Abstract
Wee1 is a critical component of the G2/M cell cycle checkpoint control and mediates cell cycle arrest by regulating the phosphorylation of CDC2. Inhibition of Wee1 by a selective small molecule inhibitor MK1775 can abrogate G2/M checkpoint resulting in premature mitotic entry and cell death. MK1775 has recently been tested in preclinical and clinical studies of human carcinoma to enhance the cytotoxic effect of DNA damaging agents. However, its role in mesenchymal tumors, especially as a single agent has not been explored. Here we studied the cytotoxic effect of MK1775 in various sarcoma cell lines and patient-derived tumor explants ex vivo. Our data demonstrate that MK1775 treatment at clinically relevant concentrations leads to unscheduled entry into mitosis and initiation of apoptotic cell death in all sarcomas tested. In MK1775 treated cells CDC2 activity was enhanced, as determined by decreased inhibitory phosphorylation of tyrosine-15 residue and increased expression of phosphorylated histone H3, a marker of mitotic entry. The cytotoxic effect of Wee1 inhibition on sarcoma cells appears to be independent of p53 status as all sarcoma cell lines with different p53 mutation were highly sensitive to MK1775 treatment. Finally, in patient-derived sarcoma samples we showed that MK1775 as a single agent causes significant apoptotic cell death suggesting that Wee1 inhibition may represent a novel approach in the treatment of sarcomas.
Keywords: Wee1i inhibitor, sarcoma, cell cycle checkpoint, mitosis, MK1775
INTRODUCTION
Sarcomas are rare (1) and heterogeneous forms of cancer with more than 70 recognized histologies (2). While conventional, cytotoxic chemotherapy clearly plays a role in the treatment of some sarcoma patients, it is unlikely that future escalations of conventional chemotherapy will be tolerable or improve survival and new approaches to therapy are required (3, 4). Toxicity with these agents is high, response rates remain modest, and improvement in overall survival, especially in the metastatic disease setting, remains negligible. Accordingly, new agents are needed for the treatment of this heterogeneous group of diseases (3, 4).
The characterization of important signaling pathways in cancer has led to the development of molecularly targeted therapies that interfere with pathways critical to cancer cell survival (3, 4). Targeted agents are aimed at essential components of cancer cell viability, sparing normal cells and resulting in fewer side effects (5). Inhibition of the pathways critical to tumor cell survival by molecularly targeted therapy represents an opportunity to reverse the biologic basis of tumor formation. Imatinib treatment for gastrointestinal stromal tumor (GIST) provided the proof of principle that a kinase-directed agent could be effective in a specific sarcoma subtype (6, 7).
DNA damage pathway plays an important role in survival of tumor cells upon treatment with DNA damaging agents. Thus, the regulation of the G2/M transition in eukaryotes through activation of the CDC2-Cyclin B complex offers an attractive molecular target in tumor therapy. The proper regulation of CDC2 requires an activating phosphorylation on threonine-161 and inhibitory phosphorylations on threonine-14 and tyrosine-15 (Tyr15) (8, 9). The inhibitory phosphorylation on Tyr15 maintains the CDC2-Cyclin B complex in an inactive state if there is incompletely replicated DNA or damaged DNA in the cell (10). CDC2 activation through removal of its inhibitory phosphorylation allows cells to enter the mitotic phase of the cell cycle (8–10). A critical component of this DNA checkpoint is Wee1, a tyrosine kinase that inactivates CDC2 through selective phosphorylation of its Tyr15 residue. In response to DNA damage, Wee1 causes inactivation of CDC2 and consequently leads to G2 arrest that gives tumor cells survival advantage by allowing time to repair their damaged DNA (11–13). Inhibition of Wee1 has been shown to abrogate the G2/M checkpoint forcing cancer cells with DNA damage to enter into unscheduled mitosis to undergo cell death, often referred to as mitotic catastrophe (14). Furthermore, Wee1 has also been shown to play important roles in spindle formation (15).
Several studies demonstrated that pharmacological inhibition of Wee1 by a small molecule kinase inhibitor MK1775 exerts anti-tumorigenic effects in NSCLC (16), breast (17), prostate (18), pancreatic (19), ovarian (20) and colon (21) cancer when combined with cytotoxic agents. These studies showed that MK1775 is particularly effective in p53-defective cancer cells (11, 16–21).
Here we demonstrate that Wee1 inhibition by MK1775 results in activation of CDC2, as assessed by decreased Tyr15 phosphorylation, unscheduled mitotic entry and significant cell death in sarcoma cell lines as well as in patient-derived sarcoma tumors. Our data demonstrate that MK1775 shows its cytotoxic effect in sarcoma cells as a single agent in a p53 independent manner and represents a promising therapeutic agent in treatment of sarcoma.
MATERIALS AND METHODS
Cell Culture and Experimental Treatments
MG63 (ATCC CRL-1427), SW-872 (ATCC HTB-92), SK-UT-1 (ATCC HTB-114), A673 (ATCC CRL-1598), SK-ES-1 (ATCC HTB-86), MNNG (ATCC CRL-1547), U2OS (ATCC HTB-96), and HT-1080 (ATCC CCL-121) cells (ATCC, Manassas, VA) were grown in DMEM supplemented with 10% fetal bovine serum, 1% (v/v) penicillin-streptomycin, and 1% (v/v) L-glutamine at 37°C in a 5% CO2 incubator. Stock solutions of the Wee1 inhibitor MK1775 (Selleck Chemicals, Houston, TX), was dissolved in DMSO and added to the media at the indicated concentrations. Control cells were treated with vehicle alone. Commercially obtained cells were not authenticated by the authors.
Cell Growth and Viability Assays
Cells were seeded into 384 well plates and treated with various increasing doses of MK1775 for 72 hours. Cell viability was measured by the CT-Blue assay (Promega). The EC50 was determined for each cell line using a method developed by Chou and Talalay (22). This method involves plotting dose-effect curves for each agent and their combination, using a median-effect equation: fa/fu = (D/Dm)m, where D is dose of drug, Dm is dose required for a 50% effect (equivalent to IC50), fa and fu are affected and unaffected fractions, respectively (fa = 1 − fu), and m is the exponent signifying the sigmoidicity of the dose-effect curve. The computer software Xlfit version 4.3.1 (ID Business Solutions) was used to calculate the values of Dm and m. The CI used for the analysis of the drug combinations was determined by the isobologram equation for mutually nonexclusive drugs that have different modes of action: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + [(D)1(D)2]/[(Dx)1(Dx)2], where (D)1 and (D)2 are relative concentrations of drugs 1 and 2 and x is the percentage of inhibition.
Western Blot Analysis
Both adherent and detached cells in tissue culture wells were collected in 15-mL conical tubes and centrifuged at 4°C for 5 minutes at 1000 rpm in an Eppendorf 5810R centrifuge. The supernatant was removed, and the cell pellet was rinsed with ice-cold PBS, after which ice-cold Universal Cell lysis buffer (Millipore, Billerica, MA) was added. Samples were sonicated, vortexed on ice every 10 minutes for 30 minutes, and then transferred to 1.5-mL microcentrifuge tubes and centrifuged for 10 minutes at 13,000 rpm at 4°C in an Eppendorf 5417R microcentrifuge. We used the Pierce BCA assay kit to determine protein concentrations, following manufacturer’s protocol (Thermo Fisher Scientific, Rockford, IL). Samples were heated to 95°C for 10 minutes prior to resolving on an SDS-PAGE using a 4–20% gradient gel (BioRad Industries, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Millipore) using a semi-dry transfer device (BioRad Industries). The membrane was blocked for 1 hour at room temperature in Pierce Superblock (Thermo Fisher Scientific) and probed for various antibodies. Enhanced chemiluminescent detection (ECL) was performed following manufacturer’s protocols (Thermo Fisher Scientific).
Antibodies
Rabbit γH2AX, total CDC2, CDC2 Y15P and total poly(ADP-ribose) polymerase (tPARP) antibodies were purchased from Cell Signaling Technology (Watertown, MA). Mouse p53 antibody was purchased from Calbiochem, EMD Chemicals (Gibbstown, NJ). Mouse β–Actin antibody was purchased from Sigma Aldrich Corp. (St. Louis, MO).
Determination of Annexin V Positive Cells by Flow Cytometry
U2OS, MG63, A673 or HT1080 cells were seeded in 6-well plates at a density of (1 × 105) cells/well. Cells were treated the next day with 500nM MK1775 and collected 24 hours later for analysis using the BD Annexin APC kit for Flow Cytometry kit (BD Bioscience, San Jose, CA, 559763) and counterstained with DAPI (Sigma Aldrich Corp., St. Louis, MO) per manufacturers’ protocols. Cells were detached with Accumax (Innovative Cell Technologies, Inc., San Diego, CA 92121), combined with floating cells and centrifuged for 5 minutes at 1000 rpm at 4° C in an Eppendorf Model 5417R centrifuge. Cell pellets were then rinsed 1× with ice-cold 1× DPBS and centrifuged again for 5 minutes at 1000 rpm and 4° C. Cells were then re-suspended in 1× Annexin V Binding Buffer at a concentration of 1 × 106 cells/mL. An aliquot of 100uL of this cell suspension was then stained by addition of 5uL Annexin V-APC solution and 5uL of DAPI (70ng/ml) solution and allowed to incubate for 15 minutes on ice in the dark. Positive control cells were prepared by heating an aliquot of cells to 85° C for 2 minutes. Separate aliquots of cells were prepared for Annexin V-APC only controls and DAPI only controls. Aliquots of healthy, untreated cells were added to these controls post-heating to obtain a representative profile of healthy and unhealthy populations for gating. After the 15-minute incubation was completed, 400uL of Annexin V Binding Buffer was added to each sample and mixed. Samples were analyzed within 30 minutes on a BD FACScan instrument with FlowJo (Tree Star, Inc.,Ashland, OR) software to determine the percentage of Annexin V positive cells.
Determination of phosphorylated Histone 3 Positive Cells and Cell cycle analysis by Flow Cytometry
U2OS, MG63, A673 or HT1080 cells were seeded in 100cm plates at a density of (1 × 106) cells/plate. Cells were treated the next day with 500nM MK1775 and collected 24 hours later for analysis using BD Alexa Fluor® 647 Rat anti-Histone H3 (pS28) (BD Bioscience, San Jose, CA) and counterstained with DAPI for cell cycle analysis (Sigma Aldrich Corp., St. Louis, MO) per manufacturers’ protocols. Cells were detached with Accumax (Innovative Cell Technologies, Inc., San Diego, CA) and centrifuged for 5 minutes at 1000 rpm at 4° C in an Eppendorf Model 5417R centrifuge. Cell pellets were then rinsed 1× with ice-cold 1× DPBS and centrifuged again for 5 minutes at 1000 rpm and 4° C. Cells were then fixed by re-suspending at 1 × 106 cells/mL in 100 µl BD CytofixTM fixation buffer (BD Bioscience, San Jose, CA) and incubated for 10 minutes at room temperature. The fixative was removed by centrifugation at 100rpm at and supernatant aspirated. Cells were then permeabilized by resuspending in 100 µl of −20°C BD Phosflow Perm Buffer III (BD Bioscience, San Jose, CA) incubated for 5 minutes at room temperature. The permeabilizer was then removed by centrifugation at 1000rpm and aspirating supernatant, and cells were washed twice with 100 µl of 1× PBS. The PBS was removed and cells were blocked by adding 100 µl of BD Pharmingen Stain Buffer (FBS) (BD Bioscience, San Jose, CA) to each well and incubating for 30 minutes at RT. FBS was removed and a 1:10 dilution of the antibody conjugate n in FBS was added and incubated for 1 hour at room temperature. The diluted antibody conjugate was removed and cells were washed three times with 100 µl of 1× PBS. PBS was removed and cells were counter-stained for cell cycle analysis by adding 100 ul of a 3uM solution of DAPI (Sigma Aldrich Corp., St. Louis, MO). Cells were then analyzed by flow cytometry using a fluorescence-activated cell sorter (BD Bioscience, San Jose, CA) and Cell-Quest and FlowJo (Tree Star, Inc.,Ashland, OR) analysis software, in order to gate out doublets we employed the conventional doublet discriminator gate based on fluorescence height, fluorescence area and signal width.
Fluorescence Microscopy Analyses
For pH3 and casapse-3 activation, U2OS, MG63, A673 or HT1080 cells were seeded in an 8 chamber slide at a density of (6 × 104) cells/ml overnight. Cells were treated with 500nM MK1775 for 24 hours and used for analysis with BD Alexa Fluor® 647 Rat anti-Histone H3 (pS28) (BD Bioscience, San Jose, CA), Cell Signaling Cleaved Caspase-3 Antibody Alexa Fluor® 488 Conjugate and counterstained with DAPI for cell cycle analysis (Sigma Aldrich Corp., St. Louis, MO) per manufacturers’ protocols. After 24 hour treatment, culture medium was removed and the cells were fixed by adding 100 µl of BD Cytofix™ fixation buffer (BD Bioscience, San Jose, CA) to each well and incubated for 10 minutes at room temperature (RT) and then fixative was removed. To permeabilize the cells 100 µl −20°C BD™ Phosflow Perm Buffer III (BD Bioscience, San Jose, CA) was added to each chamber and incubated for 5 minutes at RT and then removed. Cells were then washed twice with 100 µl of 1× PBS. PBS was removed and cells were and blocked by adding 100 µl of BD Pharmingen™ Stain Buffer (FBS) (BD Bioscience, San Jose, CA) and incubated for 30 minutes at RT. The FBS was removed and 100ul of a 1:50 dilution of the antibody conjugates in FBS was added to each well and incubated for 1 hour at RT. The diluted antibody conjugates were removed, and the cells were washed three times with 100 µl of 1× PBS. The nuclei were counter-stained with 100ul ProLong® Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) then viewed with a Zeiss-inverted microscope and analyzed with Axiovert software. For multinucleation, the cells were fixed and permeablized as above and nuclei stained with 100ul ProLong® Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) then viewed with a Zeiss-inverted microscope and analyzed with Axiovert software.
Patient-derived ex vivo studies
The ex vivo assays were performed as previously described (23). Briefly, tumor explants were exposed to MK1775 at 500nM concentrations for 18h and vehicles as well as MK1775-treated tissue fragments were collected for western blot and morphological studies. All experimental protocols were approved by the Animal Care and Use Committee and Institutional Review Board at the University of South Florida.
RESULTS
Sarcoma Cells are highly sensitive to the cytotoxic effect of MK1775
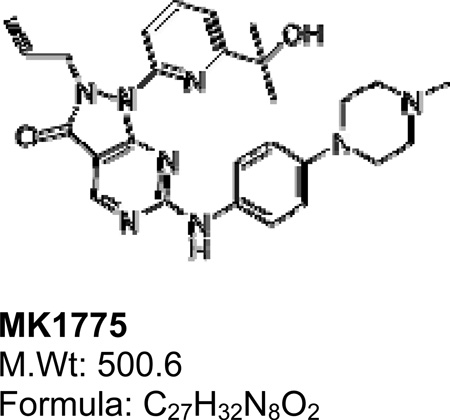
To determine the cytotoxic efficacy of MK1775 on different sarcoma cell types asynchronously growing MG63, U20S, SW872, A673, SKUT, MNNG, HT1080, and SKES cell lines were treated with MK1775 at increasing concentrations for 72 hours and CT Blue Assay was used to assess cell viability. As illustrated in Figure 1A, all sarcoma cell lines were highly sensitive to treatment with IC50 doses in the range of approximately 100–550 nM. Our data showed that this effect is independent of p53 mutational status as MK1775 showed effective cell death in both p53 wild type (U2OS and HT1080) and p53 mutant (MG63, SW872, A673, SKUT, MNNG and SKES) cell lines (24, 25) (Figure 1B).
Figure 1. MK1775 treatment induces cell death in sarcoma cell lines.
A. Cell viability assay of MG63, U20S, SW872, A673, SKUT, MNNG, HT1080 and SKES cells treated with MK1775 for 72 hours. Drug concentrations are indicated on the horizontal axis and plotted against cell viability of control wells. Error bars represent ± SD of 4 replicate wells. B. Table of eight cells lines summarizing type of sarcoma, p53 mutational status and IC50 doses for MK1775 in each of the nine cell lines calculated from the cell viability assay. Data shown as mean +/− SD n=3. C. MG63, U20S, SW872, A673, SKUT, MNNG, HT1080 and SKES cells were treated with 500nM MK1775 for 24 hours, cell extracts were prepared, and western blot analysis was performed with PARP (t: total, c: cleaved), CDC2Tyr15, total CDC2, and γH2AX. β-actin was used as a loading control. Experiments were repeated at least 3 times, and a representative experiment is shown.
MK1775 induces apoptotic cell death in sarcoma cell lines
To better understand the molecular mechanisms of cell death in the presence of MK1775, we performed western blot analysis. As illustrated in Figure 1C, all eight sarcoma cell lines showed a dramatic decrease in CDC2Tyr15 phosphorylation and a significant increase in cleaved PARP while total CDC2 levels remained the same, this demonstrates that MK1775 treatment induces apoptotic cell death in these cell lines at clinically relevant doses (26). Additionally, MK1775 treatment led to increased Serine 139 phosphorylation of γ-H2AX indicating that MK1775 may also cause DNA damage and lead to mitotic catastrophe by allowing cells with DNA damage to enter into mitosis.
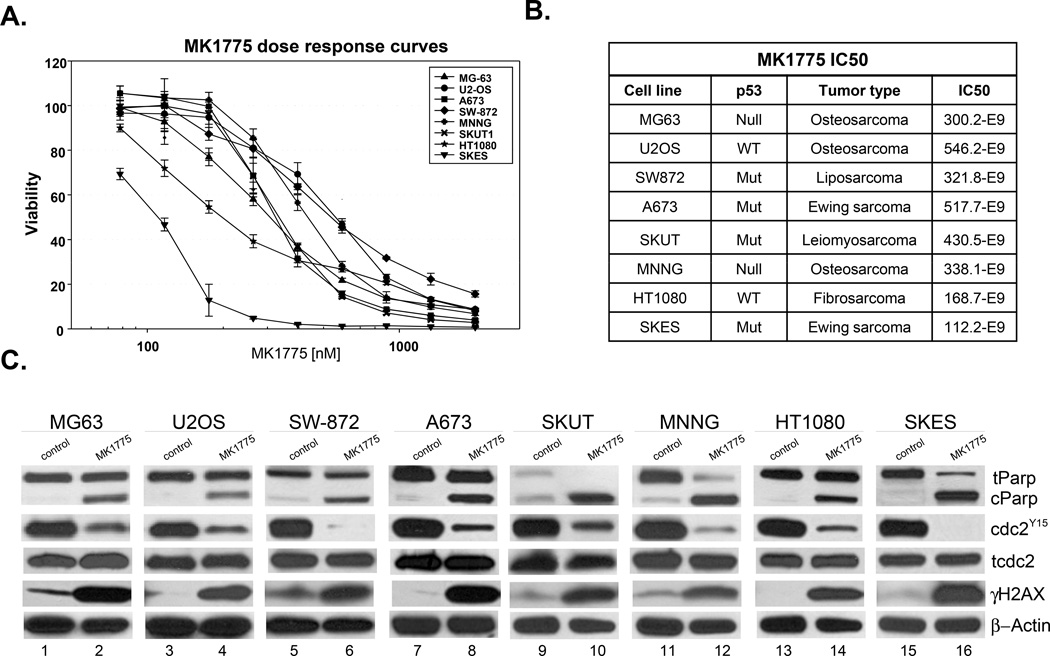
Next, we performed FACS analysis to evaluate MK1775-mediated cell death and its relationship to p53 mutation in sarcoma cells by using Annexin V and DAPI staining. For this purpose, we chose four representative sarcoma cell lines with varying p53 status including U2OS (osteosarcoma – p53 wild type), MG63 (osteosarcoma – p53 null), A673 (Ewing sarcoma – p53 mutant) and HT1080 (fibrosarcoma – p53 wild type). Apoptotic cell death was assessed by increased percentage of Annexin-V-bound cells (Figure 2A). The lower right quadrant of plots in Figure 2A shows the number of cells in early apoptosis while the upper right quadrant shows cells in late cell death. Our results demonstrate that all four cell lines underwent apoptotic cell death in response to MK1775 independent of their p53 status. MG63 cells had a nearly 15 fold increase in Annexin V positivity while HT1080, A673 and U2OS cells showed 10.2, 6.6 and 3.4 fold increase in the Annexin V positive populations, respectively, compared to vehicle treated control cells (Figure 2B). To further determine whether MK1775 treatment leads to unscheduled mitotic entry we first analyzed the microscopic features of sarcoma cells after treatment with MK1775. Cells were treated with MK1775 at indicated concentrations for 24h followed by nuclear staining with DAPI. As illustrated in Figure 2A, lower panel, MK1775 treatment caused marked nuclear abnormalities including multinucleation in all cell lines indicating abnormal mitosis with failed cytokinesis (27, 28). Interestingly, in the same multinucleated cells some of the nuclei show morphological features of apoptotic cell death consistent with mitotic catastrophe (29). Taken together, these results provide the first evidence that MK1775 monotherapy effectively induces cell death in sarcoma cells in a p53-independent manner.
Figure 2. MK1775 leads to apoptotic cell death in sarcoma cells.
A. MG63, U2OS, A673 and HT1080 cells were treated with 500nM MK1775 for 24 hours and the percentage of apoptotic cells was determined by Annexin V APC binding. Lower right quadrant of plots shows the number of cells in early apoptosis; these numbers were used in the bar graph. Upper right quadrant shows cells in late cell death. In the lower panels, MK1775 treatment results in multinucleation in sarcoma cells treated with 500nM MK1775 for 24 hours and multinucleation and micronucleation was observed using DAPI staining of nuclei and visualized using a Zeiss Upright Fluorescence microscope at 630×. B. Fold change Annexin V positive cells in control versus 500nM MK1775 treated cells for 24 hours.
MK1775 induces unscheduled mitotic entry and cell death in sarcomas
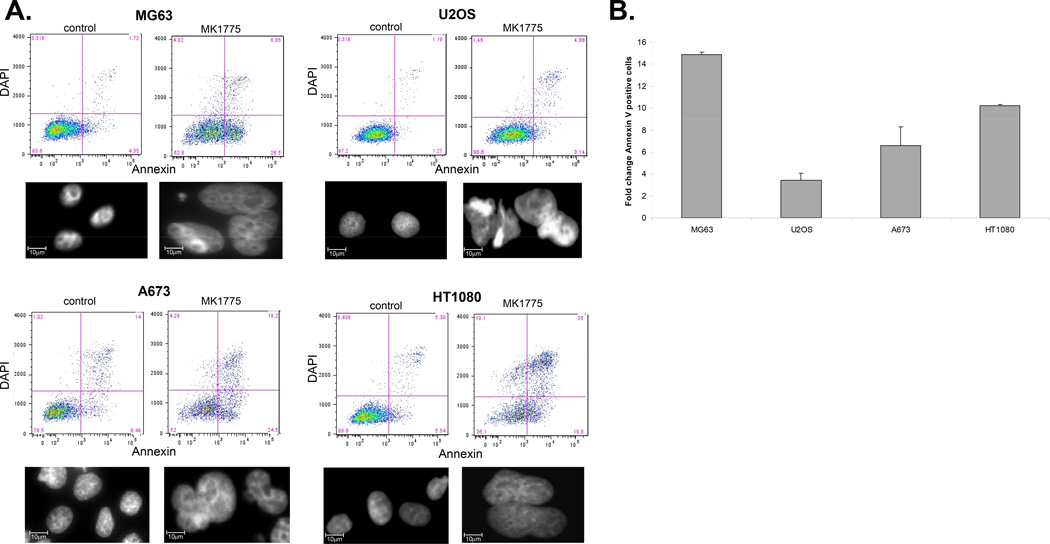
To provide further evidence that inhibition of Wee1 by MK1775 leads to mitotic cell death in sarcoma cells, we next performed immunofluorescence studies to co-localize mitosis and cell death. Mitotic entry was evaluated by phosphorylation of Histone 3 (pH3) (30, 31); while apoptotic cell death was assessed by increased expression of cleaved caspase 3. As illustrated in Figure 3A, microscopic examination of MG63, U2OS, A673 and HT1080 cells treated with MK1775 produced micro- and multi-nucleation with significantly higher pH3 staining. Furthermore, MK1775 treated cells also often showed double staining with pH3 and cleaved caspase-3 compared to vehicle treated control cells. To further quantify this increase we performed FACS analysis using pH3 antibody (Figure 3B). Cells were counterstained with DAPI to assess DNA fragmentation (data not shown). In each of the cells lines we tested there was a significant increase in the total amount of pH3 staining upon MK1775 treatment. Taken together, these data show that MK1775 treatment causes cell death associated with abnormal mitosis and failed cytokinesis, which is suggestive of mitotic catastrophe, a type of cell death that occurs during mitosis.
Figure 3. MK1775 treatment results in mitotic cell death in sarcomas.
A. MG63, U2OS, A673 and HT1080 cells were treated 500nM MK1775 for 24 hours and stained for phosphorylated Histone 3 (AlexaFluor 647, red) and cleaved caspase 3 (AlexaFluor 488, green) and counterstained nuclei with DAPI and visualized using a Zeiss Upright Fluorescence microscope. B. MG63, U2OS, A673 and HT1080 cells were treated with 500nM MK1775 for 24 hours and the percentage of total phosphorylated Histone 3 positive cells in control versus MK1775 treated was determined by FACS, fold change phospho H3 positive cells in control versus 500nM MK1775.
MK1775 treatment results in apoptotic cell death in patient derived tumor explants ex vivo
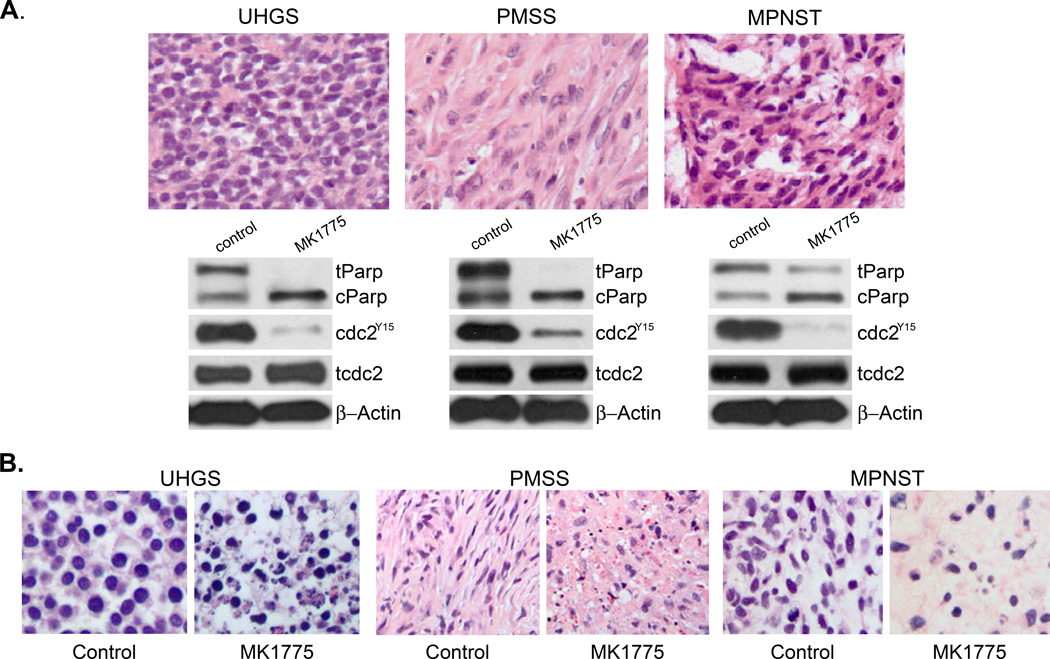
To evaluate MK1775 treatment in clinically relevant tumor samples, we tested the effect of MK1775 on patient derived tumors including undifferentiated high-grade sarcoma (UHGS), malignant peripheral nerve sheath tumor (MPNST) and pleomorphic spindle cell tumor (PMSS) (Figure 4A, upper panel). Tumor explants were treated with MK1775 ex vivo at a clinically relevant concentration, as previously described (23). After drug treatment for 24 hours tumor explants were collected for microscopic evaluation and western blot analysis to assess target inhibition and cell death (Figure 4). As illustrated in Figure 4A, lower panel, ex vivo treatment of cells with MK1775 (500 nM) caused increased cell death and activation of CDC2 in all samples, as analyzed by PARP cleavage and CDC2TYR 15 inhibition while total CDC2 levels remained the same. The histologic analysis of tumor explants confirmed that MK1775 treatment caused dramatic cell death, H&E stained paraffin section of vehicle and MK1775-treated UHGS, PMSS and MPNST explants are shown in Figure 4B.
Figure 4. MK1775 treatment results increased cleaved PARP and CDC2 phosphorylation.
A. Undifferentiated high grade sarcoma (UHGS), malignant peripheral nerve sheath tumor (MPNST) and pleiomorphic spindle cell tumor (PMSS) tumor explants were treated with MK1775 (500nM) for 24 hours. H&E-stained paraffin sections and cell extracts were prepared from tumor explants to assess microscopic features and expression levels of cPARP, CDC2Tyr15 and total CDC2 were analyzed by western blot, respectively. β-actin expression was used as loading control. B. Patient-derived UHGS, PMSS and MPNST tumor explants were treated with vehicle (control) or MK1775 (500 nM) for 24 hours. Representative H&E-stained paraffin sections were prepared to assess microscopic features of cell death.
These results further support the findings presented above with sarcoma cell lines and strongly suggest that MK1775 could be used as a single agent in the treatment of sarcoma patients in future clinical studies.
DISCUSSION
Sarcomas are heterogeneous mesenchymal tumors affecting both pediatric and adult populations (4). Approximately 10 percent of children with cancer are diagnosed with sarcomas, compared to 8 percent for young adults and 1 percent of adults (>40yrs old) diagnosed with cancer. Ewing sarcoma, osteosarcoma and rhabdomyosarcoma constitute about three quarters of all children with sarcomas whereas liposarcoma, leiomyosarcoma, undifferentiated sarcomas, synovial sarcoma and MPNST represent some of the more common histologies in the adult population (32, 33). Localized sarcomas are frequently treated with multimodal therapy including surgery, radiation therapy and anthracycline based chemotherapy with curative intent. However, disappointingly, cure rates have only been very modestly improved for recurrent and metastatic sarcomas. Multiple attempts at increasing the doses of these conventional chemotherapeutic agents have increased toxicity without improved efficacy (34, 35).
Thus, there is a pressing need to develop novel therapies to improve outcomes in sarcoma patients. Here we showed that various soft tissue and bone sarcomas including osteosarcoma, Ewing sarcoma and MPNST are highly sensitive to the cytotoxic effect of MK1775 when used as a single agent at clinically achievable concentrations.
The G2/M DNA damage checkpoint is an important cell cycle checkpoint in eukaryotic organisms ensuring that cells do not initiate mitosis before they repair damaged DNA after replication. Wee1 is a tyrosine kinase with a key role as an inhibitory regulator of the G2/M checkpoint that precedes entry into mitosis (9). There is compelling evidence that abrogation of this checkpoint through Wee1 inhibition may result in increased antitumor activity of agents that cause DNA damage such as radiation therapy or some cytotoxic agents (14).
Our data presented here show that MK1775 treatment leads to unscheduled CDC2Y15 activation and consequently premature mitotic entry and cell death of sarcoma cells. MK1775 treatment led to increased Serine 139 phosphorylation of γ-H2AX (Figure 1C) indicating that inhibition of Wee1 may also cause DNA damage and lead to mitotic catastrophe by allowing cells with DNA damage to enter into mitosis. Interestingly, in previous studies acute deletion of Wee1 has been demonstrated to cause profound growth defects and cell death in mouse models (36). Wee1 deficient cells have been shown to display chromosome aneuploidy and DNA damage providing evidence that mammalian Wee1 also plays a critical role in maintaining genome integrity (36).
Previous reports using MK1775 and other Wee1 inhibitors in combination with DNA damaging agents or radiotherapy showed that p53 deficient cancer cells are more sensitive to Wee1 inhibition (14, 16–20, 37). These studies indicated that defective p53 pathway allows cells to pass G1/S checkpoint making them largely dependent on G2/M arrest to repair damage by cytotoxic agents. Therefore, p53 mutation has been proposed as a predictor of tumor response to Wee1 inhibitors in clinical trials (14, 38). Our data demonstrate that p53 status is not predictive of MK1775 response in sarcoma cells. We showed that MK1775 is effective as monotherapy in sarcoma cells independent of their p53 status, as we observed a similar degree of cell death in sarcoma cells with wild type p53, mutant p53 and null p53. However, the role of p53 in response of sarcoma cells to combination treatment with MK1775 and DNA damaging agents remains to be elucidated.
Recent studies showed that MK1775 is a well tolerated drug that has not demonstrated a clear dose limiting toxicity (39). The data presented here together with the high safety profile of this drug strongly suggest that MK1775 can be used as a potential therapeutic agent in the treatment of both adult as well as pediatric sarcoma patients.
Acknowledgement
This study was generously supported in part by the V Foundation for Cancer Research.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher Christopher DM, MD, Unni K Krishnan, MD, Mertens Fredrik., MD, editors. Organization WH. World Health Organization Classification of Tumours. Lyon: IARC; 2002. [Google Scholar]
- 3.Loeb DM. Targeted Therapies for Sarcomas: The Next Generation of Treatments. ELECTRONIC SARCOMA UPDATE NEWSLETTER [serial on the Internet] 2007;4(4) Available from: http://sarcomahelp.org/research_center/targeted_therapies.html. [Google Scholar]
- 4.Joseph Ludwig MaJCT., MD, PhD . Targeted Therapy of Sarcoma. In: Maurie Kurzrock RM., editor. Targeted Cancer Therapy. Humana Press; 2008. pp. 317–329. [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. Embo J. 1997;16(3):545–554. doi: 10.1093/emboj/16.3.545. PMCID: 1169658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry JA, Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. PMCID: 1868713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134(4):963–970. doi: 10.1083/jcb.134.4.963. PMCID: 2120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1(+)-like gene in human cells. Nature. 1991;353(6339):80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- 12.McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. Embo J. 1993;12(1):75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. PMCID: 413177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257(5078):1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 14.De Witt Hamer PC, Mir SE, Noske DP, Van Noorden CJ, Wurdinger T. WEE1 kinase targeting combined with DNA damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 15.Garcia K, Stumpff J, Duncan T, Su TT. Tyrosines in the kinesin-5 head domain are necessary for phosphorylation by Wee1 and for mitotic spindle integrity. Curr Biol. 2009;19(19):1670–1676. doi: 10.1016/j.cub.2009.08.013. PMCID: 2762001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol. 2004;15(2):252–256. doi: 10.1093/annonc/mdh073. [DOI] [PubMed] [Google Scholar]
- 17.Murrow LM, Garimella SV, Jones TL, Caplen NJ, Lipkowitz S. Identification of WEE1 as a potential molecular target in cancer cells by RNAi screening of the human tyrosine kinome. Breast Cancer Res Treat. 2010;122(2):347–357. doi: 10.1007/s10549-009-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiviharju-af Hallstrom TM, Jaamaa S, Monkkonen M, Peltonen K, Andersson LC, Medema RH, et al. Human prostate epithelium lacks Wee1A-mediated DNA damage-induced checkpoint enforcement. Proc Natl Acad Sci U S A. 2007;104(17):7211–7216. doi: 10.1073/pnas.0609299104. PMCID: 1855358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17(9):2799–2806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuarai S, Yamanaka K, Itadani H, Arai T, Nishibata T, Hirai H, et al. Discovery of gene expression-based pharmacodynamic biomarker for a p53 context-specific anti-tumor drug Wee1 inhibitor. Mol Cancer. 2009;8:34. doi: 10.1186/1476-4598-8-34. PMCID: 2700070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9(7):514–522. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Brazelle W, Kreahling JM, Gemmer J, Ma Y, Cress WD, Haura E, et al. Histone deacetylase inhibitors downregulate checkpoint kinase 1 expression to induce cell death in non-small cell lung cancer cells. PLoS One. 2010;5(12):e14335. doi: 10.1371/journal.pone.0014335. PMCID: 3001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caron de Fromentel C, Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- 25.Souss T. The 2010_R1 release of the UMD_TP53 Mutation database. 2010 [updated 2010 July 2010; cited]; Available from: http://p53.free.fr/index.html. [Google Scholar]
- 26.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 27.Akakura S, Nochajski P, Gao L, Sotomayor P, Matsui S, Gelman IH. Rb-dependent cellular senescence, multinucleation and susceptibility to oncogenic transformation through PKC scaffolding by SSeCKS/AKAP12. Cell Cycle. 2010;9(23):4656–4665. doi: 10.4161/cc.9.23.13974. PMCID: 3048035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utani K, Kohno Y, Okamoto A, Shimizu N. Emergence of micronuclei and their effects on the fate of cells under replication stress. PLoS One. 2010;5(4):e10089. doi: 10.1371/journal.pone.0010089. PMCID: 2851613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caruso R, Fedele F, Luciano R, Branca G, Parisi C, Paparo D, et al. Mitotic catastrophe in malignant epithelial tumors: the pathologist's viewpoint. Ultrastruct Pathol. 2011;35(2):66–71. doi: 10.3109/01913123.2010.543753. [DOI] [PubMed] [Google Scholar]
- 30.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106(6):348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 31.Su TT, Sprenger F, DiGregorio PJ, Campbell SD, O'Farrell PH. Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes Dev. 1998;12(10):1495–1503. doi: 10.1101/gad.12.10.1495. PMCID: 316833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anfinsen K, Devesa SS, Bray F, Troisi R, Jonasdottir T, Bruland O, et al. Age-Period-Cohort Analysis of Primary Bone Cancer Incidence Rates in the United States (1976 – 2005) Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 33.Zahm SH, Fraumeni JF., Jr The epidemiology of soft tissue sarcoma. Semin Oncol. 1997;24(5):504–514. [PubMed] [Google Scholar]
- 34.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27(15):2536–2541. doi: 10.1200/JCO.2008.19.1478. PMCID: 2684856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Cesne A, Judson I, Crowther D, Rodenhuis S, Keizer HJ, Van Hoesel Q, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18(14):2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 36.Tominaga Y, Li C, Wang RH, Deng CX. Murine Wee1 plays a critical role in cell cycle regulation and pre-implantation stages of embryonic development. Int J Biol Sci. 2006;2(4):161–170. doi: 10.7150/ijbs.2.161. PMCID: 1483124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8(11):2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 38.Indovina P, Giordano A. Targeting the checkpoint kinase WEE1: selective sensitization of cancer cells to DNA-damaging drugs. Cancer Biol Ther. 2010;9(7):523–525. doi: 10.4161/cbt.9.7.11276. [DOI] [PubMed] [Google Scholar]
- 39.Leijen JHS S, Shapiro G, Pavlick AC, Tibes R, Demuth T, Viscusi J, Cheng JD, Xu Y, Oza AM. A phase I pharmacological and pharmacodynamic study of MK-1775, a Wee1 tyrosine kinase inhibitor, in monotherapy and combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. 2010;28(15s) [Google Scholar]