Abstract
Fragile X syndrome (FXS) results from a genetic mutation in a single gene, yet produces a phenotypically complex disorder with a range of neurological and psychiatric problems. Efforts to decipher how perturbations in signaling pathways lead to the myriad alterations in synaptic and cellular functions have provided insights into the molecular underpinnings of this disorder. From this large body of data the theme of circuit hyperexcitability has emerged as a potential explanation for many of the neurological and psychiatric symptoms in FXS. The mechanisms for hyperexcitability range from alterations in the expression or activity of ion channels to changes in neurotransmitters and receptors. Contributions of these processes are often brain region- and cell type-specific, resulting in complex effects on circuit function that manifest as altered excitability. Here, we review the current state of knowledge of the molecular, synaptic and circuit-level mechanisms underlying hyperexcitability and their contributions to the FXS phenotypes.
Keywords: audiogenic seizures, autism, Fmr1, FMRP, GABA, hyperarousal, ion channels, intrinsic excitability, tactile defensiveness
Introduction
Fragile X syndrome (FXS) is the most common inherited form of human intellectual disability and also the leading inherited cause of autism. FXS most often results from an expansion of a CGG repeat sequence in the 5’ untranslated region of the gene Fmr1 that causes hyper-methylation, transcriptional silencing, and loss of expression of the Fragile X mental retardation protein (FMRP) (Penagarikano et al., 2007). FMRP is a polyribosome-associated RNA binding protein that regulates the translation of a large number of messenger RNAs, many of which encode synaptic proteins (Darnell et al., 2011). Because of the numerous genes affected by the loss of FMRP-regulated translational control, multiple neuronal signaling pathways involved in local translation are under investigation as potential therapeutic targets, including signaling pathways associated with group I (GpI) metabotropic glutamate receptors (mGluR), ERK, mTOR, GSK3, PI3K, GABA receptors, matrix metalloproteinases, and several others (Gross et al., 2012). This dysregulated protein translation is proposed to perturb neuronal development and function by disrupting synaptic maturation and plasticity, and eventually altering network activity throughout the brain. Thus, a broad understanding of the functional alterations in circuits at multiple levels (i.e., molecular, synaptic, cellular, network) will be necessary to determine both the direct pathophysiological effects of FMRP loss and the pleiotropic compensatory changes that together make up the FXS phenotype.
Over the last decade, many studies in the Fmr1 knockout (KO) mouse have begun to approach this problem revealing alterations at multiple levels in the brain, from molecules to networks. This rapidly growing body of data about molecular, synaptic, and circuit dysfunction in FXS has yielded novel research directions and potential targets for therapeutic intervention. However, the prevailing view thus far has put a large emphasis on correcting the core molecular signature of the disorder, that is the loss of translational control in neurons in the absence of FMRP, in particular as it relates to mGluR dysfunction (Darnell and Klann, 2013). Recent setbacks in several clinical trials warrant consideration of alternative therapeutic avenues (Mullard, 2015). Indeed, loss of FMRP has multiple pleiotropic effects as a consequence of dysregulation of potentially thousands of mRNA targets and altered patterns of protein synthesis (Ascano et al., 2012; Darnell et al., 2011) that are cell type- and even compartment-specific (Wang et al., 2014). Another concern is that strategies that target distinct signaling cascades might not lead to useful treatments because interfering with core molecular pathways will likely lead to side effects and variable levels of cellular adaptation. Similar approaches based on genetics to treat neurodegenerative disorders have thus far not yielded successful treatments. Thus, until better molecular therapies for FXS emerge, it becomes imperative to develop, in parallel, interventions aimed at the underlying circuit-level abnormalities, in order to treat the symptoms of the disorder.
The possibility that correcting circuit level hyperexcitability might rectify many of the symptoms of autism in general, and FXS in particular, has emerged as a complementary approach to molecular-based therapies (Rubenstein and Merzenich, 2003). This approach could provide a viable strategy for treating the circuit level symptoms of FXS, such as hyperactivity, seizures, or sensory hypersensitivity. Here, we review accumulating evidence that elevations in neuronal and circuit excitability are prevalent in several brain regions of Fmr1 KO mice, and that these defects could explain many behavioral abnormalities in the FXS mice. We also highlight evidence that changes in network excitability result from multiple mechanisms, including alterations in the intrinsic properties of neurons caused by loss of FMRP, which ultimately result in altered synaptic plasticity, exaggerated neuronal firing, abnormally high synchrony of neural networks, and exaggerated sensory-evoked activity.
Symptoms in FXS and parallel phenotypes in the mouse model that suggest hyperexcitability
The clinical features of FXS are quite complex with multiple physical signs and a variety of neuropsychiatric symptoms, including low IQ, learning disabilities, perseverative behaviors, hyperactivity/impulsivity/aggression, language deficits, and disrupted sleep (Lozano et al., 2014). These cognitive and behavioral alterations are debilitating for the affected individuals and also represent a significant burden for parents, caregivers and teachers alike. Many affected individuals also have a number of symptoms of autism, including social anxiety (excessive shyness), gaze aversion, increased time to initiate social interaction, and difficulty forming meaningful peer relationships. In addition, children with FXS exhibit a group of core sensory alterations that range from hypersensitivity to sensory stimuli and hyperarousal to seizures. These last symptoms are particularly relevant to the focus of this review, as they seem to reflect elevated excitability in different brain regions.
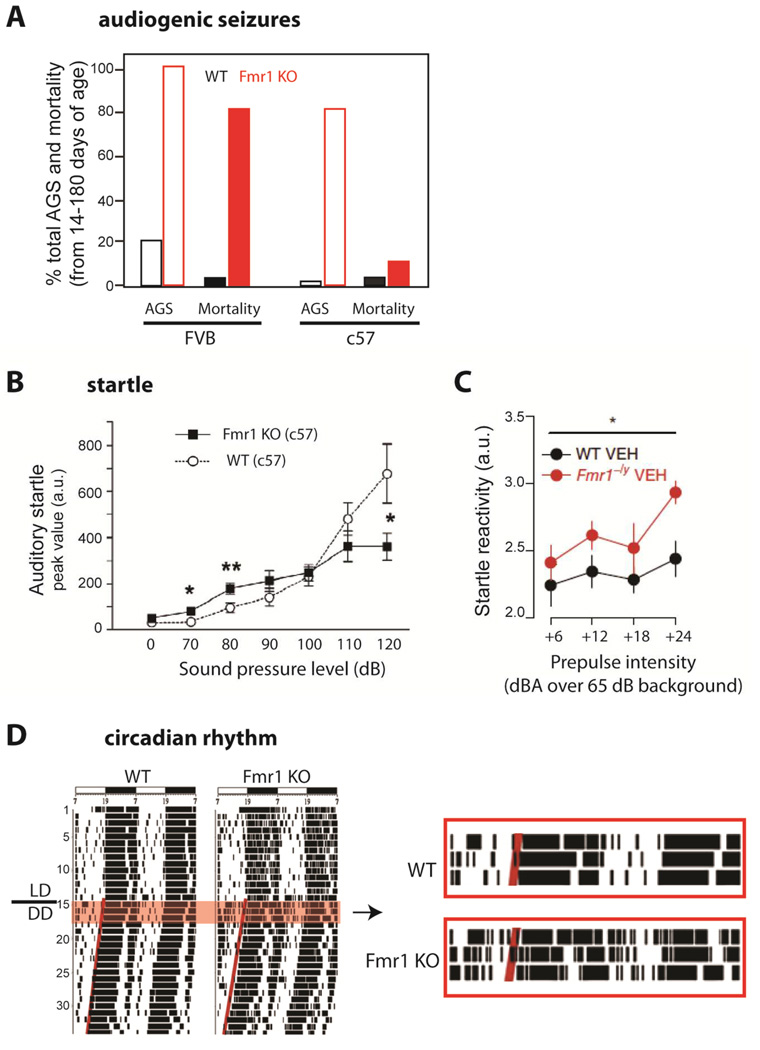
Seizures are reported in 10–20% of FXS individuals, usually as benign focal epilepsy of childhood (Berry-Kravis, 2002; Musumeci et al., 1999). These can readily be controlled with medication and, in the majority of cases, resolve spontaneously during childhood (Berry-Kravis et al., 2010). A seizure phenotype is also observed in Fmr1 KO mice, which exhibit a reduced threshold for audiogenic seizures (Fig. 1A)(Chen and Toth, 2001; Musumeci et al., 2000; Yan et al., 2005). These seizures are possibly triggered by elevated responsiveness of neurons in the auditory cortex to sound (Rotschafer and Razak, 2013), providing another example of how increased neuronal excitability in sensory cortices underlies a behavioral phenotype. Similar increases in responses in the auditory cortex are observed in the event related brain potential (ERP) recorded in the EEG in humans with FXS (Castren et al., 2003), consistent with hyperexcitability of auditory circuits associated with loss of FMRP in both mice and humans.
Figure 1. Phenotypes in Fmr1 KO mice that suggest hyperexcitability.
-
(A)Audiogenic seizures (AGS) are easier to elicit in Fmr1 KO mice of the FVB background. Percentage of animals showing AGS (light bars) and percentage mortality from status epilepticus (dark bars) after exposure to a high intensity siren delivering an average sound pressure level of 125 dB at 11cm for up to 15 min. [Adapted from Yan et al., 2005]
-
(B)Auditory startle is enhanced in Fmr1 KO mice. Fmr1 KO mice in c57 background (and FVB, not shown) show greater startle amplitude responses (measured as whole-body flinches) to low intensity acoustic stimuli (<90 dB) and lower responses to high intensity stimuli (>110 dB) compared to WT mice at 12–15 weeks of age. (from Nielsen et al., 2002)
-
(C)Whole-body startle response to 20 ms auditory stimuli (values over 65 dB background white noise) is exaggerated in 9–16 week-old Fmr1−/y mice compared to WT mice. [from Zhang et al., 2014]
-
(D)Disrupted circadian rhythms in Fmr1 KO mice. Left, Representative locomotor activity records of WT and KO mice. Activity records are double-plotted. Times of activity (wheel running) are indicated by black vertical marks, during exposure to a 12:12 light/dark cycle and after release into constant darkness (LD/DD). Right, Enlarged record showing clear disruption of circadian locomotor activity in the mutant animal, including frequent awakenings during the day. [from Zhang et al., 2008]
Sensory hypersensitivity is a prominent symptom of FXS (shared with other autism spectrum disorders) that could result from excessive neuronal or circuit hyperexcitability. In the context of auditory processing, the responsiveness to sound can be measured using prepulse inhibition (PPI), which is an attenuation of the startle reflex and a robust test of sensorimotor gating. There are well-characterized alterations in PPI responses and the startle reflex in both FXS individuals and in the mouse model, although, for reasons that are unclear, the disruptions are in opposite directions; PPI is reduced in humans but enhanced in mice (Fig. 1B–C)(Frankland et al., 2004). In the somatosensory system, hypersensitivity to touch is another sensory gating defect that manifests behaviorally as tactile defensiveness and avoidance of (or negative response to) otherwise neutral tactile stimuli (Ayres, 1964; Baranek et al., 1997). Similarly, unusual sensitivity to visual stimuli (or gaze aversion) occurs in >90% of males with FXS (Merenstein et al., 1996), which is also a manifestation of sensory hypersensitivity in response to eye contact (Cohen et al., 1989). Such excessive perceptions of otherwise normal sensory stimuli (Miller et al., 1999) could lead to autistic-like symptoms in FXS children, including abnormal approach/withdrawal behaviors seen in FXS (Cohen et al., 1991). Sensory hyper-reactivity could also bring about a hyperarousal state characterized by disruptions in circadian rhythms, including frequent awakenings from sleep seen in children with FXS (Kronk et al., 2010) and in Fmr1 KO mice (Fig. 1D)(Zhang et al., 2008).
There have been significant advances recently in elucidating the circuit basis for altered behavior in FXS, with a number of studies in the mouse model of FXS that are beginning to bridge the gap between molecular and systems level observations. Here, we discuss how known defects in excitability, from the single-molecule to the whole-network level, might inform our understanding of the neurologic symptoms and behavioral alterations. Below, we provide an overview of experimental research studies, many of them in the mouse model of FXS, that support the notion that loss of FMRP, through distinct molecular, synaptic, cellular and circuit defects, results in neuronal, network and sensory hyperexcitability.
Intrinsic excitability: expression and modulation of ion channels
FMRP functional domains and regulatory activity
FMRP is a 632aa protein that contains multiple RNA-binding and protein-protein interaction domains. The primary characterized function of FMRP is in translational control, but a growing list of novel non-canonical functions of FMRP have been uncovered recently that do not involve protein synthesis (Brager and Johnston, 2014). Prior work had described three RNA binding sites on the FMRP protein; two KH motifs and a RGG box (Ashley et al., 1993). More recently it was demonstrated that the amino-terminus domain of FMRP comprises two Agenet (Tudor) motifs, which are believed to serve as a platform for protein-protein interactions, and a novel KH0 motif that enables interaction with mRNAs (Myrick et al., 2015a; Myrick et al., 2015b). The amino terminus is capable of supporting protein-protein interactions which can directly regulate properties of ion channel proteins, such as the (potassium) K+ channels Slack and BK (Brown et al., 2010; Deng et al., 2013), while the carboxyl-terminus of FMRP is able to directly interact with calcium (Ca2+) channels (Ferron et al., 2014). FMRP can therefore potentially influence neuronal excitability through multiple mechanisms: by regulating translation of a diverse array of proteins that indirectly set neuronal excitability, and through a translation-independent role by interacting directly with a number of membrane ion channels to alter cellular excitability.
Ion channels under FMRP translational control
FMRP is primarily recognized as an RNA-binding protein that controls RNA trafficking and local translation in the dendrites. Assays of RNA binding combined with analysis of mRNA translational profiles altered in Fmr1 KO mice have revealed a wide variety of pre- and postsynaptic targets of FMRP that are normally involved in regulating neuronal excitability (Fig. 2A)(Brown et al., 2001; Darnell et al., 2011; Miyashiro et al., 2003). Quantitative proteomic analysis of cortical neurons cultured from Fmr1 KO mice directly confirmed that the levels of many pre- and postsynaptic proteins are affected by loss of FMRP (Liao et al., 2008), including many proteins affecting membrane excitability, ionic homeostasis, AP generation and propagation, neurotransmitter release, and postsynaptic receptor signaling.
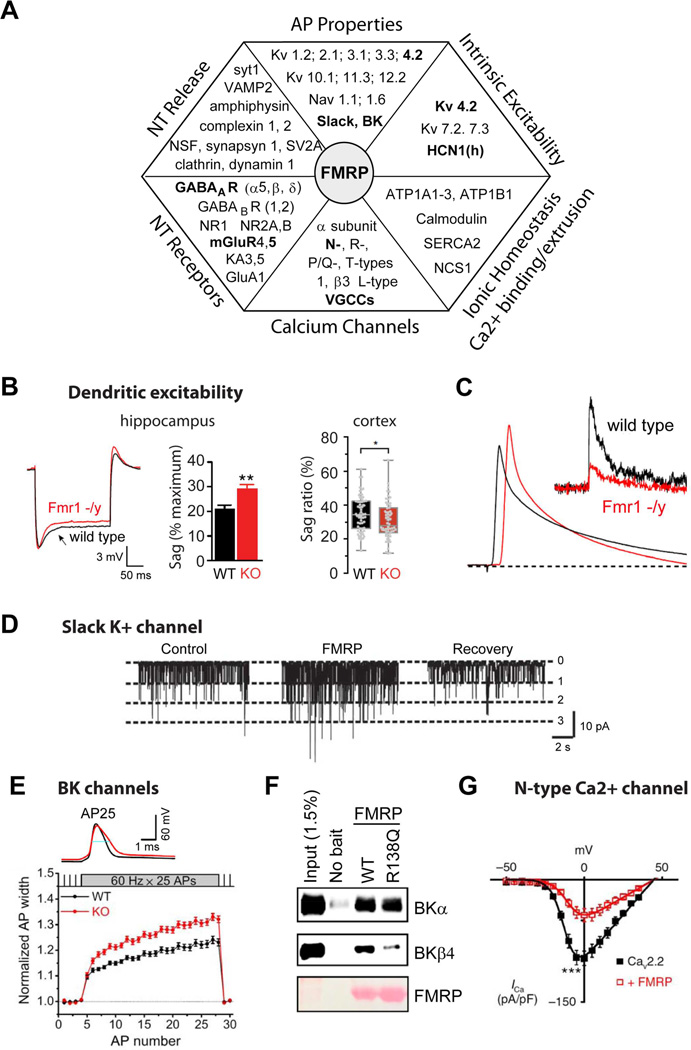
Figure 2. FMRP regulation of neuronal excitability: molecular and cellular mechanisms.
-
(A)Chart representing various aspects of neuronal excitability affected by FMRP loss and corresponding changes in the absence of FMRP in expression of proteins regulating these processes. Interactions that have been functionally validated and demonstrated to have an effect on excitability in Fmr1 KO mice are shown in BOLD.
-
(B-C)FMRP regulates dendritic excitability.
-
(B)Dendritic sag associated with the Ih current in Fmr1 KO mice is either higher (Left, hippocampus) or lower (Right, neocortex) than in WT mice. [from Brager et al., 2012 and Zhang et al., 2014, respectively]
-
(C)Back-propagating APs are larger in dendrites of hippocampal pyramidal neurons of Fmr1 KO mice due to a reduction in dendritic A-type K+ current (insert) [from Routh et al., 2013].
-
(D–G)FMRP regulates neuronal excitability via direct modulation of ion channel properties independently of its role in translational regulation.
-
(D)FMRP binds to and directly regulates gating of a K+ channel Slack [from Brown et al., 2010].
-
(E)FMRP directly regulates activity of BK channels via interactions with the channel auxiliary β4 subunit. As a result AP duration is longer in hippocampal and cortical excitatory neurons of Fmr1 KO mice [from Deng et al. 2013].
-
(F)FMRP missense mutation R138Q found in a patient with a partial FXS (intellectual disability and seizures) strongly reduces FMRP-BK β4 subunit interactions and renders FMRP unable to regulate AP duration (from Myrick et al., 2015).
-
(G)FMRP directly binds to and regulates surface expression of presynaptic N-type Ca2+ channels in DRG neurons [from Ferron et al., 2014].
A number of K+ channels that function at the resting potential have been identified as FMRP targets (Darnell et al., 2011), suggesting possible changes in intrinsic membrane properties. Abnormally elevated intrinsic membrane excitability has been observed in layer (L) 4 excitatory cortical neurons of Fmr1 KO mice, due to an increase in input resistance and decrease in cell capacitance (Gibson et al., 2008). In contrast, the resting membrane potential is normal in excitatory hippocampal neurons and in L5 pyramidal neurons in entorhinal cortex of young Fmr1 KO mice (Deng et al., 2013). Also, all intrinsic excitability parameters, including input resistance, membrane time constant and threshold potential are unaffected in the soma of L5 pyramidal neurons in somatosensory cortex of Fmr1 KO animals (Desai et al., 2006; Zhang et al., 2014). Similarly, analysis of neurons derived from FXS human embryonic stem cells (Telias et al., 2013), which provide an interesting perspective into human neuron dysfunction, have found that passive membrane properties are similar to those from neurons derived from control subjects.
Recent measurements of intrinsic excitability in the distal dendrites of cortical L5 pyramidal neurons of Fmr1 KO mice found significant differences in resting membrane potential, input resistance, and the membrane time constant, all of which are consistent with a hyperexcitable state of the dendritic membrane in Fmr1 KO mice (Zhang et al., 2014). Moreover, back-propagating APs have larger amplitudes and evoke greater calcium influx in the dendrites of Fmr1 KO neurons (Fig. 2B) (Routh et al., 2013; Zhang et al., 2014). The abnormal excitability of dendrites has been linked to the altered expression of several ion channels, including reduction in Kv4.2 (Gross et al., 2011; Lee et al., 2011; Routh et al., 2013), BK channels (Zhang et al., 2014) and altered expression of HCN1 (h) channels (Brager et al., 2012; Zhang et al., 2014). Notably, the changes in dendritic properties caused by FMRP loss appear to be brain region specific. For instance, h-channel expression is increased (and input resistance reduced) in dendrites of hippocampal neurons of Fmr1 KO mice (Brager et al., 2012), which is opposite to what is observed in the dendrites of cortical L5 neurons (Zhang et al., 2014) (Fig. 2C). These findings emphasize the non-uniformity of excitability changes caused by FMRP loss, which affects intrinsic membrane excitability in a brain region-, cell type- and compartment-specific manner.
The above-mentioned changes in excitability and AP properties have been observed predominantly in excitatory pyramidal neurons. The true impact of these defects on the circuit excitability ultimately depends on the combination of changes in excitatory neurons and inhibitory GABAergic interneurons. So far there is little evidence for altered excitability of inhibitory neurons in FXS mice. One study found no alterations in intrinsic excitability and AP properties in L4 fast spiking interneurons (Gibson et al., 2008). Further studies of different subpopulations of inhibitory interneurons and at different developmental time points are clearly needed to determine how the interplay of cellular changes in excitatory and inhibitory neurons impact circuit hyperexcitability in FXS.
Voltage-gated Ca2+ channels (VGCCs) are expressed in axons (predominantly N-, R-, P/Q-and T- types) and in dendrites (mainly the L-type) to regulate excitability, synaptic transmission and various forms of plasticity. mRNAs coding for the pore-forming subunits of most major VGCCs have been found among FMRP targets (Brown et al., 2001; Darnell et al., 2011; Miyashiro et al., 2003). L-type VGCCs are often localized to dendrites and spines where they contribute to synaptic plasticity. Major changes in dendritic Ca2+ channels are triggered by loss of FMRP, such as the striking absence of any L-type VGCC activity in spines of prefrontal cortex (Meredith et al., 2007) and reduced mRNA for L-type Ca2+ channels in several brain regions (Chen et al., 2003). These alterations have been linked to changes in the threshold for the induction of spike timing-dependent synaptic plasticity in Fmr1 KO mice (Meredith et al., 2007).
Translation-independent regulation of ion channels and excitability by FMRP
A number of synaptic FMRP actions arise from direct protein-protein interactions between FMRP and some ion channels, independently of FMRP’s traditional role in translational regulation (Brown et al., 2010; Deng et al., 2013; Ferron et al., 2014; Myrick et al., 2015a). Initial evidence for direct modulation of ion channel function by FMRP came from studies by Kaczmarek and colleagues which demonstrated that FMRP interacts directly with the sodium-activated K+ channel Slack, and modulates its gating in the auditory brainstem (Fig. 2D) (Brown et al., 2010). The N-terminal aa1-298 fragment of FMRP, which lacks ability to interact with ribosomes, is sufficient for this interaction. Slack channels play major roles in excitability in many brain regions by regulating adaptation of firing during sustained activity and by setting a high temporal accuracy of APs, essential in many forms of sensory processing (Kim et al., 2014). Mutations in the Slack channel have been linked to childhood seizures and severe forms of intellectual disability (Kim and Kaczmarek, 2014), implicating altered activity of this channel in the absence of FMRP as an important contributor to excitability defects in FXS (Zhang et al., 2012).
FMRP also regulates excitability of hippocampal and cortical pyramidal neurons by directly modulating another K+ channel, the BK channel (Deng et al., 2013; Myrick et al., 2015a). Owing to their dual voltage- and Ca2+ dependence, BK channels influence excitability by controlling the AP duration and the AHP during sustained firing, thereby regulating both neurotransmitter release efficiency and the neuron’s ability to generate high-frequency spiking (Contractor, 2013). Loss of FMRP reduces BK channel activity and causes excessive AP broadening (Fig. 2E), leading to elevated presynaptic Ca2+ influx and increased glutamate release during repetitive activity in both hippocampal and cortical pyramidal neurons (Deng et al., 2013). This study determined that FMRP regulates BK channel activity by interacting with the channel’s auxiliary β4 subunit, which explains its reduced Ca2+ sensitivity in the absence of FMRP. This interaction is necessary for FMRP regulation of AP duration since FMRP-dependent AP broadening could not be induced in mice lacking BK β4 subunit. Together these findings suggest that FMRP regulation of AP duration is translation-independent; indeed, the excessive AP broadening in Fmr1 KO mice can be rapidly rescued and mimicked in the absence of protein synthesis (Deng et al., 2013).
The translation-independent role of this FMRP function in FXS excitability defects has been revealed in a recent study of an Fmr1 missense mutation R138Q, which is in the KH0 RNA binding domain of FMRP, and was found in a patient with a partial FXS phenotype (Myrick et al., 2015a; Myrick et al., 2015b). This patient has a history of global developmental delay, intellectual disability, and intractable seizures, but no other features typical of FXS. While the R138Q mutation was found to preserve the canonical mRNA binding and translation-regulation capabilities of FMRP, the mutation rendered FMRP unable to modulate AP duration in both hippocampal and cortical pyramidal neurons (Myrick et al., 2015a). The R138Q mutation also almost fully abolished the interaction of FMRP with the BK channel β4 subunit (Fig. 2F). This finding provides further evidence for the role of FMRP-BK channel interactions in regulation of the AP properties and suggests that this translation-independent function of FMRP is linked to a specific subset of FXS phenotypes.
Another non-canonical aspect of FMRP’s effects on excitability is that it directly binds to presynaptic N-type VGCCs and regulates their surface expression (Fig. 2G) (Ferron et al., 2014). Unlike the interactions of FMRP with the Slack and BK channels, there is no effect of FMRP loss on N-type channel activity. This interaction of FMRP also differs from interactions with other channels in that it is mediated by the carboxyl-terminal domain, rather than the amino-terminus of FMRP (Ferron et al., 2014). Loss of this interaction has a major impact on neurotransmitter release in DRG neurons, expanding the known range of FMRP functions that affect excitability and neurotransmitter release. Loss of direct protein-protein interactions of FMRP with ion channels is therefore emerging as a major contributor to excitability defects in FXS.
Synaptic and Cellular Disruptions
Although many of the mRNA targets regulated by FMRP encode for synaptic proteins (Fig. 2A), many reported effects of loss of FMRP on synapse function are perhaps surprisingly modest. This likely reflects the fact that, because synaptic transmission is a fundamental process, robust adaptive mechanisms can compensate for synaptic mRNA dysregulation in FXS. The earliest reported synaptic defect in FXS was the overabundance of morphologically immature dendritic spines in cortical pyramidal neurons in human postmortem tissue (Hinton et al., 1991; Rudelli et al., 1985). Similar changes in spine morphology have been described in Fmr1 KO mice, although this remains controversial (He and Portera-Cailliau, 2013). It is difficult to directly draw conclusions about how spine changes might contribute to excitability, or whether instead their immature morphology and instability (Cruz-Martin et al., 2010)(Padmashri et al., 2013; Pan et al., 2010) simply reflect the immaturity of circuits in the absence of FMRP. However, these spine morphological perturbations are likely to impact connectivity and could indirectly contribute to the excitability changes in FXS.
In addition to alterations in synaptic structure there are multiple described perturbations in synaptic function, many of them quite subtle. But for the purposes of this review we will focus on those perturbations that are likely to have effects on cellular or network excitability.
Excitatory synapses and plasticity
Glutamatergic synapses provide the principal excitatory input to all neurons in the brain, such that gross alterations in their functional properties will greatly influence cellular and circuit excitability. Mechanisms that favor or hinder plasticity of excitatory synapses could also affect neuronal excitability. As with other aspects of the deficits found in FXS, there seems to be considerable heterogeneity in the literature, and there are likely multiple factors (e.g., brain region, developmental stage), that can influence the exact endophenotypes observed. In addition, because excitability of the neurons themselves can affect plasticity induction, we discuss both the changes in excitatory synapses and the alterations in plasticity threshold that are clearly a result of changes in intrinsic excitability. Since regulated trafficking of AMPA receptors (AMPA-R) underlies most long-term forms of synaptic plasticity, effects of FMRP on the complex pathways that control glutamate receptor insertion and removal from the synaptic membrane will, by themselves, have a large effect on synaptic strength and ultimately excitability.
Metabotropic glutamate receptors and long term depression
The most prominent form of synaptic plasticity that has been studied in relation to FXS is group 1 (Gp1) mGluR-dependent long term depression (LTD). It was first reported more than a decade ago that the amplitude of mGluR-LTD was elevated in hippocampal CA1 (Huber et al., 2002). As this form of LTD results from AMPA-R internalization and requires protein synthesis, there has been considerable interest in the intersection between signaling pathways underlying mGluR-LTD and the known role of FMRP in translational control.
While it is unclear whether exaggerated mGluR-LTD directly affects neuronal excitability, signaling through mGluRs can more directly influence excitability by activating intrinsic neuronal conductances (Bianchi et al., 2009). For instance in the hippocampus, Gp1 mGluR-mediated coupling to an excitatory conductance is enhanced in Fmr1 KO mice (Bianchi et al., 2009). Gp1 mGluRs are also known to mobilize endocannabinoids (eCBs) and recent evidence suggests that disruption of coupling of mGluRs (Jung et al., 2012) to their normal signaling pathways can contribute to elevated excitability in FXS (Tang and Alger, 2015).
Although the interaction between mGluR signaling and FMRP is complex, the prevailing model suggests that FMRP acts as a break on mGluR-mediated protein translation (Bear et al., 2004). There may also be a translation-dependent mechanism underlying epileptogenesis, as activation of Gp1 mGluRs can upregulate proteasome degradation of FMRP, thereby removing the brake on translation and increase neuronal activity and triggering seizures (Zhao et al., 2011).
Long-term potentiation
Initial studies in the FXS mouse model found no alteration in LTP in the CA1 area of the hippocampus (Godfraind et al., 1996; Larson et al., 2005; Li et al., 2002); in contrast impairments were found in LTP in certain neocortical regions (Larson et al., 2005; Li et al., 2002). Subsequently, differences in hippocampal CA1 LTP threshold emerged when less stringent induction methods were used (Hu et al., 2008; Lauterborn et al., 2007). The difference in the threshold for the induction of LTP could arise from changes in cellular excitability. Although there was no evidence for this in the initial characterization of hippocampal LTP, recent studies have examined this more closely. In particular, there is growing evidence of the connection between altered excitability of dendrites and alterations in synaptic plasticity thresholds (Meredith and Mansvelder, 2010).
The ion channels that regulate excitability of the dendrites are key modulators of synaptic plasticity. As noted above, several channels that control excitability and AP propagation are altered in Fmr1 KO mice through both translation-dependent and translation-independent signaling of FMRP. APs that back-propagate into dendritic regions of the neuron provide an associative signal to active synapses by depolarizing the membrane and facilitating divalent ion unblock of synaptic NMDA receptors to allow active synapses to potentiate. The K+ channel Kv4.2 is very influential in hippocampal neurons because it underlies the dendritic A-type K+ current and normally suppresses AP-backpropagation into dendrites (Chen et al., 2006). In fact, Kv4.2 knockout mice have a reduced threshold for LTP induction (Chen et al., 2006). Analysis of both protein and mRNA levels revealed that Kv4.2 expression is reduced in Fmr1 KO mice, suggesting that FMRP normally upregulates Kv4.2 expression (Gross et al., 2011). This is a non-canonical action for FMRP, which is generally characterized as an inhibitor of translation. Consistent with this a direct measurement of the A-type current by dendritic recordings confirmed that there is a reduction in current density in the dendrites of CA1 pyramidal neurons (Routh et al., 2013). Moreover, the Ca2+ influx associated with backpropagating APs in the dendrites was enhanced and, as a result, LTP induced by a weak theta burst stimulation of EPSPs on the most distal part of the dendritic tree was also increased (Routh et al., 2013).
All these findings are consistent with increased excitability of pyramidal neuron dendrites in the hippocampus of Fmr1 KO mice, but are not consistent with the elevated threshold for LTP induction that was previously reported (Lauterborn et al., 2007). In direct contrast to these findings, it has also been reported that the expression of Kv4.2 is upregulated in Fmr1 KO mice (Lee et al., 2011). However, that study used immunohistochemical and surface biotinylation analysis to demonstrate that Kv4.2 protein was elevated, rather than a direct measurement of dendritic Kv4.2 current density. Moreover, the threshold for LTP using a theta burst induction was elevated in slices from Fmr1 KO mice (similar to the original findings in CA1), but could be restored to normalcy with a selective Kv4.2 inhibitor (Lee et al., 2011). The diametrically opposed findings in these studies are difficult to reconcile. In both cases biochemical techniques were used to address the expression levels of Kv4.2 protein in CA1 (Chen et al., 2006; Gross et al., 2011; Lee et al., 2011) and in both cases theta burst stimulation was used to determine LTP threshold (Lee et al., 2011; Routh et al., 2013). A difference in background strain of the Fmr1 KO mouse would be an unlikely possibility in such a fundamental molecular process. It is more likely that there are differences between synapses at the most distal parts of the dendrites compared to those more proximal to the soma. Kv4.2 expression will likely have the biggest impact at those more distal synapses (recorded by Routh et al.). Future work will be needed to define how the excitability of the dendritic tree affects plasticity thresholds, or whether altered excitability of dendrites may have consequences for oscillatory or seizure activity in hippocampal networks.
Dendritic excitability regulated by Ih
Another channel that is prominent in regulating dendritic excitability is the hyperpolarization activated cyclic nucleotide-gated (HCN) channel. HCN channels are non-selective cation channels that are active at rest and therefore contribute to the resting membrane potential and to membrane resistance (Shah, 2014). They are prominently expressed in the dendrites of hippocampal and cortical pyramidal neurons with a gradient of increasing density towards the most distal regions. This channel gradient has been demonstrated to affect the integration of synaptic events occurring at different dendritic locations (Magee, 2000). The current through dendritic HCN channels (Ih) is increased in CA1 neuron dendrites from Fmr1 KO mice (Fig. 2C, left) (Brager et al., 2012). Ih has a complex effect on dendritic excitability, but an overall increase in current density would lead to a decrease in input resistance of the dendrites and actually make the dendrites less excitable (Brager et al., 2012). However, just as with Kv4.2 (see above), the net effect on dendritic excitability is difficult to estimate. Additionally, in L5 neurons of somatosensory cortex, dendritic Ih is abnormally reduced in Fmr1 KO mice, where it was associated with a higher than normal input resistance (Fig. 3B, right) (Zhang et al., 2014). This points, once again, to what are likely brain region-specific differences in FXS phenotypes. At least in the neocortex, reduced Ih was coupled to changes in BK channels that clearly increase the excitability of L5 pyramidal neuron dendrites, leading to increased synaptic summation and AP backpropagation.
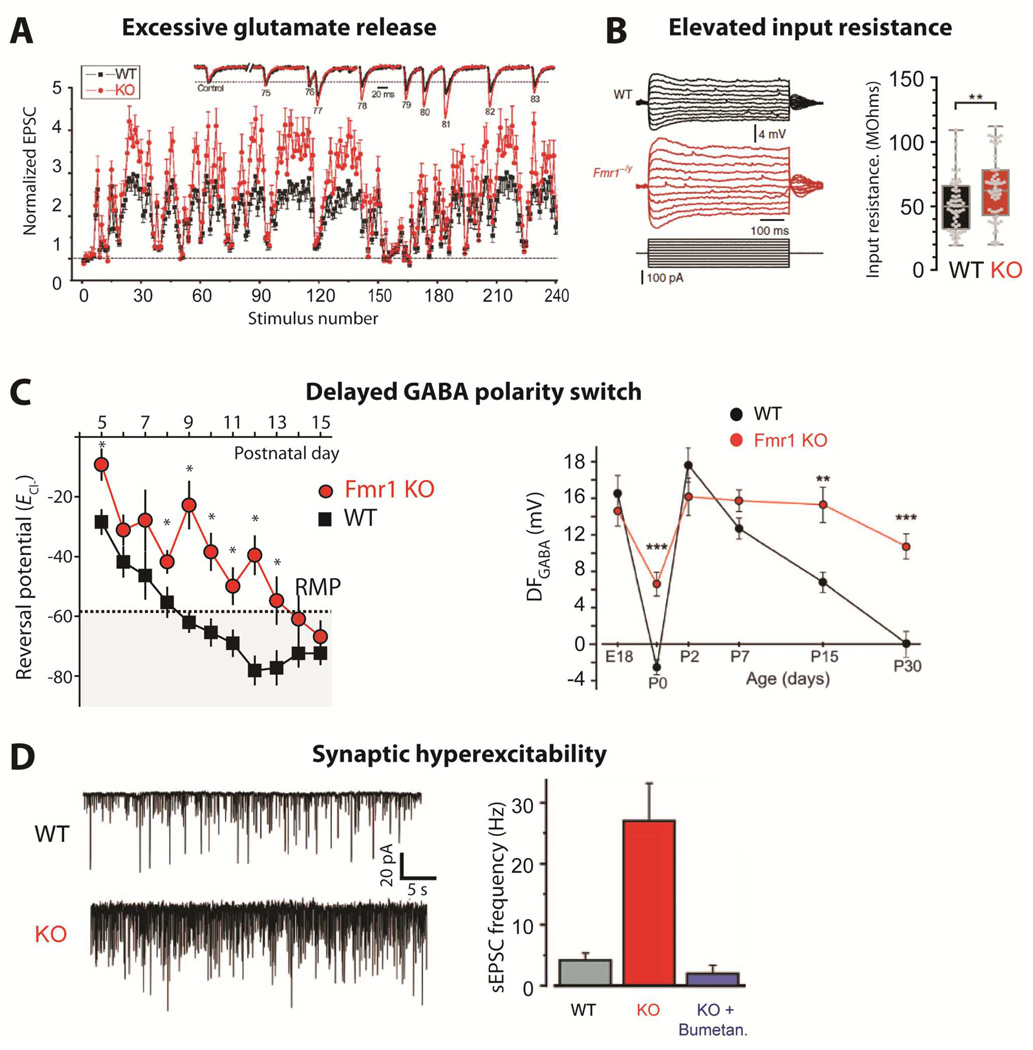
Figure 3. Synaptic hyperexcitability.
-
(A)Changes in synaptic strength during natural stimulus trains plotted as a function of stimulus number for WT and Fmr1 KO mice. Inset shows EPSCs 75–83 during the natural stimulus trains, scaled to their own controls for comparison. (from Deng et al., 2011).
-
(B)Dendritic input resistance is increased in cortical L5 pyramidal neurons of Fmr1 KO mice [from Zhang et al., 2014].
-
(C)Delayed GABA polarity switch. Left, ECl- remains depolarized in Fmr1 KO mice during cortical development. Average ECl- calculated from individual recordings plotted against the age of the mouse. The resting membrane potential (RMP) measured at P10 is denoted by the dashed line and shaded area represents points at which GABA would have a mature hyperpolarizing response. *p < 0.05 [from He et al., 2014]. Right, Age-dependence of the driving force of GABA-A receptor (DFGABA) in neurons from control and Fmr1 KO mice [from Tyzio et al., 2014]
-
(D)Spontaneous activity is increased in Fmr1 KO mice at P15. Left, representative traces of whole-cell voltage clamp recordings of sEPSCs at –70 mV from individual hippocampal CA3 pyramidal neurons in acute brain slices. Right, Average values of sEPSC frequencies are higher in Fmr1 KO mice, but are normalized by treatment with bumetanide at birth. [from Tyzio et al., 2014]
Spike timing plasticity
In neurons of the medial prefrontal cortex of Fmr1 KO mice, the threshold for the induction of spike timing-dependent plasticity is also altered (Meredith et al., 2007). While in this case it was found that single APs failed to produce a large Ca2+ signal in the dendrites, a detailed investigation of dendritic excitability was not performed. Regardless, these findings are in line with evidence that increased neuronal activity is required to overcome the plasticity threshold in Fmr1 KO mice. Further detailed studies of dendritic excitability and the link to synaptic plasticity are required to determine if different cell types have more or less excitable dendritic trees, and whether these have significant effects on synaptic plasticity thresholds, which may underlie some of the cognitive dysfunction in FXS.
GABA, inhibitory synapses and tonic inhibition
Inhibitory synapses, acting primarily through the release of the neurotransmitter GABA, are essential to providing inhibitory control of neuronal networks. The principal receptors at these synapses are the ionotropic GABAA receptors and the metabotropic GABAB receptors. There is evidence for both reduction in the mRNA encoding for many of the GABAA receptor subunits in Fmr1 KO mice (D’Hulst et al., 2006; Gantois et al., 2006) and for regional and age-dependent alterations in protein levels of certain GABAA subunits (El Idrissi et al., 2005). Reductions in the levels of various GABAA receptor subunits α5, δ, β have been reported in different brain regions of Fmr1 KO mice (Curia et al., 2009; El Idrissi et al., 2005; Gantois et al., 2006). Interestingly, all three Drosophila GABAA receptor subunits are downregulated in the fly model of FXS (D’Hulst et al., 2006). In addition, expression of glutamic acid decarboxylase, the rate-limiting GABA synthesis enzyme, is also reduced not only in the mutant flies (Gatto et al., 2014) but also in the amygdala of Fmr1 KO mice (Olmos-Serrano et al., 2010). Despite this, the functionally characterized alterations in phasic GABAA-mediated transmission are quite modest and may be regionally restricted. Both the frequency and amplitude of miniature IPSCs and spontaneous IPSCs are reduced in the mature amygdala (Olmos-Serrano et al., 2010), as well as during development (Vislay et al., 2013). However, in the subiculum and L2/3 of somatosensory cortex there are no reported alterations in postsynaptic GABA signaling (Curia et al., 2009; Paluszkiewicz et al., 2011). Similarly, the amplitude of GABA currents was not altered in striatal projection neurons of Fmr1 KO mice, despite a lower density of GABAergic synapses (Centonze et al., 2008), suggesting that synaptic GABAA receptors are not altered. Instead, the frequency of spontaneous and miniature events was reduced in these neurons, indicating a presynaptic deficit in GABA release. In addition to subtle alterations in synaptic GABAA signaling, recent evidence has demonstrated that the tonic GABA current (present in some neurons and mediated by extrasynaptic receptors) is reduced in principal neurons of the basolateral amygdala (Martin et al., 2014). Interestingly, this reduction in the tonic GABA current did not adversely impact the overall synaptic excitation/inhibition (E/I) ratio, but did affect the relative timing of excitatory inputs and feedforward inhibition, as well as the integration of the total conductance (Martin et al., 2014). It remains to be determined whether alterations in tonic GABA currents are present throughout the brain; however, a GABA-R agonist that is relatively selective for extrasynaptic GABAA subunits can reduce hyperactivity in Fmr1 KO mice and improve their response to PPI (Olmos-Serrano et al., 2011).
Depolarizing GABA and effects on excitability
The neurotransmitter GABA acting on GABAA receptors can produce excitatory, depolarizing actions on cortical neurons. This occurs primarily during the first few postnatal weeks in rodents and is determined by a changing Cl− gradient in neurons (Cherubini et al., 2011). Intracellular Cl− is relatively elevated during embryonic and early postnatal development. This higher intracellular Cl− concentration in juvenile neurons results in a reversal potential for GABAA channels that is relatively depolarized compared to adult neurons. This relatively depolarized reversal potential reduces the driving force for Cl− through GABAA receptors and can be sufficiently depolarized in some neurons that GABA can be excitatory. Numerous roles have been ascribed to depolarizing GABA, including trophic roles during development (Ganguly et al., 2001), and when this process goes awry the resulting increased excitability is thought to contribute to childhood seizures (Ben-Ari et al., 2012).
A recent study demonstrated that the developmental switch in GABA polarity, from depolarizing to hyperpolarizing, is delayed in the cortex of Fmr1 KO mice (Fig. 3C) (He et al., 2014). The reversal potential for GABAA receptors (EGABA) matures over the first postnatal week in L4 neurons in the cortex with the resulting maturation of GABA responses by the end of this period. In Fmr1 KO mouse, EGABA remains relatively depolarized at these early postnatal ages. This likely has a significant impact on the development of the synaptic and circuit properties in the cortex, which are undergoing a critical period of plasticity and rewiring (Crair and Malenka, 1995).
EGABA is largely controlled by the actions of two Cl− co-transporters, NKCC1 and KCC2. NKCC1 is the juvenile Cl− co-transporter whose expression is relatively abundant during the perinatal period. This transporter actively regulates the intrusion of Cl− into the cell. KCC2 expression is elevated later in postnatal development and this co-transporter actively extrudes Cl− from neurons. Therefore, the intracellular Cl− homeostatic balance is regulated by the relative ratio of the expression and functional properties of these two transporters. In FXS mice, NKCC1 protein expression is elevated at later times in the cortex and this directly correlates with the relatively depolarized EGABA (He et al., 2014). This finding suggests that inhibiting NKCC1 could potentially rectify the imbalance in chloride homeostasis.
A delay in the developmental switch in GABA polarity has also been reported in hippocampus, where the driving force for GABA is elevated in hippocampal CA3 neurons from Fmr1 KO mice, compared to WT mice (Tyzio et al., 2014) (Fig. 3D). This study also reported a rapid and transient hyperpolarization of EGABA during parturition that was blunted in FXS mice and in the valproate-induced rat model of autism. Pre-birth maternal treatment with a NKCC1 inhibitor, the loop diuretic bumetanide, could rectify the driving force changes and rescue the elevated excitability in CA3 neurons (Fig. 3E) (Tyzio et al., 2014). How does exposure of the mother to bumetanide in the drinking water for one day prior to birth have such a dramatic effect on Cl− homeostasis? The authors proposed that the transient switch in EGABA during parturition is controlled by oxytocin (Tyzio et al., 2006). Indeed, antagonizing oxytocin during birth produced altered chloride homeostasis similar to that seen in Fmr1 KO mice. While many mechanistic questions remain, these studies have provided a strong rationale for assessing disrupted chloride homeostasis during development in FXS and other neurodevelopmental disorders.
Local circuits and networks
The molecular changes in ion channels, transporters and neurotransmitter receptors brought about by loss of FMRP that result in increased neuronal excitability would naturally be predicted to trigger disruptions in network activity. Unfortunately, compared to our current level of understanding of the defects in FXS at the molecular, synaptic and cellular levels, we know much less about circuit alterations in this disorder. And yet, bridging this knowledge gap is critical for developing therapies for FXS because individual symptoms (or behavioral impairments in mice) can be linked more directly to specific alterations in circuits than to defects at the molecular level.
Circuit level alterations in humans with FXS
As stated earlier, many of the symptoms of FXS (e.g., hyperactivity, hyperarousal, seizures, hypersensitivity to sensory stimuli) fit well with the notion of neuronal hyperexcitability. However, it is challenging to determine whether there are similar changes in neuronal excitability in individuals with FXS because of the limitations or invasiveness of recording techniques. Still, approaches like EEG, visual evoked potentials (VEP), and functional magnetic resonance imaging (fMRI), are currently being used and might provide such evidence. It also stands to reason that different symptoms could be ascribed to circuit alterations in specific brain regions. For example, anxiety, memory deficits and tactile defensiveness might correlate with circuit alterations in the amygdala, hippocampus or somatosensory cortices, respectively. Here, we summarize and discuss studies in humans that provide support for the concept of network hyperexcitability in FXS.
Magnetic resonance imaging
Several anatomical studies of the brain have been performed in children and adults with FXS, and many of these have found that certain brain structures are abnormally large. For example, enlarged gray matter volume (GMV) has been reported in FXS individuals in the caudate nucleus and thalamus, as well as in the frontal, cingulate and fusiform gyri (Bray et al., 2011; Hallahan et al., 2011; Hazlett et al., 2012; Hoeft et al., 2010; Wilson et al., 2009). The enlargement of the caudate nucleus, which is perhaps the most reproducible neuroanatomical abnormality in FXS, has been implicated in dysfunction of frontostriatal circuitry in this disorder. The larger caudate size is already apparent in young affected children (Hoeft et al., 2010) and this persists into adulthood (Hallahan et al., 2011). Interestingly, two separate structural MRI studies have hinted that GMV of the caudate nuclei and cerebellum were larger in FXS relative to other individuals with autism who did not have FXS (Hazlett et al., 2012; Wilson et al., 2009).
The cause of these enlarged brain regions found in FXS individuals is not known. In mice, there are known alterations in the proliferation and differentiation of glia and neurons in both embryonic stem (ES) cells and adult neural stem cells (Luo et al., 2010). Still, the thickness of cortical layers and the total number of neurons in Layer 4 are both normal in Fmr1 KO mice, at least in barrel cortex (Bureau et al., 2008; Till et al., 2012). Given that caudate enlargement is already present in young children with FXS, it could be caused by a failure in developmental pruning of superfluous axonal projections, as reported in mice (Bureau et al., 2008).
It is not known how an increase in the size of certain brain structures might affect behavioral/cognitive function in FXS. Recent studies have begun to examine how alterations in white matter structural connectivity (with diffusion tensor imaging; DTI), and in functional connectivity (with fMRI) correlate with cognition and behavior during normal and abnormal brain development (Dennis and Thompson, 2013). One DTI study found that young males with FXS exhibited increased density of reconstructed fibers compared to typically developing subjects, particularly in the left ventral frontostriatal pathway (Haas et al., 2009). Interestingly, greater relative fiber density was found to be associated with lower IQ in affected individuals.
Few fMRI studies have been performed on FXS subjects, and these provide only partial support to the theory of hyperexcitability. For instance in one study where subjects performed a facial-emotion discrimination task, the FXS group showed fusiform gyrus hypoactivation compared with the typically developing control group (Dalton et al., 2008). In contrast, FXS subjects displayed significantly greater activation than controls subjects in the left hippocampus, left superior temporal gyrus, right insula, and left postcentral gyrus. A different study from the same group found that FXS individuals had decreased activation of prefrontal regions associated with complex social cognition, including the medial and superior frontal cortex, during successful face encoding (Holsen et al., 2008). The authors concluded that social anxiety in FXS might be related to the inability to successfully recruit higher-level social cognition regions during the initial phases of memory formation.
Electroencephalography (EEG)
One might expect that the higher propensity for seizures in FXS correlates with EEG abnormalities. Unfortunately, there is a paucity of EEG studies in this disorder. In a small group of FXS children with parent-reported behaviors resembling seizures, abnormal EEG findings included slowing of background rhythm and epileptiform discharges, although the EEG was completely normal in some of the subjects (Heard et al., 2014). Another study of resting-state EEG demonstrated a decrease in global functional connectivity in FXS males for upper alpha and beta frequency bands, but increased connectivity in long-range (fronto-posterior) and short-range (frontal-frontal and posterior-posterior) clusters (van der Molen et al., 2014). The study also provided evidence for increased path length in the theta band, which is consistent with immature topological organization of neuronal networks governing theta synchronization. Indeed, because longer path length likely reflects excess neuronal connectivity, these defects could result in uncoordinated information transfer within brain networks in FXS.
Sensory evoked potentials
The neural correlates of hypersensitivity to sensory stimuli can be studied with evoked potentials. Two studies of auditory evoked potentials (AEPs) and VEPs found that the amplitudes in both modalities were increased in FXS compared to both chronological (VEP only) and developmental (both VEP and AEP) control groups (Knoth et al., 2014). This profile suggests disruptions in sensory processing in FXS and supports the notion of a hyper-reactive nervous system.
In summary, neuroimaging studies in humans indicate subtle anatomical defects in FXS. Whether the slightly larger size of any given brain region correlates with maladaptive hyperfunction of that region, perhaps because of hyperconnectivity, is a question that future studies will need to address. In particular, efforts at combining different MRI modalities with EEG and other physiological measures of activity may help to determine whether circuit hyperactivity in FXS individuals is a pervasive feature of the disorder.
Circuit defects in mice
Linking specific symptoms of FXS to alterations in channel or neurotransmitter receptor expression is not always intuitive. By considering each of the symptoms in FXS (and autism in general) as alterations in network function in specific brain regions, we can begin to bridge the circuit to behavior gap. This is certainly true for other neurological and psychiatric disorders in which particular symptoms can be attributed to specific types of network dysfunction. For example, altered patterns of activity in the basal ganglia and motor circuitry causes the motor symptoms of Parkinson disease, while runaway excitation in the hippocampus triggers temporal lobe seizures. Conceivably, many symptoms in FXS can be understood on the basis of alterations in cortical circuits too. But this could be a difficult task in FXS due to the complexity of neuropsychiatric symptoms, the distributed nature of circuits underlying those symptoms, and the fact that experimental approaches for recording network activity in awake behaving rodents are technically challenging, time-consuming, and inherently low-throughput. Concentrating on a single symptom that is more tractable, such as sensory alterations, might be a more fruitful path. Indeed, circuit hyperexcitability in the somatosensory or auditory cortex might explain why children with FXS show hypersensitivity to sensory stimuli.
Just like changes in the expression of channels or neurotransmitter receptors can lead to neuronal and synaptic hyperexcitability, they are also expected to lead to increased network activity. This could be reflected as a simple increase in firing rates for neurons during spontaneous activity (i.e., resting state) or as a higher proportion of neurons being recruited to network events. In the context of hypersensitivity to sensory stimuli, increased neuronal excitability might lead neurons to fire more APs in response to sensory stimulation, to a greater proportion of sensory stimuli eliciting neural responses, or to a greater proportion of neurons in sensory cortex responding to a given stimulus. As a result, neurons might be expected to have broader tuning. Such alterations may be detectable in electrophysiological studies in vitro, or might be apparent only in vivo, when naturalistic stimuli can be used to interrogate intact circuits with preserved brainstem neuromodulation and sensory inputs.
Abnormal UP states
Over the last decade, a number of studies have provided evidence of network dysfunction in Fmr1 KO mice. In the somatosensory cortex, network hyperexcitability first manifested as a decrease in excitatory drive onto fast-spiking interneurons and prolonged UP states in some cortical pyramidal neurons (Gibson et al., 2008) (Fig. 4A). UP states are short periods (<2 sec) of persistent membrane depolarization that reflect overall local network activity (Sanchez-Vives and McCormick, 2000; Steriade et al., 1993). These network phenomena are mostly present during periods of rest, sleep or quiet wakefulness and tend to disappear when the animal is in a state of engaged arousal. When recorded extracellularly in brain slices, L4 and L5 excitatory neurons from Fmr1 KO mice exhibit longer thalamically evoked UP states (Gibson et al., 2008) and longer spontaneously occurring UP states, compared to WT mice (Hays et al., 2011). The same was seen in cell-attached recordings in vivo, in adult FXS mice anesthetized with urethane (Hays et al., 2011).
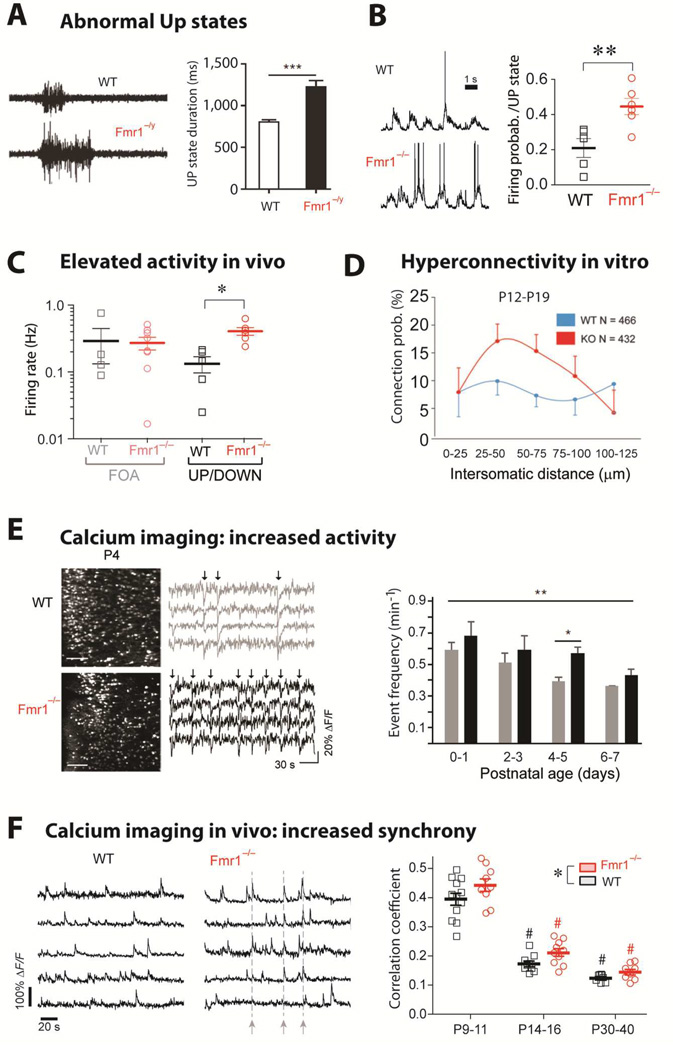
Figure 4. Circuit hyperexcitability: UP states, firing, synchrony.
-
(A)Left, Representative extracellular multiunit recordings from L4 in slices from barrel cortex of WT and Fmr1−/y mice. Right, Group averages showing prolonged UP state duration in slices from Fmr1−/y mice (n = 22). [from Ronesi et al., 2012]
-
(B)Left, Sample traces from whole-cell patch-clamp in vivo recordings of L2/3 neurons during UP/DOWN states in unanesthetized WT and Fmr1−/− mice. Right, The mean firing probability during any given Up state (active or silent) was higher in Fmr1−/− mice (**p < 0.01, t-test). In contrast, the frequency and duration of Up states were the same in WT and mutant mice (not shown). [from Goncalves et al., 2013]
-
(C)Firing rates for L2/3 neurons are higher in Fmr1−/− mice compared to WT mice during in vivo whole-cell recordings showing UP/DOWN states (typical of sleep or quiet wakefulness), but not during fast oscillatory activity (FOA; typical of awake brain state). *p < 0.05. [from Goncalves et al., 2013]
-
(D)Hyperconnectivity of L5 pyramidal neurons in prefrontal cortex of 2–3 week old Fmr1 KO mice. Direct connections between neurons were tested at a range of distances using hexa-patch electrode recordings in brain slices. Connection probability distributions were significantly higher for clusters of Fmr1 KO neurons than for those of WT neurons (p < 0.01). The same analysis in 3–5 week-old mice did not reveal any significant differences. (from Testa-Silva et al., 2011)
-
(E)Hyperactive postnatal brain networks in Fmr1 KO mice. Left, representative two-photon images of acute coronal sections of the cortex at P4 from WT and Fmr1 KO mice that were loaded with the fluorescent calcium indicator Fura2-AM and corresponding sample traces for 4 neurons. Arrows indicate epochs of synchronous firing in the recording. Right, frequency of activity during development in WT and Fmr1 KO mice (**p = 0.024 across ages, and *p = 0.002 at P4–5) (from El Fata et al., 2014)
-
(F)Left, Calcium traces for 5 representative L2/3 neurons in barrel cortex of unanesthetized WT mice and Fmr1−/− mice at P14-16 showing synchronous bursts of cell firing (dashed lines) in the Fmr1−/− mouse. Right, Mean correlation coefficients for all cell pairs within 100 µm of each other for WT and Fmr1−/− mice at different postnatal ages. Both age and genotype significantly affected correlation coefficients,*p < 0.05). [from Goncalves et al., 2013]
A different study used whole-cell recordings of L2/3 neurons in vivo in unanesthetized Fmr1 KO mice and found a 2-fold higher than normal probability of neuronal firing in UP states, again supporting the interpretation of cortical network hyperexcitability (Fig. 4B) (Goncalves et al., 2013). But in contrast to the Hays et al., UP state duration was normal in Fmr1 KO mice. Potential explanations for this discrepancy include the different cell types (L2/3 neurons normally have shorter UP states than L4 or L5 neurons), the fact that different layers have different densities of interneurons (which dictate the duration of UP states), the age of the animal (P14-P16 for Gonçalves et al. study vs. adult for Hays et al.), the criteria for defining an UP state, or the method of recording (cell-attached in urethane-anesthetized mice vs. whole-cell in unanesthetized mice).
While investigating the mechanisms involved in this defect, Hays et al. (2011) reported that selective deletion of Fmr1 in cortical excitatory neurons was sufficient to cause prolonged UP states, whereas deletion in inhibitory neurons had no effect. Genetic reduction or pharmacological blockade of mGluR5 can rescue the prolonged UP state phenotype suggesting that excess mGluR5 signaling contributes to the longer UP states (Hays et al., 2011). Moreover, when Homer1a is deleted from Fmr1 KO mice the prolonged UP state phenotype is rescued (Ronesi et al., 2012), suggesting that disruption of mGluR5-Homer interactions might mediate the prolonged UP states in Fmr1 KO mice.
Increased firing rates
The higher intrinsic excitability of neurons lacking FMRP would also be expected to lead to abnormally high firing rates during spontaneous activity in vivo. In line with this, cortical L2/3 neurons of unanesthetized Fmr1 KO animals at P14-P16 have 3-fold higher firing rates compared to WT neurons, but only during periods when animals were at rest (asleep or in quiet wakefulness) (Fig. 4C) (Goncalves et al., 2013). This brain state-dependent defect in modulating spontaneous network activity is interesting because abnormally high firing during sleep could interfere with coordinated replay of ensemble-level patterns of activity and the normal process of memory consolidation (Ji and Wilson, 2007), or result in a state of hyperarousal during sleep as is hypothesized to occur in FXS (Kronk et al., 2010).
Local hyperconnectivity and higher network synchrony
Besides elevated firing rates, what are other potential consequences of increased neuronal excitability at the circuit or network level? Using multiple patch-clamp recordings of neighboring L5 cortical pyramidal neurons in acute slices of prefrontal cortex from Fmr1 KO mice, a recent study demonstrated that such clusters of neurons are hyperconnected (Fig. 4D) (Testa-Silva et al., 2012). Importantly, this defect was transient and occurred only during a critical period in mouse brain development (2nd and 3rd postnatal weeks), which may be particularly important for FXS pathogenesis, since it coincides with a time when Fmr1 KO mice exhibit a variety of other transient synaptic or brain circuit alterations (Bureau et al., 2008; Cruz-Martin et al., 2010; Goncalves et al., 2013; Harlow et al., 2010; He et al., 2014; Meredith et al., 2011).
Recent studies that used two-photon Ca2+ imaging to record network activity in large ensembles of neurons during development have shown that cortical circuits in Fmr1 KO mice show abnormally high activity, as well as abnormally high synchronous firing of neuronal ensembles as early as P4 (Fig. 4E) (La Fata et al., 2014) and throughout the first 3–4 postnatal weeks (Fig. 4F) (Goncalves et al., 2013). Elevated network activity in Fmr1 KO mice can also be demonstrated pharmacologically. Bath application of the GABAA and GABAB antagonists picrotoxin and CGP55845 led to abnormally prolonged bursts of activity in acute brain slices from Fmr1 KO compared to WT mice (Hays et al., 2011). Previously, others had shown that blocking GABAergic synapses with bicuculline led to persistent network activity in hippocampal slices from Fmr1 KO mice (Chuang et al., 2005).
Exaggerated sensory responses and impaired sensory learning
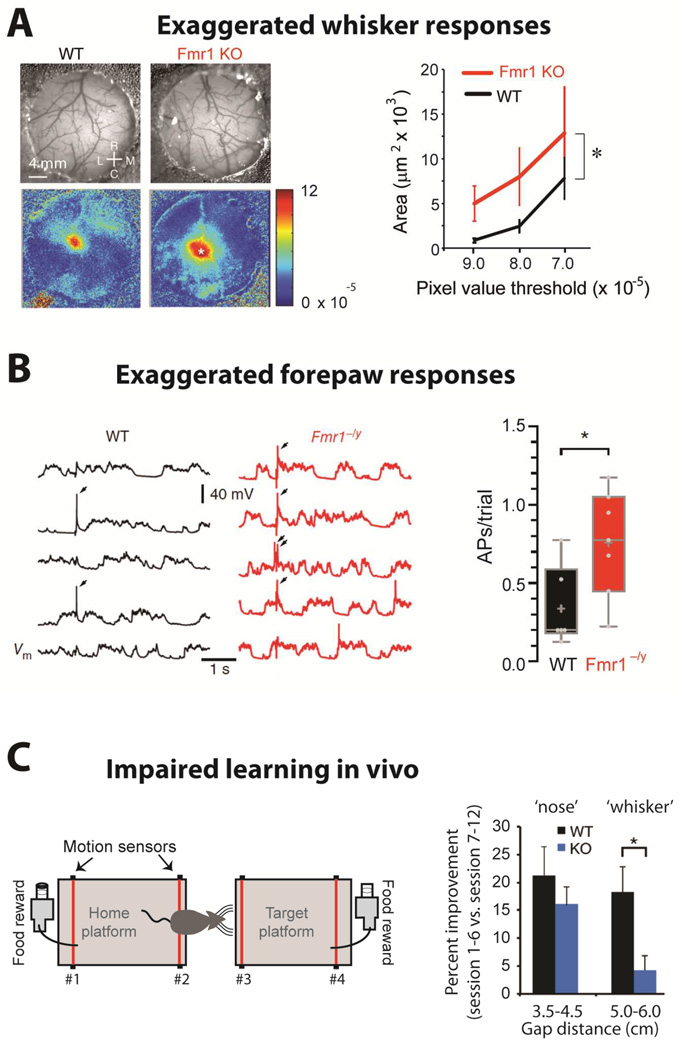
Intuitively, abnormally elevated spontaneous network activity and hyperexcitability might be expected to cause exaggerated responses to sensory stimuli in Fmr1 KO animals. Recent studies are beginning to address this important issue. A study using intrinsic signal imaging through cranial windows reported that whisker stimulation in Fmr1 KO mice resulted in a larger sensory map in barrel cortex than in WT mice (Arnett et al., 2014) (Fig. 5A). In a similar experiment using voltage sensitive dye imaging in vivo, a different group reported that whisker stimulation resulted in a faster spread of depolarization in barrel cortex of Fmr1 KO mice compared to WT mice (Zhang et al., 2014). In addition, sensory-evoked activity in response to forepaw stimulation was exaggerated in somatosensory cortex, which may be the first demonstration of how increased neuronal excitability results in an overwhelming sensory response in the Fmr1 KO cortex (Fig. 5B). Interestingly, the exaggerated whisker-evoked responses in barrel cortex likely interfere with sensory processing in the Fmr1 KO mice, because they exhibit impaired learning in a whisker-dependent ‘gap crossing’ test (Arnett et al., 2014)(Fig. 5C).
Figure 5. Sensory hypersensitivity and impaired learning.
-
(A)Left, Images of the vasculature through the cranial window (top) and intrinsic signal images (bottom) collected from a WT and a Fmr1 KO mouse after single whisker stimulation. Rostral (R), Caudal (C), Lateral (L) and Medial (M). Right, The region of response with DR/R magnitudes greater than the threshold is larger for Fmr1 KO than for WT mice (n= 10 each; WT vs. KO, p= 0.011). [from Arnett et al., 2014]
-
(B)Left, Five consecutive somatic responses to contralateral hindpaw stimulation (2 ms, 30 mA) recorded from L2/3 pyramidal neurons of S1 in anesthetized WT and Fmr1−/y littermate mice (APs indicated by arrows, top). Right, The average number of APs per trial of hindpaw stimulation was increased in neurons from Fmr1−/y mice (p < 0.05). [from Zhang et al., 2014]
-
(C)Left, Schematic of the gap-crossing apparatus (left). Successful localization of the object and gap-crossing was rewarded appetitively. For short distances, mice use their noses, whereas for long gaps (>4.5 cm) they use whiskers. Right, Percent improvement from first 6 sessions to last 6 sessions for short (nose) and long distances (whiskers). WT mice display significantly greater improvement at whisker-dependent distances than Fmr1 KO mice (p= 0.02) [from Arnett et al., 2014]
Therefore, a variety of systems-level alterations in Fmr1 KO mice have been reported over the last few years that are beginning to provide a foundation from which to understand neurologic and psychiatric symptoms in individuals with FXS. Because recordings of network activity in awake behaving animals are increasingly mainstream, we anticipate that even more direct links between circuit alterations and impairments in learning, sensory processing and cognition in rodent models of FXS will soon be established.
SUMMARY
In presenting evidence for the theory of hyperexcitability in FXS, we have hopefully gone beyond arguing for a simple excitation/inhibition imbalance by providing specific examples of disruptions at the molecular, synaptic and circuit levels in Fmr1 KO mice (Fig. 6). The idea that hyperexcitability might explain symptoms like seizures or hypersensitivity to sensory stimuli in the most common genetic cause of autism could also mean that other genetic or sporadic forms of autism might also have altered neuronal and circuit excitability. Certainly, the concept of hyperexcitability fits well with the Intense Word Theory of autism (Markram and Markram, 2010), which posits that hyper-functioning of local neural microcircuits leads to the core cognitive features of autism, including hyper-perception, hyper-attention, hyper-emotionality.
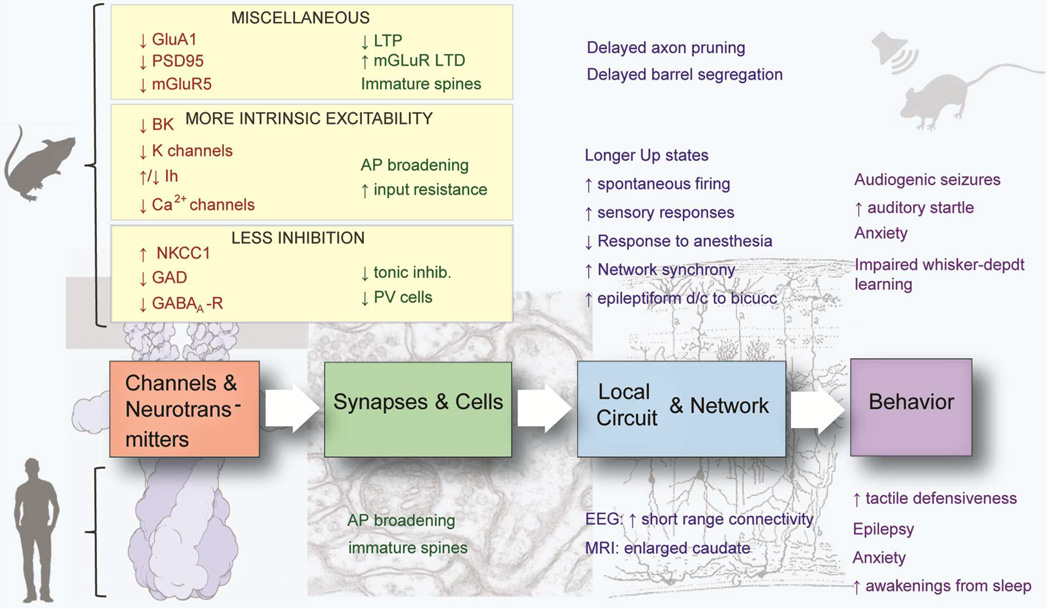
Figure 6. Summary diagram.
Loss of FMRP results in a delay in brain maturation, which is especially obvious in the first 2 postnatal weeks in mice, in line with the peak expression of FMRP in neocortex at P7 (Till et al., 2012). This might explain the observed delay in the maturation of neuronal structure (dendritic spines; overelaboration of axons) and function (e.g., reduced cortical LTP). In addition, FMRP directly regulates the expression of a number of channels, transporters and synaptic proteins that lead to either increased intrinsic excitability of neurons or to diminished inhibition. Eventually, these defects coincide in creating hyperexcitability at the circuit and network level, which leads to impairments in behavior (attention deficit/hyperactivity, anxiety, frequent awakening from sleep), sensory hypersensitivity seizures, or impaired learning and cognition.
It remains unclear whether some or all of the neuronal and circuit alterations discovered in adult Fmr1 KO mice are caused by the absence of FMRP during specific critical periods of brain development, or whether instead they reflect the absence of steady-state functions of FMRP in mature circuits. As described above several studies have pointed to delayed maturation of synaptic and neuronal structure in Fmr1 KO mice (Fig. 6). How this might lead to hyperexcitability is easier to conceive for some phenotypes (e.g., delayed expression of GluA1 or delayed downregulation of NKCC1) than for others (e.g., delayed spine stabilization). Nevertheless, the notion of delayed maturation in FXS is of critical importance and could explain differences in phenotypes across brain regions (e.g., cortex vs. hippocampus) based on their relative timing of maturation.
Clearly, the fact that many of these disruptions are already present in early postnatal animals, and the fact that peak expression of FMRP occurs around the 1st and 2nd postnatal weeks, favor the former scenario. The future use of conditional genetics in mice to knockdown Fmr1 at specific developmental stages will help distinguish between these two possibilities. Moreover, conditional restoration of FMRP expression in Fmr1 KO mice during development or in adulthood will address critical questions regarding the timing and possible outcomes of human therapies. We anticipate that in the next few years a growing number of studies will attempt to link specific alterations in cellular or network excitability with impaired behavior. One recent study came close to providing such a full-circle hyperexcitability view of FXS, by demonstrating that Fmr1 KO mice exhibit exaggerated responses to whisker stimuli and learning impairments in tactile discrimination, as assessed by the gap crossing test (Arnett et al., 2014). Another comprehensive study showed that pharmacological correction of a channelopathy in Fmr1 KO mice (using a drug that works as a BK channel opener in vitro), could rescue exaggerated acoustic startle in vivo (Zhang et al., 2014). We are optimistic that in coming years, strategies that target the symptoms of FXS by correcting the alterations in neuronal and circuit excitability will provide promising directions towards new therapies for this disorder.
Acknowledgments
We thank Drs. Dean Buonomano, Molly Huntsman and Aaron McGee, as well as members of our laboratories for their critical reading of this review. We also thank Erica Arroyo for help with Figure 6. This study was supported by grants from NICHD 2R01HD054453-06 (CP-C), NINDS R01 NS081972 (VAK) & R01 NS089449 (VAK), Simons Foundation Autism Research Initiative grant 295438 (CP-C), Department of Defense AR130187P2 (AC and CP-C), NIMH R01 MH099114-03 (AC) and FRAXA research foundation (AC)
Abbreviations
- ADP/AHP
after depolarization/hyperpolarization potential
- AP
action potential
- eCB
endocannabinoid
- FMRP
fragile X mental retardation protein
- FXS
fragile X syndrome
- GpI
Group I
- KO
knockout
- L
layer
- LTD/LTP
long-term depression/potentiation
- mGluR
metabotropic glutamate receptor
- P
postnatal day
- PPI
prepulse inhibition
- S1
primary somatosensory cortex
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnett MT, Herman DH, McGee AW. Deficits in tactile learning in a mouse model of fragile X syndrome. PloS one. 2014;9:e109116. doi: 10.1371/journal.pone.0109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Ayres AJ. Tactile Functions. Their Relation to Hyperactive and Perceptual Motor Behavior. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1964;18:6–11. [PubMed] [Google Scholar]
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 1997;51:91–95. doi: 10.5014/ajot.51.2.91. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends in neurosciences. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E. Epilepsy in fragile X syndrome. Developmental medicine and child neurology. 2002;44:724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. American journal on intellectual and developmental disabilities. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3497–3507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(−/y) mouse model of fragile X syndrome. Cell reports. 2012;1:225–233. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Channelopathies and dendritic dysfunction in fragile X syndrome. Brain research bulletin. 2014;103:11–17. doi: 10.1016/j.brainresbull.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, Hirt M, Jo B, Hall SS, Lightbody AA, Walter E, Chen K, Patnaik S, Reiss AL. Aberrant frontal lobe maturation in adolescents with fragile X syndrome is related to delayed cognitive maturation. Biological psychiatry. 2011;70:852–858. doi: 10.1016/j.biopsych.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nature neuroscience. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren M, Paakkonen A, Tarkka IM, Ryynanen M, Partanen J. Augmentation of auditory N1 in children with fragile X syndrome. Brain topography. 2003;15:165–171. doi: 10.1023/a:1022606200636. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, Bagni C. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biological psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Yun SW, Seto J, Liu W, Toth M. The fragile X mental retardation protein binds and regulates a novel class of mRNAs containing U rich target sequences. Neuroscience. 2003;120:1005–1017. doi: 10.1016/s0306-4522(03)00406-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Griguoli M, Safiulina V, Lagostena L. The depolarizing action of GABA controls early network activity in the developing hippocampus. Molecular neurobiology. 2011;43:97–106. doi: 10.1007/s12035-010-8147-z. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Parent-child dyadic gaze patterns in fragile X males and in non-fragile X males with autistic disorder. Journal of child psychology and psychiatry, and allied disciplines. 1989;30:845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. American journal of medical genetics. 1991;38:498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- Contractor A. Broadening roles for FMRP: big news for big potassium (BK) channels. Neuron. 2013;77:601–603. doi: 10.1016/j.neuron.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cerebral cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain research. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism research : official journal of the International Society for Autism Research. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nature neuroscience. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Typical and atypical brain development: a review of neuroimaging studies. Dialogues in clinical neuroscience. 2013;15:359–384. doi: 10.31887/DCNS.2013.15.3/edennis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. Journal of neurophysiology. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neuroscience letters. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nature communications. 2014;5:3628. doi: 10.1038/ncomms4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Molecular psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiology of disease. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Pereira D, Broadie K. GABAergic circuit dysfunction in the Drosophila Fragile X syndrome model. Neurobiology of disease. 2014;65:142–159. doi: 10.1016/j.nbd.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. Journal of neurophysiology. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D’Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Long-term potentiation in the hippocampus of fragile X knockout mice. American journal of medical genetics. 1996;64:246–251. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nature neuroscience. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Berry-Kravis EM, Bassell GJ. Therapeutic strategies in fragile X syndrome: dysregulated mGluR signaling and beyond. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:178–195. doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Barnea-Goraly N, Lightbody AA, Patnaik SS, Hoeft F, Hazlett H, Piven J, Reiss AL. Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Developmental medicine and child neurology. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, Robertson D, Murphy KC, Murphy DG. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. NeuroImage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, Piven J. Trajectories of early brain volume development in fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:921–933. doi: 10.1016/j.jaac.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. 2013;251:120–128. doi: 10.1016/j.neuroscience.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile X mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard TT, Ramgopal S, Picker J, Lincoln SA, Rotenberg A, Kothare SV. EEG abnormalities and seizures in genetically diagnosed Fragile X syndrome. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2014;38:155–160. doi: 10.1016/j.ijdevneu.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. American journal of medical genetics. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. NeuroImage. 2008;43:592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature neuroscience. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature communications. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Kaczmarek LK. Emerging role of the KCNT1 Slack channel in intellectual disability. Frontiers in cellular neuroscience. 2014;8:209. doi: 10.3389/fncel.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Kronengold J, Barcia G, Quraishi IH, Martin HC, Blair E, Taylor JC, Dulac O, Colleaux L, Nabbout R, Kaczmarek LK. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell reports. 2014;9:1661–1672. doi: 10.1016/j.celrep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth IS, Vannasing P, Major P, Michaud JL, Lippe S. Alterations of visual and auditory evoked potentials in fragile X syndrome. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2014;36:90–97. doi: 10.1016/j.ijdevneu.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB., Jr Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010;33:679–687. doi: 10.1093/sleep/33.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fata G, Gartner A, Dominguez-Iturza N, Dresselaers T, Dawitz J, Poorthuis RB, Averna M, Himmelreich U, Meredith RM, Achsel T, et al. FMRP regulates multipolar to bipolar transition affecting neuronal migration and cortical circuitry. Nature neuroscience. 2014;17:1693–1700. doi: 10.1038/nn.3870. [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, Gall CM. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Ge WP, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Molecular and cellular neurosciences. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P, Yates JR., 3rd Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15281–15286. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Rosero CA, Hagerman RJ. Fragile X spectrum disorders. Intractable & rare diseases research. 2014;3:134–146. doi: 10.5582/irdr.2014.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS genetics. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nature reviews Neuroscience. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Frontiers in human neuroscience. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala. Journal of neurophysiology. 2014;112:890–902. doi: 10.1152/jn.00597.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RM, de Jong R, Mansvelder HD. Functional rescue of excitatory synaptic transmission in the developing hippocampus in Fmr1-KO mouse. Neurobiology of disease. 2011;41:104–110. doi: 10.1016/j.nbd.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Mansvelder HD. STDP and Mental Retardation: Dysregulation of Dendritic Excitability in Fragile X Syndrome. Frontiers in synaptic neuroscience. 2010;2:10. doi: 10.3389/fnsyn.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, Hagerman RJ. Molecular-clinical correlations in males with an expanded FMR1 mutation. American journal of medical genetics. 1996;64:388–394. doi: 10.1002/(SICI)1096-8628(19960809)64:2<388::AID-AJMG31>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. American journal of medical genetics. 1999;83:268–279. [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Mullard A. Fragile X disappointments upset autism ambitions. Nature reviews Drug discovery. 2015;14:151–153. doi: 10.1038/nrd4555. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, et al. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proceedings of the National Academy of Sciences of the United States of America. 2015a;112:949–956. doi: 10.1073/pnas.1423094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Hashimoto H, Cheng X, Warren ST. Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human molecular genetics. 2015b;24:1733–1740. doi: 10.1093/hmg/ddu586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Corbin JG, Burns MP. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Developmental neuroscience. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R, Reiner BC, Suresh A, Spartz E, Dunaevsky A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19715–19723. doi: 10.1523/JNEUROSCI.2514-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. Journal of neurophysiology. 2011;106:2264–2272. doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annual review of genomics and human genetics. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, Hu JH, Worley PF, Gibson JR, Huber KM. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nature neuroscience. 2012;15:431–440. doi: 10.1038/nn.3033. S431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer S, Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain research. 2013;1506:12–24. doi: 10.1016/j.brainres.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Brager DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19442–19450. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta neuropathologica. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Shah MM. Cortical HCN channels: function, trafficking and plasticity. The Journal of physiology. 2014;592:2711–2719. doi: 10.1113/jphysiol.2013.270058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile x syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telias M, Segal M, Ben-Yosef D. Neural differentiation of Fragile X human Embryonic Stem Cells reveals abnormal patterns of development despite successful neurogenesis. Developmental biology. 2013;374:32–45. doi: 10.1016/j.ydbio.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cerebral cortex. 2012;22:1333–1342. doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till SM, Wijetunge LS, Seidel VG, Harlow E, Wright AK, Bagni C, Contractor A, Gillingwater TH, Kind PC. Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Human molecular genetics. 2012;21:2143–2156. doi: 10.1093/hmg/dds030. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- van der Molen MJ, Stam CJ, van der Molen MW. Resting-state EEG oscillatory dynamics in fragile X syndrome: abnormal functional connectivity and brain network organization. PloS one. 2014;9:e88451. doi: 10.1371/journal.pone.0088451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7548–7558. doi: 10.1523/JNEUROSCI.2764-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Smith SJ, Mourrain P. Fmr1 KO and fenobam treatment differentially impact distinct synapse populations of mouse neocortex. Neuron. 2014;84:1273–1286. doi: 10.1016/j.neuron.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry research. 2009;174:138–145. doi: 10.1016/j.pscychresns.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fang Z, Jud C, Vansteensel MJ, Kaasik K, Lee CC, Albrecht U, Tamanini F, Meijer JH, Oostra BA, Nelson DL. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. American journal of human genetics. 2008;83:43–52. doi: 10.1016/j.ajhg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, Frick A. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nature neuroscience. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brown MR, Hyland C, Chen Y, Kronengold J, Fleming MR, Kohn AB, Moroz LL, Kaczmarek LK. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with slack potassium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15318–15327. doi: 10.1523/JNEUROSCI.2162-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chuang SC, Bianchi R, Wong RK. Dual regulation of fragile X mental retardation protein by group I metabotropic glutamate receptors controls translation-dependent epileptogenesis in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:725–734. doi: 10.1523/JNEUROSCI.2915-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]