Abstract
Following the recognition that hematopoietic stem cells improve the outcome of myocardial infarction in animal models, bone marrow mononuclear cells, CD34-positive cells and mesenchymal stromal cells have been introduced clinically. The intracoronary or intramyocardial injection of these cell classes has been shown to be safe and to produce a modest but significant enhancement in systolic function. However, the identification of resident cardiac stem cells in the human heart (hCSCs) has created great expectation concerning the potential implementation of this category of autologous cells for the management of the human disease. Although phase 1 clinical trials have been conducted with encouraging results, the search for the most powerful hCSC for myocardial regeneration is in its infancy. This manuscript discusses the efforts performed in our laboratory to characterize the critical biological variables that define the growth reserve of hCSCs. Based on the theory of the immortal DNA template, we propose that stem cells retaining the old DNA represent one of the most powerful cells for myocardial regeneration. Similarly, the expression of insulin-like growth factor-1 receptors in hCSCs recognizes a cell phenotype with superior replicating reserve. However, the impressive recovery in ventricular hemodynamics and anatomy mediated by clonal hCSCs carrying the “mother” DNA underscores the clinical relevance of this hCSC class for the treatment of human heart failure.
Keywords: immortal DNA strand hypothesis, clonal expansion, IGF-1-IGF-1 receptor system, myocardial regeneration
Work performed in the last decade has challenged the generally accepted but never proven paradigm that the heart is a post-mitotic organ characterized by a predetermined number of parenchymal cells, which is defined at birth and is preserved throughout life till death of the organism. Several lines of evidence have been obtained in favor of the regeneration potential of the adult and failing myocardium. These results have offered a more biologically valid interpretation of the growth reserve of the decompensated heart and of its myocyte population. Activation of the components of the cell cycle machinery, BrdU incorporation, and expression of markers of cell replication, Cdc6, Ki67, MCM5 and cyclin B1, have been detected in cardiac myocytes. The impressive documentation of the mitotic spindle, with the bipolar shooting out of chromosomes, and the recognition of the contractile ring, as the last narrow bond between two dividing daughter cells, karyokinesis and cytokinesis, have unequivocally shown that cardiomyocyte replication occurs in the fully-developed mature heart.1-4 These observations have imposed a reinterpretation of the growth mechanisms of the myocardium, which has resulted in the identification of a compartment of resident multipotent cardiac stem cells (CSC).5-8 However, the search for the most powerful human CSC for myocardial regeneration is in its infancy, and this manuscript discusses the efforts performed in our laboratory to characterize the critical biological variables that define the growth reserve of this novel cardiac cell category.
Mechanisms of Stem Cell Division
The immortal DNA strand hypothesis advanced by John Cairns in 19759 raised the possibility that stem cell division is characterized by asymmetric segregation of chromatids so that one daughter cell contains only the old intact DNA templates and the other daughter cell contains chromatids composed exclusively of the newly synthesized DNA strands (Figure 1).10 The process of non-random segregation of DNA templates would attenuate the accumulation of spontaneous mutations9,11,12 and in the event that deleterious mutations have been acquired, stem cells would undergo replicative senescence and apoptosis13-16 having a reduced capacity to repair DNA damage.9,17,18 The recent reconsideration of the immortal strand theory12,19-28 has promoted intense debate in the scientific community9,11,29-31 adding a new level of complexity to the recognition and understanding of stem cell function in adult solid organs.
Figure 1.

Schematic representation of DNA segregation with stem cell division. A, With asymmetric chromatid segregation, one dividing mother stem cell (DNA strands, blue) synthesizes new DNA (red) during S-phase. The two sets of chromosomes are separated in anaphase and then the two daughter stem cells are generated, one carrying only the mother DNA (blue) and the other only the newly-synthesized DNA (red). In each chromosome, the two sister chromatids are held together at their centromere (green dot). B, With symmetric chromatid segregation, one dividing mother stem cell (DNA strands, blue) synthesizes new DNA (red) during S-phase and generates two daughter stem cells, each carrying the mother DNA (blue) and the newly-synthesized DNA (red). (Adapted from Kajstura et al, Circ Res 2012). Ref 10
If Cairns' hypothesis is correct, telomeric shortening dictated by DNA replication would affect only partly the actual stem cells retaining immortal strands. Telomere attrition would be largely restricted to the newly synthesized strands when they become templates in subsequent descendants.20,21 Additionally, the validity of the long-term label-retaining assay, employed for the identification of stem cells in various organs, would be problematic.32-37 This protocol is based on the notion that stem cells divide rarely and/or have a very long cell cycle time. Therefore, the long-term label-retaining property of a cell would document its stemness while the progressive dilution of the label would identify the generated progeny. However, this assumption would not be valid: “real” stem cells which incorporate bromodeoxyuridine (BrdU) would lose the labeled DNA by the second division,9-11,29 challenging the recognition and quantification of the stem cell compartment by this methodology (Figure 2). According to Cairns' model, chromosomes are segregated asymmetrically in a population of stem cells which represent the most primitive cell pool that controls cell turnover of organs in a steady state. Whether these progenitor cells are involved in rapid tissue repair following damage is uncertain; theoretically, these cells cannot divide symmetrically and each form two committed cells.
Figure 2.

One grandparent stem cell (DNA strands, blue) entering the cell cycle synthesizes new DNA during S-phase, incorporating BrdU (red); two parent stem cells are formed, each carrying the unlabeled grandparent DNA (blue) and the BrdU-labeled newly-synthesized DNA (red). Subsequent division of each parent stem cell results in the generation of one daughter stem cell, carrying the old, unlabeled grandparent DNA (blue), and one daughter stem cell, carrying only the newly-synthesized, BrdU-labeled DNA. Newly-synthesized unlabeled DNA (black). (Adapted from Kajstura et al, Circ Res 2012).
As emphasized by Lansdorp,30 however, several questions have been raised concerning asymmetric segregation of chromatids during stem cell division:38-40 they include the extremely high number of DNA lesions occurring every day in both DNA strands which are successfully repaired;41 the low rate of stem cell turnover in organs such as the bone marrow and the gut;42,43 the non-primitive progenitor state of cells showing immortal strand segregation due to their high rate of division;30 the presence of epigenetic marks promoting in one sister chromatid, and suppressing in the other, the expression of selective genes;30 the impossibility to prevent telomeric shortening at the 5′ end of the immortal DNA template;30 and the lack of estimates of the fraction of cells showing asymmetric versus symmetric chromatid segregation.30,38-40 These variables impose a reevaluation of the strategies required for the identification, characterization and quantification of CSCs.
Based on the reconsideration of this modality of stem cell replication and its potential role in CSCs, the possibility exists that the loss of the most primitive stem cell compartment of the myocardium might be causally related to organ and organism aging and lifespan, and to the onset of ventricular dysfunction and its progression to end-stage heart failure (HF). The limits in the recovery process of the adult human heart remain undefined and the cellular processes that condition the progression of cardiac pathologies to overt HF have not been characterized yet. However, defects in progenitor cell function may determine the decompensated cardiac phenotype.13,15,44-47 On this premise, the role of symmetric stem cell division and fate, i.e., random DNA template segregation, and asymmetric stem cell division and fate, i.e., non-random DNA template segregation, needs to be determined to define whether changes in human CSC growth and differentiation contribute to the impairment in ventricular performance of the diseased heart. In both cases, fate refers to the destiny of the daughter cells and whether they retain an undifferentiated state or acquire a specialized cell phenotype.
Division of Human Cardiac Stem Cells
According to the immortal DNA strand hypothesis, cells carrying the old DNA function as stem cells and preserve the stem cell pool of the organ, while cells containing the newly synthesized DNA undergo lineage specification.9 Thus, asymmetric segregation of chromatids has been considered equivalent to asymmetric stem cell division. This view questions that symmetric division of stem cells may form two daughter stem cells or two daughter committed cells. However, human CSCs divide symmetrically and asymmetrically in vitro and in vivo generating daughter cells with identical or divergent fate.48 These patterns of human CSC division have been determined based on the localization of the cell fate determinants numb and α-adaptin, which condition the function of the Notch receptor critical for the pattern of stem cell replication and destiny of the daughter cells (Figure 3).24,36,49-52 Additionally, clonogenic growth with generation of a cluster of stem cells derived from division of individual parent cells would have to be reinterpreted. Only parent cells would be undifferentiated while all other clonal daughter cells would represent a committed progeny. Our data do not support this view.10 Cells within the clones only rarely express nuclear and cytoplasmic proteins of cardiac lineages, indicating the primitive state of clonal cells, rather than the commitment of the entire pool with the exception of the parent cell. These data suggest that human CSC behavior may be more dynamic than projected by Cairns' theory.
Figure 3.
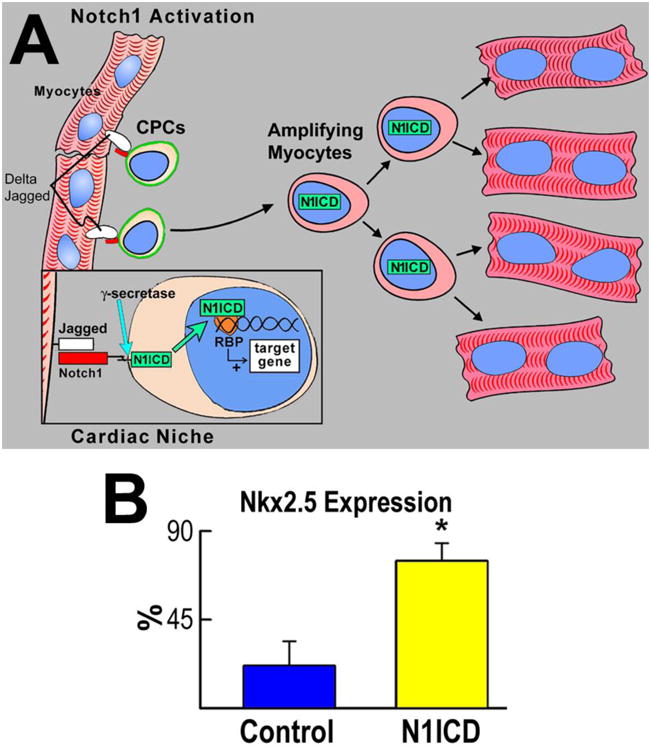

Notch inhibition induces a dilated myopathy. A, Schematic representation of the interaction between Jagged1 and Notch1 within the cardiac stem cell niches. B, Fraction of Nkx2.5-positive CSCs in the absence (control) and presence of N1ICD overexpression (N1ICD). Results are means±SD. *P<0.05. C and D, B-mode and M-mode echocardiography of vehicle (left) and γ-secretase inhibitor–injected (right) mice. E and F, γ-secretase inhibition led to ventricular dilation, depressed fractional shortening and ejection fraction, and increased mortality. G, With respect to a control heart (left panel), ventricular dilation and wall thinning are apparent in γ-secretase inhibitor–treated mice (central and right panels). H, Effects of γ-secretase inhibition on cardiac anatomy. Results are means±SD. *P<0.05.(Adapted from Urbanek et al, Circ Res 2010). Ref 52
The approach required to discriminate random from non-random DNA template segregation during human CSC division is complex; in vivo studies in humans cannot be performed and the primitive or partially committed state of progenitor cells cannot be definitely ascertained in any system in vivo.30,39 This limitation questions the accuracy and interpretation of in vivo results.53 If the immortal DNA strand hypothesis is correct, the ancient “grandparent” DNA cannot be targeted by exogenously delivered thymidine analogs and the co-existence of old and newly synthesized labeled “parent” DNA is lost in the second generation.12,19,24,29 making it impossible to follow in vivo the destiny of human CSCs carrying the immortal DNA (see Figure 2).
Although stem cell division by asymmetric chromatid segregation prevents the accumulation of mutation errors during DNA replication, eventually decreasing the incidence of cancer,11 the application of this concept to the heart is less clear. Whether human CSCs dividing by asymmetric chromatid segregation can eventually result in cancer formation has not been established, but this is an unlikely possibility. In fact, this mechanism of cell replication prevents defects in the DNA that might favor the acquisition of a tumorigenic stem cell phenotype. Malignant neoplasms are rare in the adult myocardium and this modality of stem cell duplication may be highly relevant if it reflects the preservation of the most powerful pool of cardiac progenitors for the homeostatic control of the organ and tissue repair following injury. If the theory is correct, the hypothesis may be advanced that non-random chromatid segregation during human CSC division may generate a daughter stem cell, which retains all the properties of the mother cell, and a daughter committed cell that forms a progeny of young functioning cells. In contrast, random chromatid segregation during human CSC division would not have a similar effect. The daughter stem cell and the daughter committed cell should lose in part their original growth and differentiation potential, triggering the process of cellular senescence. Therefore, the age of the progeny derived from activation, division and differentiation of human CSCs may vary whether the mother cell carries the old or the newly synthesized DNA.
Stem Cell Division and Myocardial Pathobiology
The implications of the immortal DNA strand theory of stem cell proliferation in the myocardium has been based on the premise that throughout the lifespan of the organ, human CSCs consist of two subsets of primitive cells, which divide according to the non-random and random segregation of chromatids, respectively. If human CSCs which retain immortal DNA strands are superior cells in terms of growth and differentiation, changes in the relative proportion of progenitor cell subsets may occur with aging and HF. Human CSCs carrying old DNA templates may predominate during the postnatal growth of the heart, but their number may progressively decrease with aging alone and together with cardiac diseases, attenuating with time the intrinsic growth reserve of the decompensated heart. This prospect has several important implications: a) it would support or question the validity of the immortal DNA strand hypothesis for the heart; b) it would support or question a biological and clinical role of the immortal DNA strand hypothesis for myocardial aging and HF; c) it would support or question the notion that the aging myopathy and HF are a stem cell disease; and d) it would support or question the view that the aging myopathy and HF may be partially reversed by activation of the residual compartment of human CSCs that have retained the old mother DNA.
Understanding how primitive cells decide to acquire a particular cell phenotype is of major relevance biologically and clinically.54 The fate of human CSCs may be dictated by their gene expression profile that may condition their preferential lineage specification, together with inductive signals from neighboring cells.7 The relevance of cell-to-cell interaction in the activation and differentiation of cardiac progenitors into mature myocytes has been well-documented.51,55-57 However, we cannot exclude that the acquisition of cell identity may be purely stochastic and independent from the environment and history of the cell.
Whether human CSCs divide by non-random and/or random segregation of chromatids can only be shown by clonal assay of BrdU tagged human CSCs (Figure 4). In fact, clonal cells formed by division of BrdU positive parent human CSCs carrying the immortal DNA will show only one BrdU-labeled cell (newly synthesized strands of parent DNA), while all other human CSCs in the clone will be BrdU-negative, being the descendants of the mother cell retaining the entire old DNA template. In contrast, clones formed by division of human CSCs with random segregation of chromatids will be composed of cells that are all BrdU-positive, although dilution of BrdU will occur with clonal expansion.10
Figure 4.

Schematic representation of clonal assay. Unlabeled grandparent human CSCs (hCSCs; DNA strands, blue), undergoing cell division in the presence of BrdU, generate parent hCSCs which incorporate the halogenated nucleotide in the newly synthesized DNA (red). BrdU-tagged parent hCSCs (one DNA strand, red) can divide by non-random and random segregation of chromatids, giving rise to two types of clones: one contains only one BrdU-positive hCSC (one red nucleus) and the other is formed by hCSCs all BrdU-positive (all red nuclei). (Adapted from Kajstura et al, Circ Res 2012).
This protocol has been applied and it has been able to underscore at the stem cell level, i.e., clonogenicity, that hCSCs derived from the same sample of human myocardium divide by non random or random chromatid segregation, strongly suggesting that the resident stem cell pool is composed of two cellular subsets with differential growth properties.10 The number of clonal cells is higher and the population doubling time is shorter in clonal cells carrying the mother DNA during replication. Additionally, dramatic differences have been found in terms of the number of senescent p16INK4a-positive human CSCs and fraction of dying apoptotic cells. These parameters were all in favor of a superior growth reserve of human CSCs carrying the immortal DNA (Figure 5).
Figure 5.

Growth characteristics of hCSCs. A, Clonal growth and population doubling time of hCSCs dividing by asymmetrical and symmetrical chromatid segregation, respectively. B, Rate of apoptosis and senescence in hCSC classes. Results are means±SD. *P<0.05 versus new DNA. (Adapted from Kajstura et al, Circ Res 2012).
Importantly, the BrdU-labeled cell in the clones generated by human CSCs dividing by asymmetric chromatid segregation was analyzed to determine possible alternatives to the immortal DNA strand hypothesis:10,30 a) the BrdU-positive human CSC may have reached replicative senescence and growth arrest early in the formation of the clone; b) the non-senescent BrdU-labeled sister cell may be responsible for the generation of the clone with dilution of BrdU, which became undetectable by immunolabeling; and c) the BrdU-positive human CSC may reflect a cell blocked in S-phase, due to replication errors and activation of the DNA repair machinery. To address these potential confounding variables, 40 clones, each containing one BrdU-positive human CSC, were stained for the senescence-associated protein p16INK4a that prevents permanently the reentry of stem cells into the cell cycle.10 In these clones, BrdU-positive and BrdU-negative cells did not express p16INK4a, excluding that the human CSC that inherited the newly-synthesized DNA reached growth arrest. With the exception of one BrdU-positive human CSC, these small clones were composed of BrdU-negative cells, indicating that an extreme level of dilution of BrdU did not occur in clonal cells with multiple divisions. Moreover, proteins indicative of DNA repair, p53 and ATM kinase,10 were absent in BrdU-positive cells, which showed a diploid DNA content. Taken together, these data affirm that division of human CSCs by asymmetric chromatid segregation does not involve acquisition of the senescent phenotype of the BrdU-labeled cell, excessive dilution of BrdU in dividing cells carrying the old DNA, or transient blockade in S-phase of the BrdU-positive human CSC undergoing DNA repair.
In vivo strategies can define whether asymmetric or symmetric stem cell division occurs,58 but this critical aspect of stem cell growth cannot be interpreted in the context of non-random or random chromatid segregation.39,59 Chromosome orientation-fluorescence in situ hybridization (CO-FISH) of metaphase spreads, or tissue sections, allows the discrimination of the pattern of DNA segregation at the single chromatid level,60,61 but the presence or absence of markers of stemness and commitment remains to be defined. This limitation applies to the multi-isotope imaging mass spectrometry method59 and to the partial asymmetric chromatid segregation shown in mouse cardiac progenitor cells.62
The mitotic spindle uses dynamic microtubules and mitotic motors to drive the movements that underlie “search and capture” of chromosomes, and their alignment and segregation.63 In an attempt to understand the molecular basis of the biased segregation of chromatids in human CSCs, the left-right dynein motor protein (LRD),64 which regulates the modality of cell division and embryonic left-right body axis asymmetry, was studied.10,65 LRD mRNA was highly expressed in human CSCs, and to a lesser extent in human myocardium (Figure 6).10
Figure 6.

Left-right dynein motor protein (LRD). Representative tracings of transcript for LRD in hCSCs and human myocardium (hMyo). PCR products had the correct molecular size. (Adapted from Kajstura et al, Circ Res 2012).
BrdU-labeled human CSCs were then transduced with a plasmid or lentivirus containing siRNA for LRD and EGFP, FACS-sorted for EGFP, and plated for clonal assay. Control human CSCs, transduced with scrambled siRNA, expressed normal levels of LRD. Nearly 5% of clones generated by control human CSCs showed a single BrdU bright cell, reflecting the expected fraction of human CSCs dividing by ACS. Conversely, downregulation of LRD in human CSCs led to clones formed almost exclusively by SCS, suggesting that LRD is implicated in asymmetric chromatid segregation during mitosis.10 Thus, these results provide evidence that the human heart possesses a pool of human CSCs that, during division, undergo asymmetric chromatid segregation, generating two daughter stem cells which retain the old and new DNA, respectively. This pattern of stem cell replication is conserved in subsequent divisions, preserving exponential cell growth and attenuating, partly, the processes implicated with replicative senescence and growth arrest. Selective partitioning of chromatids in cycling human CSCs may be regulated by the LRD protein, which is required for the biased segregation of the leading and lagging strands of the DNA in daughter cells.10
Stem Cells and Human Heart Failure
It is difficult to anticipate how aging, HF and the unpredictable path of cardiac diseases in the elderly influence human CSC function and myocardial reserve. Ischemic cardiomyopathy and hypertension are the major causes of chronic HF.66 However, myocardial infarction and high blood pressure lead to HF by mechanisms that are initially distinct and continue to vary during the progression of the pathologic state, although their differences may be attenuated in the terminal phases of cardiac decompensation in both animals and humans. Large myocardial infarcts result in acute ventricular dysfunction, while with smaller infarcts, the segmental loss of myocardium conditions chronic cardiac remodeling and the clinical course of the myopathy. The post-infarcted heart typically shows cavitary dilation, wall thinning, decreased systemic blood pressure and an increase in both diastolic and systolic wall stress.
Conversely, systemic hypertension is characterized by preservation of chamber volume with thickening of the wall. The hypertensive heart is faced initially by an increased systolic load only; diastolic dysfunction is a minor factor promoted by the increase in myocardial stiffness. Long-term, left ventricular end-diastolic pressure increases and chamber volume expands. Myocyte death affects clusters of myocytes and foci of replacement fibrosis together with interstitial fibrosis become apparent.66 Myocardial perfusion is impaired; minimal coronary vascular resistance increases and coronary reserve decreases. These abnormalities are prominent in hypertensive hypertrophy but are also detected in the post-infarcted heart. Valvular defects are commonly associated with a combination of pressure and volume overload and anatomical remodeling is dictated by the prevailing load. Complex is our understanding of how the diabetic myopathy and/or aging influences human CSCs;44,67 experimentally, oxidative stress is the major determinant of progenitor cell death and HF with uncontrolled diabetes.68-70
Ischemic heart disease, hypertension, diabetes and valvular defects are frequently associated with myocardial aging and HF. However, the phenotypic architecture and loading of the heart vary significantly among patients raising two challenging questions: a) is the human CSC compartment depleted in the decompensated heart or no matter how long and severe is HF a pool of functionally-competent progenitors is present in the myocardium and can be employed therapeutically? and b) is myocardial aging alone and in combination with cardiac pathology affecting more human CSCs which replicate retaining the old or the new DNA? These determinants may condition the duration of the disease and severity of the clinical manifestations. HF of genetic origin has to be excluded. Autologous human CSCs will carry the genetic defect and will differentiate in myocytes and coronary vessels with an abnormal phenotype. If present, the therapeutic efficacy of the expanded human CSCs will be short-term and limited in scope. To answer these challenging questions, we need to establish the biological and clinical import of human CSCs which divide by symmetric and asymmetric chromatid segregation in a variety of patients to define the complexity of the human disease. The broad inclusion of cases aims at the identification of human CSC function in each individual and, thereby, the recognition of the most effective progenitor cell pool for myocardial regeneration in each case. The objective is to advance the field of human CSC biology and introduce a unique analysis of the growth and differentiation properties of the patient's own human CSC classes. By this strategy, we may achieve a targeted, selective treatment of the patient's specific pathologic state.
Some data are available and strongly suggest that myocardial aging is characterized by a decrease in the percentage of clones derived from human CSCs carrying the old DNA and by an increase in the fraction of clones generated by human CSCs carrying the newly synthesized DNA (Figure 7A). Of great relevance, in hearts 1 to 14 years of age, telomere length varies from 6.0 to 12 kbp in both human CSC classes, regardless whether they divide by asymmetric or symmetric chromatid segregation (Figure 7B); however, in hearts 46 to 83 years old, telomere length ranges from 5.0 to 12 kbp and from 2.0 to 11 kbp in human CSCs carrying the old and newly synthesized DNA, respectively. More importantly, telomere length decreases linearly with age only in human CSCs dividing by symmetric chromatid segregation (Figure 7C); its preservation in human CSCs harboring the old DNA may reflect the protection of telomeric DNA at the 3′ chromosomal end during S-phase. Additionally, telomerase activity is comparable in these stem cell classes (Figure 7C). Epigenetic marks in the longer telomeres of human CSCs with old DNA may account for the more effective maintenance of chromosomal ends in this stem cell pool.30 This mechanism is operative in stem cells with high self-renewing potential, a property found in human CSCs harboring the old DNA. Thus, human CSCs dividing by asymmetric chromatid segregation constitute a stem cell pool with high degree of growth reserve and self-renewing ability, critical variables for effective cardiac homeostasis and repair.
Figure 7.



Growth properties of hCSCs. A, Clones derived from hCSCs carrying the old (red) and new (blue) DNA in various patients. Linear relationship between age and number of clones formed by hCSCs carrying the old (red) and new (blue) DNA. B, Distribution of telomere length in hCSC subsets; in hearts 46 to 83 years old (y), telomere length of hCSCs carrying the old DNA is shifted toward higher values. C, Telomere length decreases with age only in hCSCs carrying the new DNA. Telomerase activity in hCSC subsets measured by qPCR. Data are presented as individual values or as mean±SD. (Adapted from Kajstura et al, Circ Res 2012).
Stem Cells and Myocardial Regeneration
The discovery that hematopoietic stem cells (HSCs) can acquire cell lineages different from the organ of origin has started a scientific revolution.71,72 The behavior of HSCs has surprised and distressed many of us; they disobey the dogma of embryonic specification and undergo unexpected metamorphoses.73 However, to recompose a safe, comfortable, and orderly view, studies reporting negative findings and affirming the impossibility to reproduce published positive results have suddenly become very popular and have found easy hospitality in high impact journals.4 This confusion is feeding a fire that has divided the scientific community in supporters and opponents of cell therapy for the damaged heart. Despite the controversy, bone marrow mononuclear cells (BM-MNCs) have been implemented clinically, but rather than attenuating the debate this strategy has increased the dispute among physician scientists creating a significant level of uncertainty in the field.
Historically, the recognition, nearly 13 years ago, that c-kit-positive HSCs have the inherent ability to repair the infarcted myocardium in experimental models71 has profoundly affected cardiovascular research and clinical cardiology; c-kit is the receptor of stem cell factor. These observations raised the possibility that HSCs retain a remarkable degree of developmental plasticity being able to generate cardiomyocytes and coronary vessels, a process that seems to contrast with their assumed predetermined lineage specification. During prenatal life, stem cells undergo a hierarchical progressive restriction of developmental options, and this mechanism of embryonic determination was thought to be irreversible and inviolable in adulthood.1,73 The unanticipated plasticity of adult HSCs to form cells beyond their own tissue boundary has become the driving force of a series of clinical studies in which BM-MNCs have been introduced as an experimental therapy in the management of the acutely infarcted or chronically failing heart.74 Additionally, CD34-positive cells, and BM or adipose tissue-derived mesenchymal stem cells (MSCs) have been used clinically, in general with positive results, but the absence of benefit with these strategies has also been reported.75
Although a recent meta-analysis strongly supports the view that various classes of BM cells interfere with left ventricular dysfunction, infarct size, ventricular remodeling and mortality in patients with ischemic heart disease,74 the inconsistency in clinical outcome observed in some studies has attenuated the enthusiasm for this experimental therapeutic approach. Some trials showed no benefit while others have provided unequivocal beneficial outcomes.74,76 Several variables may be acknowledged in an attempt to reconcile these differences, but the simplest and most probable explanation could be related to the preparation and characteristics of the BM-MNCs used in these trials. Importantly, BM-MNCs should not be confused with HSCs; only an undetermined, minute number of cells in the BM-MNC pool may possess properties of HSCs. Experimentally, remarkable levels of myocardial regeneration after infarction have been obtained with HSCs, not with BM-MNCs.71,72,77
The concept that factors released from BM-MNCs or MSCs activate resident human CSCs, inducing indirectly cardiac repair, is an attractive possibility. This growth response, however, may expand the surviving myocardium where viable human CSCs are present.78 It is unlikely that the potential paracrine effect mediated by BM-MNCs or MSCs promotes the migration of human CSCs from the spared myocardium to the necrotic/scarred tissue, initiating a process capable of restoring the structural and functional integrity of the infarcted heart. Similarly, the recruitment of circulating progenitors is modest, at best, pointing to the delivered cells as the critical determinant of a successful clinical trial. A phase 3 clinical trial is ongoing in Europe with mortality as an end-point, and this study will answer definitely the question whether BM-MNCs have to be implemented in the management of human HF.
In addition to the plasticity of HSCs and their ability to regenerate damaged organs, the function of resident stem cells is to maintain tissue homeostasis in response to perturbations. The documentation of the existence of organ-specific adult stem cells has created great expectations concerning their utilization as a novel strategy for the treatment of the human disease.1-4 If the goal of medicine has been to treat symptoms of diseases and possibly remove their causes, scientists and physicians point now to a more ambitious target. The primary objective of regenerative medicine is the complete structural and functional recovery of the injured organ. Regeneration coincides with tissue homeostasis and involves the replacement of cells lost by normal wear and tear and, ideally, following injury. The regenerative capacity of organs is a property of particular significance in organisms with long lifespan; in fact, the preservation of the components of each tissue and their functional integration is essential for survival.
Damage creates a barrier to restitutio ad integrum and promotes the initiation of a repair process that leads to the formation of a scar. Scar formation is crucial for rapid handling of the damage, to seclude the lesion from healthy tissue and to prevent a cascade of uncontrolled deleterious events.1 However, the scar does not possess the biochemical, physical and functional properties of the uninjured tissue and, therefore, negatively affects the overall performance of the organ. To the best of our knowledge, the c-kit-positive human CSC is the only one which has been shown experimentally to replace scarred infarcted myocardium with functioning tissue composed of newly-formed cardiomyocytes and coronary vessels.15,79-81 This structural recovery restores in part the hemodynamic performance of the injured heart.
A dilemma is emerging in clinical cardiology. The human heart contains a pool of human CSCs that can be harvested from small samples of myocardium and, following their expansion in vitro, delivered back to patients by intracoronary infusion. This approach has been implemented in a phase I clinical trial comprising a cohort of 20 patients with chronic HF of ischemic origin;82,83 this study is in its stage of completion with encouraging results. Additionally, functionally-competent human CSCs for subsequent autologous delivery can be obtained from myocardial biopsies of patients with advanced HF, undergoing either cardiac transplantation or left ventricular assist device implantation.84 Thus, autologous human CSC therapy is feasible and can be considered for research evaluation in patients with advanced HF. The predicament is whether tissue-specific adult stem cells are superior, equally effective, or inferior to HSCs, BM-MNCs and MSCs. Cardiospheres contain a core of c-kit-positive human CSCs and their beneficial effects clinically85 may be largely dependent on the small pool of primitive cells delivered together with a large number of mesenchymal cells.
Human Cardiac Stem Cell Classes
Multiple variables interfere with the function of human CSCs; diabetes, aging and HF alone or in combination alter the stem cell compartment and affect negatively the growth of human CSCs. Moreover, the isolation and expansion of c-kit-positive human CSCs from small samples of human myocardium yields a heterogeneous cell population composed of stem cell subsets with a considerably different ability to divide and differentiate in vitro and in vivo. Based on clinical and animal data on aging and ischemic HF,15,86 the insulin-like growth factor (IGF) system and the renin-angiotensin system (RAS) have been defined in human CSCs.87 A series of in vitro and in vivo assays have been conducted to evaluate the independent and combined function of IGF-1 receptor (IGF-1R), IGF-2R and angiotensin type 1 receptor (AT1R) and their respective ligands in human CSC growth and repair capacity. The expression of IGF-1R in human CSCs recognizes a young cell phenotype characterized by long telomeres, high telomerase activity, enhanced cell proliferation and attenuated apoptosis. In addition to IGF-1, IGF-1R-positive-human CSCs secrete IGF-2 that promotes myocyte differentiation. Conversely, the presence of IGF-2R and AT1R, in the absence of IGF-1R, identified senescent human CSCs with impaired growth reserve and increased susceptibility to apoptosis. The expression of IGF-1, IGF-2, and IGF-1R decreased linearly with age, while the expression of IGF-2R, AT1R and angiotensin II (Ang II) increased with age (Figure 8A). Additionally, diabetes affected further the level of expression of IGF-2R and AT1R (Figure 8B). More importantly, IGF-1R-positive-human CSCs improve experimentally cardiomyogenesis and vasculogenesis, resulting in a significant potentiated recovery of the infarcted myocardium.87 Pretreatment of IGF-1R-positive-human CSCs with IGF-2 results in the formation of more mature myocytes and superior recovery of ventricular structure, pointing to this human CSC subset as an ideal candidate cell for the management of human HF.
Figure 8.


Effects of age and diabetes on hCSCs. A, Relationships of aging to receptor and ligand expression in hCSCs. B, Diabetes affected further IGF-2R and AT1R expression. *P<0.05 vs. non-diabetic patients. C, hCSCs with old DNA have a greater impact on left ventricular end-diastolic pressure (LVEDP), LV systolic pressure (LVSP), LV developed pressure (LVDP), positive and negative dP/dt, and calculated diastolic wall stress than hCSCs carrying the new DNA. (A and B, adapted from D'Amario et al, Circ Res 2011; C, adapted from Kajstura et al, Circ Res 2012)
Thus, the association of c-kit with distinct proteins on the membrane of human CSCs conditions functional differences within an apparently uniform cell compartment. The behavior of human CSCs is dictated by a specific surface phenotype which permits the selective isolation of young highly dividing human CSCs from the pool of c-kit-positive cells. Different membrane receptors affect the phenotypic plasticity of human CSCs and their ability to compensate myocyte loss by forming new efficiently contracting parenchymal cells. Additionally, the IGF-1-IGF-1R system in human CSCs provides critical information concerning the recovery of the myocardium following successful revascularization of patients suffering from chronic coronary artery disease.88 Negative left ventricular remodeling after coronary bypass surgery is not observed if the human CSC compartment, prior to surgery, retains a significant growth reserve. The decline in the replicative potential of human CSCs is paralleled by alterations in ventricular wall thickening, together with chamber dilation and reduction in left ventricular mass-to-chamber volume ratio. The expression of IGF-1R has dramatic effects on CSC division, survival, and in the generation of a differentiated specialized progeny.87,88 And the high correlation between human CSC growth and the size and shape of the heart strongly suggests that the behavior of human CSCs is implicated in the positive or negative outcome of the surgically treated ischemic cardiomyopathic heart.88
In a manner comparable to IGF-1R-positive human CSCs, human CSCs carrying the old DNA have long telomeres and generate a large pool of non-senescent cells. This high self-replicating potential exceeds significantly the growth of human CSCs that possess only the newly-synthesized DNA, making the former class of stem cells a more desirable progenitor for myocardial regeneration.10 When implemented in vivo, human CSCs with old DNA lead experimentally to a restoration of the infarcted myocardium, which is structurally and functionally superior to that induced by human CSCs with new DNA.10 The replacement of the entire infarcted region of the wall with newly-formed cardiomyocytes and coronary vessels has never been seen before with any cardiac and non-cardiac stem cells. The impressive recovery in ventricular hemodynamics (Figure 8C) and anatomy mediated by clonal human CSCs carrying the “mother” DNA underscores the importance of this stem cell category for the management of ischemic and non-ischemic HF.
Conclusion
In conclusion, IGF-1R-positive human CSCs and human CSCs carrying the old DNA represent important subsets of the complex pool of stem cells nested in the adult human myocardium. A direct comparison of the regenerative capacity of these two human CSC classes remains to be done, leaving unanswered the question whether human CSCs retaining the “immortal DNA” are superior, equal or inferior to IGF-1R-positive humahn CSCs in restoring the integrity of the injured myocardium. Future studies will resolve this conundrum, which may have critical implications in the understanding of the pathobiology of the decompensated heart and the search for the most powerful human CSC for the treatment of advanced HF.
Acknowledgments
This work was supported by grants from the NIH and American Heart Association.
References
- 1.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006;47:1769–76. doi: 10.1016/j.jacc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Kajstura J, Rota M, et al. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–61. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Unno K, Jain M, Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–63. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey D, Han L, Bauer M, et al. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–62. doi: 10.1161/CIRCRESAHA.112.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohsin S, Khan M, Nguyen J, et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–79. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairns J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc Natl Acad Sci U S A. 2002;99:10567–70. doi: 10.1073/pnas.162369899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajstura J, Bai Y, Cappetta D, et al. Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium. Circ Res. 2012;111:894–906. doi: 10.1161/CIRCRESAHA.112.273649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Cairns J. Cancer and the immortal strand hypothesis. Genetics. 2006;174:1069–72. doi: 10.1534/genetics.104.66886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–24. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 14.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez A, Rota M, Nurzynska D, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 16.Goichberg P, Chang J, Liao R, et al. Cardiac Stem Cells: Biology and Clinical Applications. Antioxid Redox Signaling. 2014 Apr 10; doi: 10.1089/ars.2014.5875. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–21. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 18.Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987;55:113–23. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merok JR, Lansita JA, Tunstead JR, et al. Cosegregation of chromosomes containing immortal DNA strands in cells that cycle with asymmetric stem cell kinetics. Cancer Res. 2002;62:6791–5. [PubMed] [Google Scholar]
- 20.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–8. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 21.Karpowicz P, Morshead C, Kam A, et al. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J Cell Biol. 2005;170:721–32. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rambhatla L, Ram-Mohan S, Cheng JJ, et al. Immortal DNA strand cosegregation requires p53/IMPDH-dependent asymmetric self-renewal associated with adult stem cells. Cancer Res. 2005;65:3155–61. doi: 10.1158/0008-5472.CAN-04-3161. [DOI] [PubMed] [Google Scholar]
- 23.Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–7. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- 24.Shinin V, Gayraud-Morel B, Gomes D, et al. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–87. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 25.Del Re DP, Sadoshima J. Enhancing the potential of cardiac progenitor cells: pushing forward with Pim-1. Circ Res. 2012;110:1154–6. doi: 10.1161/CIRCRESAHA.112.269183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charville GW, Rando TA. The mortal strand hypothesis: non-random chromosome inheritance and the biased segregation of damaged DNA. Sem Cell Dev Biol. 2013;24:653–60. doi: 10.1016/j.semcdb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadlapalli S, Yamashita YM. DNA asymmetry in stem cells - immortal or mortal? J Cell Sci. 2013;126:4069–76. doi: 10.1242/jcs.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh YH, Cohen J, Sherley JL. Higher 5-hydroxymethylcytosine identifies immortal DNA strand chromosomes in asymmetrically self-renewing distributed stem cells. Proc Natl Acad Sci U S A. 2013;110:16862–7. doi: 10.1073/pnas.1310323110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–43. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Lansdorp PM. Immortal strands? Give me a break Cell. 2007;129:1244–7. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Lew DJ, Burke DJ, Dutta A. The immortal strand hypothesis: how could it work? Cell. 2008;133:21–3. doi: 10.1016/j.cell.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Braun KM, Niemann C, Jensen UB, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 33.Braun KM, Watt FM. Epidermal label-retaining cells: background and recent applications. J Investig Dermatol Symp Proc. 2004;9:196–201. doi: 10.1111/j.1087-0024.2004.09313.x. [DOI] [PubMed] [Google Scholar]
- 34.Potten CS. Keratinocyte stem cells, label-retaining cells and possible genome protection mechanisms. J Investig Dermatol Symp Proc. 2004;9:183–95. doi: 10.1111/j.1087-0024.2004.09305.x. [DOI] [PubMed] [Google Scholar]
- 35.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth BW, Boulanger CA, Smith GH. Selective segregation of DNA strands persists in long-label-retaining mammary cells during pregnancy. Breast Cancer Res. 2008;10:R90. doi: 10.1186/bcr2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroki T, Murakami Y. Random segregation of DNA strands in epidermal basal cells. Jpn J Cancer Res. 1989;80:637–42. doi: 10.1111/j.1349-7006.1989.tb01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiel MJ, He S, Ashkenazi R, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–42. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waghmare SK, Bansal R, Lee J, et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–20. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffer P. The mutagenic potential of DNA double-strand break repair. Toxicol Lett. 1998;96-97:119–29. doi: 10.1016/s0378-4274(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 42.Lansdorp PM. Self-renewal of stem cells. Biol Blood Marrow Transplant. 1997;3:171–8. [PubMed] [Google Scholar]
- 43.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–64. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chimenti C, Kajstura J, Torella D, et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–13. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 45.Goichberg P, Kannappan R, Cimini M, et al. Age-associated defects in EphA2 signaling impair the migration of human cardiac progenitor cells. Circulation. 2013;128:2211–23. doi: 10.1161/CIRCULATIONAHA.113.004698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Olivetti G, Melissari M, Capasso JM, et al. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 47.Leri A, Franco S, Zacheo A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–9. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong W. Diversifying neural cells through order of birth and asymmetry of division. Neuron. 2003;37:11–4. doi: 10.1016/s0896-6273(02)01178-9. [DOI] [PubMed] [Google Scholar]
- 50.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 51.Boni A, Urbanek K, Nascimbene A, et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–34. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Urbanek K, Cabral-da-Silva MC, Ide-Iwata N, et al. Inhibition of notch1-dependent cardiomyogenesis leads to a dilated myopathy in the neonatal heart. Circ Res. 2010;107:429–41. doi: 10.1161/CIRCRESAHA.110.218487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–74. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 54.Wu JC. Molecular imaging: antidote to cardiac stem cell controversy. J Am Coll Cardiol. 2008;52:1661–4. doi: 10.1016/j.jacc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–71. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosoda T, Zheng H, Cabral-da-Silva M, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–96. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Ferreira-Martins J, Rondon-Clavo C, Tugal D, et al. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res. 2009;105:764–74. doi: 10.1161/CIRCRESAHA.109.206698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99. doi: 10.1101/gad.1850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinhauser ML, Bailey AP, Senyo SE, et al. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–9. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, et al. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–25. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 61.Falconer E, Chavez E, Henderson A, et al. Chromosome orientation fluorescence in situ hybridization to study sister chromatid segregation in vivo. Nat Protoc. 2010;5:1362–77. doi: 10.1038/nprot.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundararaman B, Avitabile D, Konstandin MH, et al. Asymmetric chromatid segregation in cardiac progenitor cells is enhanced by Pim-1 kinase. Circ Res. 2012;110:1169–73. doi: 10.1161/CIRCRESAHA.112.267716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunet S, Vernos I. Chromosome motors on the move. From motion to spindle checkpoint activity. EMBO Rep. 2001;2:669–73. doi: 10.1093/embo-reports/kve158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armakolas A, Klar AJ. Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science. 2007;315:100–1. doi: 10.1126/science.1129429. [DOI] [PubMed] [Google Scholar]
- 65.Sapienza C. Molecular biology. Do Watson and Crick motor from X to Z? Science. 2007;315:46–7. doi: 10.1126/science.1137587. [DOI] [PubMed] [Google Scholar]
- 66.Anversa P, Olivetti G. Handbook of Physiology, Section 2. New York, NY: Oxford University Press; 2002. The Cardiovascular System: The Heart; pp. 75–144. [Google Scholar]
- 67.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 68.Cesselli D, Jakoniuk I, Barlucchi L, et al. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89:279–86. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 69.Rota M, LeCapitaine N, Hosoda T, et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 70.Camici GG, Schiavoni M, Francia P, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–22. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leri A, Kajstura J, Anversa P. Identity deception: not a crime for a stem cell. Physiology. 2005;20:162–8. doi: 10.1152/physiol.00005.2005. [DOI] [PubMed] [Google Scholar]
- 74.Jeevanantham V, Butler M, Saad A, et al. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leri A, Kajstura J, Anversa P. Chapter 16 - Regenerative Therapy for Heart Failure. In: Sabatine EMAS, editor. Cardiovascular Therapeutics: A Companion to Braunwald's Heart Disease. Fourth. Philadelphia: W.B. Saunders; 2013. pp. 322–31. [Google Scholar]
- 76.Loughran JH, Chugh AR, Ismail I, et al. Stem cell therapy: promising treatment in heart failure? Curr Heart Fail Rep. 2013;10:73–80. doi: 10.1007/s11897-012-0128-2. [DOI] [PubMed] [Google Scholar]
- 77.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 78.Leri A, Anversa P. Stem cells: bone-marrow-derived cells and heart failure--the debate goes on. Nat Rev Cardiol. 2013;10:372–3. doi: 10.1038/nrcardio.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–16. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Padin-Iruegas ME, Misao Y, Davis ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–87. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D'Amario D, Fiorini C, Campbell PM, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–61. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Opie LH, Commerford PJ, Gersh BJ, et al. Controversies in ventricular remodelling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 87.D'Amario D, Cabral-Da-Silva MC, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–81. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.D'Amario D, Leone AM, Iaconelli A, et al. Growth properties of cardiac stem cells are a novel biomarker of patients' outcome after coronary bypass surgery. Circulation. 2014;129:157–72. doi: 10.1161/CIRCULATIONAHA.113.006591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


