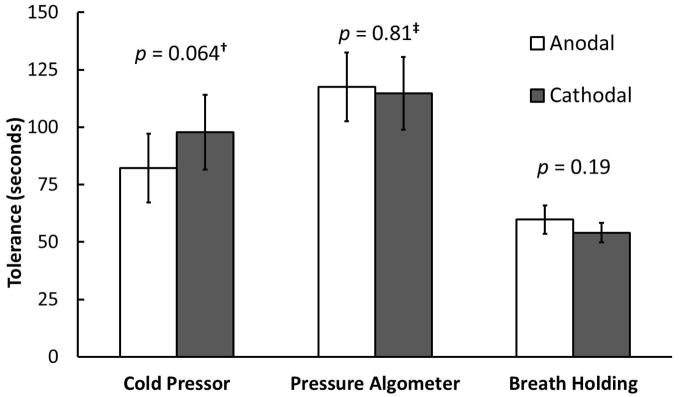
Forty healthy volunteers received tDCS targeting left dACC in two testing blocks. Cathodal tDCS was delivered in one block and anodal in the other, assigned in randomized and counterbalanced fashion. During active tDCS, pain-related distress tolerance was assessed with the cold pressor, pressure algometer, and breath holding tasks. Mixed ANOVA revealed that mean cold pressor tolerance was higher with cathodal versus anodal stimulation, but the difference did not reach significance (F(1,38) = 3.6, p = 0.064). Stimulation polarity did not significantly affect tolerance to the pressure algometer (F(1,38) = 0.056, p = 0.81) or breath holding (F(1,38) = 1.8, p = 0.19). †However, if the five participants who “maxed out” the cold pressor task (see text) were excluded, significance improved to p = 0.055 (F(1,33) = 3.9). ‡The stimulation order x polarity interaction was significant (F(1,38) = 8.1, p = 0.0071), suggesting task-related sensitization. Error bars represent ±1 standard errors of the mean.
Tolerance to the pressure algometer exhibited a significant stimulation order (cathodal-first vs. anodal-first) x polarity interaction (F(1,38) = 8.1, p = 0.0071), indicating a strong order effect (irrespective of stimulation polarity) with overall shorter tolerance in the second testing block; this suggests task-related sensitization. There was no significant main effect of stimulation polarity for the pressure algometer (F(1,38) = 0.056, p = 0.81, cathodal mean 114.7 seconds, anodal mean 117.6 seconds) or breath holding (F(1,38) = 1.8, p = 0.19, cathodal mean 54.1 seconds, anodal mean 59.8 seconds). There were no other significant main or interaction effects for any task (all F(1,38) < 1.3, all p > 0.27).
Endurance
Mixed ANOVA for cold pressor endurance revealed no significant main or interaction effects (all F(1,38) < 1.9, all p > 0.17, cathodal mean 61.9 seconds, anodal mean 57.4 seconds). Pressure algometer endurance showed a significant stimulation order x polarity interaction (F(1,38) = 8.5, p = 0.0061), again demonstrating an order effect with overall shorter endurance in the second testing block, which suggests task-related sensitization. Pressure algometer endurance showed no other significant main effects (all F(1,38) < 0.21, all p > 0.65, cathodal mean 81.4 seconds, anodal mean 85.2 seconds). Removing the same five participants who “maxed out” the cold pressor task did not change the results.
DVPRS
Pain ratings rose from baseline values by (mean ± standard deviation) 3.6 ± 2.4 and 2.9 ± 2.3 for the cold pressor and pressure algometer tasks, respectively, using paired samples t-tests (both t > 11, both p < 0.001), but not for breath holding (0.3 ± 1.8, t = 1.67, p = 0.099). Mixed ANOVA revealed no statistically significant main effect of polarity on DVPRS difference (cold pressor F(1,38) = 3.4, p = 0.072; all other polarity and interaction effects: F(1,38) < 0.72, p > 0.4). The DVPRS rose less for cathodal (3.3 ± 2.3) than anodal (3.9 ± 2.5) cold pressor trials.
Discussion
This was a first study with the primary goal of using tDCS to attempt to modulate pain distress tolerance. Stimulation was generally well-tolerated by participants. Due to the preliminary nature of this pilot study, there were several limitations, and ultimately our results did not reach formal statistical significance. Nonetheless, the trends in our data were in the expected directions from our hypotheses. They suggest that noninvasive tDCS targeting midline cortical structures for pain neuromodulation warrants further investigation, ideally in the form of double-blinded clinical trials in chronic pain patients.
Cold pressor tolerance (total time in ice water bath) tended to be longer after cathodal tDCS targeting left dACC versus anodal tDCS targeting the same region, although this difference was not statistically significant. In contrast, performance on the pressure algometer and breath holding tasks did not differ between cathodal and anodal tDCS. We used a validated self-report, the DVPRS, to measure pain intensity. As expected, DVPRS ratings increased acutely after both the cold pressor and pressure algometer tasks, but not after breath holding. For the cold pressor, DVPRS pain ratings tended to rise less after cathodal versus anodal tDCS, although this also did not reach statistical significance. These pilot tDCS findings on acute reactions to painful tasks in healthy participants can be compared to prior neurosurgical outcomes in individuals with chronic pain. Early work with cingulotomy (11,12) and recent work with DBS (15) demonstrated that chronic pain patients felt less bothered by their pain even though pain intensity did not necessarily decrease following these invasive interventions.
Cingulotomy for intractable pain is performed bilaterally. In our study, unilateral left-sided dACC stimulation was chosen because of (1) limitations of the single-channel stimulator and (2) the fact that all participants were right-handed. Cold pressor testing was performed on the right hand and arm, contralateral to the hemisphere receiving tDCS. Pressure algometer testing was performed on the left hand (to eliminate confounding effects from a prior cold pressor trial to the same hand), and exhibited task-related sensitization. The contribution of laterality to the effects of brain stimulation on pain processing remains understudied. In rodents, a study of the antinociceptive effects of electrical stimulation of anterior cingulate cortex showed no effect of side of stimulation despite there being a significant main effect of stimulation (39). Attempting to compare left- and right-sided dACC stimulation in the present pilot study would have complicated an already-complex design and reduced statistical power. Clearly, future studies of tDCS targeting dACC for pain must specifically investigate the role of stimulation laterality.
A limitation of our study was the focality of stimulation. By its nature, tDCS is non-focal and volumes of cortex adjacent to dACC were most likely also affected by stimulation. It is therefore necessary to pair active tDCS with neuroimaging or experimentally validated field modeling before conclusively attributing any effects to neuromodulation only of dACC.
Another limitation was the comparison of two active stimulation conditions without a sham condition. This was again done to simplify an already-complex pilot study design, also avoiding blinding difficulties noted with a 2 mA stimulation amplitude (25). We were also specifically interested in investigating polarity-dependent effects of tDCS targeting dACC. However, this design also precluded detection of any polarity-independent general effects of stimulation.
A further limitation was that our cold pressor apparatus did not use a water circulator as some other investigators have done (35,40). Rather, our apparatus used a cylindrical mesh screen to separate the ice from the rest of the water bath and prevent direct contact between the ice and participants’ skin. While we are confident that this design provided adequate temperature uniformity (as confirmed by our close monitoring of water temperature), the use of a temperature-controlled water circulator would likely have provided superior uniformity. Additionally, the cold pressor and pressure algometer tasks were terminated by the experimenters after five minutes if a participant had not yet stopped the task voluntarily. This limit was imposed for participant safety, but it also tended to underestimate the true tolerance of these participants, which would be expected to exceed the recorded value of 300 seconds. The sub-analyses performed after removing these participants would concomitantly tend to bias the dataset towards lower-tolerance individuals.
A potential concern with tDCS--particularly if contemplated as a chronic pain treatment--is the duration of effect once active stimulation ends. The present study comprised only two sessions of tDCS given on the same day. However, a prior study of tDCS over motor cortex demonstrated increases in excitability that persisted for up to 90 minutes after stimulation ended (21). Imaging studies have also demonstrated changes in cerebral blood flow (CBF) both during and after active tDCS (41,42). Interestingly, one study using functional near-infrared spectroscopy (fNIRS) showed negligible effects after cathodal tDCS with bifrontal electrodes (41) while the other using whole-brain arterial spin labeling showed a decrease in CBF following both cathodal and anodal stimulation targeting left DLPFC (42). This apparent discrepancy may be due to the different tDCS electrode montages used, as the electric field imposed on the cortex depends on electrode placement (43,44).
Several studies of anodal tDCS over motor cortex have demonstrated long-lasting reductions in various types of pain up to 12 weeks following multiple tDCS sessions (18,45–51). Whereas the effects of a single tDCS session may be transient, those of multiple sessions seem to persist far longer. Thus, finding any difference after only two active tDCS sessions--at opposing polarities--is a strength of our study.
A final consideration is that the DVPRS scale includes facial representations of pain in addition to numerical, color, and text descriptions (28). These ratings therefore likely capture some affective weight, which may explain why cold pressor DVPRS tended to rise less with cathodal versus anodal stimulation -- similar to how cold pressor tolerance tended to increase with cathodal tDCS. In future clinical practice, strictly separating the perceptual and affective components of pain may not be feasible or desirable. However, dissociating these pain dimensions as best as possible will help reveal the underlying neural circuitry of chronic pain and identify ideal cortical targets for neuromodulation.
Conclusions
Chronic pain is an entity that remains poorly understood and inadequately treated. Our results suggest that cathodal tDCS (thought to reduce cortical excitability) targeting left dACC may increase pain distress tolerance in the cold pressor task. These results follow those of prior studies utilizing cingulotomy and DBS for treating pain. Our results remain preliminary in nature, and subsequent studies investigating laterality effects and involving neuroimaging, field modeling, and chronic pain populations are necessary to refine our understanding of the underlying neural circuitry and to measure clinical efficacy. Stimulation of other identified cortical targets associated with affective or attentional aspects of pain is also an important next step.
Acknowledgments
The authors thank Nicole McLaughlin, Ph.D., Ganaelle Joseph, and Ignacio Perez Pozuelo for their assistance during the research. NM assisted with study logistics. GJ and IP assisted with data collection.
This work was supported by internal funding from Butler Hospital, NIMH R25 MH101076, the Brown Institute for Brain Science, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service and the Center of Excellence for Neurorestoration and Neurotechnology at the Providence VA Medical Center. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
The authors report no conflicts of interest or other disclosures.
References
- 1.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002 Dec;18(6):355–65. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hooten WM, Mantilla CB, Sandroni P, Townsend CO. Associations between heat pain perception and opioid dose among patients with chronic pain undergoing opioid tapering. Pain Med Malden Mass. 2010 Nov;11(11):1587–98. doi: 10.1111/j.1526-4637.2010.00962.x. [DOI] [PubMed] [Google Scholar]
- 3.Suzan E, Eisenberg E, Treister R, Haddad M, Pud D. A negative correlation between hyperalgesia and analgesia in patients with chronic radicular pain: is hydromorphone therapy a double-edged sword? Pain Physician. 2013 Jan;16(1):65–76. [PubMed] [Google Scholar]
- 4.Eisenberg E, Suzan E, Pud D. Opioid-Induced Hyperalgesia (OIH): A Real Clinical Problem or Just an Experimental Phenomenon? J Pain Symptom Manage. 2014 Aug 12; doi: 10.1016/j.jpainsymman.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012 Jul;28(6):475–83. doi: 10.1097/AJP.0b013e3182385392. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter KM, Stoner SA, Mundt JM, Stoelb B. An online self-help CBT intervention for chronic lower back pain. Clin J Pain. 2012 Jan;28(1):14–22. doi: 10.1097/AJP.0b013e31822363db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012 Aug;15(8):1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013 Oct;154(10):2160–8. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philos Trans R Soc Lond B Biol Sci. 2014 Jan 5;369(1633):20130146. doi: 10.1098/rstb.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt BA, Wiley RG, Jensen EL. Localization of Mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of Mu regulation. Exp Neurol. 1995 Oct;135(2):83–92. doi: 10.1006/exnr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 11.Ballantine HT, Cassidy WL, Flanagan NB, Marino R. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967 May;26(5):488–95. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 12.Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962 Feb;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 13.Spangler WJ, Cosgrove GR, Ballantine HT, Cassem EH, Rauch SL, Nierenberg A, et al. Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery. 1996 Jun;38(6):1071–6. discussion 1076–8. [PubMed] [Google Scholar]
- 14.Aghion DM, Cosgrove G Rees. Chapter 5 - Surgical Interventions for Pain. In: Saab CY, editor. Chronic Pain and Brain Abnormalities [Internet] San Diego: Academic Press; 2014. pp. 75–93. [cited 2014 Sep 3] Available from: http://www.sciencedirect.com/science/article/pii/B9780123983893000054. [Google Scholar]
- 15.Boccard SGJ, Fitzgerald JJ, Pereira EAC, Moir L, Van Hartevelt TJ, Kringelbach ML, et al. Targeting the affective component of chronic pain: a case series of deep brain stimulation of the anterior cingulate cortex. Neurosurgery. 2014 Jun;74(6):628–35. doi: 10.1227/NEU.0000000000000321. discussion 635–7. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008 Jul;1(3):206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimulat. 2012 Jul;5(3):175–95. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fregni F, Boggio PS, Lima MC, Ferreira MJL, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006 May;122(1–2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain Off J Am Pain Soc. 2011 May;12(5):610–7. doi: 10.1016/j.jpain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition With Psychotic Screen. New York, New York: Biometrics Research Department, New York State Psychiatric Institute; 2007. (SCID-I/P W/PSYCHOTIC SCREEN, 1/2007 revision) [Google Scholar]
- 21.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001 Nov 27;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan L, Asaad WF, Ginat DT, Gale JT, Dougherty DD, Williams ZM, et al. Action initiation in the human dorsal anterior cingulate cortex. PloS One. 2013;8(2):e55247. doi: 10.1371/journal.pone.0055247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pittaccio S, Viscuso S, Tecchio F, Zappasodi F, Rossini M, Magoni L, et al. Assessment of the Peripheral Performance and Cortical Effects of SHADE, an Active Device Promoting Ankle Dorsiflexion. In: Lim CT, Goh JCH, editors. 13th International Conference on Biomedical Engineering [Internet]; Berlin Heidelberg: Springer; 2009. pp. 961–5. [cited 2014 Jun 24] Available from: http://link.springer.com/chapter/10.1007/978-3-540-92841-6_238. [Google Scholar]
- 24.Puzzo I, Cooper NR, Vetter P, Russo R. EEG activation differences in the pre-motor cortex and supplementary motor area between normal individuals with high and low traits of autism. Brain Res. 2010 Jun 25;1342:104–10. doi: 10.1016/j.brainres.2010.04.060. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, et al. Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PloS One. 2012;7(10):e47514. doi: 10.1371/journal.pone.0047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci Off J Soc Neurosci. 2007 Nov 14;27(46):12500–5. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoch D, Nitsche MA, Fischbacher U, Eisenegger C, Pascual-Leone A, Fehr E. Studying the neurobiology of social interaction with transcranial direct current stimulation--the example of punishing unfairness. Cereb Cortex N Y N 1991. 2008 Sep;18(9):1987–90. doi: 10.1093/cercor/bhm237. [DOI] [PubMed] [Google Scholar]
- 28.Buckenmaier CC, Galloway KT, Polomano RC, McDuffie M, Kwon N, Gallagher RM. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med Malden Mass. 2013 Jan;14(1):110–23. doi: 10.1111/j.1526-4637.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 29.Zettle R, Hocker T, Mick K, Scofield B, Petersen C, Song H, et al. Differential Strategies in Coping with Pain as a Function of Level of Experiential Avoidance. Psychol Rec [Internet] 2005;55(4) Available from: http://opensiuc.lib.siu.edu/tpr/vol55/iss4/1. [Google Scholar]
- 30.Hezel DM, Riemann BC, McNally RJ. Emotional distress and pain tolerance in obsessive-compulsive disorder. J Behav Ther Exp Psychiatry. 2012 Dec;43(4):981–7. doi: 10.1016/j.jbtep.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. J Pain Symptom Manage. 1994 Oct;9(7):462–73. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 32.Hooley JM, Delgado ML. Pain insensitivity in the relatives of schizophrenia patients. Schizophr Res. 2001 Mar 1;47(2–3):265–73. doi: 10.1016/s0920-9964(00)00064-5. [DOI] [PubMed] [Google Scholar]
- 33.Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002 Feb;111(1):180–5. [PubMed] [Google Scholar]
- 34.Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2009 May;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodes RL, Howland EW, Lightfoot N, Cleeland CS. The effects of distraction on responses to cold pressor pain. Pain. 1990 Apr;41(1):109–14. doi: 10.1016/0304-3959(90)91115-Y. [DOI] [PubMed] [Google Scholar]
- 36.Pud D, Golan Y, Pesta R. Hand dominancy--a feature affecting sensitivity to pain. Neurosci Lett. 2009 Dec 31;467(3):237–40. doi: 10.1016/j.neulet.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Ren Z-Y, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology (Berl) 2009 Jun;204(3):423–9. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigil JM, Rowell LN, Alcock J, Maestes R. Laboratory personnel gender and cold pressor apparatus affect subjective pain reports. Pain Res Manag J Can Pain Soc J Société Can Pour Trait Douleur. 2014 Feb;19(1):e13–8. doi: 10.1155/2014/213950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senapati AK, Lagraize SC, Huntington PJ, Wilson HD, Fuchs PN, Peng YB. Electrical stimulation of the anterior cingulate cortex reduces responses of rat dorsal horn neurons to mechanical stimuli. J Neurophysiol. 2005 Jul;94(1):845–51. doi: 10.1152/jn.00040.2005. [DOI] [PubMed] [Google Scholar]
- 40.Turk DC, Meichenbaum D, Genest M. Pain and Behavioral Medicine: A Cognitive-Behavioral Perspective. Guilford Press; 1986. p. 472. [Google Scholar]
- 41.Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, et al. Prefrontal hemodynamic changes produced by anodal direct current stimulation. NeuroImage. 2010 Feb 1;49(3):2304–10. doi: 10.1016/j.neuroimage.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 42.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci Off J Soc Neurosci. 2013 Jul 10;33(28):11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Im C-H, Park J-H, Shim M, Chang WH, Kim Y-H. Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Phys Med Biol. 2012 Apr 21;57(8):2137–50. doi: 10.1088/0031-9155/57/8/2137. [DOI] [PubMed] [Google Scholar]
- 44.Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Front Psychiatry. 2012;3:83. doi: 10.3389/fpsyt.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auvichayapat P, Janyacharoen T, Rotenberg A, Tiamkao S, Krisanaprakornkit T, Sinawat S, et al. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J Med Assoc Thail Chotmaihet Thangphaet. 2012 Aug;95(8):1003–12. [PubMed] [Google Scholar]
- 46.Antal A, Terney D, Kühnl S, Paulus W. Anodal Transcranial Direct Current Stimulation of the Motor Cortex Ameliorates Chronic Pain and Reduces Short Intracortical Inhibition. J Pain Symptom Manage. 2010 May;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006 Dec;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 48.Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, et al. Randomized, Sham Controlled Trial of Transcranial Direct Current Stimulation for Painful Diabetic Polyneuropathy. Ann Rehabil Med. 2013;37(6):766. doi: 10.5535/arm.2013.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori F, Codecà C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of Anodal Transcranial Direct Current Stimulation on Chronic Neuropathic Pain in Patients With Multiple Sclerosis. J Pain. 2010 May;11(5):436–42. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–61. [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon EJ, Kim YK, Kim H-R, Kim SE, Lee Y, Shin HI. Transcranial Direct Current Stimulation to Lessen Neuropathic Pain After Spinal Cord Injury: A Mechanistic PET Study. Neurorehabil Neural Repair. 2014 Mar 1;28(3):250–9. doi: 10.1177/1545968313507632. [DOI] [PubMed] [Google Scholar]



