Abstract
Rationale
Dopamine (DA) in the ventral striatum (VST) has long been implicated in addiction pathologies, yet its role in temporal decision-making is not well-understood.
Objectives
To determine if VST DA D2 receptor availability corresponds with greater impulsive choice in both non treatment-seeking alcoholics (NTS) and social drinkers (SD).
Methods
NTS subjects (n=10) and SD (n=13) received PET scans at baseline with the D2/D3 radioligand [11C]raclopride (RAC). Outside the scanner, subjects performed a delay discounting procedure with monetary rewards. RAC binding potential (BPND) was estimated voxelwise, and correlations were performed to test for relationships between VST BPND and delay discounting performance. Self-reported impulsivity was also tested for correlations with BPND.
Results
Across all subjects, greater impulsive choice for $20 correlated with lower BPND in the right VST. NTS showed greater impulsive choice than SD, and were more impulsive by self-report. Across all subjects, the capacity of larger rewards to reduce impulsive choice (the magnitude effect) correlated negatively (p= 0.028) with problematic alcohol use (AUDIT) scores. Self-reported impulsivity did not correlate with BPND in VST.
Conclusions
Preference for immediate reinforcement may reflect greater endogenous striatal DA or lower D2 number, or both. Alcoholic status did not mediate significant effects on VST BPND, suggesting minimal effects from alcohol exposure. The apparent lack of BPND correlation with self-reported impulsivity highlights the need for objective behavioral assays in the study of the neurochemical substrates of behavior. Finally, our results suggest that the magnitude effect may be more sensitive to alcohol-induced problems than single discounting measures.
Introduction
Alcoholism, and addiction in general, can be characterized as a pattern of impaired decision-making; specifically, one that selects immediate gratification over future adverse consequences. As a trans-disease phenomenon, steep temporal discounting (i.e., impulsive choice) appears to underlie addiction pathologies (for review, see Bickel et al, 2014). Human and animal studies implicate impulsive choice as a trait that precedes and predicts addiction behaviors. For example, preschool-age performance on a delay-of-reward paradigm predicted adult drug use 20 years later (Ayduk et al, 2000), and in adolescents, predicted abstinence in a smoking cessation program (Krishnan-Sarin et al, 2007). Selective breeding for an alcohol-preferring phenotype also selects for high impulsive choice, as demonstrated in independent lines of rats and mice (Oberlin and Grahame, 2009; Wilhelm and Mitchell, 2008). In non-selectively-bred animals, impulsive choice predicts later cocaine self-administration (Perry et al, 2005) and drug reward (amphetamine conditioned place preference; Yates et al, 2012). The neurological basis for impulsive choice is not well understood, but converging evidence suggests an important role for dopamine (DA).
Increasing synaptic DA with therapeutic doses of psychostimulants decreases impulsive choice in both humans (e.g., de Wit et al, 2002) and generally, in animals (Richards et al, 1999)1. However, psychostimulant modulation of impulsive choice appears to be biphasic, with low doses decreasing, and higher or prolonged doses increasing impulsive choice (Richards et al, 1999). Additionally, hyperdopaminergic states in humans are associated with more impulsive behavior (Grosset et al, 2006; Moore et al, 2014). This suggests the possibility that optimal DAergic tone is required for normal adaptive temporal discounting, such that too little or too much DA can result in a heightened preference for immediate rewards.
Positron emission tomography (PET) studies utilizing [11C]raclopride (RAC) indicate reduced striatal DA D2/D3 receptor binding in detoxified alcoholics compared to controls (e.g., Volkow et al, 1996). Additionally, non-alcoholic subjects with a familial risk for alcoholism showed higher BPND in the ventral striatum than subjects without familial risk, raising the possibility of a protective effect from high D2 receptor availability (Volkow et al, 2006). Together, these findings may reflect alcoholism risk conferred by lower D2 receptor availability. There is currently a dearth of information relating specific behavioral phenotypes that confer alcoholism risk with D2 availability. Given the robust association of impulsive choice with alcoholism, and of reduced RAC BPND with alcoholism, we hypothesized that greater impulsive choice would negatively correlate with baseline VST RAC BPND, such that preference for smaller immediate rewards will correspond with lower D2 receptor availability.
Materials and Methods
Subjects
All procedures were approved by the Indiana University Institutional Review Board, and all subjects signed informed consent prior to study procedures. Twenty-five subjects participated in all procedures reported herein. Subjects were recruited from the community and were right-handed drinkers in good self-reported physical and mental health. Subjects underwent an in-person structured interview that included the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA: Bucholz et al, 1994), and the Timeline Followback interview (90 day TLFB: Sobell et al, 1986) to quantify recent drinking. The Structured Clinical Diagnostic Interview for DSM-IV disorders (SCID) I Substance Use Disorders section (Module E) was used to screen for substance use disorders when illicit drug use was endorsed. The Alcohol Use Disorder Identification Test (AUDIT: Saunders et al, 1993) was used to assess drinking problem severity. Social drinkers (SD) did not meet DSM-IV criteria for either alcohol abuse or dependence. Nontreatment-seeking alcoholics (NTS) met DSM-IV criteria for alcohol dependence, and neither had received treatment for alcohol use disorders in the last 12 months, nor were interested in receiving treatment. Exclusionary criteria for both groups included: current or past dependence on illicit drugs; self-report of diagnoses of past or current major DSM-IV Axis I psychiatric disorders; history of neurological disease; head injury resulting in loss of consciousness for > 20 minutes; current use of psychotropic medications; or contraindication(s) for magnetic resonance imaging. Nicotine dependence and sporadic marijuana use (less than 2 “joints” per week) were permitted in both groups.
Procedure
The morning of the study day, tobacco smokers received a nicotine patch to prevent nicotine withdrawal (Yoder et al, 2012). Cigarette withdrawal was monitored periodically throughout the day with the Cigarette Withdrawal Scale. NTS subjects were screened periodically for alcohol withdrawal using the CIWA-Ar (Sullivan et al, 1989), with scores > 8 triggering formal evaluation for hospitalization. Subjects received an anatomic magnetic resonance imaging (MRI) scan in the morning and a baseline (“resting”) condition RAC-PET scan in the early afternoon. All subjects completed the Impulsiveness Questionnaire (I7: with Impulsiveness, Venturesomeness, and Empathy subscales; Eysenck et al, 1985) and the Sensation Seeking Scale (SSS-V: with Boredom Susceptibility, Disinhibition, Thrill/Adventure Seeking, and Experience Seeking subscales; Zuckerman et al, 1978). Shortly after the baseline RAC-PET scan, subjects performed the DD task outside the scanner environment (see below).
Delay discounting (DD)
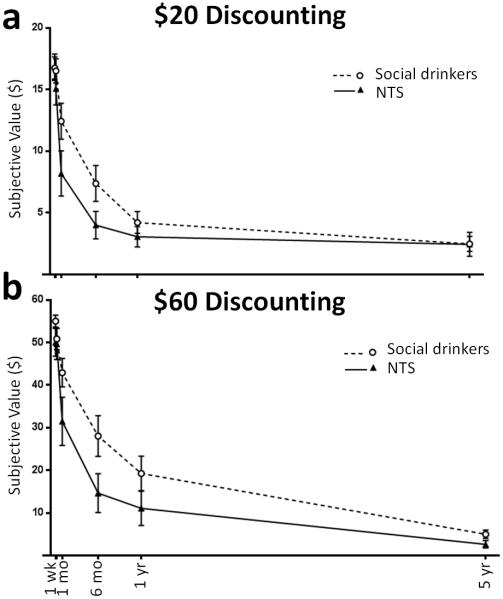
Subjects performed a delay discounting task for immediate or delayed monetary rewards in an adjusting-amount paradigm based on Du et al, (2002). After the baseline RAC-PET scan, subjects were seated in a comfortable chair in a patient room. The task and payment contingencies were verbally described, and a sample trial was shown to the subject on a laptop computer. Tasks were coded in E-Prime® software (Psychology Software Tools, Inc; Sharpsburg, PA), and the payment contingencies were explained. The verbal instructions included, “You should make every choice as though it is real, because one of your choices will be real. One choice will be selected at random by the computer and paid out according to your choice of amount and delay.” This approach is consistent with the commonly-employed strategy of random single trial reinforcement. Reward amounts of $20 and $60 were used with delays of 2 days, 1 week, 1 month, 6 months, 1 year, and 5 years. Every trial was presented as a binary choice in the form of, “Which would you prefer: $30 today OR $60 after 6 months”. For each delay, the amounts were initially presented with the delayed option of either $20 or $60, along with 50% of those amounts for the immediate option, i.e. $10 and $30, respectively. For every subsequent trial, the immediate amount adjusted up or down in halves based on the previous choice for that delay. In the current example, if the subject chose “$60 after 6 months”, the options for the next trial (within that amount/delay combination) would be “$45 today OR $60 after 6 months”. In this manner, the adjustment procedure converges on the point of indifference for a given delay and amount using a staircase method. Amount/delay combinations and the side of the screen that the options were presented on were pseudorandomized. The total trial number was 60; five trials for each of the six delays, duplicated for two amounts. The task took approximately 10 minutes to complete; choice behavior did not alter task length. In addition to a traditional analysis of indifference points at each delay, we also calculated area under the curve (AUC) for each subject at both amounts. This was derived from the resulting indifference points for the $20 and $60 tasks to quantify discounting for each amount (Figure 1), and permits capturing discounting behavior in a single, standardized metric. Use of AUC obviates concerns about theoretical model assumptions and yields normally distributed measures amenable to parametric testing (Myerson et al, 2001). AUC values could theoretically range from zero to one, with zero representing complete discounting (all immediate choice), and one representing no discounting (all delayed choice).
Fig. 1.
Delay discounting. (a) Social drinkers (n = 13, open circles) and nontreatment-seeking alcoholics (NTS; n = 10, filled triangles) discounted $20 or (b) $60 across a range of delays (2 day delay unlabeled). Group × Delay interaction demonstrated NTS’ preference for immediate monetary reward. Subjective value = mean indifference points ± SEM by Group.
Image Acquisition
A 3D magnetization prepared rapid acquisition gradient echo (MP-RAGE) magnetic resonance imaging (MRI) volume was acquired for all subjects using a Siemens 3T Trio-Tim (160 sagittal slices, 1.0 × 1.0 × 1.2 mm3 voxels, FOV = 256 × 256 mm, TR=2300 ms, TE = 2.91 ms, FA = 9°, duration 9:14) for structural coregistration with PET images. RAC synthesis was as described previously (Fei et al, 2004). RAC-PET scans were initiated with the IV infusion of 522 ± 44 MBq RAC (mass dose 0.14 ± 0.06 nmol/kg, mean ± SD) over 1.5 min; dynamic data were acquired for 50 min (Yoder et al, 2012). PET scans were acquired on a Siemens EXACT HR+ (3-D mode; septa retracted). Dynamic PET images were generated using Siemens manufacturer’s software for Fourier rebinning (FORE) and filtered backprojection algorithms, including corrections for attenuation, random coincidences, scattering, and dead time. Image processing and analyses utilized SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and were procedurally similar to that described previously (Oberlin et al, 2013; Yoder et al, 2012). Each subject’s anatomic MRI was used as a reference to which an early mean PET image (containing a mixture of blood flow and specific binding) was co-registered. To facilitate motion-correction, all PET frames were then co-registered to the early mean PET image (in native MRI space). After co-registration, rigid body realignment to the early mean PET image was applied to minimize spatial variance across frames and to evaluate residual motion. Each subject’s MRI was spatially transformed into Montreal Neurological Institute (MNI) space; the transformation parameters were applied to all motion-corrected dynamic PET data. All subsequent analyses were performed in MNI space. For each subject, a reference region was created from cerebellar gray matter, excluding the vermis. Time-activity curves for the cerebellar region were generated from dynamic RAC data using scripted commands (AFNI; http://afni.nimh.nih.gov/afni/). D2/D3 receptor availability was indexed by binding potential (BPND), operationally defined as the specifically bound RAC concentration relative to non-displaceable RAC concentration (Innis et al, 2007). BPND was estimated at each voxel within the striatum using the multilinear reference tissue model (MRTM2; Ichise et al, 2003), utilizing the cerebellar time-activity curve as the input function, and smoothed with an 8 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. The current investigation is limited to the striatum, as the signal to noise characteristics of RAC make interrogation of extrastriatal regions unreliable (Yoder et al, 2011).
Statistics
To detect group differences in discounting behavior, a mixed-effects ANOVA was performed with the between-subjects factor of Group (Social drinkers, NTS), and within-subjects factors of Amount ($20, $60) and Delay (2d, 1wk, 1mo, 6mos, 1yr, 5yrs). Individual indifference points were divided by their respective large delayed amounts ($20 or $60) and analyzed as a percentage of the theoretical maximum. Significant interactions were followed by Holm-Bonferroni corrected t-tests at each delay. The magnitude effect (difference in discounting of different amounts) was calculated for each subject by subtracting AUC $20 from AUC $60. To test for a significant magnitude effect across all subjects, a one-sample t-test relative to zero was used; group differences in magnitude effect were assessed with independent sample t-tests. One-sample t-tests were used within group to verify the presence of a significant magnitude effect. To determine relationships between monetary discounting and drinking behavior, AUC was correlated with drinking behavior (drinks per week, drinks per drinking day, and heavy drinking days per week). Pearson product-moment correlation was assessed between AUC $20, AUC $60, and magnitude effect and drinking behavior. Spearman’s rank-order was used to assess correlations between AUDIT and discounting measures, as AUDIT scores are ordinal. Personality measures were assessed for normality with the Lilliefors test; each measure was tested for group differences with independent samples t-test if normally-distributed, and the Mann-Whitney U test if non-normal. Non-imaging statistical procedures utilized SPSS v17.0 (IBM® Corp; Armonk, New York).
Voxel-wise group differences (t-test) in BPND and correlations between task performance and personality measures with BPND were evaluated with random effects models in SPM8. Discounting behavior was assessed for correlations with baseline BPND using a priori search regions of L and R VST (spatially-defined by Mawlawi et al, 2001). Exploratory analyses were also performed using search regions of L and R caudate, putamen, and pallidum (defined by AAL atlas; Tzourio-Mazoyer et al, 2002). All voxelwise tests used an uncorrected threshold of p < 0.01, and peak voxel significance was set to p < 0.05, corrected for family-wise error (pFWE) within the search region (Oberlin et al, 2013).
Results
Subjects
Drinking groups differed on all drinking measures ts(21) > 5.9, ps < 0.001, as well as number of smokers [c2(1, N = 23) = 5.1, p = 0.024], but not in age (p > 0.98, t-test) or group membership by sex or race (ps > 0.6, Chi-Square test; Table 1). NTS showed a trend of lower education, p = 0.063. Two subjects were excluded from all analyses for nonsystematic responding on the delay discounting task (i.e. failing criteria defined by Johnson and Bickel (2008) for both monetary amounts). Two other subjects’ imaging data were excluded for poor image quality. Therefore, the behavioral data include 23 subjects and the imaging data include 21 subjects.
Table 1.
Subject Characteristics.
|
Social Drinkers n=13
|
NTS n=10
|
|||||
|---|---|---|---|---|---|---|
| Mean ± (SD) | Range | n (%) | Mean ± (SD) | Range | n(%) | |
|
|
|
|||||
| Age | 33.2 (7.4) | 23-49 | 33.3 (6.8) | 23-41 | ||
| Male | - | - | 7 (54%) | - | - | 7 (70%) |
| Caucasian | - | - | 9 (69%) | - | - | 7 (70%) |
| Smokers | - | - | 3 (23%)+ | - | - | 7 (70%) |
| Education | 15.1 (1.9) | 12-18 | 13.3 (2.5) | 8-16 | ||
| Drinks/ weeka | 3.6 (3.6)* | 0-12 | 37.3 (12.6) | 17-55 | ||
| Drinks/ drinking daya | 2.0 (1.3)* | 0-5 | 8.3 (2.5) | 4-12 | ||
| Heavy drinking days/ weeka,b | 0.1 (0.1)* | 0-0.2 | 3.9 (1.5) | 2-6 | ||
| AUDITc | 4.3 (2.3)* | 1-10 | 19.6 (8.9) | 8-37 | ||
NTS = Non Treatment Seeking alcoholic.
From the Timeline Followback Interview.
Greater than 4 or 3 drinks per day for males or females, respectively.
Alcohol Use Disorder Identification Test.
p < 0.05,
ps < 0.001 between groups, ps > 0.05 for other comparisons.
Delay discounting behavior
Discounting by Group
ANOVA in the whole sample (n = 23) revealed main effects of Amount F(1,21) = 15.4, p = 0.001 and Delay F(5,105) = 162.0, p < 0.001, but not Group (p = 0.13). A significant interaction of Group × Delay was detected, F(5,105) = 2.6, p = 0.030, but t-tests between groups at individual indifference points did not reach significance when corrected for multiple comparisons (Figure 1 illustrates discounting by Amount).
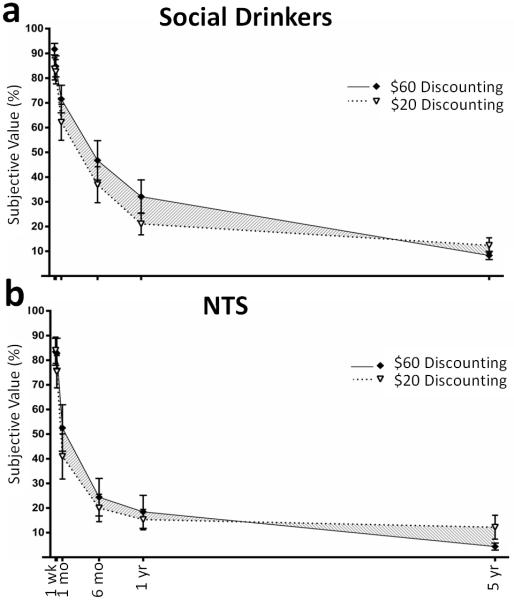
Magnitude effects
Social drinkers showed a positive magnitude effect t(12) = 2.5, p = 0.026, while NTS did not differ from zero (p > 0.6), means 0.047 ± 0.019 and −0.007 ± 0.016, respectively. The magnitude effect in social drinkers was significantly larger than that for NTS, t(21) = 2.14, p = 0.044; shown graphically in Figures 2a and b. There was a weak trend of magnitude effect across all subjects (p = 0.096).
Fig. 2.
Magnitude effects. Shading illustrates the difference in area under the curve between amounts. (a) Magnitude effects were detected in social drinkers (n = 13). (b) No magnitude effects were detected in non treatment seeking alcoholics (NTS; n = 10). y-axis: subjective value is shown as a percentage of the larger delayed amount. Delays are as Fig. 1.
Magnitude effects and drinking
The magnitude effect correlated negatively with drinking problem severity as measured by the AUDIT, ρ(21) = −0.46, p = 0.028. The range and distribution of AUDIT scores in all subjects is shown in Figure 3a. Larger scores corresponded with smaller differences in discounting by amount (Figure 3b). Importantly, this correlation was not driven by discounting in either single condition (AUC $20, AUC $60, or the mean of these), ps > 0.26. Discounting behavior, i.e., AUC $20 and $60, did not correlate directly with other drinking measures (ps > 0.07).
Fig. 3.
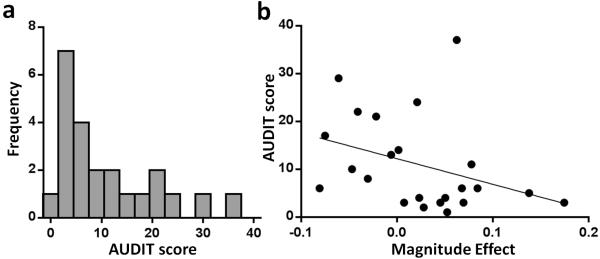
Histogram (a) Alcohol Use Disorders Identification Test (AUDIT) score distribution in all subjects (n = 23) spans virtually the entire range (0-40 possible). Correlation (b) The magnitude effect (illustrated in Fig. 2) negatively correlates with AUDIT; i.e. larger differences in discounting by amount corresponds with less alcohol-related problems.
PET Imaging; voxel-wise correlations with discounting
Baseline BPND positively correlated with AUC $20 (note that larger AUC means less impulsivity) in the R VST (n = 21, peak voxel [12 6 −8], Z = 2.62, pFWE = 0.044) and showed a trend-level correlation in the R anterior putamen, (pFWE = 0.071); the cluster of voxels showing correlation is shown in Figure 4a. This correlation means that greater impulsivity corresponds with lower RAC BPND. For illustrative purposes, extracted values within the correlation-defined cluster are shown in Figure 4b. There was no correlation between BPND and AUC $20 in caudate, but L posterior putamen showed a trend (pFWE = 0.064). AUC $60 did not show significant correlation in the VST search regions, although a 6-voxel cluster exceeding the p < 0.01 threshold was detected just posterior to the R VST search region (the ROI caudal boundary was y=6 and this cluster extended rostrally to y=4). Baseline BPND was not negatively correlated with either AUC $20 or $60 in any region.
Fig. 4.
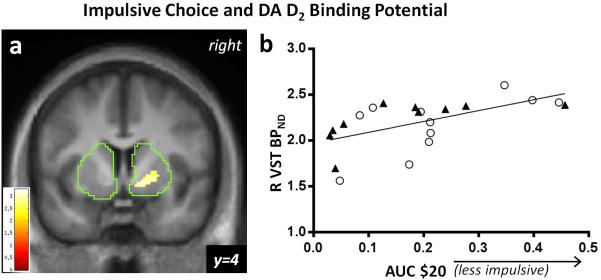
(a) [11C]raclopride baseline scan. Right ventral striatal (R VST) DA D2 binding potential (BPND) positively correlates with area under the curve (AUC) in choice behavior for $20 across delays in all subjects with scans (n = 21; pFWE < 0.05 corrected for R VST anatomical volume). The boundaries of the striatal mask are shown in green. The color bar indicates the voxel-wise t statistic; display threshold at p < 0.01, uncorrected. (b) The nature of the correlation is illustrated by mean BPND extracted values (threshold p < 0.01), indicating that increased binding potential corresponds with increased preference for delayed monetary rewards. NTS (n = 10, closed triangles), Social Drinkers (n = 11, open circles).
Personality measures
Results from all measures were normally distributed within Group (ps > 0.06) except I7:Venturesomeness and SSS-V:Thrill/Adventure Seeking; neither of which differed between Groups (Mann-Whitney U, ps > 0.6).
Impulsiveness
NTS endorsed higher Eysenck I7 Impulsiveness scores than social drinkers, t(21) = 3.0, p = 0.007. Means ± SEM were 11.4 ± 1.5 and 5.8 ± 1.1, respectively. The two other subscales did not differ (ps > 0.3).
Sensation seeking
NTS showed greater SSS-V Boredom Susceptibility than social drinkers, t(21) = 2.5, p = 0.019; 4.7 ± 0.9 and 2.2 ± 0.5, respectively. Disinhibition differed similarly, t(21) = 2.5, p = 0.020; 7.5 ± 0.7 and 4.8 ± 0.8, respectively. There were no group differences within the other two subscales (ps > 0.16).
Intercorrelations
AUC $20 did not correlate with the self-report measures of Impulsiveness, Boredom Susceptibility, or Disinhibiiton (ps > 0.6), but these self-report measures all positively correlated with each other, rs > 0.56, ps < 0.006.
PET Imaging; other analyses
A voxelwise t-test revealed no differences in baseline BPND between social drinkers and NTS in any region; t-test between smoking groups (ns = 10, 13 for smokers and nonsmokers, respectively) similarly failed to detect differences. To determine if group differences in personality measures were related to baseline BPND, voxelwise correlations were performed on the personality subscales that differed by group: Eysenck Impulsiveness, and Zuckerman Boredom Susceptibility and Disinhibition. These measures showed no positive or negative correlation in the a priori VST search regions. Exploratory analyses revealed a strong trend of positive correlation between Eysenck Impulsiveness and BPND in left posterior putamen (pFWE = 0.05), and a trend of positive correlation between Zuckerman Disinhibition and BPND in right anterior putamen (pFWE = 0.079).
Discussion
This study was intended to integrate two lines of inquiry important to alcohol research— impulsive choice and DA receptor availability in ventral striatum. To our knowledge, this is the first demonstration of a relationship between intertemporal decision-making and in vivo ventral striatal RAC binding. Our primary finding was a negative correlation between impulsive choice and DA D2 receptor availability in the right VST. That is, greater impulsive choice corresponded with lower DA receptor availability. We also corroborated the extant literature on differences in discounting behavior between alcoholic and social drinkers. Finally, we found an unexpected relationship between magnitude effects (decreased impulsivity by larger amounts) and alcohol drinking problems.
DA was strongly implicated in addiction when early studies suggested that the abuse liability of a drug may be related to its ability to provoke striatal DA release (Di Chiara and Imperato, 1988). More recently, however, striatal DA is known to be involved in signaling salience and several other cognitive and behavioral functions related to the acquisition and maintenance of addiction. Especially relevant to addiction processes is the incentive salience hypothesis, which proposes that drug-conditioned stimuli acquire enhanced motivational power that is largely gated by VST DA (for review, see Berridge, 2007). Presentation of cues previously paired with drug availability induces VST DA release (Weiss et al, 1993) and drug-seeking behavior (Chaudhri et al, 2008), which is consistent with the prominent role that drug-conditioned cues play in human relapse to addiction (O'Brien et al, 1990). In heavy drinkers, the flavor of a preferred drink, absent intoxication, is sufficient to induce VST DA release (Oberlin et al, 2013). In animals, enhancing VST DA increases cue-induced responding for rewards (Peciña and Berridge, 2013), while D2 antagonism reduces alcohol seeking and drinking (Czachowski et al, 2001), implicating VST DA in cue-reactivity and reward-seeking behavior. In that context, the impulsive choice of a stimulus representing available immediate reward is related to ventral striatal responding (for fMRI meta-analysis see Carter et al, 2010).
Individuals suffering from alcoholism have reduced striatal D2 availability compared to healthy controls (Martinez et al, 2005; Volkow et al, 1996). Given that alcoholism is associated with impaired impulse control, the generalized finding of lower D2 availability could be interpreted to suggest a negative correlation between D2 availability and impulsivity. Prior animal studies substantiate this idea. For example, D2 knockdown mice will eat normally when fed regular chow, yet when exposed to a highly rewarding diet for > 40 days, engage in compulsive eating (resistant to punishment), and show increases in brain stimulation reward thresholds (Johnson and Kenny, 2010). Similarly, subjects with lower VST D2 receptor availability reported greater craving to alcohol images (Heinz et al, 2004). These findings suggest a mechanism by which lower D2 availability could interact with exposure to highly salient rewards to produce addiction-like behaviors. If D2 availability is indeed a risk factor for development of addiction disorders (as suggested by Volkow et al, 2006), then it is also possible that the dopamine system may mediate errant signaling that improperly modulates salience attribution and impulsive choice with respect to rewarding stimuli. A parsimonious summary of the large body of literature on DA and incentive salience could then be that aberrant DA signaling corresponds to the formation and manifestation of addiction-related behaviors. Within the framework of incentive salience, one could postulate that an immediate reward may take on heightened, or even pathological, significance with aberrant ventral striatal dopaminergic tone.
Pharmacological studies indicate that the effect of DA on intertemporal choice may depend on particular experimental and subject factors (see footnote in Introduction) as well as dose of the dopaminergic agent. Hyperdopaminergic states can result in behaviors analogous to impulsive choice. Among Parkinson’s patients being treated with DA agonists (pramipexole, ropinirole, pergolide), rates of compulsive gambling increased to over ten times the typical rate in the U.S. (4.4% and 0.42%, respectively; Grosset et al, 2006). A large retrospective disproportionality study found pathological gambling, hypersexuality, and compulsive shopping to be the three most-reported impulse control events following DA agonist intervention (Moore et al, 2014); importantly, these types of impulse control events were also observed in patients without Parkinson’s, e.g. restless legs syndrome patients. Given the robust increase in DA transmission over baseline that these agents provoke, it may be concluded that enhanced DA tone leads to increased impulsivity. In healthy subjects, L-dopa increases impulsive choice (Pine et al, 2010), in contrast to the effects of low-dose psychostimulants, which generally decrease impulsive choice (e.g., de Wit et al, 2002). These observations, although apparently discrepant, suggest a biphasic effect of DA on impulsivity. This idea comports with animal data suggesting that optimal intertemporal choice behavior is achieved with an intermediate DA tone (Richards et al, 1999).
The present data show that lower D2 availability is related to greater impulsive choice across both social drinkers and NTS groups. These results were obtained with estimates of baseline RAC BPND, which is sensitive to both D2 receptor number and D2 receptor occupancy by endogenous DA. Therefore we cannot know to what extent each parameter may drive our result. However, each of these possible mechanisms engender compelling explanations. For this discussion, we will assume a priori that impulsive choice is a proxy for addiction risk (e.g. Bickel et al, 2014). The first possibility, that greater impulsive choice corresponds with lower receptor number, is consistent with previous studies that demonstrated that reduced D2 receptor density also reduced reward sensitivity (Johnson et al, 2010). Insofar as rodents selectively bred for high alcohol preference can be regarded as a high impulsive phenotype, two independent strains of high drinkers show lower D2 receptor number in the nucleus accumbens (NAcc; part of what is measured in the human VST) than their low-drinking counterparts (McBride et al, 1993; Stefanini et al, 1992). Together with the human literature inferring that reductions in D2 availability correspond to propensity for addiction disorders, the current findings could be construed to support a ‘reward deficiency syndrome’ (Blum et al, 1996). The second possibility, that greater sensitivity to immediate reinforcement (and therefore impulsive choice) corresponds with higher endogenous DA, is supported by studies showing that DA efflux in the NAcc immediately precedes alcohol self-administration (Doyon et al, 2005) and that enhanced DA in the NAcc increased responding to reward-paired cues (Peciña et al, 2013). Enhanced NAcc DA responses to cue-associated stimuli may indicate the strength of salience attribution to the expected reward delivery, which, as above, could be an important substrate for engaging in impulsive choice. When this work is considered alongside human studies of hyperdopaminergia, which indicate that elevated DA states increase salience of immediate rewards (Grosset et al, 2006; Moore et al, 2014; Pine et al, 2010), the data are consistent with the incentive salience hypothesis, which directly implicates striatal DA in addiction behaviors. These differing explanations are not necessarily mutually exclusive; the balance of receptor number and endogenous DA may vary to different degrees across subjects that exhibit high impulsive choice— at the same time, they may both be true within the same subject, i.e. low D2 number and high endogenous DA within an individual.
Magnitude effects can be viewed as an interaction of intertemporal choice behavior and subjective time horizon, and as such, may be more sensitive to impulsive tendencies than simple discounting measures, such as DD assessment with a single delayed amount. Previous studies have detected magnitude effects in healthy populations (reviewed in Johnson and Bickel, 2002), suggesting that it is a normal, adaptive behavior to value a future that promises a higher degree of reinforcement relative to a future that promises less (i.e. discounting behavior favors the smaller immediate choice more often when the delayed reward is $20 vs. when it is $60). Our results in social drinkers comport with those existing findings. Prior work has detected magnitude effects in alcoholics/drug abusers (e.g., Kirby et al, 1999), however in the current study, we did not detect a magnitude effect in the NTS sample. This may be explained by the fact that the difference in the delayed amounts ($20 and $60) was not as large as in most discounting studies utilizing differing delayed amounts. Detection of magnitude effects varied slightly depending on method; i.e. use of AUC detected only a trend of magnitude effects in the sample as a whole, while use of ANOVA on scaled indifference points found a significant effect of Amount. This may be due to the greater weighting of indifference points at longer delays in AUC calculations, which is not present in ANOVA. Magnitude effects quantified as differences in AUC differed between groups, but more interestingly, the magnitude effect correlated with AUDIT scores across groups, such that larger amounts had less effect on discounting in those with more alcohol-related problems. Insensitivity to increases in future reward size in alcoholics suggests a generalized indifference to future rewards. While these findings should be replicated in a larger sample for greater confidence, a dearth of current information on the relationship of magnitude effects and addiction highlights this behavior as a trait of interest for future studies.
NTS subjects scored higher on Eysenck’s I7 Impulsiveness subscale than controls, consistent with previous work (e.g., Kirby et al, 1999). Unlike that study, however, self-reported Impulsiveness did not correlate with measured discounting behavior in the current sample. Self-reported impulsivity did correlate with other self-reported measures linked to risk (Boredom Susceptibility and Disinhibition), suggesting coherence with latent risk factors independent of behavioral measures. This finding aligns with a prior report of a principal component analysis in a large, heterogeneous sample that found coherence of self-reported impulsivity, and independence of impulsive choice (Meda et al, 2009). The current data further support the idea of an independent factor rooted in a biologically-based relationship between ventral striatal D2 availability and discounting, with no such relationship in self-reported impulsivity (although note the strong trend in left posterior putamen). Moreover, the lack of a group difference in D2 availability, even in the face of large group differences in drinking, suggests that the correlation between discounting behavior and D2 availability is not mediated by alcohol, per se. A potential explanation is that impulsive choice is a biological predisposition that preceded and/or contributed to the onset of alcoholic behavior, although longitudinal studies will be required to assess the relationship of preexisting impulsive choice, D2 availability, and later development of alcoholism.
Exploratory analyses in left posterior putamen revealed trend-level correlation between BPND and AUC $20 as well as Eysenck Impulsiveness. These trends suggest the possibility of an overlapping anatomical substrate where different dimensions of impulsivity converge that is independent of the right VST. Additional power will be required to address this intriguing possibility with confidence. Surprisingly, NTS and controls did not differ in resting BDND; these groups might be expected to differ based on previous studies with similar sample sizes (alcoholic n=11; Heinz et al, 2004; alcoholic n=10; Volkow et al, 1996). This discrepancy may be explained by our use of older alcoholic subjects— these studies utilized alcoholic subjects that averaged 44 years old, while NTS in the current sample averaged 33 years old. Previous RAC-PET studies indicate that D2 receptor availability declines with age (Rinne et al, 1993), an effect that is likely exacerbated by alcohol abuse. While AUC $60 did not reach the threshold of significance within the a priori search region, the same pattern of results was observed just caudal to the posterior boundary of the R VST ROI, suggesting a similar but slighter weaker relationship.
In summary, subjects who undervalue future rewards also show low striatal D2 receptor availability— a potential biomarker for maladaptive decision-making related to addiction risk. This could be related to higher endogenous striatal DA, lower D2 number, or both. Alcoholic subjects engaged in discounting behavior that was insensitive to increases in the amount of the future reward. Alcoholics’ indifference to larger future rewards may reflect a generalized undervaluation of the future. This tendency corresponded with alcohol problem severity in the current sample, and reinforces the concept that impulsive choice is a risk factor for alcohol use disorders. Finally, these data suggest that measured discounting behavior may be more directly related to dopaminergic function than self-reported impulsiveness, particularly in the right ventral striatum.
Acknowledgements
We gratefully acknowledge Kevin Perry, Wendy Territo, Michele Beal, Courtney Robbins (Dept. of Radiology and Imaging Sciences), for technical and regulatory assistance, as well as Dr. Jaroslaw Harezlak for statistical consultation. We also thank Dr. Mark Green, Dr. Qi-Huang Zheng, Barbara Glick-Wilson and Brandon Steele for radiochemical synthesis of [11C]raclopride. Supported by R01 AA018354 to KKY, T32 AA007462 to BGO, R01 AA017661 to DAK. Additionally supported by the Indiana Clinical and Translational Sciences Institute Clinical Research Center, UL1TR001108, NIH, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
The authors declare no conflict of interest.
Amphetamine’s ability to reduce impulsive choice in animal studies is known to be sensitive to the presence of a cue bridging the delay (Cardinal et al. 2000), order of delay presentation (Tanno et al., 2014), as well as sex, strain, and baseline impulsivity differences (Eubig et al., 2014, Huskinson et al., 2012, Krebs and Anderson, 2012). Some discrepancies in this literature are likely due to these varied factors across studies.
Authors maintain full control of all primary data and agree to review by journal upon request.
References
- Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol. 2000;79(5):776–792. doi: 10.1037//0022-3514.79.5.776. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine. 1996;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152(4):362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: a review. Journal of Neuroscience, Psychology, and Economics. 2010;3(1):27–45. [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008;64(3):203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25(10):1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93(6):1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Du W, Green L, Myerson J. Cross-cultural comparisons of discounting delayed and probabilistic rewards. The Psychological Record. 2002;52(4):479–492. [Google Scholar]
- Eubig PA, Noe TE, Floresco SB, Sable JJ, Schantz SL. Sex differences in response to amphetamine in adult Long-Evans rats performing a delay-discounting task. Pharmacol Biochem Behav. 2014;118:1–9. doi: 10.1016/j.pbb.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness, and empathy in adults. Person individ Diff. 1985;6(5):613–619. [Google Scholar]
- Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, et al. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synthetic Communications. 2004;34(10) [Google Scholar]
- Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J, et al. Problematic gambling on dopamine agonists: Not such a rarity. Mov Disord. 2006;21(12):2206–2208. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Krebs CA, Anderson KG. Strain differences in delay discounting between Lewis and Fischer 344 rats at baseline and following acute and chronic administration of d-amphetamine. Pharmacol Biochem Behav. 2012;101(3):403–416. doi: 10.1016/j.pbb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23(9):1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharmacol. 2008;16(3):264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Krebs CA, Anderson KG. Preference reversals and effects of D-amphetamine on delay discounting in rats. Behav Pharmacol. 2012;23(3):228–240. doi: 10.1097/FBP.0b013e32835342ed. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10(5):387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, et al. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20(5-6):390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Mattison DR. Reports of Pathological Gambling, Hypersexuality, and Compulsive Shopping Associated With Dopamine Receptor Agonist Drugs. JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2014.5262. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15(4):355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, et al. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38(9):1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33(7):1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178(2-3):193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146(4):432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, et al. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13(2):310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: utility for alcohol research. Addict Behav. 1986;11(2):149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stefanini E, Frau M, Garau MG, Garau B, Fadda F, Gessa GL. Alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol. 1992;27(2):127–130. [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology (Berl) 2014;231(1):85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20(9):1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7(7):705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. High impulsivity in rats predicts amphetamine conditioned place preference. Pharmacol Biochem Behav. 2012;100(3):370–376. doi: 10.1016/j.pbb.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, et al. Reliability of striatal [11C]raclopride binding in smokers wearing transdermal nicotine patches. Eur J Nucl Med Mol Imaging. 2012;39(2):220–225. doi: 10.1007/s00259-011-1965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Morris ED. Assessing dopaminergic neurotransmission with PET: basic theory and applications in alcohol research. Current Medical Imaging Reviews. 2011;7:118–124. [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46(1):139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]