Abstract
BACKGROUND
Secondary acute myeloid leukemia (AML) from an antecedent myelodysplastic syndrome (MDS)/myeloproliferative neoplasm is associated with a poor prognosis. The authors evaluated predictive factors in patients with secondary AML treated with anthracycline-based induction therapy.
METHODS
This was a retrospective review of secondary AML patients treated with induction therapy. Age, International Prognostic Scoring System, Eastern Cooperative Oncology Group performance status, cytogenetics, duration of MDS/myeloproliferative neoplasm, and prior MDS/myeloproliferative neoplasm treatment were evaluated for their impact on complete response (CR), CR with low platelets, and overall survival (OS).
RESULTS
The authors evaluated 61 secondary AML patients who received induction chemotherapy; 59% (36 patients) achieved CR/CR with low platelets (95% confidence interval [CI], 46%–71%), and median OS was 6.5 (95% CI, 3.9–8.1) months. Three factors were associated with lower CR/CR with low platelets and OS: poor risk cytogenetics, prior treatment with hypomethylating agents or lenalidomide, and longer time to transformation to AML. Of those treated with hypomethylating agents or lenalidomide, 32% achieved CR/CR with low platelets versus 78% in the group not treated with a hypomethylating agent or lenalidomide (odds ratio [OR], 0.13; 95% CI, 0.04–0.42). Median OS for those treated with a hypomethylating agent or lenalidomide was 3.7 versus 10.5 months for those not treated with a hypomethylating agent or lenalidomide (P < .0001). The CR/CR with low platelets rate for those with intermediate risk cytogenetics was 70% versus 35% for those with poor risk (OR, 4.33; 95% CI, 1.38–13.6). Those with poor risk cytogenetics had a median OS of 2.8 versus 7.5 months for those with intermediate risk (P = .01).
CONCLUSIONS
Prior treatment with hypomethylating agents or lenalidomide, poor risk cytogenetics, and longer time to transformation to AML are independent negative predictive factors for response and OS in patients with secondary AML after induction therapy.
Keywords: leukemia, myeloid, acute, myelodysplastic syndromes, myelodysplastic-myeloproliferative diseases, methylation, azacitidine, decitabine, lenalidomide, cytogenetics
Acute myeloid leukemia (AML) is a malignancy characterized by a clonal proliferation of neoplastic hematopoietic progenitor cells. These malignant cells exhibit differentiation arrest, a higher proliferative index, and failure to undergo apoptosis.1 The overall incidence of AML is 3.4 cases per 100,000 people in the United States, with the median age at diagnosis being 67 years.2 Approximately 50% to 75% of adults with de novo AML achieve complete remission after induction chemotherapy with cytarabine and an anthracycline. However, only 20% to 30% of patients enjoy long-term disease-free survival.2,3
Established prognostic factors in AML include age, cytogenetic profile, and the presence of an antecedent myelodysplastic syndrome (MDS) or myeloproliferative neoplasm.4,5 Older adults with AML have a higher frequency of poor risk karyotypes and MDS/myeloproliferative neoplasm; up to 40% of elderly patients with AML had previously been diagnosed with MDS.4,6–11 Given these adverse risk factors, the 5-year overall survival (OS) rate for older adults with AML is <10%.4,5
Recently approved treatments, such as the hypomethylating agents (ie, azacitidine, decitabine), have been shown to improve survival in patients with higher risk MDS.12,13 In addition, recent data have shown that lenalidomide provides meaningful clinical responses in patients with lower risk MDS associated with deletion 5q.14 However, response rates and survival with standard induction chemotherapy after progression to AML after initial treatment with those newer modalities have not been well studied.12–14 In this study, we examined the impact of prior treatment with hypomethylating agents or lenalidomide on response rate and outcomes in patients with secondary acute myeloid leukemia in the context of other well-known prognostic factors.
MATERIALS AND METHODS
A retrospective chart review was done to evaluate adult patients with secondary AML from antecedent MDS, myeloproliferative neoplasm, or MDS/myeloproliferative neoplasm who had been treated with induction chemotherapy at H. Lee Moffitt Cancer Center and Research Institute between January 1, 2004, and April 30, 2008. Induction chemotherapy in the majority of our patients consisted of the 7 + 3 regimen, which includes cytarabine 100 mg/m2 for 7 days combined with 3 days of an anthracycline (daunorubicin or idarubicin).15 Of the 61 patients, 40 received a 7 + 3 regimen, and the remainder received a high-dose cytarabine-based regimen. Fourteen of the 61 patients received an investigational therapy combined with 7 + 3 or high-dose cytarabine.
Patient Population
The following were inclusion criteria for this study: 1) histologically proven diagnosis of AML, 2) previous histologically proven diagnosis of MDS or MDS/myeloproliferative neoplasm, and 3) receipt of induction chemotherapy at H. Lee Moffitt Cancer Center and Research Institute for secondary AML. The diagnosis of AML was defined using the World Health Organization (WHO)’s definition of >20% blasts in the bone marrow or peripheral blood.16
Data were collected from review of electronic records, pathology reports, and pharmacy records. Variables collected included the following: age at diagnosis of AML; karyotype at diagnosis of AML; prior therapy for MDS, myeloproliferative neoplasm, or MDS/myeloproliferative neoplasm; duration of MDS, myeloproliferative neoplasm, or MDS/myeloproliferative neoplasm before AML diagnosis; Eastern Cooperative Oncology Group (ECOG) performance status before initiation of induction chemotherapy for AML; WHO category of MDS or MDS/myeloproliferative disease overlap syndrome at diagnosis; and International Prognostic Scoring System score. Patient karyotypes at the time of AML diagnosis were placed into 3 categories: good risk (t[15;17], t[8;21], or inv16), poor risk (complex karyotype defined as 3 or more chromosomal abnormalities, del 5/5q, del 7/7q, trisomy 8), and intermediate risk (all those not in the poor risk group and not in the good risk group).17
Study Endpoints
The study endpoints included rate of complete response (CR) or complete response with low platelets to induction chemotherapy for secondary AML according to the International Working Group criteria, OS, and disease-free survival (DFS). DFS was defined only for patients who achieved CR or CR with low platelets, and it is the time from date of CR/CR with low platelets to date of relapse or death. If no relapse or death, DFS was censored at the time of last follow-up. OS was defined for all patients, and it is the time from date of AML induction therapy to date of death. Similarly, if no death, OS was censored at the time of last follow-up. CR status was confirmed at our institution by bone marrow biopsy and peripheral blood cell count.
Statistical Analysis
Data were summarized by using statistics such as means, medians, and ranges for continuous variables and numbers and percentages for discrete variables. Both univariate and multivariate analyses were implemented to explore relationships between various disease characteristics or demographic variables and outcomes (ie, response [CR/CR with low platelets], DFS, and OS) of the induction chemotherapy for secondary AML patients. Nonparametric methods such as Fisher exact test for binary outcomes and the log-rank test and Kaplan-Meier product limit estimate for time to event variables as well as (semi-)parametric approaches (eg, logistic regression and Cox proportional hazards regression models) were used, when appropriate, to study potential effects of these variables on the induction treatment outcome for secondary AML. Both point estimates of parameters of interest (eg, response rate, odds ratio [OR], and hazard ratio) and their 95% confidence intervals (CIs) were reported, when feasible. Stepwise regression approach was used in multivariate analyses to study which set of variables had the predictive ability for the outcome variables in this study with an entry probability of .1 and a stay probability of .05. Sample size was always taken into consideration to decide whether a particular statistical method was appropriate to use.
A P value of < .05 was considered statistically significant. No multiple testing adjustments for P value were performed because of small sample size. Because of the exploratory nature of this study, it was not powered to confirm or refute any specific hypothesis.
RESULTS
By using the criteria of treatment between January 1, 2004 and April 30, 2008, we evaluated a total of 61 patients with secondary AML. The baseline characteristics of the group are summarized in Table 1. The majority of the patients were men (67%) with a median age of 66 years. The median time to development of AML from the diagnosis of MDS/myeloproliferative neoplasm was 8 months (range, 0.5–82; mean [standard deviation]: 15 [18] months). The median follow-up time for surviving AML patients was 18 months (range, 1.4–54 months). Of note, about ⅓ of patients had an adverse cytogenetic profile, whereas the other ⅔ had an intermediate risk profile. Additional molecular information such as FMS-like tyrosine kinase 3 (FLT-3) or nucleophosmin (NPM) status was not included in this study. The majority of patients did not have this information available. For patients who received prior definitive (nongrowth factor) therapy for MDS/myeloproliferative neoplasm, the vast majority (23 of 27) received 5-azacitidine.
Table 1.
Study Population Characteristics
| Variable | No. (%) |
|---|---|
| Sex | |
| Men | 41 (67) |
| Women | 20 (33) |
| Age, y | |
| <65 | 23 (38) |
| 65–69 | 22 (36) |
| ≥70 | 16 (26) |
| ECOG performance status | |
| 0 or 1 | 48 (86) |
| ≥2 | 8 (14) |
| IPSS | |
| Low/intermediate-1 risk | 28 (57) |
| Intermediate-2/high risk | 21 (42) |
| MDS/MPN subtype | |
| RA | 4 (7) |
| RARS | 1 (2) |
| RCMD | 10 (16) |
| RAEB-1 | 14 (23) |
| RAEB-2 | 13 (21) |
| MDS NOS | 3 (5) |
| MPN | 5 (8) |
| MDS/MPN overlap | 10 (16) |
| AA | 1 (2) |
| MDS/MPN treatment | |
| Decitabine | 1 (2) |
| Azacitidine | 23 (38) |
| Lenalidomide | 3 (5) |
| Growth factor only/supportive care | 24 (39) |
| Other | 12 (20) |
| Cytogenetic risk categorya | |
| Good | 0 (0) |
| Intermediate | 40 (66) |
| Poor | 20 (33) |
ECOG indicates Eastern Cooperative Oncology Group; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; RA, refractory anemia; RARS, RA with ringed sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; NOS, not otherwise specified; AA, aplastic anemia.
Cytogenetic category at time of AML diagnosis. Good = inv16, t (15;17), t (8;21); intermediate = not good and not poor; poor = complex cytogenetics, del 5/5q, del 7/7q, trisomy 8.
The overall response rate (CR/CR with low platelets) for the entire group was 59%. Table 2 summarizes univariate analysis for various factors. In addition to the factors listed in Table 2, the time from MDS/myeloproliferative neoplasm diagnosis to the development of AML was a predictive factor for response to AML induction treatment. Univariably, time to development of AML on a log scale had an unadjusted OR of 0.53 (P = .02) for 1 U (ie, 2.7 months) of increase, favoring those who had a shorter duration of their MDS/myeloproliferative neoplasm. The type of therapy administered for prior MDS/myeloproliferative neoplasm had a significant impact on the patient’s response to AML induction therapy. Patients who received hypomethylating agents or lenalidomide for the treatment of MDS/myeloproliferative neoplasm had an inferior rate of CR/CR with low platelets to induction chemotherapy compared with patients who only received supportive care for MDS/myeloproliferative neoplasm, with a response rate of 32% versus 78% (OR, 0.13; 95% CI, 0.04–0.42; P = .001), respectively. In addition, those with poor risk cytogenetics (defined as complex karyotype, del 5/5q, del 7/7q, trisomy 8) at the time of progression to AML had an adversely affected response to induction therapy, as evidenced by a response rate of 35% versus 70% (P = .013) in the intermediate risk cytogenetic group (defined as all those not in the poor risk group and not consisting of the good risk cytogenetics: t[15;17], t[8;21], or inv16). Cytogenetics at the time of conversion to AML did not change from those reported at MDS diagnosis in the vast majority of patients. Neither the WHO subtype of prior MDS/myeloproliferative neoplasm nor the International Prognostic Scoring System score had an effect on response to induction therapy. In addition, sex, age, and ECOG performance status did not seem to predict the response rate in this study (Table 2).
Table 2.
Response Rates, ORs, and Their CIs by Patient Characteristics (n = 61)
| Variable | Response Rate, CR/CRp (%) |
Exact 95% CI |
OR (95% CI) | P |
|---|---|---|---|---|
| Overall | 36/61 (59) | 0.46, 0.71 | — | — |
| Sex | 1.73 (0.59–5.12) | .41 | ||
| Men | 26/41 (63) | 0.47–0.78 | ||
| Women | 10/20 (50) | 0.27–0.73 | ||
| Age, y | 1.65 (0.52–5.20) | .55 | ||
| <70 | 28/45 (62) | 0.47–0.76 | ||
| ≥70 | 8/16 (50) | 0.25–0.75 | ||
| ECOG PS | 1 (0.21–4.69) | 1.0 | ||
| 0 or 1 | 30/48 (63) | 0.47–0.76 | ||
| 2 | 5/8 (63) | 0.24–0.91 | ||
| IPSS | 1.92 (0.60–6.17) | .38 | ||
| 0 or 1 | 19/28 (68) | 0.48–0.84 | ||
| ≥2 | 11/21 (52) | 0.30–0.74 | ||
| MDS/MPN treatment | 0.13 (0.04–0.42) | .001 | ||
| HMA/L | 8/25 (32) | 0.15–0.54 | ||
| Supportive/othera | 28/36 (78) | 0.61–0.90 | ||
| Cytogenetics | 4.33 (1.38–13.56) | .013 | ||
| Intermediate risk | 28/40 (70) | 0.53–0.83 | ||
| Poor risk | 7/20 (35) | 0.15–0.59 |
OR indicates odds ratio; CI, confidence interval; CR, complete response; CRp, CR with low platelets; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; HMA/L, hypomethylating agent (ie, decitabine and azacitidine)/ lenalidomide.
Supportive/other = no treatment, growth factors, hydroxyurea, or imatinib.
In multivariate analysis, only 3 variables correlated with response rates to induction chemotherapy (Table 3). Treatment of prior MDS/myeloproliferative neoplasm with hypomethylating agents or lenalidomide was associated with an adjusted OR of obtaining a CR with standard AML induction therapy of 0.13 (95% CI, 0.04–0.51). Poor risk cytogenetics at the time of transformation to AML also had a negative effect, with an adjusted OR of 0.17 (95% CI, 0.04–0.69). In addition to prior therapy for MDS/myeloproliferative neoplasm and poor risk cytogenetics, the time from diagnosis of MDS/myeloproliferative neoplasm to AML transformation (on a log scale) negatively affected the likelihood of response to induction therapy, with an adjusted OR of 0.4 (95% CI, 0.19–0.82) for 1 U (ie, 2.7 months) of increase in time to AML.
Table 3.
Multivariate Analysis for Association of Response (CR/CRp) With Patient Variables
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Time to AML (1 log unit increase)a | 0.40 | 0.19–0.82 | .013 |
| MDS/MPN Treatment with HMA/L | 0.13 | 0.04–0.51 | .003 |
| Cytogenetics, poor risk | 0.17 | 0.04–0.69 | .014 |
CR indicates complete response; CRp, CR with low platelets; OR, odds ratio; CI, confidence interval; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; HMA/L, hypomethylating agent (ie, decitabine and azacitidine)/lenalidomide.
One log unit = 2.7 months.
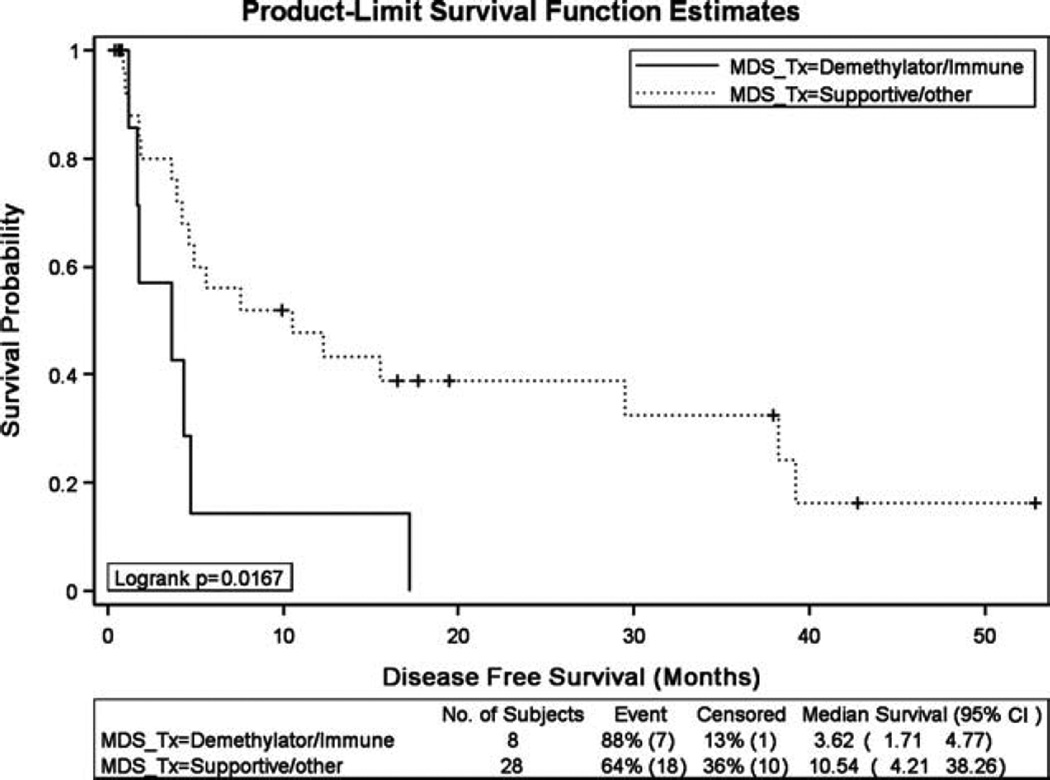
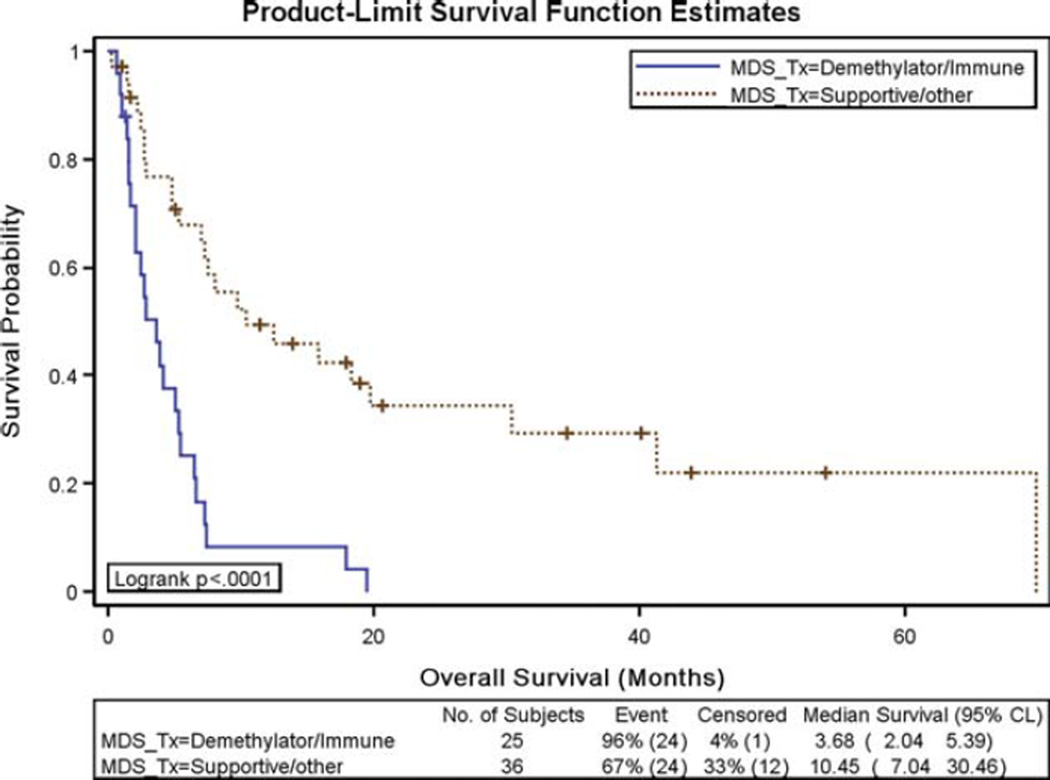
Disease-free survival and OS results for secondary AML patients treated with induction chemotherapy were also negatively affected by prior MDS/myeloproliferative neoplasm treatment with hypomethylating agents or lenalidomide. The median disease-free survival for those patients who received treatment with a hypomethylating agent or lenalidomide before developing AML was 3.6 months, compared with 10.5 months (P = .02) for those who received supportive care/other (Fig. 1). Patients who received prior hypomethylating agents or lenalidomide had a median OS of 3.7 months, compared with 10.5 months for patients who received supportive care/other (P < .0001) (Fig. 2). This difference in OS was not a reflection of early treatment-related mortality. There were 48 deaths in the entire cohort, and only 3 were within the first 30 days of induction therapy. The remaining 45 deaths occurred after the 30-day time frame, with 32 of these deaths occurring >60 days from the start of induction therapy.
Figure 1.
Disease-free survival results were compared for patients who received prior treatment (Tx) for their myelodysplastic syndrome (MDS)/myeloproliferative neoplasm with a hypomethylating agent or lenalidomide versus those who received supportive care/other. CI indicates confidence interval.
Figure 2.
Overall survival results were compared in patients who received prior treatment for their myelodysplastic syndrome (MDS)/myeloproliferative neoplasm with a hypomethylating agent or lenalidomide versus those who received supportive care/other. CI indicates confidence interval.
Poor risk cytogenetics at the time of transformation to AML was also associated with significantly inferior OS. The median OS for patients with poor risk cytogenetics was 2.8 months, compared with 7.5 months for those with intermediate risk cytogenetics (Table 4; P = .01). None of the patients in this analysis had good risk cytogenetics (Table 1).
Table 4.
Kaplan-Meier Analysis of OS by Patient Characteristics (n=61)
| Variable | Number of Deaths (%) |
Median OS (95% CI) |
P |
|---|---|---|---|
| Overall | 48/61 (79%) | 6.5 (3.9–8.1) | — |
| Sex | .59 | ||
| Men | 17/20 (85%) | 6.4 (3.9–12.6) | |
| Women | 31/41 (76%) | 6.5 (2.8–7.5) | |
| Age, y | .04 | ||
| <70 | 33/45 (73%) | 7.3 (5.0–12.6) | |
| ≥70 | 15/16 (94%) | 2.8 (1.6–5.5) | |
| ECOG PS | .87 | ||
| 0 or 1 | 36/48 (75%) | 6.5 (3.7–15.9) | |
| 2 | 7/8 (88%) | 7.2 (4.8–7.5) | |
| IPSS | .68 | ||
| 0 or 1 | 21/28 (75%) | 8.1 (4.1–18.3) | |
| ≥2 | 16/21 (76%) | 5.5 (2.8–15.9) | |
| MDS/MPN treatment | <.0001 | ||
| HMA/L | 24/25 (96%) | 3.7 (2.0–5.4) | |
| Supportive/other | 24/36 (67%) | 10.5 (7.0–30.5) | |
| Cytogenetics | .01 | ||
| Intermediate risk | 29/40 (73%) | 7.5 (5.4–18.3) | |
| Poor risk | 18/20 (90%) | 2.8 (2.2–6.5) |
OS indicates overall survival in months; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; HMA/L, hypomethylating agent (ie, decitabine and azacitidine)/lenalidomide.
In addition, time to AML transformation and age affected OS. The hazard ratio was 1.40 for each unit increase in time to transformation to AML on a log scale (ie, 2.7-month increase) for OS (95% CI, 1.06–1.85; P = .017). Older age also appeared to be marginally significant in predicting worse OS (Table 4; P = .04). No other covariates seemed to predict DFS or OS in the univariate analysis in this study.
Multivariate Cox regression analysis indicated that the same 3 factors that were associated with response were also significantly associated with OS (all P < .04). Poor risk cytogenetics, prior treatment with hypomethylating agents or lenalidomide, and longer time to transformation to AML were all associated with an inferior OS (P < .04; Table 5). Although prior MDS/myeloproliferative neoplasm treatment type was significant in the univariate analysis of DFS, because of the relatively small number of responses (36 in this study) no multiple Cox regression analysis of DFS was performed.
Table 5.
Multivariate Cox Regression Analysis for Association of Overall Survival With Patient Characteristics (n = 61)
| Variable | Hazards Ratio |
95% CI | P |
|---|---|---|---|
| Time to AML (on log scale)a | 1.42 | 1.02–1.96 | .037 |
| MDS/MPN treatment HMA/L vs other | 3.05 | 1.62–5.71 | .0005 |
| Cytogenetics, intermediate vs poor | 0.46 | 0.25–0.87 | .017 |
CI, confidence interval; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; HMA/L, hypomethylating agent (ie, decitabine and azacitidine)/lenalidomide.
One log unit = 2.7 months.
The response to prior therapy with hypomethylating agents or lenalidomide before transformation to AML could not be analyzed with any statistical significance because of the small sample size. However, it was noted that 5 of the 21 patients treated with hypomethylating agents or lenalidomide for their MDS had a response to treatment based on the International Working Group criteria.18 Of these 5, only 1 had a CR after induction therapy for AML. The remaining 16 patients did not have a response to treatment with hypomethylating agents or lenalidomide. Of these 16 patients, only 6 had a CR after induction therapy for AML.
DISCUSSION
The traditional risk factors associated with poor outcome in AML, which include age, adverse karyotype, and the presence of MDS/myeloproliferative neoplasm, are well established and validated. With the advent of novel treatments for both MDS and AML, however, it is necessary to reinvestigate the importance of these risk factors in the context of new therapies. Given the impact of such new therapies upon the natural history of MDS, it may be expected that the efficacy and/or toxicity of subsequent treatments will be affected by prior therapy within an individual patient.
In light of recent advances in the treatment of MDS that appear to affect its natural history, this study was performed to revisit the impact of several variables on the outcomes of patients with secondary AML who underwent standard induction therapy. Although traditional poor risk features such as adverse karyotype remained a negative prognostic marker, we found that the prior administration of hypomethylating agents or lenalidomide during the period of MDS/myeloproliferative neoplasm was independently and adversely associated with response rates and survival. In addition, a more prolonged time span from onset of MDS/myeloproliferative neoplasm to AML transformation negatively affected outcomes in these patients, suggesting that a protracted natural history of the MDS/myeloproliferative neoplasm may select for higher rates of chemotherapy resistance after AML develops.
The reason for the negative impact induced by prior hypomethylating agent therapy for MDS/myeloproliferative neoplasm upon induction chemotherapy outcomes in AML is unclear, although several hypotheses may be entertained. One possible hypothesis is the development of resistance via changes in critical metabolic enzymes, including deoxycytidine kinase, as well as the generation of aberrant nucleoside metabolism, which resulted in less incorporation of decitabine into DNA.19 This resulted in an impairment of hypomethylation and gene reactivation. Another hypothesis is the up-regulation of the multidrug resistance (MDR) gene. MDR has been documented as a major cause of chemotherapy resistance in AML.19 The MDR1 gene has been shown to be regulated by such epigenetic changes as methylation and acetylation.20–22 These studies have demonstrated that, in the human MDR1 gene, the promoter is always hypermethylated in drug-sensitive cells, and drug-resistant cells have a hypomethylated MDR1 promoter. A study by Kantharidis et al showed that treatment of leukemia cell lines with decitabine resulted in demethylation of the MDR1 gene and resulted in the gene’s activation.23 In our study, based upon small numbers only, the seemingly lower response rate to AML induction after previous response to hypomethylating agents or lenalidomide during MDS phase could theoretically be because of emergence of secondary resistance.
Although the results of our study are interesting, the study does have several limitations. One of the criticisms of the study is a higher than expected CR rate for the cohort. CR was confirmed at our center, and only 7 of the 36 documented CRs were CRs with low platelet counts. The rest were full CRs per the International Working Group criteria. This could be partly explained by the small sample size of our study; however, this could also be partly explained by the finding that our institution is aggressive in treating AML, so that patients frequently undergo multiple induction regimens to obtain a CR. In our study, 19 of 61 patients received a second induction therapy with a high-dose cytarabine-containing combination. Another limitation is that this is a retrospective study. The retrospective nature of the study does not allow for definitive conclusions to be made; rather, it is more conducive to generating hypotheses for future prospective studies.
In conclusion, the results of this study suggest that patients with secondary AML who receive prior therapy for their MDS/myeloproliferative neoplasm may be better served by novel approaches rather than conventional induction chemotherapy. In addition, stratification for these risk factors should be considered in future clinical trials.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
These results were presented as a poster abstract at the American Society of Clinical Oncology Annual Meeting, Orlando, Florida, May 20, 2009.
REFERENCES
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; 2007. [Accessed November 2009]. Surveillance, Epidemiology, and End Results Program. Acute Myeloid Leukemia Incidence for 2000–2004. Available at: www.seer.cancer.gov. [Google Scholar]
- 3.Litzow MR. The therapy of relapsed acute leukaemia in adults. Blood Rev. 2004;18:39–63. doi: 10.1016/s0268-960x(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, O Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 Trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 7.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 8.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 9.Lancet JE, Willman CL, Bennett JM. Acute myelogenous leukemia and aging: clinical interactions. Hematol Oncol Clin North Am. 2000;14:251–267. doi: 10.1016/s0889-8588(05)70287-2. [DOI] [PubMed] [Google Scholar]
- 10.Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–1274. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 11.Rowe JM, Andersen JW, Mazza JJ, et al. A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490) Blood. 1995;86:457–462. [PubMed] [Google Scholar]
- 12.Kantarjian HM, O’Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 13.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 14.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 15.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58:1203–1212. [PubMed] [Google Scholar]
- 16.Swerdlow SH, Campo E, Harris NL, et al. World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 17.Byrd JJC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- 19.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.List AF. Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia. Leukemia. 1996;10:937–942. [PubMed] [Google Scholar]
- 21.Desiderato L, Davey MW, Piper AA. Demethylation of the human MDR1 5-region accompanies activation of P-glycoprotein expression in a HL60 multidrug resistant subline. Somat Cell Mol Genet. 1997;23:391–400. doi: 10.1007/BF02673749. [DOI] [PubMed] [Google Scholar]
- 22.Kusaba H, Nakayama M, Harada T, et al. Maintenance of hypomethylation status and preferential expression of exogenous human MDR1/PGY1 gene in mouse L cells by YAC mediated transfer. Somat Cell Mol Genet. 1997;23:259–274. doi: 10.1007/BF02674417. [DOI] [PubMed] [Google Scholar]
- 23.Kantharidis P, El-Osta A, deSilva M, et al. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin Cancer Res. 1997;3:2025–2032. [PubMed] [Google Scholar]