Abstract
BACKGROUND
Currently, urine and blood are the only matrices authorized for antidoping testing by the World Anti-Doping Agency (WADA). Although the usefulness of urine and blood is proven, issues remain for monitoring some drug classes and for drugs prohibited only in competition. The alternative matrix oral fluid (OF) may offer solutions to some of these issues. OF collection is easy, noninvasive, and sex neutral and is directly observed, limiting potential adulteration, a major problem for urine testing. OF is used to monitor drug intake in workplace, clinical toxicology, criminal justice, and driving under the influence of drugs programs and potentially could complement urine and blood for antidoping testing in sports.
CONTENT
This review outlines the present state of knowledge and the advantages and limitations of OF testing for each of the WADA drug classes and the research needed to advance OF testing as a viable alternative for antidoping testing.
SUMMARY
Doping agents are either prohibited at all times or prohibited in competition only. Few OF data from controlled drug administration studies are available for substances banned at all times, whereas for some agents prohibited only in competition, sufficient data may be available to suggest appropriate analytes and cutoffs (analytical threshold concentrations) to identify recent drug use. Additional research is needed to characterize the disposition of many banned substances into OF; OF collection methods and doping agent stability in OF also require investigation to allow the accurate interpretation of OF tests for antidoping monitoring.
Athletes have doped to enhance sport performance for centuries (1). Ancient Greek athletes imbibed special food and stimulant potions to fortify themselves before events. In the 19th century, endurance athletes took cocaine and caffeine stimulants to improve performance. In 1928, the International Association of Athletics Federations banned the intake of all stimulants, but the first antidoping tests were not available until 1966, when introduced by the Union Cycliste Internationale and the Fédération Internationale de Football Association. After the 1998 Tour de France scandal, the International Olympic Committee, responsible for doping control, convened the 1st World Conference on Doping in Sport and created the World Anti-Doping Agency (WADA).2 The WADA code was established in 2003, harmonizing antidoping regulations across sports (2). The code undergoes revisions every 4 years, with the new code set for implementation in January 2015.
The Prohibited List, published each year since 2004, includes the list of banned substances and methods of doping (3). Substances are divided into 3 categories, forbidden at all times (in and out of competition), only in competition, or only in specified sports in competition. These 3 categories of banned substances are divided into different classes on the basis of their physicochemical and biological characteristics.
Until the 1994 Lillehammer games, when blood was first collected to detect transfusions, urine was the only specimen for antidoping testing. Urine is easily and noninvasively collected, available in sufficient volume for multiple analyses, and primarily contains drug metabolites with longer detection windows than parent drugs. Urine also has disadvantages that include the ease of adulteration and dilution, difficulty in obtaining samples due to postexercise dehydration, and windows of drug detection that may be too long for substances banned only in competition. Substances banned only in competition (classes S6–S9) can have long detection windows in urine, for example cannabinoids in the urine of chronic frequent cannabis smokers (4). Psychoactive Δ9-tetrahydrocannabinol (THC) is metabolized to inactive 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH), which may be excreted in urine for 30 days or more in chronic cannabis smokers, but in occasional smokers the period of excretion after cannabis use is approximately 5 days at the 15-μg/L cutoff. Therefore, availability of an alternative matrix with shorter detection times might better define drug use during competition.
The use of blood for the detection of drug use requires invasive collection, and the detection process is more complex than for urine owing to numerous cellular and protein constituents. Currently in antidoping laboratories, substances undetectable in urine, such as human growth hormone (hGH), are evaluated in blood. The powerful new athlete biological passport (ABP) serially monitors blood parameters in an athlete over time. Repeated measurements of hematological parameters allow athletes to serve as their own controls, reducing variability and improving doping detection (5).
Alternative matrices offer unique information on drug intake and have gained acceptance in toxicology, forensic, and clinical programs over the last 20 years (6). However, before adding a new matrix for antidoping, research comparing the advantages and limitations of any new antidoping matrix to urine and blood testing will be required owing to the multiple challenges that accompany any such change.
In this review, the current state of knowledge of oral fluid (OF) testing for each of the doping classes, and the advantages and limitations of OF for antidoping testing (Table 1) are critically discussed. Gaps in knowledge and the research needed before OF can be considered a viable alternative matrix for antidoping testing are specifically addressed.
Table 1.
Advantages, limitations, and knowledge gaps in key aspects of OF drug testing.
| Advantages | Limitations | Knowledge gaps | |
|---|---|---|---|
| OF collection | Easy, noninvasive; no need for privacy; sex neutral | Between- and within-device variability in drug recovery, stability, volume collected; dry mouth reduces OF volume; stimulation can affect test results | Establish a standardized procedure for sample collection |
| OF analysis | Basic drugs concentrate in OF; parent drug frequently present | Few stability data; low volume compared to urine; possible instrumentation issue due to OF buffer | Need to develop multianalyte LC-MS or GC-MS methods; need stability studies for all doping agents |
| Substance banned at all times | Endogenous steroids and peptide hormones detectable in OF; OF concentrations correlate better with blood than urine | Unable to differentiate endogenous production and exogenous contribution; T/E OF irrelevant; enzymatic activity in salivary glands; detection windows may be too short for synthetic steroids | Need to provide disposition data for majority of these compounds; evaluation of OF contamination when drugs given by spray; controlled administration studies needed |
| Substance banned in competition only | Several agents already extensively studied in OF; short detection windows; OF concentrations correlate well with blood | Weak bases sensitive to OF pH change; enzymatic activity in salivary glands for glucocorticosteroids | Need synthetic glucocorticosteroid data; evaluation of OF contamination when drugs given by spray (other than cannabinoids); controlled administration studies needed |
Drug Transfer into OF
The transfer of free drug from blood to OF occurs by different mechanisms dependent upon a drug's physicochemical properties (molecular weight, pKa, protein binding, liposolubility) (7). Passive diffusion occurs through the cell membrane down a concentration gradient for generally lipophilic, small (<500 Da), unconjugated molecules. Most drugs, with the exception of peptide or protein doping agents, transfer via passive diffusion. More polar metabolites, such as glucuronide and sulfate conjugates, transfer poorly into OF by this mechanism and are usually present in lower concentrations than parent drugs. High molecular weight compounds may bind to a transmembrane protein and be transferred into OF by active transport that also consumes energy (8). Small molecules <100 Da, such as ethanol, may transfer into OF by ultrafiltration through pores in the cell membrane; however, glucuronide and sulfate conjugates and larger molecules, up to 1900 Da, also can diffuse by ultrafiltration, but low OF concentrations are achieved.
Gross oral mucosal and OF contamination can occur following smoked, intranasal, or oral drug administration (9, 10). OF pH, generally 5–7, is an important factor influencing drug concentrations. Only nonionized drug crosses the membrane between blood and salivary glands, establishing equilibrium across these compartments. However, at the lower OF pH, basic drugs ionize, driving the equilibrium toward higher basic drug concentrations in OF, termed ion trapping. Stimulation of OF flow increases pH up to 8 owing to higher bicarbonate content potentially reducing ion trapping (7). This phenomenon could have practical implications for antidoping testing because many doping agents are weak bases with pKa values below 8.5. Lowering OF pH by stimulating OF flow rates with chewing gum or citric acid before sample collection could decrease drug OF concentrations of basic drugs, possibly leading to false-negative test results. For basic compounds with pKa >8.5, acidic compounds with pKa <5.5, or neutral compounds, a change of OF pH has little effect on OF concentrations because percentages of drug present as ionized or neutral forms remains unchanged with minor pH changes.
Substances Prohibited in Competition Only
The goal for antidoping testing for substances prohibited in competition only is to detect recent drug use. Samples are collected just after competition, requiring short drug detection windows to avoid identification of much earlier intake. One of the main advantages of OF for antidoping testing is the ability to define analytes and cutoffs to provide short detection windows, as shown in Table 2, which summarizes the different controlled drug administration studies. Many of this class of doping agents are illicit drugs of abuse whose OF pharmacokinetics have been characterized.
Table 2.
Oral fluid concentrations after controlled drug administration.
| Doping agent class | Drug | Doses | Administration types | OF collection durations (collection method) | Analytes | LOQ (method) | Detection windows (cutoffs) | Referencea |
|---|---|---|---|---|---|---|---|---|
| S6: Stimulant | Cocaine | 15 and 40 mg | i.v. | 5 h (expectoration) | Cocaine | 5 μg/L (GC-MS) | 5 h (LOQ) | (12) |
| 25 mg | i.v. | 12 h (expectoration) | Cocaine, BE | 1 μg/L (GC-MS) | Cocaine, 5 h; BE, 7 h (8 μg/L DHHS) | (13) | ||
| 32 mg | Intranasal | 12 h (expectoration) | Cocaine, BE | 1 μg/L (GC-MS) | Cocaine, 6 h; BE, 9 h (8 μg/L DHHS) | (13) | ||
| 42 mg | Smoked | 12 h (expectoration) | Cocaine, BE | 1 μg/L (GC-MS) | Cocaine, 4 h; BE, 5 h (8 μg/L DHHS) | (13) | ||
| Up to 2 g/day for 16 days |
Oral | 120 h (expectoration) | Cocaine, BE | 1 μg/L (GC-MS) | Cocaine, 21 h; BE, 50 h (8 μg/L DHHS) | (13) | ||
| 75 mg/70 kg body |
Subcutaneous | 48 h (expectoration) | Cocaine, BE, EME | 2.5 μg/L (GC-MS) | Cocaine, 11.5 h/8 h/8 h (LOQ/8 μg/L SAMSHA/10 μg/L DRUID); BE, 32 h/28 h/28 h (LOQ/8 μg/L SAMSHA/10 μg/L DRUID); EME, 28 h/24 h/24 h (LOQ/8 μg/L SAMSHA/ 10 μg/L DRUID) |
(14) | ||
| 150 mg/70 kg body |
Subcutaneous | 48 h (expectoration) | Cocaine, BE, EME | 2.5 μg/L (GC-MS) | Cocaine, 11.5 h/8 h/8 h (LOQ/8 μg/L SAMSHA/10 μg/L DRUID) BE, 32 h/28 h/28 h (LOQ/8 μg/L SAMSHA/10 μg/L DRUID) EME, 28 h/24 h/24 h (LOQ/8 μg/L SAMSHA/ 10 μg/L DRUID) |
(14) | ||
| 3 mg | Oral | 48 h (Salivette device) | Cocaine, EME, BE | 10 μg/L (GC-MS) | Cocaine, 1 h; EME, 3 h; BE, 10 h (LOQ) | (15) | ||
| S6: Stimulant | MDMA | 1 mg/kg body | Oral | 143 h (expectoration) | MDMA, MDA, HMA, HMMA |
MDMA, MDA, 5 μg/L (GC-MS); HMMA, HMA, 10 μg/L (GC- MS) |
MDMA, 36.5 h/23 h/23 h (LOQ/50 μg/L SAMSHA/25 μg/L DRUID) MDA, 23 h/13 h/13 h (LOQ/50 μg/L SAMSHA/25 μg/L DRUID) HMA and HMMA undetected |
(16) |
| 1.6 mg/kg body |
Oral | 143 h (expectoration) | MDMA, MDA, HMA, HMMA |
MDMA, MDA, 5 μg/L (GC-MS) HMMA, HMA, 10 μg/L (GC- MS) |
MDMA, 47 h/29 h/43 h (LOQ/50 μg/L SAMSHA/25 μg/L DRUID) MDA, 39 h/15 h/23 h (LOQ/50 μg/L SAMSHA/25 μg/L DRUID) HMA, HMMA undetected |
(16) | ||
| S6: Stimulant | METH | 10 mg | Oral | 24 h (expectoration) | METH, AMP | 2.5 μg/L (GC-MS) | METH, 24 h/11.5 h (LOQ/50 μg/L METH + AMP ≥LOQ SAMSHA) |
(17) |
| 20 mg | Oral | 24 h (expectoration) | METH, AMP | 2.5 μg/L (GC-MS) | METH, 24 h/11.5 h (LOQ/50 μg/L METH + AMP ≥LOQ SAMSHA) |
(17) | ||
| S6: Stimulant | Pseudoephedrine | 60 mg | Oral | 24 h (Salivette device) | PE, cathine | 5 μg/L (GC-MS) | PE, 12 h (LOQ); cathine, undetected | (18) |
| 120 mg | Oral | 24 h (Salivette device) | PE, cathine | 5 μg/L (GC-MS) | PE, 14 h (LOQ); cathine, undetected | (18) | ||
| S6: Stimulant | Modafinl | 100 mg | Oral | 24–18 h (Salivette device) |
Modafinl | 5 μg/L (GC-MS) | 24 h (LOQ) | (15) |
| S6: Stimulant | Seleginine | 10 mg | Oral | 24–48 h (Salivette device) |
Seleginine, AMP, METH |
Seleginine, METH, 10 μg/L, AMP, 25 μg/L (GC-MS) |
Seleginine: 3 h; METH, 15 h; AMP, undetected (LOQ) |
(15) |
| S6: Stimulant | Crotetamide, cropropamide |
50 mg each | Oral | 24–48 h (Salivette device) |
Crotetamide, cropropamide |
5 μg/L (GC-MS) | 24 h (LOQ) | (15) |
| S6: Stimulant | Pentetrazol | 100 mg | Oral | 24–48 h (Salivette device) |
Pentetrazol | 10 μg/L (GC-MS) | 8 h (LOQ) | (15) |
| S6: Stimulant | Ephedrine | 12 mg | Oral | 24–48 h (Salivette device) |
Ephedrine | 10 μg/L (GC-MS) | 48 h (LOQ) | (15) |
| S6: Stimulant | Sibutramine | 10 mg | Oral | 24–48 h (Salivette device) |
Sibutramine | 10 μg/L (GC-MS) | Undetected | (15) |
| S7: Narcotic | Morphine | 10 or 20 mg | IM | 24 h (expectoration) | Morphine | 0.6 μg/L (RIA) | 24 h (LOQ) | (18) |
| S7: Narcotic | Heroin | 12 mg | IN | 24 h (expectoration) | Heroin, 6-AM, morphine |
1 μg/L (GC-MS) | Heroin, 1 h; 6-AM, morphine, 3 h (LOQ) | (20) |
| 2.5 mg | Smoked | 24 h (expectoration) | Heroin, 6-AM, morphine | 1 μg/L (GC-MS) | Heroin, 2 h; 6-AM, 0.5 h; morphine, 2 h (LOQ) |
(21) | ||
| 10.5 mg | Smoked | 24 h (expectoration) | Heroin, 6-AM, morphine |
1 μg/L (GC-MS) | Heroin, 24 h; 6-AM, 2 h; morphine, 8 h (LOQ) |
(21) | ||
| 3 mg | i.v. | 24 h (expectoration) | Heroin, 6-AM, morphine |
1 μg/L (GC-MS) | Heroin, 5 min; 6-AM, 30 min; morphine, 2 h (LOQ) |
(21) | ||
| 12 mg | i.v. | 24 h (expectoration) | Heroin, 6-AM, morphine |
1 μg/L (GC-MS) | Heroin, 12 h; 6-AM, 4 h; morphine, 8 h (LOQ) |
(21) | ||
| S8: Cannabinoid | Cannabis | 20 mg | Oral | 5 h (expectoration) | THC, THCCOOH, CBN, CBD, 11-OH- THC |
THC, CBD, 11-OH-THC: 0.25 μg/L, CBN: 1 μg/L, THCCOOH: 5 ng/L (2DGC-MS) |
THC, THCCOOH, 5 h; CBD, CBN, 11-OH-THC undetected (LOQ) |
(25) |
| 20 mg | Oral | 5 h (Quantisal device) | THC, THCCOOH, CBN, CBD, 11-OH- THC |
THC, CBD, 11-OH-THC, 0.5 μg/ L; CBN, 1 μg/L; THCCOOH, 7.5 ng/L (2DGC-MS) |
THC, THCCOOH, 5 h; CBD, CBN, 11-OH-THC undetected (LOQ) |
(26) | ||
| 6.8% THC | Smoked | 22 h (expectoration) | THC, THCCOOH, CBN, CBD, 11-OH- THC |
THC, CBD, 11-OH-THC, 0.25 μg/L; CBN, 1 μg/L; THCCOOH, 5 ng/L (2D-GC- MS) |
THC, 6 h/6 h (2 μg/L SAMSHA/1 μg/L DRUID) THCCOOH, 6 h (20 μg/L) CBD, 2 h (1 μg/L) CBN, 4 h (LOQ) 11-OH-THC, undetected (LOQ) |
(9) | ||
| 6.8% THC | Smoked | 22 h (Quantisal device) |
THC, THCCOOH, CBN, CBD, 11-OH- THC |
THC, CBD, 11-OH-THC: 0.5 μg/ L, CBN: 1 μg/L, THCCOOH: 7.5 ng/L (2D-GC-MS) |
THC, 6 h/6 h (2 μg/L SAMSHA/1 μg/L DRUID) THCCOOH, 6 h (20 μg/L) CBD, 4 h (LOQ) CBN, 3.5 h (LOQ) 11-OH-THC, undetected (LOQ) |
(10) | ||
| S8: Cannabinoid | Mixture of synthetic cannabinoids |
500 mg | Smoked | 90 h (RapidEase device) |
JWH-018, JWH-200, JWH-210 |
0.2 μg/L (LC-MS/MS) | JWH-018, 19 h (LOQ) JWH-200, 3 h (LOQ) JWH-210, 21 h (LOQ) |
(30) |
| S8: Cannabinoid | AM2201 | 5 mg | Oral | 25 h (RapidEase device) |
AM2201 | 0.2 μg/L (LC-MS/MS) | AM2201, undetected (LOQ) | (30) |
| S1: Anabolic agent |
Testosterone | 4× 1.5 mg/kg body |
Transdermal | 3 weeks (expectoration) |
Testosterone | 1.9 ng/L (ELISA) | >1 Week after last administration (LOQ) | (39) |
| S1: Anabolic agent |
Oxymetholone | 50 mg | Oral | 24 h (Unknown method) |
Oxymetholone | 1 μg/L (GC-MS) | 2 h (LOQ) | (43) |
| S1: Anabolic agent |
Tibolone | 2.5 mg | Oral | 1 h (expectoration) | Tibolone | 0.1 μg/L (GC-MS) | >1 h (LOQ) | (44) |
| P1: Alcohol | Alcohol | 1g/kg body | Oral | 24 h (Statsure saliva sampler device) |
Ethanol, EtG | 4.4 μg/L (LC-MS/MS) | Ethanol, 5.5 h (LOQ); ethanol-G: 11.5 h (LOQ) |
(65) |
| Alcohol | 0.5g/kg body | Oral | 24 h (Statsure saliva sampler device) |
Ethanol, EtG | 4.4 μg/L (LC-MS/MS) | Ethanol, 3.5 h (LOQ); EtG, 3.5 h (LOQ) | (65) |
References referred to within this table: Milman et al. (9); Lee et al. (10); Cone et al. (12); Jufer et al. (13); Scheidweiler et al. (14); Strano-Rossi et al. (15); Barnes et al. (16); Schepers et al. (17); Strano-Rossi et al. (18); Wang et al. (20); Jenkins et al. (21); Milman et al. (25); Milman et al. (26); Kneisel et al. (30); Schönfelder et al. (39); Cardoso et al. (43); Sipoli Marques et al. (44); Hoiseth et al. (65).
S6: STIMULANTS
Stimulants, except topical imidazole derivatives and those in the WADA monitoring program, are banned in competition and currently monitored in urine in microgram-per-liter concentrations. The 5 most commonly identified stimulants in antidoping testing in 2011 were methylhexaneamine, amphetamine (AMP), cocaine, methylphenidate, and ephedrine (11).
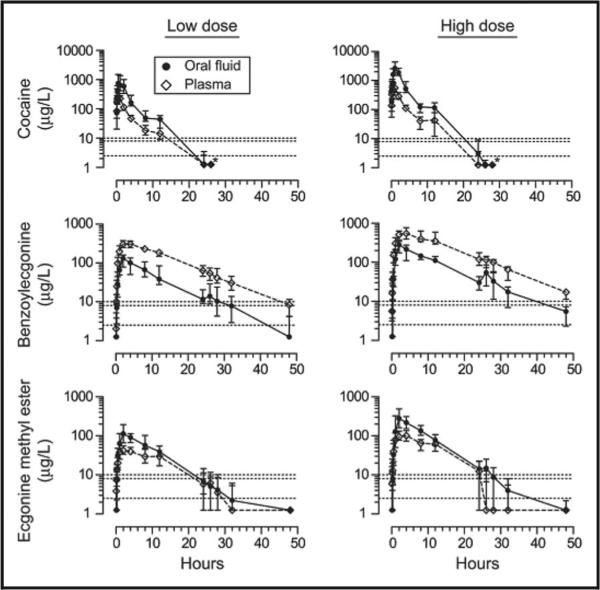
OF and plasma cocaine dispositions were explored following 15 and 40 mg intravenous (i.v.) cocaine separated by 48 h (12). With a limit of quantification (LOQ) of 5 μg/L in a GC-MS assay, OF cocaine was quantifiable throughout a 5-h period (last measurement) for both doses, yielding a cocaine half-life of 30 min. Although OF and plasma concentrations were temporally correlated, high intersubject OF variability precluded prediction of plasma concentrations from OF results. After participants received 25 mg i.v., 32 mg intranasal, or 42 mg smoked cocaine, OF and urine were collected for 12 and 72 h, respectively (13). Mean last OF detection times were 4–8 h for cocaine and benzoylecgonine (BE), the primary inactive cocaine metabolite, with 8-μg/L cutoffs. Last BE urine detection times were more than 40 h at both 100- and 150-μg/L cutoffs. Recently, after 75 and 150 mg/70 kg subcutaneous cocaine (Fig. 1), the last OF detection times (LOQ, 2.5 μg/L) were 11.5 and 17.7 h, respectively, for cocaine, 32 and 47 h for BE, and 28 and 32 h for ecgo-nine methyl ester (EME) (14). With the application of the higher Driving under the Influence of Drugs, Alcohol and Medicines (DRUID) or Substance Abuse and Mental Health Services Administration (SAMSHA) cutoffs of 10 and 8 μg/L, respectively, detection windows were 8 and 9.8 h for cocaine at low and high doses, 28 and 32 h for BE, and 24.1 and 26 h for EME. Although both cocaine and BE monitoring were essential to widen the detection window, EME contributed no additional positive samples.
Fig. 1. Median cocaine, BE, and ecgonine methyl ester OF and plasma concentrations after a single subcutaneous cocaine dose of 75 mg/70 kg (low dose n = 19 for OF, 17 for plasma) or 150 mg/70 kg (high dose, n = 14 for OF, 13 for plasma).
Bars are interquartile ranges. Asterisks indicate that all subsequent samples contained less than 2.5 μg/L cocaine. Dotted lines indicate the following cutoff concentrations: limits of quantification (2.5 μg/L), SAMHSA (8 μg/L), and DRUID (10 μg/L) Reproduced with permission from Scheidweiler et al. (14).
Participants received 5 consecutive hourly oral cocaine doses per day for 16 days, with 25-mg increasing doses to a maximum cumulative dose of 2 g (13). Large total doses could be achieved with the oral route, but dosing stopped if cardiovascular safety parameters were reached. Mean OF detection windows were 21 and 50 h for cocaine and BE, with 8-μg/L cutoffs when expectorated OF with citric acid–sour candy stimulation was collected for up to 120 h. In urine, at a 100-μg/L cutoff, BE detection windows were 105 h. After 1 female received 3 mg oral cocaine, cocaine, EME, and BE OF detection windows with 48 h of monitoring were 1, 3, and 10 h, whereas in urine they were 4, 36, and 36 h with 10-μg/L cutoffs (15).
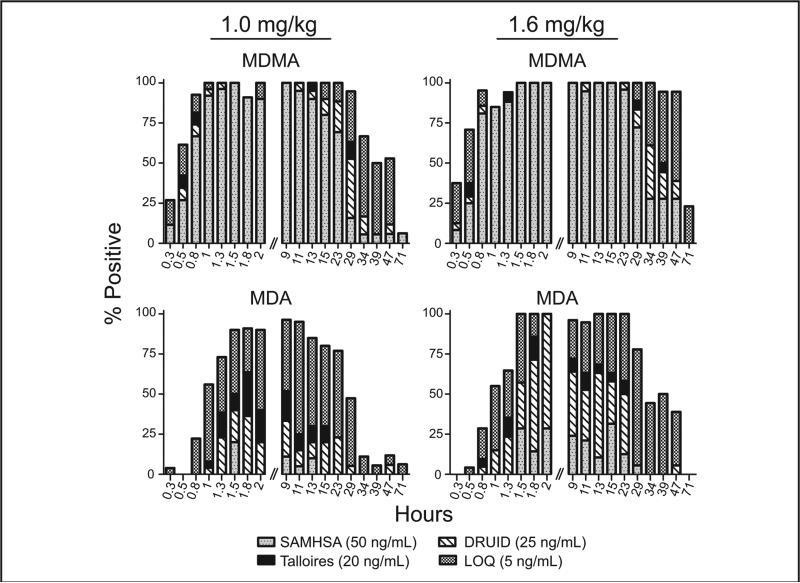
Following oral 1- and 1.6-mg/kg doses of 3,4-methylenedioxymethamphetamine (MDMA), OF MDMA and its metabolite 3,4-methylenedioxyamphetamine (MDA) were identified for 47 and 29 h, respectively, at the proposed SAMSHA 50-μg/L cutoffs, although monitoring continued for 7 days (16) (Fig. 2). These short detection time windows contributed to revised proposed SAMHSA guidelines of 25 μg/L, in accord with DRUID 25-μg/L cutoffs for stimulants. 4-Hydroxy-3-methoxymethamphetamine, and 4-hydroxy-3-methoxyamphetamine were not detected in OF.
Fig. 2. Detection rates (% positive) for MDMA and MDA in OF collected via expectoration 0.25–71 h following single oral doses of 1.0 or 1.6 mg/kg MDMA given to healthy adult MDMA users; n = 27 and 25 for doses of 1.0 and 1.6 mg/kg, respectively.
Detection rates were calculated with the LOQ (5 ng/mL) and 3 recommended cutoff concentrations, Talloires: International Meeting in Talloires, France (Sept., 2006) (20 ng/mL) (16), DRUID (25 ng/mL), and SAMHSA (50 ng/mL). Bars are not visible if the detection rate is the same as the higher cutoff concentration. Reproduced with permission from Barnes et al. (16).
After 10- and 20-mg oral methamphetamine (METH) doses, OF detection windows were 11.5 h and 24 h at the 2004 proposed SAMSHA cutoffs of METH ≥50 μg/L + AMP ≥2.5 μg/L LOQ (17). After low and high doses, 29% and 60% of OF samples were positive at 11.5 h and 0% and 20% at 24 h. When four 10- or 20-mg doses were administered within 7 days, 24 h after the last dose 33% and 80% of samples, respectively, were positive; none were positive 48 h after the last dose.
Disposition of oral modafinil (100 mg), selegiline (10 mg), crotetamide/cropropamide (50 mg each), pentetrazol (100 mg), ephedrine (12 mg), and sibutramine (10 mg) into OF for 24–48 h and urine for 72 h was evaluated in 5 females (15). All drugs except sibutramine were measureable in the first sample at 1 h. Last detection times were shorter in OF than urine (3–48 h vs 4–72 h), suggesting OF for prohibited-in-competition testing. Sibutramine and its metabolites were not detected in OF. After 4 males and 4 females received 60 and 120 mg oral pseudoephedrine, OF peak concentrations occurred in 2–4 h (18). OF pseudoephedrine detection windows with a 5-μg/L cutoff were 10–14 h and 28–32 h respectively in OF and urine, with high inter-subject variability, when OF was monitored for 24 h and urine for 32 h. Cathine was not detected in OF, but urine cathine was detected for 32 h.
All of these controlled stimulant administration studies support short OF detection windows from hours toafewdays,providinggoodevidenceoftheusefulnessof OF as a matrix to detect stimulant use in competition only. Additional research is required to characterize OF disposition in the other most commonly detected stimulants, methylhexaneamine and methylphenidate. OF detection should not be challenging, because these basic drugs should be present in high concentrations.
S7: NARCOTICS
Narcotics, including natural opiates (morphine) and synthetic opioids (oxycodone), are rarely identified in antidoping samples, representing only 0.4% of reported adverse (and atypical) analytical findings (AAF) (11). After a single 10- or 20-mg morphine intramuscular injection to 2 male study participants, maximum OF morphine concentrations (10.8 and 37.8 μg/L) occurred at 0.5 h and were detected in OF for 24 h, but in urine morphine was detected for 6 days with the same RIA and an LOQ of 0.6 μg/L (19). After a 12-mg dose of intranasal heroin, heroin, 6-acetylmorphine (6AM), and morphine were detected in OF for 1, 3, and 3 h, respectively, with a 1-μg/L LOQ (20). Detection windows depended upon dose and administration route; low 3-mg i.v. doses yielded short heroin detection windows of 5 min, whereas a 10.5-mg dose of smoked heroin was detected for 2 h. 6AM and morphine concentrations followed similar patterns, with longer detection windows for morphine than 6AM and heroin (120 vs 30 and 5 min, respectively) following i.v. dosing (21). Plasma and OF heroin concentrations were highly correlated, with a 1:1 ratio; however, in these older studies, OF was collected with citric acid stimulation, which could decrease basic OF drug concentrations, potentially shortening detection windows. Additional research is needed with OF collection devices and stabilization buffers to provide data about OF opioid stability.
S8: CANNABINOIDS
Cannabinoids are the third most common doping agents after anabolic steroids and stimulants (11). Currently, urinary THCCOOH measurement with a 15-μg/L threshold identifies cannabis intake. THCCOOH may be present in chronic, frequent cannabis smokers’ urine for 30 days or longer at this threshold (5, 22), whereas the detection time in less-than-daily cannabis smokers was approximately 5 days (23). THC-glucuronide, cannabidiol (CBD), and cannabinol (CBN) are good markers of recent cannabis smoking in urine, but are inclusionary, not exclusionary. If identified, recent smoking is indicated even in chronic cannabis smokers, but all are present for only a short time in urine and not in all individuals’ samples.
A highly sensitive analytical method was required to detect THC, CBD, and CBN in microgramper-liter and THCCOOH in nanogram-per-liter concentrations in OF. We were the first to develop a 2-dimensional GC-MS (2DGC-MS) method with LOQs of 0.25–0.5 μg/L for THC and CBD, 1 μg/L for CBN, and 5–7.5 ng/L for THCCOOH, depending on the OF collection method (24). Consecutive escalating oral 20-mg THC administrations [40–120 mg/day synthetic THC (Marinol) over 8 days] was investigated in 10 chronic daily cannabis smokers, with OF collected by expectoration or with the Quantisal™ collection device (25, 26). The highest OF THC, CBN, and CBD concentrations occurred before the first dose, reflecting previous self-administered cannabis smoking. CBN and CBD were identified for only 11 h after admission, were not present in Marinol and never identified after oral dosing. After oral THC, THCCOOH increased while THC decreased; OF samples were positive for a mean of 23 (THC) and 185 h (THCCOOH at the last collection) after the first THC dose. At 23 h after the last dose, the mean THCCOOH concentrations were 96 ng/L in expectorated OF samples and 134 ng/L in Quantisal-collected samples.
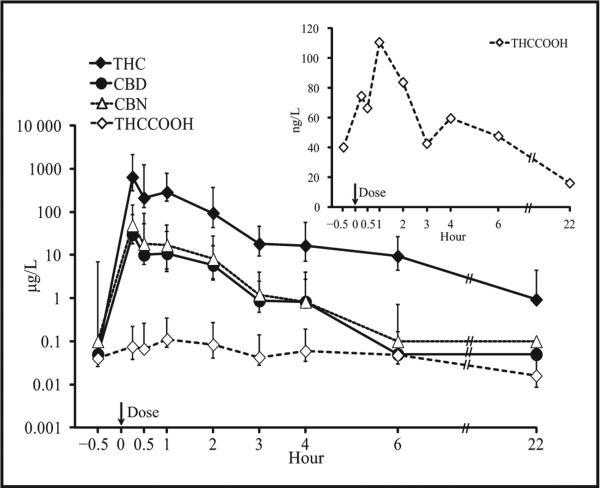
THC, CBD, CBN, and THCCOOH were quantified in 95.2%, 69.3%, 62.3%, and 94.7%, respectively, of 80 OF samples collected by expectoration and the Quantisal device up to 22 h after chronic daily cannabis users smoked a 6.8% THC cigarette (9, 10). In expectorated OF, 6 h after smoking, 8 of 10 participants were positive for THC (0.9–90 μg/L) and THCCOOH (17– 151 ng/L), when only 4 were positive for CBD (0.5–2.4 μg/L) and CBN (1–3 μg/L). At 22 h, 1, 5, 1, and 0 participants were still positive for THC, THCCOOH, CBD, and CBN, respectively. In Quantisal-collected samples, at 22 h, 4 participants were positive for THC (0.5–5.5 μg/L), 5 for THCCOOH (9–103 ng/L), and none for CBD and CBN (10). The last detection times for CBD and CBN were 6 h or less (Fig. 3). Different cannabinoid marker cutoffs were evaluated to meet the needs of different drug testing programs (see Fig. 1 in the Data Supplement that accompanies the online version of this report at http://www.clinchem.org/content/vol60/issue2). THC ≥1 or 2 μg/L and THCCOOH ≥20–30 ng/L alone or in combination produced positive results for at least for 22 h. For CBD ≥0.5 μg/L or CBN ≥1 μg/L the detection windows were 0–6 h.
Fig. 3. THC, CBD, CBN, and THCCOOH concentrations after smoking a single 6.8% THC cigarette (up to 6 h, n = 10; at 22 h, n = 6).
Error bars indicate interquartile ranges. Inset provides additional details for median THCCOOH concentrations over time. Reproduced with permission from Lee et al. (10).
Cannabinoid OF disposition was determined in 28 chronic, daily smokers during sustained abstinence on a secure research unit for 30 days with no access to cannabis. THC was not quantifiable 48 h after admission in 24 of 28 participants; CBN and CBD were detected on only admission, whereas THCCOOH was found for up to 19.6 days (27). These authors suggested that a THCCOOH/THC ratio ≤4 ng/μg could be a potential marker of recent use, but this finding requires confirmation.
As for smoked cannabis, oromucosal Sativex™ spray contaminates the oral mucosa and produces high OF THC, CBN, and CBD concentrations (28). The oral mucosal spray avoids negative smoked cannabis effects and improves THC bioavailability over oral administration; however, there is gross OF contamination for 15–60 min after administration. OF was not contaminated by oral administration of dronabinol, a encapsulated synthetic THC in sesame oil (25).
A major limitation to the implementation of OF analyses of cannabinoids may be passive contamination from environmental cannabis smoke. In 10 participants exposed to cannabis smoke for 3 h in Dutch coffee shops, and with OF collected outdoors, THC was quantifiable in all samples at 3 h with LOQs of 0.5 μg/L for THC and CBN, 1 μg/L for CBD, and 2 ng/L THCCOOH, and at the proposed 2-μg/L SAMSHA cutoff (29) for 70% of samples. At 12–22 h after THC exposure, 20% of samples were positive at the LOQ but negative at the 2-μg/L cutoff. CBN was detected at 3 h in 3 of 10 study participants; CBD was never detected, possibly owing to low CBD content in Netherlands’ cannabis. THCCOOH, which is not present in cannabis smoke, was never detected, providing a cannabinoid analyte that we believe avoids the passive environmental contamination issue.
The research described above provides a database for OF cannabinoid test interpretation and approaches to identify recent cannabis intake that are important for DUID testing, accident investigations, and antidoping in competition testing. Synthetic cannabinoids (e.g., JWH-018, AM2201) are also problematic anti-doping agents and can be identified in urine and OF (30). After 3 puffs of a mixture of 500 mg commercial herbal products containing several synthetic cannabinoids of unknown concentration and 500 mg tobacco, substances were quantifiable for at least 6 h in OF collected with the Biophor RapidEASE device over 90 h with a 0.2-μg/L LOQ. Research is needed to characterize OF disposition of the many synthetic cannabinoids.
S9: GLUCOCORTICOSTEROIDS
These relatively polar steroid hormones are largely abused in sports because of their antiinflammatory and fatigue-reducing effects, and they are currently monitored in urine. The problem is to differentiate endogenous from exogenous administrated natural glucocorticosteroids, cortisone and cortisol, as well as to identify synthetic glucocorticosteroid abuse. Cortisol diffuses freely into salivary glands, with little dependence on flow rate (9). OF cortisol concentrations are low (1–10 μg/L) because of the presence of 11β-hydroxysteroid dehydrogenase, which oxidizes cortisol to cortisone. Also, OF cortisol is subject to exercise variation; in young soccer athletes, salivary cortisol increased from 19–21 nmol/L after a 90-min training session (31). Research on natural glucocorticosteroid OF concentrations and controlled exogenous administration is necessary for antidoping OF testing to establish cortisone or cortisol abuse.
WADA reported in 2011 that all glucocorticosteroid AAFs were synthetic drugs, with budesonide the most prevalent. After administration of 200 mg inhaled budesonide to 5 healthy volunteers, urine budesonide metabolite was detected for up to 12 h, with a 5-μg/L LOQ (32). Two healthy volunteers receiving 3 mg oral budesonide were positive for metabolites in urine for 24–48 h at the same LOQ. To date, no OF data are available for synthetic glucucorticosteroids, but synthetic glucocorticosteroids may also be present after administration, due to a depot of drug in the mouth. Consequently, research is needed to identify synthetic glucocorticosteroid pharmacokinetics in OF to distinguish recent use.
Substances Prohibited at All Times
S0: NONAPPROVED SUBSTANCES
A nonapproved substance is any drug not currently approved for human therapeutic use by any government authority. S0 includes drugs under development or eliminated from further consideration and veterinary drugs. Consequently, antidoping testing for S0 substances requires new assay development for all matrices. We are not aware of any published data on analysis of S0 substances in OF. There could be an advantage for measuring S0 drugs in OF rather than blood if their pKa is basic, because these drugs would be expected to concentrate in OF, improving detection. Another potential advantage of monitoring this drug class in OF is that the parent drug is most likely to be the target analyte, because metabolism is often unknown at the time new drugs appear as doping agents, making target analyte identification in urine problematic.
S1: ANABOLIC AGENTS
Anabolic agents include synthetic and endogenous anabolic androgenic steroids and other agents with similar properties that increase protein retention and muscle growth. Anabolic agents are one of the commonest doping substances found in antidoping testing (11).
Detection of endogenous steroid doping is challenging. Currently, testosterone, epitestosterone, and other steroids are quantified in urine. Elevated testosterone/epitestosterone (T/E) ratios of >4 (WADA threshold) requireconfirmationbygaschromatography/ combustion/isotope ratio mass spectrometry (GC/C/IRMS), a technique that precisely differentiates carbon or hydrogen isotope ratios (13C/12C or 2H/1H) of exogenous and endogenous testosterone.
T/E ratios naturally vary; in athletes with naturally low ratios, doping may not produce a T/E >4, yielding false-negative presumptive results. In athletes with a naturally high T/E, false-positive presumptive test results could be obtained, but will be confirmed as negative by GC/C/IRMS (33). Urine steroid passports, which are individualized steroid profiles collected over time, are being created, similar to the blood passport (34). The steroid profile enables the detection of changes in steroid concentrations due to reduced variability in individual concentrations. The passport approach may better identify changes within athletes with naturally low or high T/E ratios.
The IRMS method is accurate, but time-consuming and expensive, and may require larger urine sample volumes due to analytical sensitivity limitations; 18 mL of urine containing 5–10 μg/L analyte was required to accurately determine the δ13C (35). Because of low steroid OF concentrations and reduced OF volume, it is currently improbable that OF GC/C/IRMS analysis could successfully confirm endogenous steroid abuse, given that IRMS analysis requires a large urine volume to obtain a sufficient analyte concentration to detect isotope abundances. Endogenous OF steroid concentrations, diurnal rhythms, and intra- and intersubject variability were extensively studied (9, 36). Free unbound steroids [testosterone, dehydroepiandrosterone (DHEA)] enter OF from blood via passive diffusion, whereas phase II steroid metabolites (DHEA-sulfate) are restricted to the ultra-filtration route (9, 37). For adult women, the correlation between OF and blood steroids is weak, with OF concentrations <57 ng/L (37); however, in adult men the OF:serum total testosterone ratio was approximately 1:90, with OF testosterone concentrations of 72–172 ng/L. 17β-Hydroxysteroid dehydrogenase in salivary glands may convert testosterone to androstenedione, reducing the testosterone OF concentration. Salivary steroids (testosterone, DHEA, and sulfated DHEA) vary in athletes from different sports related to specific physical exercise (9). In 10–16-year-old soccer players, OF testosterone increased significantly from 97 to 118 ng/L after a 90-min training session (31), whereas professional rugby players [25 (3) years old] showed no significant changes in salivary testosterone after resistance exercises, results that highlighted interindividual testosterone variation (38).
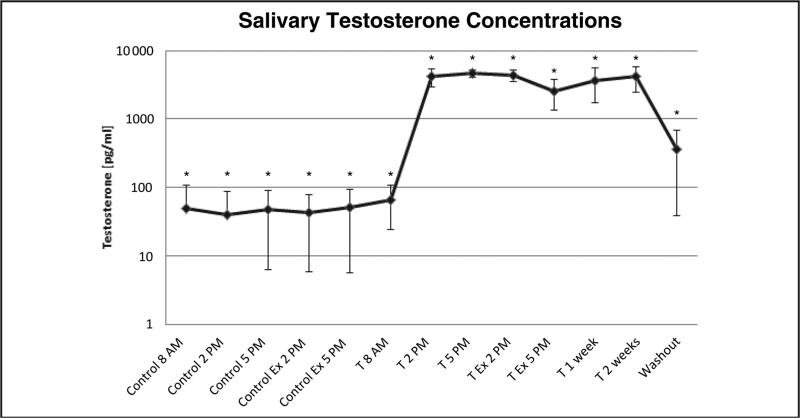
OF testosterone concentrations and urine T/E ratios were evaluated in 19 healthy males who exercised before and after receiving 1 transdermal 1.5-mg/kg testosterone gel dose per week for 3 weeks (39). OF testosterone increased significantly, from 50 ng/L at baseline to 5000 ng/L after the first testosterone gel application, and was increased for 3 weeks of dosing and for at least 1 week after the last dose (Fig. 4). The mean urinary T/E ratio increased but never exceeded 4; a significant but moderate correlation was observed between OF testosterone concentration and urinary T/E ratio. Moreover, exercise had no effect on OF testosterone concentrations determined by ELISA at baseline or after dosing. A more specific analytical technique such as LC-MS is necessary to confirm OF results because other steroids (DHEA) may cross-react with testosterone antibodies.
Fig. 4. Mean (SD) testosterone concentrations in salivary samples.
Ex, exercise; T, testosterone application (1.5 mg/kg body weight). Asterisks (*) indicate significant differences (P < 0.05). Adapted with permission from Schönfelder et al. (39).
Simultaneous OF testosterone, epitestosterone, dihydrotestosterone, DHEA, androsterone, etiocholanolone, and α- and β-androstanediol quantification collected with the Quantisal device were presented, but never published (40). Low LOQs of 10 ng/L were achieved for all analytes except α- and β-androstanediol (40 ng/L). In males, concentrations were 12–32 ng/L testosterone and 24–52 ng/L DHEA. In females, only DHEA was detected, with concentrations of 28–520 ng/L. All other analytes, including epitestosterone, were not quantifiable in OF from either sex.
These first research results constituted a proof of concept for detecting natural steroid abuse for anti-doping with OF. However, due to the undetectable concentration of epitestosterone in OF, the T/E ratio would be irrelevant to measure in OF. Additional research is needed to define criteria for positive OF testosterone tests and to develop LC-MS methods for OF steroid profiles. This effort can build on extensive steroid profiling already accomplished in urine (41), although increased analytical sensitivity is required. Finally, in blood, limited steroid concentration variability over time was observed (42); due to the correlation between OF and blood concentrations, one potential advantage for OF steroid testing is the probable low variability over time.
Synthetic androgenic anabolic steroids are generally detected in urine, although there are few reports of OF detection (43, 44). Oxymetholone (50 mg), a synthetic testosterone derivative, was orally administered to 3 women [28 (3.5) years old], with plasma and OF collection for 24 h, and oxymetholone quantification by GC-MS (43). At the first OF collection at 30 min, oxymetholone concentrations were 11.3 μg/L, but were negative by 1 h. Plasma concentrations were positive for 24 h. Recently, new urinary oxymetholone metabolites were identified in 3 males receiving 50 mg/day oral oxymetholone for 5 consecutive days, enabling detection windows in urine for up to 20 days (45). Short detection windows for OF oxymetholone, and perhaps other synthetic steroids, suggest that OF may not be a useful matrix for these doping agents.
[14C] tibolone, a 19-norsteroid–like nandrolone, was orally (2.5 mg) administered to 3 healthy post-menopausal females to investigate urinary tibolone pharmacokinetics. Radioactivity was noted for up to 192 h, documenting long urinary detection times (46). Only 1 study provided OF tibolone data (44). Tibolone (2.5 mg) was orally administered to one 27-year-old woman, with OF collected every 5 min for 1 h after dosing. The maximum tibolone OF concentration 5 min after dosing was 4 μg/L; all OF samples were positive for 1 h.
S2: PEPTIDE HORMONES, GROWTH FACTORS, AND RELATED SUBSTANCES
The OF proteome was extensively studied (47), with some peptide hormones, growth factors, and related substances detectable in OF (48). Erythropoiesis-stimulating agents such as recombinant erythropoietin (rhEPO) or continuous erythropoietin receptor activator (CERA), also known as a third-generation EPO, are usually detectable in urine and blood in low concentrations (49). Exogenous EPO is differentiated from endogenous EPO by isoelectric focusing. The analytical sensitivity of the rhEPO or CERA applied to the iso-electric focusing gel test is <40 pg. This corresponds to 50 ng/L in 1 mL serum or 3 ng/L in 18 mL urine. Natural OF EPO was measureable in adults and infants, correlating well with serum concentrations (OF:serum ratio, 1:4) (50). It is not known whether EPO is transferred into OF by direct secretion from salivary glands or by active transport. Research is needed to determine if rhEPO and CERA can be identified in OF. Expected low OF concentrations and limited OF volume suggest that detection with the current isoelectric method would be difficult.
Recombinant human growth hormone (rhGH) also must be differentiated from endogenous hGH in blood by using specific antibodies that recognize and bind to different hGH isoforms. In 2004 at the Athens Olympics, hGH testing in blood was introduced. Total isoform concentration is compared to the 22-kD iso-form concentration, which is the only recombinant form (51). When the ratio of the 22-kD isoform relative to all other isoforms is greater than the previously established threshold or reference range based on results from a demographically diverse population, the test is considered positive for rhGH abuse, unless rhGH is <0.1 μg/L. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods were developed to detect rhGH but currently lack the required analytical sensitivity (51).
OF hGH was measured in 23 men and 28 women (52). Women had significantly higher concentrations (12.8 mU/L) than men (3.4 mU/L), and the OF:serum hGH ratio was approximately 1:1000. Because of these low concentrations and low OF/serum ratio, it appears that hGH is probably not secreted into OF but derives from passive diffusion or active transport from blood. For antidoping purposes, current methods are not sensitive enough to quantify rhGH and hGH in OF at expected low nanogram per liter concentrations.
The biomarker method is another approach to detect rhGH abuse. It is based on the measurement of other biomolecules affected by the administration of rhGH, such as insulin-like growth factor-1 (IGF-1) (51). In human OF, free IGF-1 was quantified at low μg/mL concentrations, but no correlation was found with circulating plasma concentrations, suggesting that IGF-1 can be secreted directly in OF as observed in rats (53, 54).
Insulin is another peptide that may be misused in sport. In clinical OF measurements, insulin has been quantified by an immunoassay with high analytical sensitivity (1.2 ng/L) (55). In mice, insulin can be synthesized by the salivary glands; however, in humans, the good correlations between plasma and OF insulin concentrations indicate that its presence in OF is most likely due to ultrafiltration from blood (56, 57). Insulin abuse in sports involves administration of synthetic or animal insulin that differs in amino acid sequence from natural human insulin (58). Thus, in current OF clinical immunoassays, cross-reactivity to the different types of insulin may occur, making it difficult to differentiate between endogenous and exogenous insulin. In fact, 2.5 h after taking insulin, the OF insulin concentration of type I diabetes patients increased, reaching concentrations up to 1.2 μg/L. However, these data were obtained by immunoassay and included contributions from endogenous and exogenous insulin (59). In doping control, insulin detection in urine or plasma is done by mass spectrometric detection, which allows differentiation of exogenous (presumably abused) and endogenous insulin (58). Such methods need to be developed for OF. Although OF data are not available for many S2 substances, expected low concentrations require more sensitive methods than those currently available. The blood ABP, and perhaps OF passports in the future, provide another advantageous method for monitoring these substances or their markers over time.
S3: β-2 AGONISTS
β-2 agonists are pharmacotherapies for asthma and other pulmonary diseases. The most common β-2 agonists are salbutamol, formoterol, and salmeterol. β-2 agonists are prohibited, except when prescribed for therapeutic purposes by inhalation. An AAF occurs if the urine concentration exceeds 1 mg/L for salbutamol and 40 μg/L for formoterol. Several controlled salbutamol administration studies with asthmatic and nonasthmatic elite athletes showed that no urine samples were above the WADA-established threshold concentration after inhalation of maximal recommended therapeutic doses (1600 mg for 24 h) (60, 61). After a prohibited oral dose, urine salbutamol concentrations (up to 3000 μg/L) greatly exceeded the threshold. Single and repeated 18-μg formoterol inhalations by asthmatic and nonasthmatic participants did not produce urine concentrations above 40 μg/L (62).
To date, there are no published data on OF β-2 agonist concentrations. These drugs are relatively basic because of their amine functional groups and are expected to have detectable OF concentrations; however, gross oral mucosa contamination could occur following β-2 agonist inhalation, making this matrix inappropriate for testing immediately after dosing. Research is needed to determine OF concentrations over time, establishing relationships between blood and OF, defining potential threshold concentrations, and determining duration of OF detection of β-2 agonists by different routes to differentiate systemic from inhalation administration.
S4: HORMONES AND METABOLIC MODULATORS
This class includes aromatase inhibitors (e.g., androstatrienedione), selective estrogen receptor modulators (e.g., tamoxifen), antiestrogenic substances (e.g., clomiphene), myostatin inhibitors, and metabolic modulators (e.g., peroxisome proliferator-activated receptor δ agonists). Tamoxifen had the highest AAF prevalence within this class in 2011, accounting for almost 50% of AAF (11). Tamoxifen, taken by male athletes to induce androgenic steroid production, is currently determined by direct tamoxifen metabolite detection in urine by LC-MS/MS or GC-MS and by steroid profiling (63). There are currently no data for tamoxifen or other S4 drugs in OF; however, based on amine group presence in tamoxifen, the drug should be detectable in OF. Much new research is needed to characterize S4 drug OF concentrations and detection windows.
S5: DIURETICS AND OTHER MASKING AGENTS
Diuretics increase urination and dilute urine; their effect on OF volume is unexplored. Plasma volume expanders (e.g., mannitol) are other masking agents misused to vary the blood ABP by regulating hemoglobin concentrations and increasing blood volume and oxygen transport in muscles and tissues. These agents may not directly enhance sport performance but may mask doping with other substances in urine. It is possible that there are distinct masking agents in OF that might require monitoring. Most S5 drugs are highly polar, making urine an excellent specimen for their detection by LC-MS at analyte concentrations >100 μg/L (64). Because of the high polarity of these agents, it may be difficult for masking agents to passively transfer into OF. Additional research is needed to characterize the OF concentrations of these agents and to determine if OF is the appropriate matrix for monitoring.
Substances Prohibited in Particular Sports
P1: ALCOHOL
Alcohol accounted for only 5 AAF in antidoping testing in 2011 (11). Alcohol (ethanol) detection is efficiently analyzed in breath and/or blood; however, OF is also an appropriate matrix. For example, after drinking one 0.5-g/kg ethanol dose, OF ethanol was detectable in OF as long as in blood, up to 3.5 h with a 0.06-g/L cutoff (65). After the same dose, the glucuronide conjugate of ethanol (EtG) had shorter OF detection windows than in blood (3.5 h vs 11.5 h) at a 4.4-μg/L cutoff. With a higher 1 g/kg dose, ethanol OF detection windows were also shorter than those in blood (5.5 h vs 8.5 h), whereas EtG detection windows in OF and blood were similar at 11.5 h. Given the volatility of ethanol, EtG would be anticipated to be the better analyte for monitoring.
P2: β-BLOCKERS
β-Blockers are forbidden in some high-risk events such as ski jumping, freestyle skiing, and snowboarding and in sports where tremor and movement are detrimental, such as archery and shooting. β-Blockers reduce heart rate, adrenaline release, and tremor, which offers advantages in such sports. Detection is performed in urine by LC-MS/MS or GC-MS with LOQs below 100 μg/L, WADA's minimum required performance concentration (66). There are no published studies involving OF analyses for these substances. These are relatively lipophilic compounds that should readily enter OF. The ban applies only, unless otherwise specified, to in-competition use, so short detection windows in OF would be helpful for detecting recent use.
General Limitations and Advantages of Drug Testing
DRUG STABILITY IN OF
OF drug stability is an important factor affecting test results, as athletes have the right to later request a second sample analysis. Drug stability is an advantage for collecting OF with a collection device and its stabilizing buffer. In authentic nonfortified OF cortisol stability for 3 months at –20 °C and 1 year at –80 °C (OF collection with Salivette), and testosterone stability for 1 month were demonstrated at –20 °C (expectorated OF) (67, 65).
In fortified OF, THC stability depended on the type of collection device (69). In authentic OF collected with the Quantisal device, THC and other cannabinoids were stable for 1 month at 4 °C, whereas with expectoration much poorer cannabinoid stability was observed; THC decreased and CBN increased (70) because of oxidation of THC to CBN. A complicating factor for OF test interpretation is drug degradation, e.g., 6-AM to morphine or cocaine to BE (71). Preservatives, such as pH 4 citrate buffer and 0.1% sodium azide, can inhibit such degradation.
When OF was collected with the Cozart Rapiscan collection pad, methadone was reported to be stable for 2 months at 4 °C, whereas its 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolinium metabolite decreased with time (72).
Short-term stability of insulin in fortified OF collected by expectoration also was investigated (55). After 7 days at 2–8 °C, insulin concentrations decreased by 29.8%; however, no loss was observed after storage for the same period at –20 °C.
No other stability data are available for other doping agents, and additional research is needed to evaluate OF stability for all doping agents to correctly interpret test results. Long-term stability (≥1 year) is also needed, permitting sample reanalysis after this time.
OF COLLECTION
One of the biggest advantages of OF testing compared to urine or blood is ease of collection. OF can be collected under direct supervision by nonmedical staff of both sexes without compromising privacy. These factors decrease the risks of postcollection adulteration or sample substitution. Several methods exist to collect OF; however, due to serious drawbacks (distastefulness for donors and collectors and lack of stability in expectorated OF), passive drool and expectoration are not recommended. There is high interdevice variability in collected OF volume and drug recovery from the device (69). Dry mouth following stimulant intake and some therapeutic drugs may decrease OF flow rate (9, 10). Dehydration after vigorous exercise such as athletic competition might, in theory, have similar effects. OF collection is a critical part of the OF testing process. There is currently no standardized procedure to collect OF. Before OF is routinely incorporated into antidoping testing, more research is needed to define best collection practices. For example, in the same way that urinary specific gravity is employed to normalize drug concentrations in urine, can a parameter be found to normalize OF concentrations?
OF ANALYSIS
Mass spectrometry is commonly employed for anti-doping testing because it allows unambiguous drug or metabolite identification. GC-MS assays often require derivatization that can be laborious, time-consuming, and expensive. LC-MS also permits, in a limited volume, simultaneous confirmation of multiple compounds without derivatization (73). Highly sensitive and specific analytical instrumentation has reduced the problem of achieving accurate quantification of low OF concentrations. The analytical technology is now available to accomplish accurate, sensitive, routine OF analysis in support of antidoping programs.
OF testing results might be influenced by food, drink, mouthwash, or toothpaste. A study of the effect of mouthwash, orange juice, toothpaste, brewed coffee, soy milk, and tap water on the Immunalysis sweat/OF direct ELISA immunoassay found no difference between authentic blank OF and OF provided by volunteers 5 and 30 min after consumption of these products (74). Another study on healthy volunteers showed that coffee, Coca-Cola, fruit juice, oranges, spicy food, toothpaste, and vinegar could give a presumptive positive result for samples tested with the Orasure micro-plate immunoassay when OF was collected with the Intercept® device immediately after consumption (75). However, there were no false-positive results with mass spectrometry confirmation of presumptive positive OF screening test results. A third study demonstrated that mouthwash decreased 2- or 3-fold the concentrations of opiates in OF collected with an Oratect® or Intercept device 30 min after rinsing of the mouth, which was 30 min after administration of 10 mg codeine or 1 h after eating a 180-g poppy seed muffin (76). Finally, the OF elution buffer that stabilizes analytes and improves drug recovery from the collection pad can require frequent changes of GC-MS septa, liners, columns, and sources. In LC-MS, the buffer can produce high matrix effects when there is inadequate sample preparation (69, 77).
Conclusions
In this review we describe the available OF data for each class of doping agents. Table 2 summarizes the controlled drug administration studies involving OF analysis and highlights the fact that for compounds banned only in competition (e.g., cannabinoids and stimulants), there is convincing evidence that OF could be a valuable matrix because short detection times following drug intake can be addressed with appropriate analytes and cutoffs. However, for other classes of doping agents, little is known and research is required to evaluate OF as a useful alternative matrix for doping testing. Recently, hair testing was proposed as an alternative matrix for doping testing (78). However, the possibility of hair contamination from drugs in the environment, intersubject variability in drug concentrations due to hair color differences, and widely different drug detection windows made adoption of hair testing for antidoping control problematic. Thus the advantages, limitations, and knowledge gaps for OF testing and its potential usefulness in antidoping monitoring were addressed. These factors are summarized in Table 1. Just as for urine and blood testing, for which there are advantages and limitations for specific doping agents, OF testing offers solutions for some, but not all, antidoping problems. As for hair testing, this could be the major issue that prevents OF implementation in antidoping control. Indeed, only a relatively small number of banned substances have been to date quantified in OF. Adding OF as a matrix in doping control also would require the collection of a third specimen type and could lead to interpretation difficulties when results from urine, blood, and OF are disparate. A clear directive would be needed on which matrix results determined an adverse analytical finding for specific doping analytes.
Finally, several questions remain before of analysis becomes routine:
How should OF be collected? Should a standard procedure (including a specific collection device, transportation conditions, storage conditions, collected volume, OF concentration normalization) be established, as has been done for urine?
Which prohibited substances would best be monitored in OF?
What is the appropriate OF threshold concentration for each substance?
Is OF adulteration a significant risk?
Research is needed in multiple domains to fill these knowledge gaps. OF collection must be standardized to obtain accurate results and OF stability established for all doping agents. Controlled drug administration studies and OF disposition research are needed to determine drug detection windows and markers of recent use; however, OF appears to offer substantial advantages to urine monitoring, especially for agents prohibited in competition only.
Acknowledgments
Research Funding: Intramural Research Program of the National Institute on Drug Abuse, NIH.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Nonstandard abbreviations: WADA, World Anti-Doping Agency; THC, Δ9-tetrahydrocannabinol; THCCOOH, 11-nor-9-carboxy-Δ9-tetra-hydrocannabinol; hGH, human growth hormone; ABP, athlete biological passport; OF, oral fluid; i.v., intravenous; AMP, amphetamine; LOQ, limit of quantification; BE, benzoylecgonine; EME, ecgonine methyl ester; DRUID, Driving Under Influence of Drugs, Alcohol and Medicines; SAMSHA, Substance Abuse and Mental Health Services Administration; MDMA, 3,4-methylenedioxymeth-amphetamine; MDA, 3,4-methylenedioxyamphetamine; METH, methamphetamine; AAF, adverse (and atypical) analytical finding; 6AM, 6-acetylmorphine; CBD, cannabidiol; CBN, cannabinol; 2DGC-MS, 2-dimensional GC-MS; T/E, testosterone/epitestosterone; GC/C/IRMS, gas chromatography/ combustion/isotope ratio mass spec-trometry; DHEA, dehydroepiandrosterone; rhEPO, recombinant human erythropoietin; CERA, continuous erythropoietin receptor activator; rhGH, recombinant human growth hormone; LC-MS/MS, liquid chromatography–tandem mass spectrometry; IGF-1, insulin-like growth factor-1; EtG, ethanol glucuronide.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.WADA Website [July 2013];A brief history of anti-doping. http://www.wada-ama.org/en/about-wada/history/
- 2.WADA Website [July 2013];World anti-doping code. http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-The-Code/WADA_Anti-Doping_CODE_2009_EN.pdf.
- 3.WADA Website [July 2013];The 2013 prohibited list. http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/2013/WADA-Prohibited-List-2013-EN.pdf.
- 4.Huestis MA, Mazzoni I, Rabin O. Cannabis in sport: anti-doping perspective. Sports Med. 2011;41:949–66. doi: 10.2165/11591430-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sottas PE, Robinson N, Rabin O, Saugy M. The athlete biological passport. Clin Chem. 2011;57:969–76. doi: 10.1373/clinchem.2011.162271. [DOI] [PubMed] [Google Scholar]
- 6.Vearrier D, Curtis JA, Greenberg MI. Biological testing for drugs of abuse. EXS. 2010;100:489–517. doi: 10.1007/978-3-7643-8338-1_14. [DOI] [PubMed] [Google Scholar]
- 7.Aps JK, Martens LC. Review: the physiology of saliva and transfer of drugs into saliva. Forensic Sci Int. 2005;150:119–31. doi: 10.1016/j.forsciint.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Gatti R, De Palo EF. An update: salivary hormones and physical exercise. Scand J Med Sci Sports. 2011;21:157–69. doi: 10.1111/j.1600-0838.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 9.Milman G, Schwope DM, Gorelick DA, Huestis MA. Cannabinoids and metabolites in expecto-rated oral fluid following controlled smoked cannabis. Clin Chim Acta. 2012;413:765–70. doi: 10.1016/j.cca.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D, Schwope DM, Milman G, Barnes AJ, Gore-lick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58:748–56. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WADA Website [July 2013];Laboratory testing figures. 2011 http://www.wada-ama.org/Documents/Resources/Testing-Figures/WADA-2011-Laboratory-Testing-Figures.pdf.
- 12.Cone EJ, Kumor K, Thompson LK, Sherer M. Correlation of saliva cocaine levels with plasma levels and with pharmacologic effects after intravenous cocaine administration in human subjects. J Anal Toxicol. 1988;12:200–6. doi: 10.1093/jat/12.4.200. [DOI] [PubMed] [Google Scholar]
- 13.Jufer R, Walsh SL, Cone EJ, Sampson-Cone A. Effect of repeated cocaine administration on detection times in oral fluid and urine. J Anal Toxicol. 2006;30:458–62. doi: 10.1093/jat/30.7.458. [DOI] [PubMed] [Google Scholar]
- 14.Scheidweiler KB, Spargo EA, Kelly TL, Cone EJ, Barnes AJ, Huestis MA. Pharmacokinetics of cocaine and metabolites in human oral fluid and correlation with plasma concentrations after controlled administration. Ther Drug Monit. 2010;32:628–37. doi: 10.1097/FTD.0b013e3181f2b729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strano-Rossi S, Colamonici C, Botre F. Parallel analysis of stimulants in saliva and urine by gas chromatography/mass spectrometry: perspectives for “in competition“ anti-doping analysis. Anal Chim Acta. 2008;606:217–22. doi: 10.1016/j.aca.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Barnes AJ, Scheidweiler KB, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, Huestis MA. MDMA and metabolite disposition in expectorated oral fluid after controlled oral MDMA administration. Ther Drug Monit. 2011;33:602–8. doi: 10.1097/FTD.0b013e3182281975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepers RJ, Oyler JM, Joseph RE, Jr, Cone EJ, Moolchan ET, Huestis MA. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49:121–32. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- 18.Strano-Rossi S, Leone D, de la Torre X, Botrè F. Analysis of stimulants in oral fluid and urine by gas chromatography mass spectrometry II: pseudophedrine. J Anal Toxicol. 2010;34:210–5. doi: 10.1093/jat/34.4.210. [DOI] [PubMed] [Google Scholar]
- 19.Cone EJ. Testing human hair for drugs of abuse. I. Individual dose and time profiles of morphine and codeine in plasma, saliva, urine, and beard compared to drug-induced effects on pupils and behavior. J Anal Toxicol. 1990;14:1–7. doi: 10.1093/jat/14.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Wang WL, Darwin WD, Cone EJ. Simultaneous assay of cocaine, heroin and metabolites in hair, plasma, saliva and urine by gas chromatography-mass spectrometry. J Chromatogr B Biomed Appl. 1994;660:279–90. doi: 10.1016/0378-4347(94)00309-2. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins AJ, Oyler JM, Cone EJ. Comparison of heroin and cocaine concentrations in saliva with concentrations in blood and plasma. J Anal Toxicol. 1995;19:359–74. doi: 10.1093/jat/19.6.359. [DOI] [PubMed] [Google Scholar]
- 22.Saugy M, Avois L, Saudan C, Robinson N, Giroud C, Mangin P, Dvorak J. Cannabis and sport. Br J Sports Med. 2006;40(Suppl 1):i13–5. doi: 10.1136/bjsm.2006.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19:443–9. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 24.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–21. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milman G, Barnes A, Schwope D, Schwilke E, Goodwin R, Kelly D, et al. Cannabinoids and metabolites in expectorated oral fluid after 8 days of controlled around-the-clock oral THC administration. Anal Bioanal Chem. 2011;401:599–607. doi: 10.1007/s00216-011-5066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, et al. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56:1261–9. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–36. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 28.Lee D, Karschner EL, Milman G, Barnes AJ, Goodwin RS, Huestis MA. Can oral fluid cannabinoid testing monitor medication compliance and/or cannabis smoking during oral THC and oromucosal Sativex administration? Drug Alcohol Depend. 2013;130:68–76. doi: 10.1016/j.drugalcdep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, et al. Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–30. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Kneisel S, Speck M, Moosmann B, Corneillie TM, Butlin NG, Auwarter V. LC/ESI-MS/MS method for quantification of 28 synthetic cannabinoids in neat oral fluid and its application to preliminary studies on their detection windows. Anal Bioanal Chem. 2013 doi: 10.1007/s00216-013-6887-0. [DOI] [PubMed] [Google Scholar]
- 31.Di Luigi L, Baldari C, Gallotta MC, Perroni F, Romanelli F, Lenzi A, Guidetti L. Salivary steroids at rest and after a training load in young male athletes: relationship with chronological age and pubertal development. Int J Sports Med. 2006;27:709–17. doi: 10.1055/s-2005-872931. [DOI] [PubMed] [Google Scholar]
- 32.Deventer K, Mikulcikova P, Van Hoecke H, Van Eenoo P, Delbeke FT. Detection of budesonide in human urine after inhalation by liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2006;42:474–9. doi: 10.1016/j.jpba.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Van Renterghem P, Van Eenoo P, Geyer H, Schanzer W, Delbeke FT. Reference ranges for urinary concentrations and ratios of endogenous steroids, which can be used as markers for steroid misuse, in a Caucasian population of athletes. Steroids. 2010;75:154–63. doi: 10.1016/j.steroids.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Sottas PE, Saugy M, Saudan C. Endogenous steroid profiling in the athlete biological passport. Endocrinol Metab Clin North Am. 2010;39:59–73. viii–ix. doi: 10.1016/j.ecl.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Polet M, Van Gansbeke W, Deventer K, Van Eenoo P. Development of a sensitive GC-C-IRMS method for the analysis of androgens. Biomed Chromatogr. 2013;27:259–66. doi: 10.1002/bmc.2785. [DOI] [PubMed] [Google Scholar]
- 36.Schurmeyer T, Wickings EJ, Freischem CW, Nieschlag E. Saliva and serum testosterone following oral testosterone undecanoate administration in normal and hypogonadal men. Acta Endocrinol. 1983;102:456–62. doi: 10.1530/acta.0.1020456. [DOI] [PubMed] [Google Scholar]
- 37.Wood P. Salivary steroid assays: research or routine? Ann Clin Biochem. 2009;46(Pt 3):183–96. doi: 10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- 38.Beaven CM, Gill ND, Cook CJ. Salivary testosterone and cortisol responses in professional rugby players after 4 resistance exercise protocols. J Strength Cond Res. 2008;22:426–32. doi: 10.1519/JSC.0b013e3181635843. [DOI] [PubMed] [Google Scholar]
- 39.Schönfelder M, Hofmann H, Anielski P, Thieme D, Oberhoffer R, Michna H. Gene expression profiling in human whole blood samples after controlled testosterone application and exercise. Drug Test Anal. 2011;3:652–60. doi: 10.1002/dta.360. [DOI] [PubMed] [Google Scholar]
- 40.Slawson MH, Nielsen SM, Rollins DE, Wilkins DG. Analysis of endogenous steroids in oral fluid using gas chromatography-tandem mass spectrometry.. Paper presented at: SOFT 2010 Annual Meeting; Richmond, VA.. October 18–22. [Google Scholar]
- 41.Van Renterghem P, Van Eenoo P, Van Thuyne W, Geyer H, Schanzer W, Delbeke FT. Validation of an extended method for the detection of the misuse of endogenous steroids in sports, including new hydroxylated metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:225–35. doi: 10.1016/j.jchromb.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 42.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–13. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardoso CR, Marques MAS, Talhas IB, Caminha RC, Aquino Neto FR. [September 2013];Determination of oxymetholone in human plasma and saliva by high resolution gas chromatography-mass spectrometry. http://doping-info.de/proceedings/proceedings_10_pdf/10_217.pdf.
- 44.Sipoli Marques MA, Gualberto Pereira HM, De Oliveira MC, Talhas IB, Aquino Neto FR. [September 2013];Validation of the determination of tibolone in human urine and saliva using GC-MS for doping control and clinical studies. http://doping-info.de/proceedings/proceedings_12_pdf/12_403.pdf.
- 45.Sobolevsky T, Rodchenkov G. Mass spectrometric description of novel oxymetholone and desoxymethyltestosterone metabolites identified in human urine and their importance for doping control. Drug Test Anal. 2012;4:682–91. doi: 10.1002/dta.1407. [DOI] [PubMed] [Google Scholar]
- 46.Vos RM, Krebbers SF, Verhoeven CH, Delbressine LP. The in vivo human metabolism of tibolone. Drug Metab Dispos. 2002;30:106–12. doi: 10.1124/dmd.30.2.106. [DOI] [PubMed] [Google Scholar]
- 47.Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groschl M. Current status of salivary hormone analysis. Clin Chem. 2008;54:1759–69. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- 49.Lasne F, Martin L, Martin JA, de Ceaurriz J. Detection of continuous erythropoietin receptor activator in blood and urine in anti-doping control. Haematologica. 2009;94:888–90. doi: 10.3324/haematol.2009.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung GY, Jeng MJ, Lin CY, Soong WJ, Hwang B. The relationship between serum and saliva erythropoietin concentrations in adults, full-term and premature infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1998;39:380–5. [PubMed] [Google Scholar]
- 51.Bidlingmaier M. New detection methods of growth hormone and growth factors. Endocr Dev. 2012;23:52–9. doi: 10.1159/000341748. [DOI] [PubMed] [Google Scholar]
- 52.Rantonen PJ, Penttila I, Meurman JH, Savolainen K, Narvanen S, Helenius T. Growth hormone and cortisol in serum and saliva. Acta Odontol Scand. 2000;58:299–303. doi: 10.1080/00016350050217163. [DOI] [PubMed] [Google Scholar]
- 53.Antonelli G, Cappellin E, Gatti R, Chiappin S, Spinella P, De Palo EF. Measurement of free IGF-I saliva levels: perspectives in the detection of GH/IGF axis in athletes. Clin Biochem. 2007;40:545–50. doi: 10.1016/j.clinbiochem.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Ryan J, Mantle T, McQuaid S, Costigan DC. Salivary insulin-like growth factor-I originates from local synthesis. J Endocrinol. 1992;135:85–90. doi: 10.1677/joe.0.1350085. [DOI] [PubMed] [Google Scholar]
- 55.Fabre B, Maccallini G, Oneto A, Gonzalez D, Hirschler V, Aranda C, Berg G. Measurement of fasting salivary insulin and its relationship with serum insulin in children. Endocr Connect. 2012;1:58–61. doi: 10.1530/EC-12-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerr M, Lee A, Wang PL, Purushotham KR, Chegini N, Yamamoto H, Humphreys-Beher MG. Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice. Effects of type 1 diabetes mellitus. Biochem Pharmacol. 1995;49:1521–31. doi: 10.1016/0006-2952(95)00017-t. [DOI] [PubMed] [Google Scholar]
- 57.Marchetti P, Grossi C, Giannarelli R, Masoni A, Cristofani R, Giannecchini M, Navales R. Salivary immunoreactive insulin: a new entry in clinical chemistry? Clin Chem. 1988;34:1478–80. [PubMed] [Google Scholar]
- 58.Thevis M, Thomas A, Schanzer W. Doping control analysis of selected peptide hormones using LCMS(/MS). Forensic Sci Int. 2011;213:35–41. doi: 10.1016/j.forsciint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 59.Pasic J, Pickup JC. Salivary insulin in normal and type I diabetic subjects. Diabetes Care. 1988;11:489–94. doi: 10.2337/diacare.11.6.489. [DOI] [PubMed] [Google Scholar]
- 60.Mareck U, Guddat S, Schwenke A, Beuck S, Geyer H, Flenker U, et al. Determination of salbutamol and salbutamol glucuronide in human urine by means of liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2011;3:820–7. doi: 10.1002/dta.367. [DOI] [PubMed] [Google Scholar]
- 61.Elers J, Pedersen L, Henninge J, Hemmersbach P, Dalhoff K, Backer V. The pharmacokinetic profile of inhaled and oral salbutamol in elite athletes with asthma and nonasthmatic subjects. Clin J Sport Med. 2012;22:140–5. doi: 10.1097/JSM.0b013e31823513e1. [DOI] [PubMed] [Google Scholar]
- 62.Deventer K, Pozo OJ, Delbeke FT, Van Eenoo P. Quantitative detection of inhaled formoterol in human urine and relevance to doping control analysis. Drug Test Anal. 2012;4:449–54. doi: 10.1002/dta.418. [DOI] [PubMed] [Google Scholar]
- 63.Mazzarino M, Bragano MC, de la Torre X, Molaioni F, Botre F. Relevance of the selective oestrogen receptor modulators tamoxifen, toremifene and clomiphene in doping field: endogenous steroids urinary profile after multiple oral doses. Steroids. 2011;76:1400–6. doi: 10.1016/j.steroids.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Deventer K, Delbeke FT, Roels K, Van Eenoo P. Screening for 18 diuretics and probenecid in doping analysis by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2002;16:529–35. doi: 10.1002/bmc.201. [DOI] [PubMed] [Google Scholar]
- 65.Hoiseth G, Yttredal B, Karinen R, Gjerde H, Mor-land J, Christophersen A. Ethyl glucuronide concentrations in oral fluid, blood and urine after volunteers drank 0.5 and 1.0 g/kg doses of ethanol. J Anal Toxicol. 2010;34:319–24. doi: 10.1093/jat/34.6.319. [DOI] [PubMed] [Google Scholar]
- 66.Pujos E, Cren-Olive C, Paisse O, Flament-Waton MM, Grenier-Loustalot MF. Comparison of the analysis of beta-blockers by different techniques. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:4007–14. doi: 10.1016/j.jchromb.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Garde AH, Hansen AM. Long-term stability of salivary cortisol. Scand J Clin Lab Invest. 2005;65:433–6. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- 68.Matsui F, Koh E, Yamamoto K, Sugimoto K, Sin HS, Maeda Y, et al. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous measurement of salivary testosterone and cortisol in healthy men for utilization in the diagnosis of late-onset hypogonadism in males. Endocr J. 2009;56:1083–93. doi: 10.1507/endocrj.k09e-186. [DOI] [PubMed] [Google Scholar]
- 69.Langel K, Engblom C, Pehrsson A, Gunnar T, Ariniemi K, Lillsunde P. Drug testing in oral fluid-evaluation of sample collection devices. J Anal Toxicol. 2008;32:393–401. doi: 10.1093/jat/32.6.393. [DOI] [PubMed] [Google Scholar]
- 70.Lee D, Milman G, Schwope DM, Barnes AJ, Gore-lick DA, Huestis MA. Cannabinoid stability in authentic oral fluid after controlled cannabis smoking. Clin Chem. 2012;58:1101–9. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lund HM, Oiestad EL, Gjerde H, Christophersen AS. Drugs of abuse in oral fluid collected by two different sample kits: stability testing and validation using ultra performance tandem mass spec-trometry analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3367–77. doi: 10.1016/j.jchromb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Fucci N, De Giovanni N. Stability of methadone and its main metabolite in oral fluid. Drug Metab Lett. 2008;2:125–9. doi: 10.2174/187231208784040979. [DOI] [PubMed] [Google Scholar]
- 73.Thevis M, Thomas A, Pop V, Schanzer W. Ultra-high pressure liquid chromatography-(tandem) mass spectrometry in human sports drug testing: possibilities and limitations. J Chromatogr A. 2012;1292:38–50. doi: 10.1016/j.chroma.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 74.Schwope DM, Milman G, Huestis MA. Validation of an enzyme immunoassay for detection and semiquantification of cannabinoids in oral fluid. Clin Chem. 2010;56:1007–14. doi: 10.1373/clinchem.2009.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reichardt EM, Baldwin D, Osselton MD. Effects of oral fluid contamination on two oral fluid testing systems. J Anal Toxicol. 2013;37:246–9. doi: 10.1093/jat/bkt009. [DOI] [PubMed] [Google Scholar]
- 76.Wong RC, Tran M, Tung JK. Oral fluid drug tests: effects of adulterants and foodstuffs. Forensic Sci Int. 2005;150:175–80. doi: 10.1016/j.forsciint.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 77.Samyn N, Laloup M, De Boeck G. Bioanalytical procedures for determination of drugs of abuse in oral fluid. Anal Bioanal Chem. 2007;388:1437–53. doi: 10.1007/s00216-007-1245-8. [DOI] [PubMed] [Google Scholar]
- 78.Kintz P, Samyn N. Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Ther Drug Monit. 2002;24:239–46. doi: 10.1097/00007691-200204000-00006. [DOI] [PubMed] [Google Scholar]