Abstract
Background
The changing epidemiology of cardiac allograft rejection has prompted many to question the yield of surveillance endomyocardial biopsy (EMB) in heart transplant (HT) patients. We sought to determine the yield of EMB in the modern era.
Methods
We evaluated 2,597 EMBs in 182 consecutive HT patients who survived to their first EMB. EMBs were categorized as asymptomatic or clinically driven and were compared based on era of antiproliferative therapy use at our center (early azathioprine era: 1990–2000 vs. modern mycophenolate era: 2000–2011).
Results
In the modern era, patients had a higher prevalence of risk factors for developing rejection (≥ ISHLT grade 2R); however the frequency of rejection was decreased at all times (0–6 months: 60.2% vs. 21.5%, P < 0.001, 6–12 months: 26.8% vs. 1.8%, P < 0.001, 12–36 months: 32.3% vs. 10.5%, P = 0.006). The yield of asymptomatic EMB decreased in the modern era between 0–6 months (10.9% vs. 3.12%), 6–12 months (17% vs. 0 %), and years 2–3 (6.1% vs. 1.5%). In the early era, the odds ratio of rejection during asymptomatic EMB compared to a clinically driven EMB was 0.47 (95% CI: 0.31–0.71) and was decreased in the modern era (0.17[0.07–0.42], P = 0.04). The probability of detecting rejection on asymptomatic EMB was significantly reduced in the modern era, even after adjustment for tacrolimus and induction therapy (1% vs. 8%, P < 0.001).
Conclusions
The clinical yield of surveillance EMB has decreased in the modern era. EMB in asymptomatic patients >6 months after HT warrants further scrutiny.
Keywords: endomyocardial biopsy, surveillance, heart transplantation, allograft rejection
In the early years of cardiac transplantation, the prevalence of cardiac allograft rejection was significant and early detection and treatment was critical for patient survival. The development of the bioptome and techniques for endomyocardial biopsy (EMB) improved upon an otherwise non-specific clinical evaluation that assessed symptomatology, nonspecific laboratory markers and magnitude of voltage decrease on the electrocardiogram.(1)
Several concerns, however, have brought doubt to the utility of EMB in the modern era. With advancements in immune modulating therapies, patient/donor selection and organ preservation/surgical techniques, there has been a marked reduction in the incidence of acute allograft rejection.(2) In addition to causing patient discomfort, EMBs have been criticized for sampling error, poor sample quality and a high degree of inter-observer variability for the pathologic grading of tissue samples.(3) Furthermore, non-invasive tools for identifying rejection ranging from blood based biomarkers and gene expression profiling to imaging modalities, are increasing in prevalence.
Therefore, standardization of surveillance techniques and protocols has been controversial and there is marked variability in surveillance EMB protocols among transplant programs. Some centers discontinue protocol EMBs as early as 6 months while others continue through the first decade and beyond.(4) The changing epidemiology of allograft rejection has prompted many to question the utility of invasive EMB, therefore we examined application and utility of EMB at our institution since the introduction of the International Society of Heart and Lung Transplantation (ISHLT) biopsy grading criteria. We hypothesized that the clinical yield of surveillance EMB for detecting biopsy proven rejection decreased in the modern era.
Results
Patient Characteristics
We evaluated 2,597 EMBs performed in 182 patients in the first three years after undergoing heart transplantation. There were differences in established risk factors for cardiac allograft rejection when comparing patients from each era. Compared to patients from the early era (n = 89), patients from the modern era (n = 93) were older, had longer ischemic times and were more likely to be African American. Patients in the modern era were less likely to have had a female donor; however they were more likely to have been bridged to transplantation with a mechanical circulatory support device or received induction therapy (Table 1).
Table 1.
Clinical characteristics comparing patients in the early era to the modern era.
| All patients (N=187) | Early Era (N=89) | Modern Era (N=93) | P Value | |
|---|---|---|---|---|
| Age (years, mn±SD) | 45 ± 17 | 43 ± 18 | 48 ± 13 | 0.03 |
| African American (%) | 39% | 26% | 54% | 0.02 |
| Non-ischemic (%) | 67% | 62% | 72% | 0.13 |
| Female (%) | 26% | 31% | 23% | 0.19 |
| Female Donor (%) | 32% | 42% | 24% | 0.009 |
| Ischemic Time (min, mean ± SD) | 182 ± 60 | 162 ± 52 | 201 ± 53 | <0.001 |
| Maintenance Therapy | < 0.001 | |||
| CS + AZA (%) | 47% | 92% | 4% | |
| TAC + AZA (%) | 10% | 5% | 16% | |
| TAC + MMF (%) | 42% | 3% | 80% | |
| Induction Therapy | 59% | 44% | 80% | <0.001 |
| ATG (%) | 41% | 29% | 58% | |
| IL2-inhibitor (%) | 11% | 0% | 22% | |
| OKT3 (%) | 7% | 15% | 0% | |
| MCS (%) | 40% | 11% | 69% | <0.001 |
| Donor-Recipient Race | 47% | 38% | 55% | 0.03 |
| Mismatch (%) | ||||
| CMV Status | 0.3 | |||
| Donor − / Recipient − | 23% | 27% | 24% | |
| Donor + / Recipient + | 47% | 59% | 50% | |
| Donor − / Recipient + | 11% | 10% | 14% | |
| Donor + / Recipient − | 7% | 4% | 11% | |
ATG = anti-thymocyte globulin; CMV = cytomegalovirus; IL2 = interleukin 2; MCS = mechanical circulatory support; OKT3 = muromonab-CD3; SD = standard deviation.
Patients in the early era had increased prevalence of allograft rejection. The prevalence of biopsy proven rejection (ISHLT grade ≥ 2R) was increased in the early era compared to the modern era, at all examined time intervals (0–6 months: 60.2% vs. 21.5%, P < 0.001, 6–12 months: 26.8% vs. 1.8%, P < 0.001, 12–36 months 32.3% vs. 10.5%, P = 0.004). When adjusted for covariates known to or suspected of decreasing the incidence of a significant rejection (use of tacrolimus, older recipient age, Caucasian race, no VAD prior to transplant, use of induction therapy, male donor and lower ischemia time) we still found that at each time period the era was an independent predictor of biopsy proven rejection; for 0–6 months the adjusted P = 0.004, for 6–12 months the adjusted P= 0.044 and for 12–36 months the adjusted P= 0.016. No clinical characteristics were statistically different when comparing patients who suffered a rejection vs. patients without rejection within each era.
Biopsy Analysis
Patients underwent an average of 14.1 ± 8.6 biopsies in the first three years after heart transplantation (8.7 ± 3.7 in months 0–6 months, 2.0 ± 2.1 in months 6–12 and 3.5± 4.0 in months 12–36). The frequency of EMB in the early era was significantly higher when compared to the modern era, at all examined time points (all time points: 18.4 ± 10.3 vs. 9.9 ± 3.1; months 0–6: 10.4 ± 4.7 vs. 7.3 ± 1.5; months 6–12: 3.6 ± 2.2 vs. 1.1 ± 1.0; months 12–36: 7.2 ± 3.4 vs. 1.9 ± 1.8, All P’s < 0.001).
In the first year after transplantation, 8.5% (167/1964) of all EMBs performed yielded rejection. The percentage of EMBs yielding rejection in the early era was higher when compared to the modern era, at 0–6 months (12.3% [115/932] vs. 4.0% [27/683]) and 6–12 months (9.4% [24/255] vs. 1.1% [1/94]). For years two and three, 7.0% (44/633) of EMBs performed yielded rejection and the percentage of EMBs yielding rejection was similar in the early era and the modern era (7.6%, [37/487] vs 4.8%, [7/146]). The yield of EMB for detecting rejection is summarized in Table 2 and simple statistical comparisons were not performed on the raw proportions due to the variable number of repeated measures between cases.
Table 2.
Yield of biopsies for detecting rejection stratified by era and biopsy type.
| Time | All patients | Early Era | Modern Era | |||
|---|---|---|---|---|---|---|
| Biopsies (N) | % with ACR (N) | Biopsies (N) | % with ACR (N) | Biopsies (N) | % with ACR (N) | |
| All Biopsies | ||||||
| 0–6 mo | 1615 | 8.8% (142) | 932 | 12.3% (115) | 683 | 4.0% (27) |
| 6–12 mo | 349 | 7.2% (25) | 255 | 9.4% (24) | 94 | 1.1% (1) |
| 12–36 mo | 633 | 7.0% (44) | 487 | 7.6% (37) | 146 | 4.8% (7) |
| Surveillance (Asymptomatic) Biopsies | ||||||
| 0–6 mo | 1416 | 7.3% (104) | 774 | 10.9% (84) | 642 | 3.1 % (20) |
| 6–12 mo | 293 | 5.8% (17) | 215 | 7.9% (17) | 78 | 0% (0) |
| 12–36 mo | 535 | 5.0% (27) | 407 | 6.1% (25) | 128 | 1.6 % (2) |
| Clinically Indicated (Symptomatic) Biopsies | ||||||
| 0–6 mo | 199 | 19.1% (38) | 158 | 19.6%(31) | 41 | 17.1% (7) |
| 6–12 mo | 56 | 14.3% (8) | 40 | 17.5% (7) | 16 | 6.3% (1) |
| 12–36 mo | 98 | 38.6% (17) | 80 | 15.0% (12) | 18 | 27.8% (5) |
ACR = acute cellular rejection; mo = month.
To compare the yield of biopsies by era, a least squared means plot for the GLIMMIX analysis was utilized to accommodate the repeated observations (multiple biopsies) on subjects over time.
In the 0–6 month time period, there was a statistically significant difference in the probability of a positive biopsy between the two eras (11% in early era vs. 4% in modern era, P < 0.0001) and a trend towards statistical significance for the 6–12 month period (P = 0.058). There was no difference in the estimated probability of a positive biopsy for the 12–36 month time interval when comparing era (P=0.5).
Surveillance Biopsies
When selecting for only surveillance (asymptomatic) biopsies, 8.3% (159/1907) of all EMBs performed yielded rejection in the first year after transplantation. The percentage of surveillance EMBs yielding rejection in the early era was higher compared to the modern era for all time intervals examined (months 0–6:10.9% [84/774] vs. 3.1% [20/642]; months 6–12: 7.9% [17/215] vs. 0 % [0/78]; months 12–36: 6.1%, [25/407] vs. 1.6%, [2/128]). In the modern era, only two asymptomatic EMBs detected biopsy proven rejection more than 6 months after heart transplantation (Figure 1). Due to differences in the frequency of biopsies between patients (repeated measures), simple comparisons of the raw proportions were not performed, rather odds ratios and a general linear model analysis is presented below.
Figure 1.
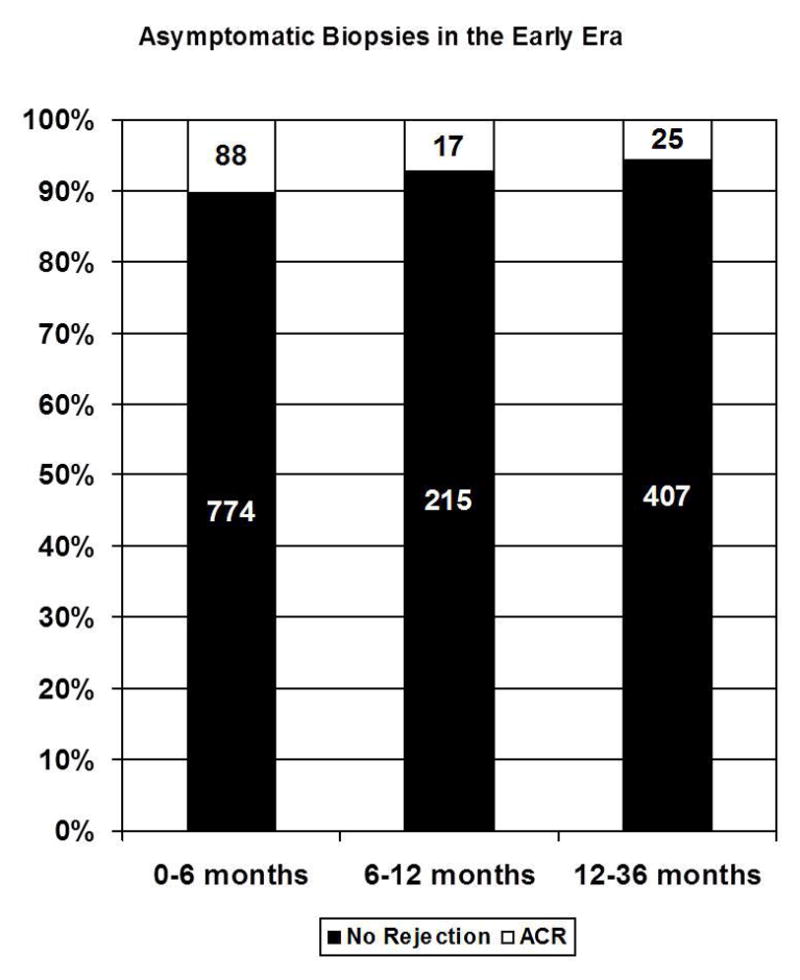
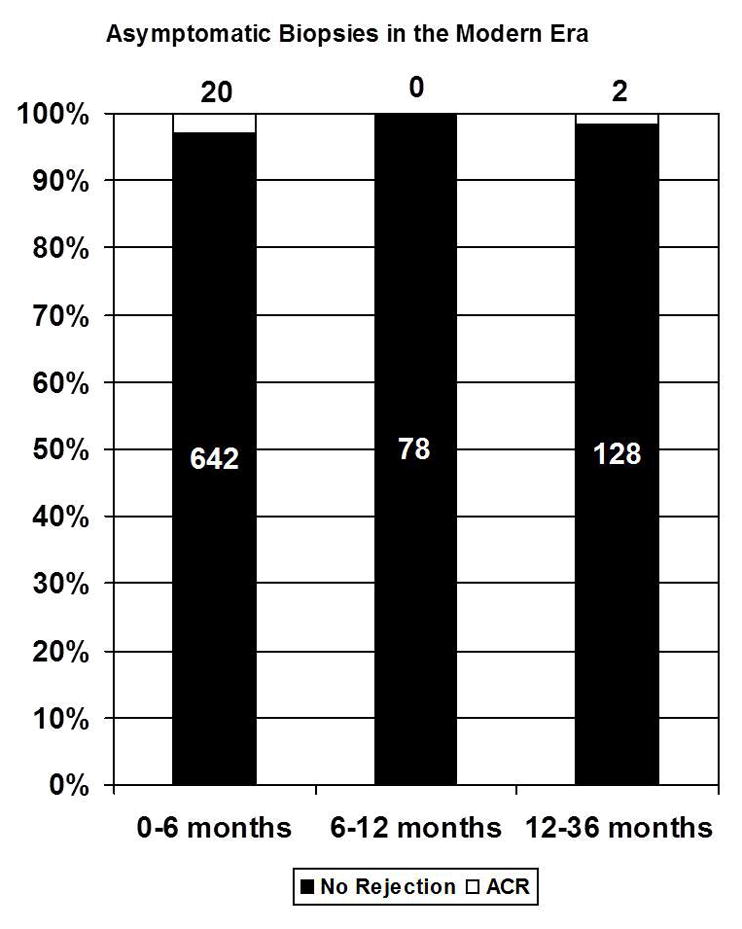
Stacked bar graphs showing the proportion of asymptomatic biopsies that yielded rejection in the (A) early and (B) modern era.
In the first year after heart transplantation, the odds ratio of detecting biopsy proven rejection during surveillance (asymptomatic) biopsy compared to a clinically indicated (symptomatic) biopsy was 0.47 (95% CI: 0.31–0.71) and was decreased in the modern era (0.17[0.07–0.42], P = 0.04) for each comparable time interval.
To compare the yields of surveillance and clinically indicated EMB by era, an additional least squares mean plot for a second GLIMMIX model was created (Figure 2). There was a significant era by biopsy type interaction, indicating that the difference between biopsy type cannot be interpreted without taking era into account. Post hoc tests revealed that there was no difference between the yields for clinically indicated biopsies in the two eras (P = 0.9), however there was a lower yield for surveillance biopsies in the modern era (P < 0.0001). Even after adjustment for use of induction therapy and tacrolimus, there was a significantly lower yield of surveillance EMB in the modern era (1% vs. 8%, P < 0.001)
Figure 2.
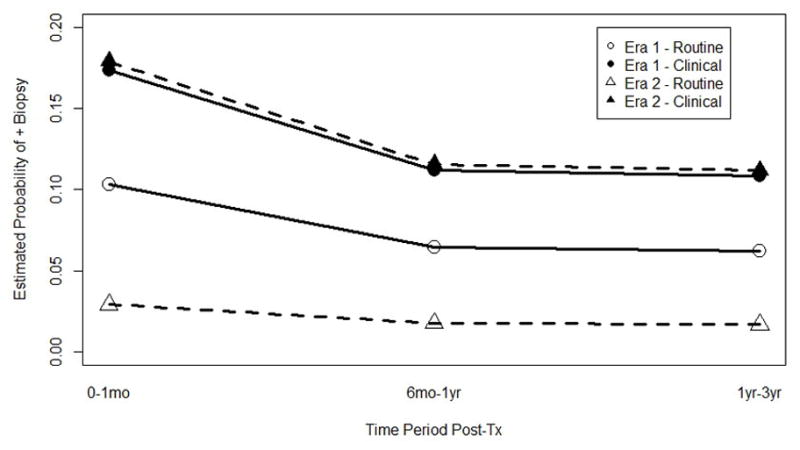
A least squared means plot for the GLIMMIX analysis shows the predicted probability of a positive biopsy by asymptomatic versus clinically indicated for the two era over the three time points.
Discussion
Since the introduction of MMF, the diagnostic yield and probability of EMB has decreased. This has occurred despite a decreasing frequency of EMB and an increasing risk profile for rejection in contemporary patients. We found the yield to be reduced to nearly nil in asymptomatic patients undergoing routine surveillance more than 6 months after heart transplantation.
These data describing the decreased incidence of biopsy proven rejection is consistent with single center studies and registry data.(5,6) In our study, the yield of asymptomatic EMB was low in the modern era despite a high proportion of African-American patients or transplanted from mechanical circulatory support, both considered risk factors for allograft rejection. The decrease in observed rejection may partly be explained by contemporary immunosuppression and the greater prevalence of induction therapy.
Previous prospective studies have proven that contemporary maintenance immune modulating therapies reduce the incidence of observed rejection early after transplantation. In addition to being better tolerated with less myelosuppression, MMF was shown to improve survival and reduce the incidence of rejection in the first year after HT when compared prospectively to azathioprine.(7,8) Similarly, tacrolimus reduced the incidence of moderate or severe rejection when compared to cyclosporine.(9) In our program, MMF and tacrolimus were used as standard therapy immediately after FDA approval in 1998 and 2006, respectively. Not surprisingly, the majority of the patients in the modern era who had less rejection were treated with the combination of MMF and tacrolimus.
The increased utilization of induction therapy in the modern era could also have contributed to the reduction in rejection. An increase in programmatic use of induction therapy occurred after an analysis of the Cardiac Transplant Research Database (CTRD) revealed a possible survival benefit with induction therapy for patients with known risk factors including younger age, African-American race, prolonged ventricular assist device support and ≥4 HLA mismatches.(10) In the modern era, the demographic composition of our patients has changed to that of a higher risk profile for developing rejection, thus protocol driven use of induction was increased. As the utility of induction therapy remains poorly defined and awareness of long term complication has increased,(11) enthusiasm for use in our practice is now limited only to pre-sensitized patients.
Advances in immune therapies alone likely do not completely explain the observed reduction in rejection. We found in this analysis that even after adjusting for type of calcineurin inhibitors or induction therapy, the low yield of asymptomatic rejection in the modern era persisted. In addition to modern immunosuppression, other difficult to quantify factors including more sensitive immunophenotyping and matching techniques, center experience and improved recipient and donor selection likely have impacted survival over time. The current generation of surgeons and cardiologists has built a knowledge base on the pioneering experiences of their predecessors.
Irrespective of the reasons, contemporary HT is associated with a low yield of detecting rejection on biopsies performed on patients without symptoms. Despite decades of utilizing EMB for HT, the current recommendations put forth by the ISHLT are nonspecific and are supported only with level C evidence (i.e. consensus opinion of experts or standard of care).(12) A few key studies have previously examined the utility of EMB for surveillance after heart transplantation, however none in a prospective and randomized design. In the largest available analysis, Stehlik and colleagues evaluated multi-institutional data from the CTRD comparing outcomes at various centers stratified by duration of biopsy surveillance.(4) The investigators concluded that there was no benefit of surveillance EMB beyond 5 years after transplant and that EMB should be continued in years 2 through 5 for high risk patients and African-American recipients. While this analysis represents the most comprehensive data on EMB after HT, the heterogeneous nature of the data did not allow adjustment for intensity or type of immunosuppression, nor did the analysis distinguish between clinically driven versus asymptomatic biopsies.
In contrast to the data published from the CTRD, we observed a low frequency of rejection in a population considered higher risk (high prevalence of African-Americans and mechanically supported patients). This may be explained by the fact that the CTRD findings predate the widespread acceptance of MMF and tacrolimus for cardiac transplantation thus may not reflect contemporary care.
Controlled studies defining the benefit of surveillance EMB have not been done. Recently, Orrego et al. shared their center’s experience with a conservative biopsy protocol, showing that complete discontinuation of EMB in asymptomatic patients at 6 months after heart transplant was feasible with acceptable outcomes.(13) Without a contemporaneous control group, the investigators could not define the effect and safety of their strategy.
Surveillance with EMB was compared to gene-expression profile testing in the multi-center Invasive Monitoring Attenuation through Gene Expression (IMAGE) trial.(14) Although the trial only included patients at low risk for rejection and was criticized for the clinical applicability of the results, there was no survival benefit observed in patients randomized to the EMB arm.(15) In light of the findings from the IMAGE trial, our study raises the question whether any asymptomatic patient should undergo EMB, or any rejection surveillance beyond 6 months after HT.
Rejection surveillance protocols differ from center to center as well as country to country. Protocols are often dictated by historic practice. A catastrophic event (late death from rejection) or a series of unanticipated rejection episodes may cause practitioners to be more conservative in approach. Frequency of EMB may be spurred by financial motivation. A fee for service system incentivizes the use of billable procedures, whereas a single payer model may explain the reduced enthusiasm for late EMB in Europe.
The present study has several limitations. This is only a single center report, molded by the practice biases of one institution and limited to the regional patient population it serves. On the other hand, this study design allows a focused evaluation of the effect of a single variable (in this case EMB) without the variability in practice patterns that limit interpretation of data aggregated from multiple transplant centers with heterogeneous practice patterns. The analysis was retrospective and observational in nature, thus we do not define harm or benefit for EMB due to the absence of a control group. Furthermore, subtle variations in immunosuppression regimens and patient adherence could not be fully accounted for in the analysis.
Conclusions
We found that the yield of surveillance EMB was decreased after the introduction of MMF, especially greater than 6 months after heart transplantation. The study cohort included a high prevalence of African-Americans and patients supported with mechanical circulatory support devices, both groups traditionally considered high risk for rejection. These data cast further doubt on the long held routine of long term surveillance with invasive and costly EMB. Based on these and previously published data, we would discourage performing surveillance EMB for asymptomatic patients greater than 6 months after transplantation. We believe that future prospective and comparative study of a biopsy-free surveillance protocol is warranted.
Methods and Materials
This was a single center retrospective study which included 208 consecutive patients who underwent heart transplantation in the calcineurin inhibitor era between January 1, 1990 and August 1, 2011. Twenty-six patients were not included in the study, leaving 182 for the biopsy analysis. The Virginia Commonwealth University Institutional Review Board reviewed and approved this project.
The patients were divided into two groups based on era of antiproliferative therapy. The early era (1990–May 4, 2000) was patients treated with azathioprine (AZA) and the modern era (May 5, 2000-present) included patients who were initially treated with mycophenolate mofetil (MMF). Early era patients received cyclosporine and azathioprine. Cyclosporine was initially unmodified (Sandimmune®). All patients were converted to the modified form of cyclosporine when it became available (1993–1994). Target trough levels were based on time post transplant and generally were greater than 250 ng/dl. Azathioprine was initially dosed at 150 mg/daily and adjusted to maintain a white blood cell count greater than 3.5K/uL. All patients received high dose prednisone initially with the intention to wean prednisone dose to zero or the lowest dose needed to prevent cellular rejection by one year after heart transplantation. Modern era patient received either modified cyclosporine or tacrolimus, once approved by the Food and Drug Administration for heart transplant in 2006. Mycophenolate mofetil was approved for heart transplant in 1998 and replaced azathioprine in the immunosuppression regimen. Initial tacrolimus levels for the first year were 10–15ng/dl and adjusted downward based on time post-transplant and rejection history. Twelve patients transplanted in the early era were excluded. Two patients were re-transplanted for primary graft failure and ten died prior to their initial biopsy. In the modern era fourteen patients were excluded. Two patients were transferred to the care of another transplant program and twelve patients died prior to an initial biopsy. We abstracted pertinent clinical data from the medical record and the patients’ information was de-identified. Data included patient demographics, medical history, laboratory data, donor demographics, and pathological grade for cellular rejection of all biopsies performed in the first three years after transplantation.
In the early era biopsies were routinely performed weekly for the first four weeks, every other week for the next two months, monthly for six months and then every other month until the patient’s first anniversary (approximately fourteen EMB). After the first year patients underwent EMB on a quarterly basis. This schedule persisted until death or loss of venous access. In the modern era the EMB schedule was the same for the first year and then quarterly until year five. Only clinically driven EMB were performed after that.
Pathological grades of all endomyocardial biopsies performed were maintained in each patient’s individual chart. Biopsy scores from the 1990 ISHLT scoring system were converted to the 2004 ISHLT scoring system. Therefore, a score of 1A, 1B or 2 was considered a 1R and a score of 3A or 3B was considered a 2R. Biopsies were further classified as “routine” if the biopsy was a result of scheduled surveillance or “clinical” if it was symptom driven or a biopsy following a rejection episode. Initial abstraction of categorization of biopsy data was performed by G.M. and adjudicated independently by M.P.F. and K.B.S.
Statistical Analysis
The PASW Statistics, version 17 (Chicago, Illinois) software package was used for most analyses. SAS version 9.3 was used for the generalized linear mixed model analysis presented. Continuous data are expressed as mean and standard deviations and categorical variables are expressed as percentages. A two tailed Student’s t-test was used to compare continuous variables. Chi-square analysis was used to compare discrete variables. Survival analysis comparing eras was performed using the Kaplan-Meier method and the log rank test was used to compare survival distributions. A generalized linear mixed model (GLIMMIX) with a binary distribution and logit link was used to analyze the difference between eras over three time points (0–6 months, 6 months–1 year and 1 year–3 year) on biopsy results (positive/negative). The GLIMMIX model had one between subject factor (Era: early era, modern era) and one within subjects factor (Time: 0–6 months, 6 –12 months, 12–36 months) and was utilized to accommodate the repeated observations (multiple biopsies) on subjects over time. For all analyses a P value < 0.05 was considered significant.
Abbreviations
- AZA
azathioprine
- CTRD
Cardiac Transplant Research Database
- EMB
endomyocardial biopsy
- FDA
Food and Drug Administration
- GLIMMIX
generalized linear mixed model
- HLA
human leukocyte antigen
- HT
heart transplant
- IMAGE
Invasive Monitoring Attenuation through Gene Expression
- ISHLT
International Society for Heart and Lung Transplantation
- MMF
mycophenolate mofetil
- VAD
ventricular assist device
Footnotes
Author Participation
Keyur B. Shah: research design, data collection, data analysis and manuscript formulation. No disclosures, no conflict of interest.
Maureen P. Flattery: data collection, data analysis and manuscript formulation. No disclosures, no conflict of interest.
Melissa C. Smallfield: data analysis and manuscript formulation. No disclosures, no conflict of interest.
Grace Merinar: data collection. No disclosures, no conflict of interest.
Daniel G. Tang: data analysis and manuscript formulation. No disclosures, no conflict of interest.
Emily H. Sheldon: statistical analysis.. Dr. Sheldon’s effort on this work was supported by NIH grant T32 ES07334.
Leroy R. Thacker: Statistical analysis. Dr. Thacker’s effort on this work was supported by NIH grant # UL1TR00058 from The National Center for Advancing Translational Sciences.
Vigneshwar Kasirajan: data analysis and manuscript formulation. No disclosures, no conflict of interest.
Richard H. Cooke: data analysis and manuscript formulation. No disclosures, no conflict of interest.
Michael L. Hess: data analysis and manuscript formulation. No disclosures, no conflict of interest.
References
- 1.Lower RR, Kontos HA, Kosek JC, Sewell DH, Graham WH. Experiences in heart transplantation. Technic, physiology and rejection. The American Journal of Cardiology. 1968;22:766. doi: 10.1016/0002-9149(68)90171-9. [DOI] [PubMed] [Google Scholar]
- 2.John R, Rajasinghe HA, Chen JM, et al. Long-term outcomes after cardiac transplantation: an experience based on different eras of immunosuppressive therapy. The Annals of Thoracic Surgery. 2001;72:440. doi: 10.1016/s0003-4975(01)02784-9. [DOI] [PubMed] [Google Scholar]
- 3.Marboe CC, Billingham M, Eisen H, et al. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. The Journal of Heart and Lung Transplantation. 2005;24:S219. doi: 10.1016/j.healun.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Stehlik J, Starling RC, Movsesian MA, et al. Utility of long-term surveillance endomyocardial biopsy: a multi-institutional analysis. The Journal of Heart and Lung Transplantation. 2006;25:1402. doi: 10.1016/j.healun.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Hamour IM, Burke MM, Bell AD, Panicker MG, Banerjee R, Banner NR. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation. 2008;85:969. doi: 10.1097/TP.0b013e318168d571. [DOI] [PubMed] [Google Scholar]
- 6.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report—2011. The Journal of Heart and Lung Transplantation. 2011;30:1078. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Ensley RD, Bristow MR, Olsen SL, et al. The use of mycophenolate mofetil (RS-61443) in human heart transplant recipients. Transplantation. 1993;56:75. doi: 10.1097/00007890-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa J, Miller L, Renlund D, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 9.Grimm M, Rinaldi M, Yonan NA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. American Journal of Transplantation. 2006;6:1387. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins R, Kirklin JK, Brown RN, et al. To induce or not to induce: do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy? The Journal of Heart and Lung Transplantation. 2005;24:392. doi: 10.1016/j.healun.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Aliabadi A, Grömmer M, Zuckermann A. Is induction therapy still needed in heart transplantation? Current Opinion in Organ Transplantation. 2011;16:536. doi: 10.1097/MOT.0b013e32834a8c61. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo MR, Costanzo MR, Dipchand A, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. The Journal of Heart and Lung Transplantation. 2010;29:914. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Orrego CM, Cordero-Reyes AM, Estep JD, Loebe M, Torre-Amione G. Usefulness of routine surveillance endomyocardial biopsy 6 months after heart transplantation. The Journal of Heart and Lung Transplantation. 2012;31:845. doi: 10.1016/j.healun.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. The New England Journal of Medicine. 2010;362:1890. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Parameshwar J. Gene expression profiling and cardiac allograft rejection monitoring: Is IMAGE just a mirage? The Journal of Heart and Lung Transplantation. 2010;29:599. doi: 10.1016/j.healun.2010.04.010. [DOI] [PubMed] [Google Scholar]


