Abstract
Microparticles (MPs) are submicron vesicles released from the plasma membrane of eukaryotic cells in response to activation or apoptosis. MPs are known to be involved in numerous biologic processes, including inflammation, the immune response, cancer metastasis, and angiogenesis. Their earliest recognized and most widely accepted role, however, is the ability to promote and support the process of blood coagulation. Consequently, there is ongoing interest in studying MPs in disorders of hemostasis and thrombosis. Both phosphatidylserine (PS) exposure and the presence of tissue factor (TF) in the MP membrane may account for their procoagulant properties, and elevated numbers of MPs in plasma have been reported in numerous prothrombotic conditions. To date, however, there are few data on true causality linking MPs to the genesis of thrombosis. A variety of methodologies have been employed to characterize and quantify MPs, although detection is challenging due to their submicron size. Flow cytometry (FCM) remains the most frequently utilized strategy for MP detection; however, it is associated with significant technological limitations. Additionally, pre-analytical and analytical variables can influence the detection of MPs by FCM, rendering data interpretation difficult. Lack of methodologic standardization in MP analysis by FCM confounds the issue further, although efforts are currently underway to address this limitation. Moving forward, it will be important to address these technical challenges as a scientific community if we are to better understand the role that MPs play in disorders of hemostasis and thrombosis.
Keywords: microparticles, microvesicles, coagulation, thrombosis, hemostasis, flow cytometry
Introduction
Historical Perspective
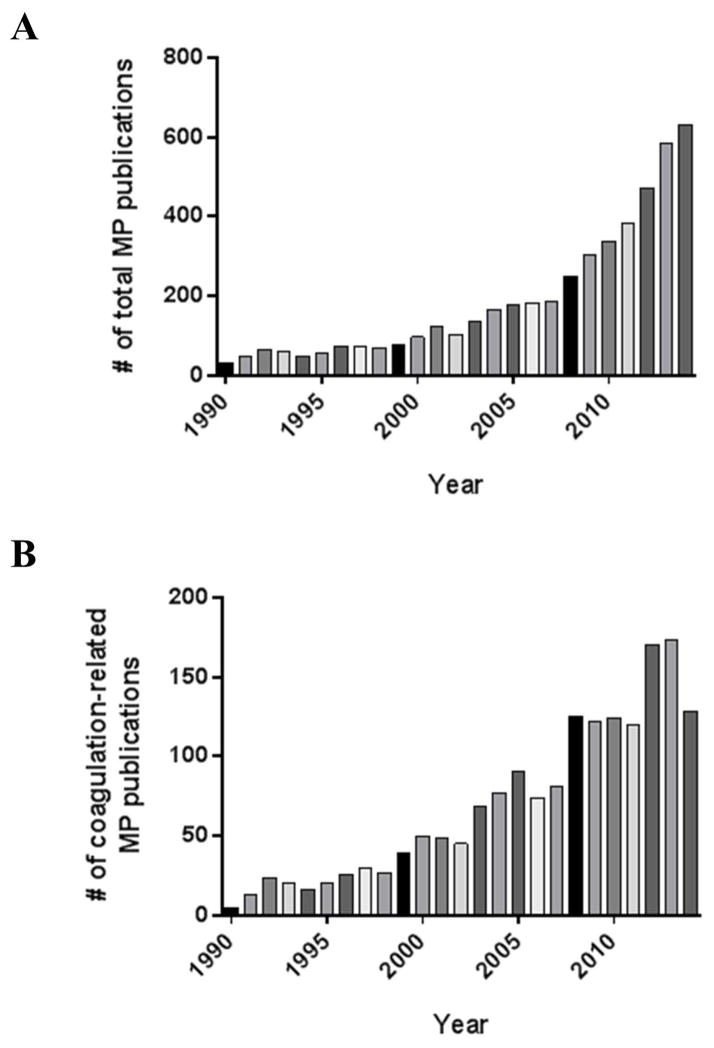
Microparticles (MPs) were first described by Chargaff and West [1] in the mid-20th century as a “precipitable factor” present in plasma that could promote coagulation processes. Wolf [2] in 1967 described “platelet dust” that was formed as a result of platelet shedding, which also exhibited procoagulant activity and was detectable in the 0.1 to 0.3 μm size range by transmission electron microscopy in the precipitate of ultracentrifuged plasma. Now understood to be platelet MPs (PMPs), this observation has led to an exponential growth in the study of MPs derived from platelets and other cell types, and with it a greater understanding of their overall biologic relevance. Although the study of MPs has now expanded beyond the realm of coagulation and into other areas of (patho)physiology, significant research effort remains focused on this aspect of MP function. As depicted in Figure 1, total MP publications have increased steadily over the past decade, with coagulation-related MP publications showing a similar increase and continuing to represent a significant portion of the total MP publications.
Figure 1.
Number of (A) total MP publications, and (B) specific coagulation-related MP publications by year since 1990. Total MP publication numbers were acquired utilizing a PubMed search for articles from 1990–2014 with keywords “microparticles or microvesicles”, while excluding studies related to pharmacology, drug delivery and non-biological entities. Coagulation-related MP publication numbers were acquired utilizing a PubMed search for articles from 1990–2014 with keywords “microparticles or microvesicles” in conjunction with coagulation specific terms such as “thrombosis” and “hemostasis”.
Definition
MPs are defined as heterogeneous, submicron (0.1 to 1 μm) vesicles released from cell membranes in response to specific stimuli or apoptosis. They have an intact phospholipid membrane and express membrane antigens specific to their cell of origin [3]. The working definition of a MP generally includes both the size discrimination, as well as the presence of externalized phosphatidylserine (PS) on the membrane [4, 5]. Newer evidence, however, supports the notion that not all MPs expose PS on their surface [6–10], and that PS content may vary depending on the cell of origin and stimulus or mechanism by which they are formed [11]. Whether this is due to a true lack of PS exposure, or whether PS expression is below the detection threshold of conventional techniques, particularly on smaller MP subsets, is unclear [12]. To complicate matters, it has also been theorized that the presence of a cell-specific antigen on the surface of a MP does not necessarily identify its cell of origin. Soluble antigens from other cell types may adhere to MPs, or fusion may occur between MPs from one cell type with the cellular membrane of a different cell, thereby allowing the detection of a MP expressing an “adopted” antigen [13, 14].
MPs must also be distinguished from two other bioactive vesicles released from cells. Exosomes are preformed vesicles < 100 nm that are generated in endocytic multivesicular bodies and released via exocytosis. They are more homogeneous in size than MPs, carry different membrane antigens, and play an important role in the immune response [15–19]. Conversely, apoptotic bodies (AptB) are produced during the latter stages of cell apoptosis [20]. They are typically larger than MPs (1–3 μm), although a few may be smaller (0.5 μm) [21]. Similar to MPs, they express PS on their surface; however, in contrast to MPs, AptB carry DNA and histones, which is one of their hallmarks [21, 22]. It should be pointed out that the term “extracellular vesicles” is increasingly being used in the scientific literature and is a term that encompasses MPs exosomes, and AptB [23]. Additionally, the term microvesicle is frequently encountered and in general is synonymous and interchangeable with the term MP [24].
Cellular Sources and Formation
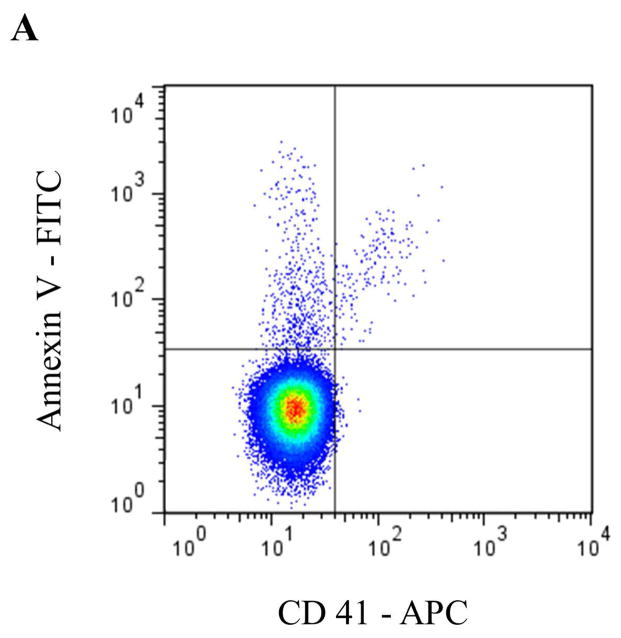
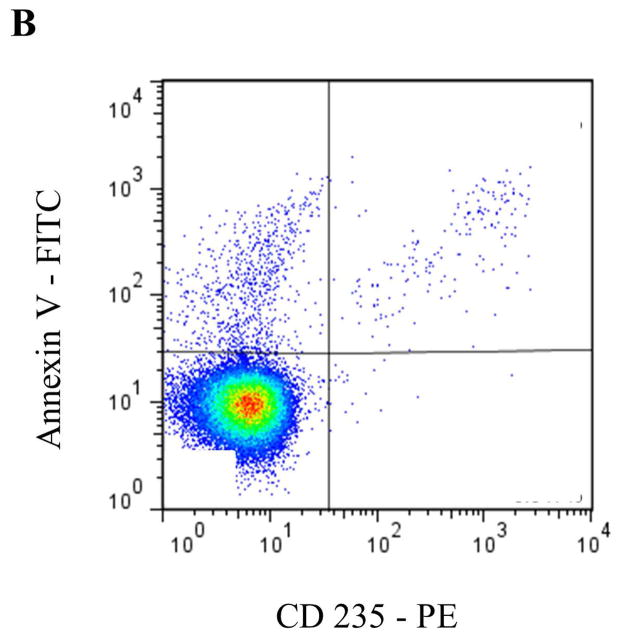
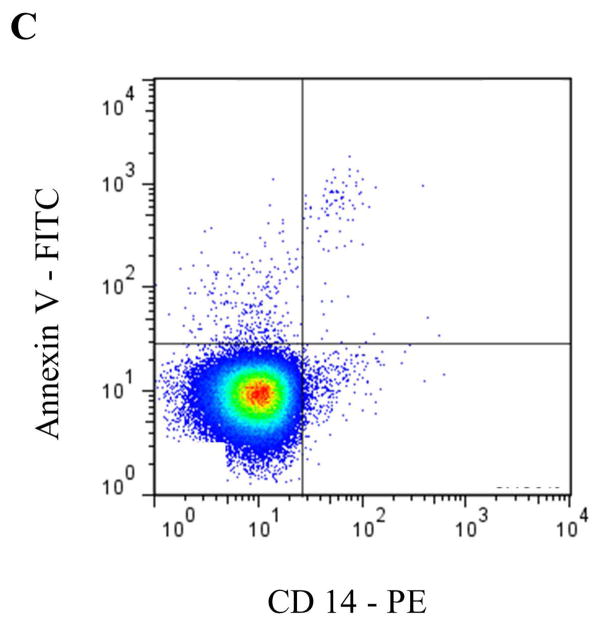
Circulating MPs are most commonly derived from blood and endothelial cells, although other sources, such as tumor cells [25], are capable of producing MPs that appear in blood. In healthy individuals, PMPs have generally been accepted to be the most abundant MP subtype [26, 27]. More recent data, however, suggest that a significant portion of PMPs may actually be derived from megakaryocytes in bone marrow [28]. To identify specific MP subsets by flow cytometry (FCM) according to their cell of origin, antibodies to common antigens of the parent cell are typically used (Table 1 and Figure 2), often in combination with Annexin V or another marker for PS, such as lactadherin.
Table 1.
Common antigens used to stain and identify specific MP subsets according to cell of origin
| MP subtype | Antigen | Alternative Name (if applicable) |
|---|---|---|
| Platelet microparticles (PMP) | CD41 CD42a CD42b CD61* CD62P* |
GPIIb GPIX |
Figure 2.
Representative staining and gating strategies for A) platelet microparticles (PMP), B) red blood cell microparticles (RMP), and C) monocyte microparticles (MMP) analyzed in platelet free plasma on a Stratedigm S1000Ex flow cytometer. Fluorescent gating was performed within the MP size gate of 200–900 μm, which was initially set utilizing polystyrene beads (data not shown). PMP = dual positive Annexin V/CD41 events. RMP = dual positive Annexin V/CD235 events. MMP = dual positive Annexin V/CD14 events.
The formation and release of MPs from cells typically occurs upon stimulation or induction of apoptosis. It is considered a broad primitive response to stress shared by all eukaryotic cells [29] and is thought to reflect a dynamic balance between cell proliferation, stimulation and death [30]. Specific stimuli known to induce MP formation include activation by substances such as endotoxin or cytokines, and partial or complete lysis such as by complement, oxidative injury, and high shear stress [31, 32]. Mechanistically, evidence continues to emerge regarding the cellular processes that lead to formation and release of MPs. In brief, loss of cellular membrane phospholipid asymmetry with resultant PS exposure appears to be a critical component of MP formation [33]. This process is governed by several phospholipid transporters (“flippase”, “floppase” and “scramblase”), which under basal conditions preserve the normal phospholipid asymmetry of the cellular membrane, with the negatively charged PS confined primarily to the inner leaflet. Calcium influx also appears to be a necessary prerequisite for MP formation, as it contributes to both PS externalization, as well as membrane cytoskeleton remodeling through activation of calpains and caspases necessary for cleavage of cytoskeletal proteins [34]. Upon stimulation, the loss of phospholipid asymmetry along with cytoskeletal disruption eventually leads to membrane blebbing and MP formation and release.
Biological Functions of Microparticles
Microparticles and Coagulation – Mechanistic Insight
This section of the review will briefly summarize what is known about MPs and their contribution to coagulation processes, as this information provides a backdrop for better understanding their potential relevance in disorders of hemostasis and thrombosis. This topic has also been extensively reviewed recently [35]. In basic terms, coagulation refers to the processes that regulate blood clot formation, whether it be under physiologic conditions to prevent hemorrhage (hemostasis) or under pathologic conditions (thrombosis). Additionally, coagulation processes can be further divided into those that promote blood clotting (procoagulant) and those that counterbalance or regulate blood clotting (anticoagulant and fibrinolytic).
The potential procoagulant function of MPs may be related to the presence of PS on the outer membrane, as well as the possible presence of tissue factor (TF). MP-associated PS provides a catalytic surface for the assembly of enzymatic coagulation complexes that initiate and maintain coagulation [36]. This function may underlie the contribution of MPs to both the physiologic process of hemostasis as well as the pathologic process of thrombosis [37]. Interestingly, it has been estimated that a PMP generated ex vivo has 50- to 100-fold higher procoagulant activity than the same area on an activated platelet [38], which may help account for the potential thrombogenicity of certain MPs. TF is the principal physiological initiator of coagulation in vivo through its interactions with the coagulation protease Factor VII/VIIa and is constitutively expressed by most vessel wall component cells other than endothelium [39]. It is often therefore described as a “hemostatic envelope” that surrounds the vasculature, preventing excessive hemorrhage upon injury. Circulating TF in the blood may, however, be present at very low concentration, with monocytes believed to be the primary source [40]. The presence of TF on some monocyte-derived MPs (MMPs) and tumor-derived MPs is well established; however, whether PMPs or endothelial MPs (EMPs) express biologically active TF remains a matter of debate [41, 42]. Although likely only a small fraction of total TF in the blood (most of which is likely to be cell-bound), MP-borne TF is thought to be functionally active and may thus contribute to the procoagulant nature of MPs.
More recent data also point to a role for MPs supporting coagulation independent of TF and the extrinsic pathway of coagulation. PMPs and red cell MPs (RMPs) generated ex-vivo have been shown to initiate and support thrombin generation through the intrinsic pathway in a Factor XII-dependent manner [43], meaning that the procoagulant properties of MPs are abolished when Factor XII is inhibited. Similarly, RMPs in sickle cell disease [44] and in banked units for transfusion [45] have also been shown to promote coagulation through the intrinsic pathway in a Factor XI-dependent manner, again through abolished MP procoagulant properties when Factor XI is inhibited. These findings shed new light on the procoagulant repertoire of MPs and their possible impact through alternative mechanisms in coagulation initiation, although further studies are needed for verification as well as to elucidate the mechanism by which this occurs. With the renewed interest in the possible role of the intrinsic pathway in thrombosis [46, 47], additional studies are also needed to define the role MPs might play in this context.
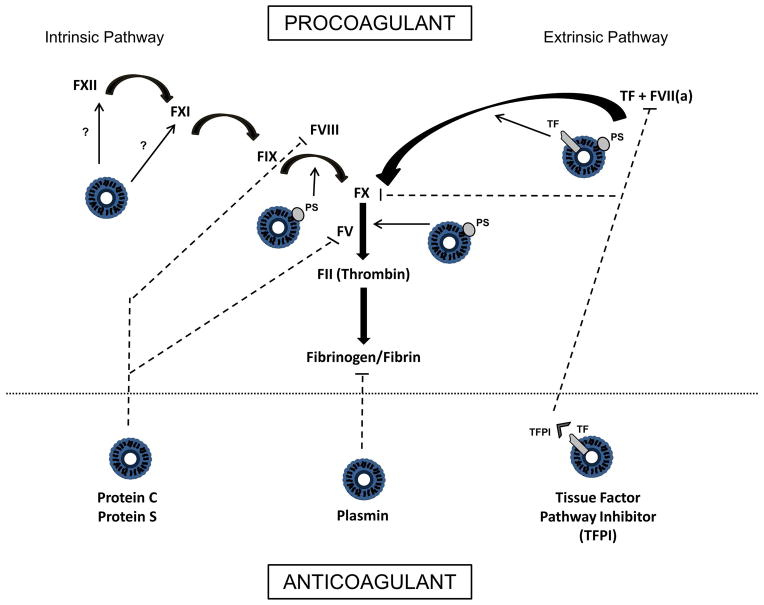
In addition to the procoagulant functions of MPs, evidence exists regarding their ability to regulate coagulation through anticoagulant or fibrinolytic mechanisms. MPs have been demonstrated to harbor functionally active tissue factor pathway inhibitor (TFPI) on their membrane [48, 49], and support activated protein C and protein S mediated regulation of coagulation [50–52], both of which are normal anticoagulant pathways in the blood. Newer evidence also establishes that MPs support plasmin generation [53, 54], another regulatory mechanism by which fibrin clots are degraded. These more recent discoveries point to a more complex role of MPs in coagulation, where it is likely that the balance between pro- and anticoagulant properties ultimately determines their net effect in hemostasis and thrombosis (Figure 3).
Figure 3. Multifaceted role of MPs in coagulation processes.
Simplified schemata of the coagulation cascade showing the different potential contributions of MPs. MPs support coagulation through exposure of phosphatidylserine (PS), which provides a catalytic surface for assembly of the coagulation complexes. Tissue factor (TF) bearing MPs can activate coagulation through the extrinsic pathway. MPs may also support coagulation through the intrinsic pathway, although the mechanism by which this occurs is not fully known. Anticoagulant properties of MPs include the ability to support Protein C/Protein S mediated regulation of coagulation, as well as tissue factor pathway inhibitor (TFPI) mediated inhibition of TF/VIIa activity and FX. MPs can also support plasmin generation, an enzyme that solubilizes and degrades clots. (Bolded arrows indicate activation steps [ie FXII activates FXI]. Dashed lines indicate inhibitory effects. Unbolded arrows emanating from MPs indicate areas of MP participation in coagulation activation processes.)
Role of Microparticles in Thrombosis
As a corollary to studies that have investigated mechanisms by which MPs may contribute to the process of coagulation, the role of MP participation directly in the process of pathologic thrombosis in vivo has also received attention. Utilizing a mouse model of arteriolar thrombosis, it has been demonstrated that TF+ MPs (presumably MMPs) accumulate at the site of thrombus formation and participate in clot propagation [55]. Other studies utilizing mouse models of venous thrombosis have additionally implicated MPs [56, 57]. Although these data are important, they have limitations due to the imperfect nature of murine models of thrombosis [58], which either use vessel injury or ligation to initiate thrombus formation. Additionally, exogenous MPs are often infused in these studies, which may also limit the applicability of these findings to human thrombotic disorders.
Thus, there is ample evidence to assert that MPs play a biologically plausible role in coagulation disorders, and in the remainder of this review we will focus on the literature addressing MP analysis by FCM in disorders of hemostasis and thrombosis, as well as the technical challenges and limitations encountered when using this approach.
Phenotypic vs Functional Assays for Microparticle Analysis
FCM remains the most commonly utilized approach for the detection and analysis of MPs [59]. This platform is advantageous in that it provides not only quantitative information but also qualitative information by immunophenotyping particles and thereby identifying their cellular origin. Numerous other modalities have also been used for MP analysis, including immunoassays, atomic force microscopy, electron microscopy, dynamic light scattering, and impedance-based FCM [60]. These methodologies will not be reviewed further; however, each has its own advantages and disadvantages. In general, though, these techniques are either not widely available, are low throughput, or do not provide both qualitative and quantitative data.
It is also worth briefly discussing functional assays that have been used in the study of MPs in coagulation disorders. Generally speaking, these assays assess MP pro- or anti-coagulant functions. MPs are isolated from plasma using either capture techniques or ultracentrifugation and are interrogated for their ability to support or inhibit coagulation using either clot-based or chromogenic endpoint assays. TF-dependent procoagulant activity can be assessed through the use of a specific blocking antibody to TF [61].
There are limited data on the correlation between functional assays and flow cytometric analysis of MPs; however, what data do exist are inconsistent. Several studies have shown a positive correlation between MPs detected by FCM and MP procoagulant activity [62, 63], while others have failed to demonstrate a correlation [13, 64, 65]. The lack of a positive correlation between FCM and functional assays is not surprising, since more sensitive assays (such as atomic force microscopy) detect upwards of 1000-fold more MPs than conventional FCM [66]. Additionally, dynamic light scattering has shown the median size of MPs to be under 300 nm, with the fraction of MPs < 200 nm in plasma accounting for at least 50% of the thrombin generating capacity [67]. Since FCM does not detect these smaller MPs, it is understandable why functional assays and FCM may fail to correlate. It also appears that the plasma centrifugation protocol may influence assay correlations, as increased numbers of contaminating platelets prior to freezing will erroneously lead to an improved correlation [68]. This is explained by the presence of a greater number of larger PMPs from fractured platelets that are then detectable by FCM. More investigation in comparing the two types of assays is warranted, but functional assays remain a useful tool in supplementing FCM when evaluating MPs in disorders of coagulation.
Microparticle Analysis in Disorders of Hemostasis
One might assume that a lack or decrease in circulating MPs could contribute to a clinically relevant bleeding phenotype, and in fact, this has been shown to be the case. Scott Syndrome is a very rare genetic disorder characterized by impaired outward transmembrane migration of PS on cell surfaces, including platelets. Individuals with this condition have a moderate bleeding tendency due to impaired ability to carry out enzymatic coagulation processes on the platelet and/or MP surface [69]. Flow cytometric analysis has demonstrated markedly decreased levels of circulating MPs in affected patients [70], which is intuitive given that PS externalization is an important step in MP formation. To what degree the lack of MPs in these patients directly contributes to their bleeding symptoms is not known, but this hypothesis seems likely given the proposed importance of PMPs in hemostasis [71].
Several studies have also examined a potential role of MPs in hemostasis in congenital bleeding disorders. Hemophilia A is a rare inherited bleeding disorder characterized by deficiency of coagulation Factor VIII (FVIII), which in its severe form results in spontaneous hemorrhage [72]. Current treatment involves replacement of the deficient coagulation factor using infusions of recombinant or plasma derived FVIII concentrates [73]. One study evaluated MPs by FCM in hemophilia A patients before and after receiving FVIII infusion for a documented clinical bleeding event. The authors observed a significant decrease in total MPs, PMPs and EMPs after treatment [74]. It was suggested that MP incorporation into a developing hemostatic plug at the site of injury explained their decreasing numbers after treatment.
Von Willebrand disease (VWD) is another congenital bleeding disorder characterized by either a qualitative or quantitative deficiency of von Willebrand factor (VWF). It is characterized primarily by mucocutaneous bleeding [75]. Therapy typically consists of infusing plasma derived VWF containing concentrates or desmopressin (aka DDVAP) during acute bleeding episodes [76]. DDAVP promotes hemostasis by increasing endogenous levels of VWF and FVIII [77], and by increasing platelet activation [78]. The number of PMPs and VWF-bound MPs increases significantly after DDAVP administration [79], in conjunction with increased VWF functional activity in plasma. Furthermore, depletion of MPs from plasma significantly decreases the VWF functional activity observed after DDAVP administration. These data provide evidence that DDAVP administration increases MP numbers, including VWF-bound MPs, and that this MP-VWF contributes to the increased VWF functional activity. Thus, MPs appear to contribute to the therapeutic efficacy of DDAVP in the treatment of VWD.
Microparticle Analysis in Thrombotic Disorders
To date, there has been much more interest in studying MPs in thrombotic disorders (as opposed to disorders of hemostasis), and increased circulating MPs have been reported in many inherently prothrombotic conditions (Table 2). Additionally, MP numbers are often correlated with markers of an activated coagulation system, and in some cases with the presence or absence of a historical thrombotic event. These studies are almost exclusively retrospective or cross-sectional, and therefore need to be interpreted with caution. While the existence of an association between elevated MP numbers and prothrombotic conditions has been repeatedly reported, a causal relationship between increased circulating MPs and thrombotic events cannot necessarily be concluded.
Table 2.
Prothrombotic conditions with reported increased microparticles.
| Prothrombotic condition | References |
|---|---|
| Sickle cell disease | [141–143] |
| Malignancy | [80, 82, 83, 86, 88–90, 93, 94, 97, 144] |
| Thrombotic thrombocytopenic purpura(TTP) | [145, 146] |
| Antiphospholipid antibody syndrome | [147–149] |
| Sepsis | [150–153] |
| Myeloproliferative disorders | [154–157] |
| Inflammatory bowel disease | [158–160] |
| Nephrotic syndrome | [161] |
| Paroxysmal nocturnal hemoglobinuria | [142, 162] |
| Systemic vasculitis | [163, 164] |
| Pregnancy/Preeclampsia | [165–168] |
| Systemic lupus erythematosus | [169–171] |
| Thrombophilia | [172, 173] |
| Trauma | [174–176] |
A specific area that has received considerable attention is cancer-associated thrombosis. In general, increased MPs have been detected using numerous methodologies, including FCM in patients with a variety of tumors [80–85]. Several studies have also shown a relative increase in MPs in cancer patients with thrombosis compared to cancer patients without thrombosis [86–89]. These data pertain primarily to procoagulant functional analysis of MPs; however, at least one study has shown increased TF+ MPs by FCM [90].
There are some prospective data in cancer that have linked elevated levels of MPs with future occurrence of thrombosis [88, 91–93]. One study showed that TF+ MPs detected by FCM was predictive of thrombosis in brain tumor patients [94]. However, other studies failed to show increased MPs as predictive biomarkers of future thrombosis [82, 95, 96]. The reason for these discrepant results is not clear, but may be related to either variability in thrombotic risk with different malignancies or differences in methodologies and analytical variables. Although prospective in nature, these data have limitations due to lack of serial MP measurements over time. In that regard, probably the most convincing evidence linking MPs to cancer-associated thrombosis comes from a study that prospectively examined serial MP TF-dependent procoagulant activity (MP-TF activity) in pancreatic cancer patients [97]. Herein, there was a significant correlation between increasing levels over time and subsequent development of thrombosis; however, the study conclusions were limited due to its small size.
There have also been quite discrepant studies evaluating MPs in the setting of thrombosis without an underlying prothrombotic condition (ie. idiopathic thrombosis). Several cross sectional studies have reported increased MP procoagulant functional activity [98, 99], while others have shown increased MPs by FCM [90, 99–102]. Still other studies failed to demonstrate an increase in MPs, either using functional assays [103–105] or FCM [106, 107]. Again, these contradictory results are most likely attributable to differences in methodologies and techniques.
Challenges in Flow Cytometric Analysis of Microparticles
Despite FCM being the most frequently utilized methodology for the analysis of MPs, numerous challenges exist, in large part related to limitations in the ability to detect submicron particles. Additionally, differences in how samples are processed and analyzed can have significant impact on the results obtained. Thus, important factors related to the analysis of MPs can be divided into pre-analytical and analytical variables (Table 3).
Table 3.
Overview of major challenges related to flow cytometric analysis of MPs.
| Important Issues in MP Analysis by Flow Cytometry | |
|---|---|
| Pre-Analytical | Analytical/Technical |
|
|
Pre-Analytical Variables in Microparticle Analysis
Numerous pre-analytical variables, most of which pertain to the collection and handling of specimens, can directly impact MP analysis. Although these variables can theoretically affect results regardless of the detection methodology, the majority of information addressing pre-analytical variables has been identified in studies utilizing FCM. Major pre-analytical variables include the method of blood collection including type of anticoagulant used, timeframe and method for processing samples, and method of sample storage.
(i) Blood Collection
Several issues regarding the method of blood collection can contribute to an artifactual elevation in MP numbers. Use of a tourniquet, traumatic venipuncture, small-diameter needles, and use of vacuum-filled containers may cause hemolysis, platelet activation or endothelial damage, all of which can falsely increase the number of MPs detected. It is therefore standard practice to discard the first several milliliters of blood to minimize these variables [11]. The type of anticoagulant used for blood collection may also have effects on MP analysis. Specifically, heparin has been shown to cause increased MP numbers compared to other anticoagulants [108]. Additionally, PMP levels can increase due to ex-vivo vesiculation of platelets in citrate, with no such increase observed in samples obtained in citrate-theophylline-adenosine-dipyridamole [109] or acid-citrate dextrose [110], although sample agitation makes this much more pronounced [111]. In general, however, citrate is most commonly used due to its wide availability and acceptable results, provided that sample agitation and delays in processing are avoided.
(ii) Sample Processing
MP analysis is generally performed on plasma samples, although can also be done in whole blood [112] or on isolated MPs [107]. Although data directly comparing MP numbers assessed in each sample type from the same individual are lacking, it is likely that the results would be variable across techniques. Particularly when MPs isolated from plasma by ultracentrifugation are re-suspended for analysis, aggregation of MPs is highly likely to occur using such high centrifugation speeds, thus changing their size profile and altering the number of detectable MPs within the appropriate size-based gate. As such, it is recommended that MP analysis be performed in plasma. Delays in plasma preparation should also be avoided to help prevent the ex-vivo generation of MPs from blood cells.
For plasma MP analysis, the centrifugation protocol is highly important. Numerous centrifugation protocols have been employed [113], resulting in either platelet-poor or platelet-free plasma. Depending on the centrifugation speed, there is either the potential for loss of MPs in the sediment or the supernatant, as well as the risk of contamination of the sample with residual platelets. During a freeze/thaw cycle, these residual platelets can be fractured, leading to artificially increased PMP numbers [111, 114]. When MP analysis is performed on fresh samples, there is probably minimal effect on the results unless the centrifugation speed is high enough to pellet a portion of the MPs with the cellular fraction. Overall, the goal is to avoid loss of MPs, thus maintaining sensitivity, without sacrificing specificity through the contamination of samples with residual platelets and resultant false increase in MP numbers.
Another potential pitfall is the possibility for “micro-clot” formation to occur in the plasma prior to analysis. This typically occurs with Annexin V staining, which requires the addition of calcium to plasma samples anticoagulated with calcium chelators (such as citrate). The addition of calcium also enables the enzymatic processes of coagulation to occur, which can lead to formation of a fibrin clot [115]. These “micro-clots” can then bind MPs, creating large aggregates that fall outside the MP size gate and thus artificially reduce the number of MPs detected. The use of heparin for blood collection has been proposed as a solution to this problem, since it inhibits coagulation and prevents “micro-clot” formation [116]. Alternatively, an anticoagulant such as Hirudin, a direct thrombin inhibitor, can be incorporated into the calcium-containing Annexin V buffer to prevent this artifact, which also avoids the known increase in MPs that occurs when blood samples are collected in heparin. This latter method is currently being utilized in the most recent standardization workshop for flow cytometric evaluation of MPs sponsored by the International Society on Thrombosis and Haemostasis (ISTH).
(iii) Handling and Storage Practices
The method of transportation of samples and potential agitation during transport are thought to influence MP numbers, particularly in blood samples anticoagulated with citrate, due to ex-vivo generation of MPs from blood cells. More widely recognized, however, is the impact of storage methods on MP analysis. Several studies have shown an increase in MP numbers when comparing frozen to fresh samples [117, 118], although this increase appears to be minimal over a 12 month period [111]. There is also the theoretical risk of fracturing MPs, causing an artificial increase in overall MP numbers [106]. Ideally, fresh plasma samples should be analyzed immediately to avoid this artifact. However, in addition to being impractical, labor-intensive and inefficient, this restriction would severely limit the ability for collaborative studies.
Analytical Variables in Microparticle Analysis
Several analytical variables can also affect the detection and enumeration of MPs by FCM. The most obvious of these is the type of flow cytometer used for MP evaluation and its intrinsic ability to discriminate submicron particles. Newer generation flow cytometers have improved detection capabilities in the 200–300 nm range and should be the preferred option when studying MPs [119], although the use of older generation flow cytometers with suboptimal resolution is still common practice. Apart from the type of flow cytometer used, other analytical variables include gating strategies for both size and fluorescence and the use of counting beads for enumeration.
(i) Size Gating
Initial gating for MP analysis is typically based upon size through the use of calibration beads. Generally, a bead that approximates 1 μm is used to set the upper size limit for MP detection, and all events smaller in size are interrogated for PS and/or cellular antigens using fluorescently labeled antibodies. Plastic beads, however, remain an imperfect model for size calibration since factors other than size can influence FSC, including relative refractive indices of both particles and suspension medium, presence of surface absorptive material, particle shape, and surface roughness [120, 121], which are not equivalent between biological entities and beads. This has led to controversy over their appropriate application and use [122–125]. A proposed solution to this problem is the use of biological entities, such as bacteria or viruses, for size calibration, although this strategy would also need standardization [123, 124].
(ii) Fluorescence Gating
Another challenging analytical variable is discriminating a positive fluorescent signal from background when using fluorescently labeled antibodies. Most laboratories use isotype controls (ITCs) to aid in setting gates for positive vs. negative events. This technique has classically been used for cellular phenotyping, but in recent years its use even in this field has been challenged [126–128]. Although intended to account for background fluorescence and nonspecific binding, different ITCs can manifest various levels of background staining depending on their concentration, degree of aggregation, and fluorophore:antibody ratio [129]. Furthermore, the degree of nonspecific binding of an ITC may or may not reflect an equivalent degree of nonspecific binding as that of the antibody of interest. Therefore, the indiscriminant use of ITCs can significantly affect the number of positive events detected [130]. Adding to the complexity is the fact that many of the antigens used for fluorescent labeling, such as those on leukocyte and endothelial MPs, are weakly expressed and do not provide a clear separation in fluorescence between positive and negative events, rendering the use of ITCs even more troublesome. As such, titration of ITCs against the specific antibody of choice at its intended concentration should always be done in an antigen free sample to match background fluorescence of the ITC to that of the specific antibody. Options for antigen free samples include MP free plasma obtained through the use of detergents to lyse MPs [131] or via ultracentrifugation. Additionally, titration of specific antibodies should also be done to ensure optimal concentration and to help limit the effects of nonspecific antibody binding [132, 133]. Lastly, all antibodies and ITCs should undergo ultracentrifugation prior to use to pellet free fluorochrome aggregates, which can be detected in the MP size gate and interpreted as a false positive fluorescent signal [134].
(iii) Counting Beads and Enumeration
Counting beads are commonly used for the enumeration of MPs by FCM. These calibrated bead solutions have a known concentration and typically fluoresce brightly in a wide range of excitation and emission wavelengths. A known volume of beads is added to a sample, and by comparing the ratio of bead events to MP events, absolute numbers of MPs can be calculated. Although a useful tool, caution is needed when using counting beads for enumeration of submicron particles. Designed primarily for cell counting, the available beads are typically in the 5–10 micron size range. Therefore, when analyzing MPs, particularly if pushing the detection threshold to its lower limits, the flow cytometer may not have the dynamic range necessary to accurately detect and count the beads. To help avoid this potential pitfall, counting beads should always be assessed using thresholds and settings for both MP analysis and large particle (ie. cellular) analysis to ensure that similar numbers of beads are counted with each set of parameters. Additionally, counting beads on the smaller end of the size spectrum should be chosen for MP analysis to help lessen the chance for error.
Accurate enumeration of MPs can also be complicated by “swarm effect” as recently described by van der Pol and colleagues [135]. Swarm effect is encountered when multiple small particles, which alone are below the detection threshold of the cytometer, are simultaneously present in the laser beam and thus generate a single event signal. In this situation, the flow cytometer-determined concentration of particles underestimates the true concentration, and the relationship between count rate and prepared concentration is non-linear. This detection of coincident events can be controlled for by optimization of flow rates and dilutions [136], and therefore it is important to use low flow rates and optimal sample dilutions in order to avoid or minimize this complication. The advent of imaging flow cytometry appears to enable accurate counting of individual MPs regardless of sample concentration [137], however this modality is still in its infancy.
The Need for Standardization
Due to the inherent difficulties associated with MP detection by FCM, as well as the numerous pre-analytical and analytical variables discussed above, it is clear that standardization of practices in flow cytometric analysis of MPs is urgently needed. To that end, an attempt at standardization of PMP enumeration was recently undertaken by the ISTH. Using a mixture of fluorescent beads of known sizes (Megamix™ – Biocytex, Marseille, France) and gating strategies to set both an upper and lower size limit (~500 nm) for MP detection, it was shown that a window of MP analysis could be reproducibly set on different cytometers of the same model to allow consistent PMP enumeration over time [138]. This protocol was then adopted as part of an ISTH workshop in an attempt to validate its use, wherein it was shown to facilitate the reproducible enumeration of PMPs across different flow cytometers and in different labs, though modifications of the protocol were required for certain cytometers [139]. As a next step, a second ISTH workshop has attempted to standardize PMP enumeration down to ~300 nm utilizing a similar bead-based gating strategy. The results of this exercise are expected in late 2014/early 2015.
In addition to standardization attempts focused on analytical variables, the Vascular Biology Scientific sub-Committee of the ISTH also organized a workshop aimed at standardizing pre-analytical variables that can critically impact MP measurements and remain a major source of variability [140]. Herein it was shown that a standardized pre-analytical protocol could reduce the inter-laboratory variability of flow cytometric evaluation of PMPs, although variability was not completely eliminated. Together, the results of these workshops are promising, although much work is still needed, particularly to help standardize the evaluation of MP subsets other than PMPs that are more challenging to detect, such as endothelial and leukocyte MPs. Hopefully, however, these efforts will serve as a first step towards continued exercises aimed at standardizing methodologies for MP analysis to allow better comparison of results across studies and promote the development of collaborative studies across centers.
Acknowledgments
MJM was supported by NIH grant K12HL087097-06.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97. [PubMed] [Google Scholar]
- 2.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–88. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 3.Tesse A, Martínez MC, Meziani F, Hugel B, Panaro MA, Mitolo V, Freyssinet JM, Andriantsitohaina R. Origin and Biological Significance of Shed-Membrane Microparticles. Endocrine, Metabolic & Immune Disorders - Drug Targets. 2006;6:287–94. doi: 10.2174/187153006778249976. [DOI] [PubMed] [Google Scholar]
- 4.Zwaal RFA, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–88. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaler J, Ay C, Pabinger I. Clinical significance of circulating microparticles for venous thromboembolism in cancer patients. Hamostaseologie. 2012;32:127–31. doi: 10.5482/ha-1164. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–80. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 7.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–35. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 8.Ahn YS, Jy W, Jimenez JJ, Horstman LL. More on: cellular microparticles: what are they bad or good for? J Thromb Haemost. 2004;2:1215–6. doi: 10.1111/j.1538-7836.2004.00797.x. [DOI] [PubMed] [Google Scholar]
- 9.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–51. [PubMed] [Google Scholar]
- 10.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 11.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 12.Key NS, Chantrathammachart P, Moody PW, Chang JY. Membrane microparticles in VTE and cancer. Thromb Res. 2010;125(Suppl 2):S80–3. doi: 10.1016/S0049-3848(10)70020-7. [DOI] [PubMed] [Google Scholar]
- 13.Zahra S, Anderson JAM, Stirling D, Ludlam CA. Microparticles, malignancy and thrombosis. Br J Haematol. 2011;152:688–700. doi: 10.1111/j.1365-2141.2010.08452.x. [DOI] [PubMed] [Google Scholar]
- 14.Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol. 2011;110:340–51. doi: 10.1152/japplphysiol.00811.2010. [DOI] [PubMed] [Google Scholar]
- 15.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 17.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 18.Théry C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–21. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–90. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 21.Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci U S A. 2011;108:20684–9. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2007;15:183–91. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 23.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.György B, Szabó T, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger É, Pap E, Kittel Á, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and Molecular Life Sciences. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dvorak HF, Quay SC, Orenstein NS, Dvorak AM, Hahn P, Bitzer AM, Carvalho AC. Tumor shedding and coagulation. Science. 1981;212:923–4. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 26.Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, Schifferli J, Guillevin L, Lesavre P, Halbwachs-Mecarelli L. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69:1416–23. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]
- 27.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 28.Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–21. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol. 2011;33:469–86. doi: 10.1007/s00281-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 30.Freyssinet JM, Toti F, Hugel B, Gidon-Jeangirard C, Kunzelmann C, Martinez MC, Meyer D. Apoptosis in vascular disease. Thromb Haemost. 1999;82:727–35. [PubMed] [Google Scholar]
- 31.Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med. 2004;53:210–30. doi: 10.2302/kjm.53.210. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–64. [PubMed] [Google Scholar]
- 33.Morel O, Jesel L, Freyssinet J-M, Toti F. Cellular Mechanisms Underlying the Formation of Circulating Microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 34.Chang CP, Zhao J, Wiedmer T, Sims PJ. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J Biol Chem. 1993;268:7171–8. [PubMed] [Google Scholar]
- 35.Lacroix R, Dubois C, Leroyer AS, Sabatier F, Dignat-George F. Revisited role of microparticles in arterial and venous thrombosis. J Thromb Haemost. 2013;11:24–35. doi: 10.1111/jth.12268. [DOI] [PubMed] [Google Scholar]
- 36.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davizon P, Lopez JA. Microparticles and thrombotic disease. Curr Opin Hematol. 2009;16:334–41. doi: 10.1097/MOH.0b013e32832ea49c. [DOI] [PubMed] [Google Scholar]
- 38.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34. [PubMed] [Google Scholar]
- 39.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–75. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 40.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 41.Camera M, Brambilla M, Toschi V, Tremoli E. Tissue factor expression on platelets is a dynamic event. Blood. 2010;116:5076–7. doi: 10.1182/blood-2010-09-307306. [DOI] [PubMed] [Google Scholar]
- 42.Bouchard BA, Mann KG, Butenas S. No evidence for tissue factor on platelets. Blood. 2010;116:854–5. doi: 10.1182/blood-2010-05-285627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Der Meijden PEJ, Van Schilfgaarde M, Van Oerle R, RennÉ T, Ten Cate H, Spronk HMH. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10:1355–62. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- 44.van Beers EJ, Schaap MCL, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, Meijers JCM, Biemond BJ. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin O, Delobel J, Prudent M, Lion N, Kohl K, Tucker EI, Tissot J-D, Angelillo-Scherrer A. Red blood cell–derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 46.Woodruff R, Sullenger B, Becker R. The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis. 2011;32:9–20. doi: 10.1007/s11239-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 47.Gruber A. The Role of the Contact Pathway in Thrombus Propagation. Thromb Res. 2014;133(Supplement 1):S45–S7. doi: 10.1016/j.thromres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Kushak RI, Nestoridi E, Lambert J, Selig MK, Ingelfinger JR, Grabowski EF. Detached endothelial cells and microparticles as sources of tissue factor activity. Thromb Res. 2005;116:409–19. doi: 10.1016/j.thromres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Steppich B, Mattisek C, Sobczyk D, Kastrati A, Schömig A, Ott I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb Haemost. 2005;93:35–9. doi: 10.1160/TH04-06-0393. [DOI] [PubMed] [Google Scholar]
- 50.Livaja Koshiar R, Somajo S, Norstrom E, Dahlback B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somajo S, Koshiar RL, Norström E, Dahlbäck B. Protein S and factor V in regulation of coagulation on platelet microparticles by activated protein C. Thromb Res. 2014;134:144–52. doi: 10.1016/j.thromres.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Tans G, Rosing J, Thomassen M, Heeb M, Zwaal R, Griffin J. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–8. [PubMed] [Google Scholar]
- 53.Lacroix R, Dignat-George F. Microparticles: new protagonists in pericellular and intravascular proteolysis. Semin Thromb Hemost. 2013;39:33–9. doi: 10.1055/s-0032-1333310. [DOI] [PubMed] [Google Scholar]
- 54.Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo S, Mezzapesa A, Anfosso F, Leroyer AS, Poullin P, et al. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012;97:1864–72. doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biro E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–8. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 57.Ramacciotti E, Hawley AE, Farris DM, Ballard NE, Wrobleski SK, Myers DD, Jr, Henke PK, Wakefield TW. Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis. Thromb Haemost. 2009;101:748–54. [PMC free article] [PubMed] [Google Scholar]
- 58.Cooley BC. Murine models of thrombosis. Thromb Res. 2012;129(Supplement 2):S62–S4. doi: 10.1016/j.thromres.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 59.Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–18. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 60.van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8:2596–607. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- 61.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 62.Connor DE, Exner T, Ma DDF, Joseph JE. Detection of the procoagulant activity of microparticle-associated phosphatidylserine using XACT. Blood Coagul Fibrinolysis. 2009;20:558–64. doi: 10.1097/MBC.0b013e32832ee915. [DOI] [PubMed] [Google Scholar]
- 63.Campello E, Spiezia L, Radu CM, Gavasso S, Woodhams B, Simioni P. Evaluation of a procoagulant phospholipid functional assay as a routine test for measuring circulating microparticle activity. Blood Coagul Fibrinolysis. 2014;25:534–7. doi: 10.1097/MBC.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 64.Haubold K, Rink M, Spath B, Friedrich M, Chun FK-H, Marx G, Amirkhosravi A, Francis JL, Bokemeyer C, Eifrig B, et al. Tissue factor procoagulant activity of plasma microparticles is increased in patients with early-stage prostate cancer. Thromb Haemost. 2009;101:1147–55. [PubMed] [Google Scholar]
- 65.Marco A, Brocal C, Marco P. Measurement of procoagulant activity of microparticles in plasma: Feasibility of new functional assays. Thromb Res. 2014;134:1363–4. doi: 10.1016/j.thromres.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 66.Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–23. doi: 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- 67.Lawrie AS, Albanyan A, Cardigan RA, Mackie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009;96:206–12. doi: 10.1111/j.1423-0410.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 68.Stagnara J, Garnache Ottou F, Angelot F, Mourey G, Seilles E, Biichlé S, Saas P, Racadot E. Correlation between platelet-derived microparticle enumeration by flow cytometry and phospholipid-dependent procoagulant activity in microparticles: The centrifugation step matters! Thromb Haemost. 2012;107:1185–7. doi: 10.1160/TH11-07-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss HJ. Scott syndrome: a disorder of platelet coagulant activity. Semin Hematol. 1994;31:312–9. [PubMed] [Google Scholar]
- 70.Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–15. [PubMed] [Google Scholar]
- 71.Burnouf T, Goubran HA, Chou M-L, Devos D, Radosevic M. Platelet microparticles: Detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Reviews. 2014;28:155–66. doi: 10.1016/j.blre.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev. 2013;27:179–84. doi: 10.1016/j.blre.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Franchini M. The modern treatment of haemophilia: a narrative review. Blood Transfus. 2013;11:178–82. doi: 10.2450/2012.0166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mobarrez F, Mikovic D, Antovic A, Antovic JP. Is a decrease of microparticles related to improvement of hemostasis after FVIII injection in hemophilia A patients treated on demand? J Thromb Haemost. 2013;11:697–703. doi: 10.1111/jth.12103. [DOI] [PubMed] [Google Scholar]
- 75.Federici AB. Clinical diagnosis of von Willebrand disease. Haemophilia. 2004;10:169–76. doi: 10.1111/j.1365-2516.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 76.Mannucci PM. Treatment of von Willebrand’s Disease. N Engl J Med. 2004;351:683–94. doi: 10.1056/NEJMra040403. [DOI] [PubMed] [Google Scholar]
- 77.Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977–2007) Haemophilia. 2008;14(Suppl 1):5–14. doi: 10.1111/j.1365-2516.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 78.Colucci G, Stutz M, Rochat S, Conte T, Pavicic M, Reusser M, Giabbani E, Huynh A, Thürlemann C, Keller P, et al. The effect of desmopressin on platelet function: a selective enhancement of procoagulant COAT platelets in patients with primary platelet function defects. Blood. 2014;123:1905–16. doi: 10.1182/blood-2013-04-497123. [DOI] [PubMed] [Google Scholar]
- 79.Trummer A, Haarmeijer B, Werwitzke S, Wermes C, Ganser A, Budde U, Tiede A. Increased amounts of von Willebrand factor are bound to microparticles after infusion of desmopressin. Haemophilia. 2013;19:236–41. doi: 10.1111/hae.12032. [DOI] [PubMed] [Google Scholar]
- 80.Tilley RE, Holscher T, Belani R, Nieva J, Mackman N. Tissue factor activity is increased in a combined platelet and microparticle sample from cancer patients. Thromb Res. 2008;122:604–9. doi: 10.1016/j.thromres.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toth B, Liebhardt S, Steinig K, Ditsch N, Rank A, Bauerfeind I, Spannagl M, Friese K, Reininger AJ. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb Haemost. 2008;100:663–9. [PubMed] [Google Scholar]
- 82.Thaler J, Ay C, Weinstabl H, Dunkler D, Simanek R, Vormittag R, Freyssinet JM, Zielinski C, Pabinger I. Circulating procoagulant microparticles in cancer patients. Ann Hematol. 2011;90:447–53. doi: 10.1007/s00277-010-1111-1. [DOI] [PubMed] [Google Scholar]
- 83.Laresche C, Pelletier F, Garnache-Ottou F, Lihoreau T, Biichle S, Mourey G, Saas P, Humbert P, Seilles E, Aubin F. Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma. J Invest Dermatol. 2014;134:176–82. doi: 10.1038/jid.2013.288. [DOI] [PubMed] [Google Scholar]
- 84.Toukh M, Siemens DR, Black A, Robb S, Leveridge M, Graham CH, Othman M. Thromboelastography Identifies Hypercoagulablilty and Predicts Thromboembolic Complications in Patients with Prostate Cancer. Thromb Res. 2014;133:88–95. doi: 10.1016/j.thromres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Trappenburg MC, van Schilfgaarde M, Bredewold EO, van Aalderen MC, Spronk HMH, ten Cate H, Leyte A, Terpstra WE. Elevated numbers and altered subsets of procoagulant microparticles in breast cancer patients using endocrine therapy. Thromb Res. 2011;127:363–9. doi: 10.1016/j.thromres.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 86.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 87.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–3. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 88.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125:511–2. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campello E, Spiezia L, Radu CM, Bulato C, Castelli M, Gavasso S, Simioni P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011;127:473–7. doi: 10.1016/j.thromres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Sartori MT, Della Puppa A, Ballin A, Saggiorato G, Bernardi D, Padoan A, Scienza R, d’Avella D, Cella G. Prothrombotic state in glioblastoma multiforme: an evaluation of the procoagulant activity of circulating microparticles. J Neurooncol. 2011;104:225–31. doi: 10.1007/s11060-010-0462-8. [DOI] [PubMed] [Google Scholar]
- 92.van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, Richel DJ, Büller HR, Sturk A, Nieuwland R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–5. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 93.Bharthuar A, Khorana AA, Hutson A, Wang J-G, Key NS, Mackman N, Iyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–4. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Sartori MT, Della Puppa A, Ballin A, Campello E, Radu CM, Saggiorato G, d’Avella D, Scienza R, Cella G, Simioni P. Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role. Thromb Haemost. 2013;110:378–85. doi: 10.1160/TH12-12-0957. [DOI] [PubMed] [Google Scholar]
- 95.Auwerda JJ, Yuana Y, Osanto S, de Maat MP, Sonneveld P, Bertina RM, Leebeek FW. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105:14–20. doi: 10.1160/TH10-03-0187. [DOI] [PubMed] [Google Scholar]
- 96.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–70. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 97.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bal L, Ederhy S, Di Angelantonio E, Toti F, Zobairi F, Dufaitre G, Meuleman C, Mallat Z, Boccara F, Tedgui A, et al. Circulating procoagulant microparticles in acute pulmonary embolism: a case-control study. Int J Cardiol. 2010;145:321–2. doi: 10.1016/j.ijcard.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 99.Ye R, Ye C, Huang Y, Liu L, Wang S. Circulating tissue factor positive microparticles in patients with acute recurrent deep venous thrombosis. Thromb Res. 2012;130:253–8. doi: 10.1016/j.thromres.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 100.Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 101.Ramacciotti E, Blackburn S, Hawley AE, Vandy F, Ballard-Lipka N, Stabler C, Baker N, Guire KE, Rectenwald JE, Henke PK, et al. Evaluation of soluble P-selectin as a marker for the diagnosis of deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17:425–31. doi: 10.1177/1076029611405032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bucciarelli P, Martinelli I, Artoni A, Passamonti SM, Previtali E, Merati G, Tripodi A, Mannucci PM. Circulating microparticles and risk of venous thromboembolism. Thromb Res. 2012;129:591–7. doi: 10.1016/j.thromres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 103.Ay C, Freyssinet J-M, Sailer T, Vormittag R, Pabinger I. Circulating procoagulant microparticles in patients with venous thromboembolism. Thromb Res. 2009;123:724–6. doi: 10.1016/j.thromres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Garcia Rodriguez P, Eikenboom HCJ, Tesselaar MET, Huisman MV, Nijkeuter M, Osanto S, Bertina RM. Plasma levels of microparticle-associated tissue factor activity in patients with clinically suspected pulmonary embolism. Thromb Res. 2010;126:345–9. doi: 10.1016/j.thromres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 105.Steppich BA, Hassenpflug M, Braun SL, Schomig K, von Beckerath O, von Beckerath N, Eckstein HH, Ott I. Circulating tissue factor and microparticles are not increased in patients with deep vein thrombosis. Vasa. 2011;40:117–22. doi: 10.1024/0301-1526/a000081. [DOI] [PubMed] [Google Scholar]
- 106.Rectenwald JE, Myers DD, Jr, Hawley AE, Longo C, Henke PK, Guire KE, Schmaier AH, Wakefield TW. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–7. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 107.Flores-Nascimento MC, Beltrame MP, De Paula EV, Montalvão SL, Pereira FG, Orsi FLA, Lorand-Metze I, Annichino-Bizzacchi JM. Microparticles in deep venous thrombosis, antiphospholipid syndrome and Factor V Leiden. Platelets. 2009;20:367–75. doi: 10.1080/09537100903096676. [DOI] [PubMed] [Google Scholar]
- 108.Shah MD, Bergeron AL, Dong J-F, López JA. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–72. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 109.Kim HK, Song KS, Lee ES, Lee YJ, Park YS, Lee KR, Lee SN. Optimized flow cytometric assay for the measurement of platelet microparticles in plasma: pre-analytic and analytic considerations. Blood Coagul Fibrinolysis. 2002;13:393–7. doi: 10.1097/00001721-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 110.György B, Pálóczi K, Kovács A, Barabás E, Bekő G, Várnai K, Pállinger É, Szabó-Taylor K, Szabó TG, Kiss AA, et al. Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb Res. 2014;133:285–92. doi: 10.1016/j.thromres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, Dignat-George F. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–46. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 112.Vidal C, Spaulding C, Picard F, Schaison F, Melle J, Weber S, Fontenay-Roupie M. Flow Cytometry Detection of Platelet Procoagulant Activity and Microparticles in Patients with Unstable Angina Treated by Percutaneous Coronary Angioplasty and Stent Implantation. Thromb Haemost. 2001;86:784–90. [PubMed] [Google Scholar]
- 113.Enjeti AK, Lincz LF, Seldon M. Detection and Measurement of Microparticles: An Evolving Research Tool for Vascular Biology. Semin Thromb Hemost. 2007;33:771–9. doi: 10.1055/s-2007-1000369. [DOI] [PubMed] [Google Scholar]
- 114.Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thromb Res. 2011;127:370–7. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 115.van der Heyde HC, Gramaglia I, Combes V, George TC, Grau GE. Flow cytometric analysis of microparticles. Methods Mol Biol. 2011;699:337–54. doi: 10.1007/978-1-61737-950-5_16. [DOI] [PubMed] [Google Scholar]
- 116.Iversen LV, Ostergaard O, Nielsen CT, Jacobsen S, Heegaard NH. A heparin-based method for flow cytometric analysis of microparticles directly from platelet-poor plasma in calcium containing buffer. J Immunol Methods. 2013;388:49–59. doi: 10.1016/j.jim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 117.Mobarrez F, Antovic J, Egberg N, Hansson M, Jörneskog G, Hultenby K, Wallén H. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb Res. 2010;125:e110–6. doi: 10.1016/j.thromres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Dey-Hazra E, Hertel B, Kirsch T, Woywodt A, Lovric S, Haller H, Haubitz M, Erdbruegger U. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag. 2010;6:1125–33. doi: 10.2147/VHRM.S13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shantsila E, Montoro-García S, Gallego P, Lip GYH. Circulating microparticles: challenges and perspectives of flow cytometric assessment. Thromb Haemost. 2014;111:1009–14. doi: 10.1160/TH13-11-0937. [DOI] [PubMed] [Google Scholar]
- 120.Shapiro HM. Practical Flow Cytometry. 4. New York: Wiley-Liss; 2003. pp. 4–5. [Google Scholar]
- 121.Zwicker JI, Trenor CC, 3rd, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31:728–33. doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chandler WL, Yeung W, Tait JF. A new microparticle size calibration standard for use in measuring smaller microparticles using a new flow cytometer. J Thromb Haemost. 2011;9:1216–24. doi: 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- 123.Mullier F, Bailly N, Chatelain C, Dogne JM, Chatelain B. More on: calibration for the measurement of microparticles: needs, interests, and limitations of calibrated polystyrene beads for flow cytometry-based quantification of biological microparticles. J Thromb Haemost. 2011;9:1679–81. doi: 10.1111/j.1538-7836.2011.04386.x. author reply 81–2. [DOI] [PubMed] [Google Scholar]
- 124.Robert S, Poncelet P, Lacroix R, Raoult D, Dignat-George F. More on: calibration for the measurement of microparticles: value of calibrated polystyrene beads for flow cytometry-based sizing of biological microparticles. J Thromb Haemost. 2011;9:1676–8. doi: 10.1111/j.1538-7836.2011.04387.x. author reply 81–2. [DOI] [PubMed] [Google Scholar]
- 125.Chandler WL, Yeung W, Tait JF. More on: calibration for the measurement of microparticles: the authors respond. Journal of Thrombosis and Haemostasis. 2011;9:1681–2. doi: 10.1111/j.1538-7836.2011.04283.x. [DOI] [PubMed] [Google Scholar]
- 126.O’Gorman MR, Thomas J. Isotype controls—time to let go? Cytometry. 1999;38:78–80. [PubMed] [Google Scholar]
- 127.Hulspas R, O’Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom. 2009;76B:355–64. doi: 10.1002/cyto.b.20485. [DOI] [PubMed] [Google Scholar]
- 128.Keeney M, Gratama JW, Chin-Yee IH, Sutherland DR. Isotype controls in the analysis of lymphocytes and CD34+ stem and progenitor cells by flow cytometry—time to let go? Cytometry. 1998;34:280–3. [PubMed] [Google Scholar]
- 129.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69A:1037–42. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 130.Trummer A, De Rop C, Tiede A, Ganser A, Eisert R. Isotype controls in phenotyping and quantification of microparticles: A major source of error and how to evade it. Thromb Res. 2008;122:691–700. doi: 10.1016/j.thromres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 131.György B, Módos K, Pállinger É, Pálóczi K, Pásztói M, Misják P, Deli MA, Sipos Á, Szalai A, Voszka I, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 132.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77A:502–14. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gelderman MP, Simak J. Functional Proteomics: Methods and Protocols. Clifton, N.J: Springer; 2008. Flow cytometric analysis of cell membrane microparticles; pp. 79–93. [DOI] [PubMed] [Google Scholar]
- 134.Aass HC, Ovstebo R, Troseid AM, Kierulf P, Berg JP, Henriksson CE. Fluorescent particles in the antibody solution result in false TF- and CD14-positive microparticles in flow cytometric analysis. Cytometry A. 2011;79A:990–9. doi: 10.1002/cyto.a.21147. [DOI] [PubMed] [Google Scholar]
- 135.van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost. 2012;10:919–30. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 136.Harrison P, Gardiner C. Invisible vesicles swarm within the iceberg. Journal of Thrombosis and Haemostasis. 2012;10:916–8. doi: 10.1111/j.1538-7836.2012.04711.x. [DOI] [PubMed] [Google Scholar]
- 137.Erdbrügger U, Rudy CK, Etter EM, Dryden KA, Yeager M, Klibanov AL, Lannigan J. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry Part A. 2014;85A:756–70. doi: 10.1002/cyto.a.22494. [DOI] [PubMed] [Google Scholar]
- 138.Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, Sampol J, Dignat-George F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–7. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 139.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–4. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 140.Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013;11:1190–3. doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tantawy AAG, Adly AAM, Ismail EAR, Habeeb NM, Farouk A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: Relation to cardiovascular complications. Platelets. 2013;24:605–14. doi: 10.3109/09537104.2012.749397. [DOI] [PubMed] [Google Scholar]
- 142.Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2004;125:804–13. doi: 10.1111/j.1365-2141.2004.04974.x. [DOI] [PubMed] [Google Scholar]
- 143.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 144.Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb Res. 2010;125(Supplement 2):S89–S91. doi: 10.1016/S0049-3848(10)70022-0. [DOI] [PubMed] [Google Scholar]
- 145.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 146.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Soderland C, Ahn YS. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003;123:896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 147.Vikerfors A, Mobarrez F, Bremme K, Holmström M, Ågren A, Eelde A, Bruzelius M, Antovic A, Wallén H, Svenungsson E. Studies of microparticles in patients with the antiphospholipid syndrome (APS) Lupus. 2012;21:802–5. doi: 10.1177/0961203312437809. [DOI] [PubMed] [Google Scholar]
- 148.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, Veit V, Combes V, Gentile S, Moal V, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–73. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 149.Jy W, Tiede M, Bidot CJ, Horstman LL, Jimenez JJ, Chirinos J, Ahn YS. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb Res. 2007;121:319–25. doi: 10.1016/j.thromres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 150.Joop K, Berckmans RJ, Nieuwland R, Berkhout J, Romijn FP, Hack CE, Sturk A. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–20. [PubMed] [Google Scholar]
- 151.Delabranche X, Boisrame-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, Grunebaum L, Lanza F, Gachet C, Freyssinet JM, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. 2013;39:1695–703. doi: 10.1007/s00134-013-2993-x. [DOI] [PubMed] [Google Scholar]
- 152.Tőkés-Füzesi M, Woth G, Ernyey B, Vermes I, Mühl D, Bogár L, Kovács GL. Microparticles and acute renal dysfunction in septic patients. J Crit Care. 2013;28:141–7. doi: 10.1016/j.jcrc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 153.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, ThM, Romijn FPH, Westendorp RGJ, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5. [PubMed] [Google Scholar]
- 154.Tan X, Shi J, Fu Y, Gao C, Yang X, Li J, Wang W, Hou J, Li H, Zhou J. Role of erythrocytes and platelets in the hypercoagulable status in polycythemia vera through phosphatidylserine exposure and microparticle generation. Thromb Haemost. 2013;109:1025–32. doi: 10.1160/TH12-11-0811. [DOI] [PubMed] [Google Scholar]
- 155.Trappenburg MC, van Schilfgaarde M, Marchetti M, Spronk HM, Cate Ht, Leyte A, Terpstra WE, Falanga A. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94:911–8. doi: 10.3324/haematol.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchetti M, Tartari CJ, Russo L, Panova-Noeva M, Leuzzi A, Rambaldi A, Finazzi G, Woodhams B, Falanga A. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am J Hematol. 2014;89:68–73. doi: 10.1002/ajh.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duchemin J, Ugo V, Ianotto J-C, Lecucq L, Mercier B, Abgrall J-F. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res. 2010;126:238–42. doi: 10.1016/j.thromres.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 158.Palkovits J, Novacek G, Kollars M, Hron G, Osterode W, Quehenberger P, Kyrle PA, Vogelsang H, Reinisch W, Papay P, et al. Tissue factor exposing microparticles in inflammatory bowel disease. J Crohns Colitis. 2013;7:222–9. doi: 10.1016/j.crohns.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 159.Deutschmann A, Schlagenhauf A, Leschnik B, Hoffmann KM, Hauer A, Muntean W. Increased procoagulant function of microparticles in pediatric inflammatory bowel disease: role in increased thrombin generation. J Pediatr Gastroenterol Nutr. 2013;56:401–7. doi: 10.1097/MPG.0b013e31827daf72. [DOI] [PubMed] [Google Scholar]
- 160.Andoh A, Tsujikawa T, Hata K, Araki Y, Kitoh K, Sasaki M, Yoshida T, Fujiyama Y. Elevated circulating platelet-derived microparticles in patients with active inflammatory bowel disease. Am J Gastroenterol. 2005;100:2042–8. doi: 10.1111/j.1572-0241.2005.50381.x. [DOI] [PubMed] [Google Scholar]
- 161.Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C, Xie R, Zhou J, Gilbert GE, Shi J. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb Haemost. 2012;107 doi: 10.1160/TH11-09-0673. [DOI] [PubMed] [Google Scholar]
- 162.Hugel B, Socié G, Vu T, Toti F, Gluckman E, Freyssinet J-M, Scrobohaci M-L. Elevated Levels of Circulating Procoagulant Microparticles in Patients With Paroxysmal Nocturnal Hemoglobinuria and Aplastic Anemia. Blood. 1999;93:3451–6. [PubMed] [Google Scholar]
- 163.Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, Arelaki S, Froudarakis M, Nakopoulou L, Giatromanolaki A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73:1854–63. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 164.Eleftheriou D, Hong Y, Klein NJ, Brogan PA. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J Thromb Haemost. 2011;9:1864–7. doi: 10.1111/j.1538-7836.2011.04434.x. [DOI] [PubMed] [Google Scholar]
- 165.Bretelle F, Sabatier F, Desprez D, Camoin L, Grunebaum L, Combes V, D’Ercole C, Dignat-George F. Circulating microparticles: a marker of procoagulant state in normal pregnancy and pregnancy complicated by preeclampsia or intrauterine growth restriction. Thromb Haemost. 2003;89:486–92. [PubMed] [Google Scholar]
- 166.Marques FK, Campos FMF, Filho OAM, Carvalho AT, Dusse LMS, Gomes KB. Circulating microparticles in severe preeclampsia. Clinica Chimica Acta. 2012;414:253–8. doi: 10.1016/j.cca.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 167.Lok CA, Jebbink J, Nieuwland R, Faas MM, Boer K, Sturk A, Van Der Post JA. Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am J Reprod Immunol. 2009;61:346–59. doi: 10.1111/j.1600-0897.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 168.Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, Nieuwland R. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:344–60. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 169.Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, Perez C, Saez C, Panes O, Matus V, et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95:94–9. [PubMed] [Google Scholar]
- 170.Nielsen CT, Ostergaard O, Johnsen C, Jacobsen S, Heegaard NH. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3067–77. doi: 10.1002/art.30499. [DOI] [PubMed] [Google Scholar]
- 171.Sellam J, Proulle V, Jungel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, et al. Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Campello E, Spiezia L, Radu CM, Bon M, Gavasso S, Zerbinati P, Woodhams B, Tormene D, Prandoni P, Simioni P. Circulating microparticles in carriers of factor V Leiden with and without a history of venous thrombosis. Thromb Haemost. 2012;108:633–9. doi: 10.1160/TH12-05-0280. [DOI] [PubMed] [Google Scholar]
- 173.Enjeti AK, Lincz LF, Scorgie FE, Seldon M. Circulating microparticles are elevated in carriers of Factor V Leiden. Thromb Res. 2010;126:250–3. doi: 10.1016/j.thromres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 174.Park MS, Owen BAL, Ballinger BA, Sarr MG, Schiller HJ, Zietlow SP, Jenkins DH, Ereth MH, Owen WG, Heit JA. Quantification of hypercoagulable state after blunt trauma: Microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–6. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morel N, Morel O, Petit L, Hugel B, Cochard JF, Freyssinet JM, Sztark F, Dabadie P. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma. 2008;64:698–704. doi: 10.1097/TA.0b013e31816493ad. [DOI] [PubMed] [Google Scholar]
- 176.Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801–9. doi: 10.1097/00005373-200105000-00005. [DOI] [PubMed] [Google Scholar]